Publisher’s version / Version de l'éditeur:
Materiaux et constructions. Materials and Structures, 1, 1, pp. 7-12, 1968-09-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Effect of organic surface agents on properties of hydrated cement
compacts
Hosek, J.; Sereda, P. J.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=0c7eff74-b3cc-4159-afd2-89cc2a58beed https://publications-cnrc.canada.ca/fra/voir/objet/?id=0c7eff74-b3cc-4159-afd2-89cc2a58beedEffect of organic surface agents on properties
of hydrated cement compacts
j.
HOSEK
( I )and
P. j. SEREDA
( 2 )RSSUMS
O n expose le traiternent d e cirnent Portland hydrate par des organosilanes, I'acide ol6ique e t I'octadecylamine, afin de r 6 d u i r e la s o r p t i o n d'eau e t les variations dirnensionnelles correspon- dantes. Les mesures d e ces variations dirnensionnelles e t de la rnicroduret6 o n t 6 t 6 faites sur des Qprouvettes de p o u d r e d e cirnent hydrate t r a i t 6 e t compact6, cornme modele de la p%te de c i m e n t hydrat6. II est apparu que les organochlorosilanes ne conviennent pas i cause de I'acide chlorhydrique p r o d u i t d u r a n t la rbaction, q u i entraine des effets secondaires ind6sirables. e t rndme, dans certains cas, augrnente I'adsorption d'eau e t I'expan- sion d u cirnent. Plus efficace, I'hexarnethyldisilazane r 6 d u i t I'adsorption de 10 a 20 % e t I'expansion d e 20 i 30 %. L'acide ol6ique agit i des concentrations en poids d e I ' o r d r e d e 25 %, rnais o n a constat6 u n e i m p o r t a n t e d i m i n u t i o n des propri6t6s m6caniques e t de la microduret6.
Hydrated portland cement consists mainly of colloi- dal-sized particles of hydrosilicates having a large surface area and a high surface-free energy. This characteristic results in the adsorption of a relatively large amount of water, with a corresponding large dimensional change for a given change in relative humidity.
From an engineering standpoint it is desirable that materials of construction have the lowest dimensional change with corresponding practical change in rela- tive humidity. There would b e great advantage if the dimensional instability of hydrated cement could b e reduced.
(I) Postdoctorate Fellow, lnorganic Building Materials Section, Division of Building Research, National Research Council of Canada.
(2) Head, lnorganic Building Materials Section, Division
of Building Research, National Research Council of Canada.
SUMMARY
This paper reports the treatment of bottle-hydrated portland cement with organosilanes, oleic acid and octadecylamine intended to reduce the sorption of water and the corresponding dimensional changes. Compacts were made from the treated hydrated cement powder to simulate cement paste and allow measurements of sorption-dimen- sional change characteristics and microhardness. I t was found that organochlorosilanes are not suitable for the treatment because the H C I released during the reaction produced undesirable side effects and i n some cases increased the adsorption and expansion of the cement. Hexamethyldisilazane was more effective, reducing adsorpt~on by 10 to 20 %. Oleic acid was effective a t concentrations as high as 25 % by weight, b u t serious loss i n mechanical property of micro- hardness was experienced.
Theoretically, it should b e possible to reduce the dimensional changes of hydrated cement by decreas- ing the surface area or by lowering the free energy. A reduction in the surface-free energy of silica compounds has been accomplished by their surface interaction with organosilanes [ l , 2, 3, 4 and 51. An attempt to achieve a similar result with hydrated cement compounds has been reported [6], but these were treated with organosilanes as additives during hydration.
The present work examines the possibility of reducing dimensional changes resulting from adsorp- tion of water by hydrated portland cement compacts by modifying the cement surface with organic surface agents. Two types of surface agents were investiga- ted : those that were absorbed on the surface to pro- vide a continuous coating, represented by oleic acid and octadecylarnine ; and organosilanes, which reac- ted with the surface to form an effective organic coating.
The use of compacts as a model of hydrated cement paste was essential because it enabled the easy contact of the agents with all of the surface of hydra- ted cement w h l e it was in the powdered state and in suspension in the solvent for the agent. By compaction the treated powder could b e formed into a porous body. Previous work [?I in this laboratory has demons- trated that this technique is successful.
For comparison of the efficiency of treatment, silica powder was used for some of the experiments, hydra- ted cement for the rest.
EXPERIMENTAL
Powdered hydrated cement was obtained by hydrating Type I portland cement (the same as was used for studies reported previously [TI) for four months in rotating polyethylene bottles at a water to cement ratio of 5 : 1. The silica was a commercial grade designated as Quso F-20 manufactured by Philadelphia Quartz Co.
In the preliminary work the powdered samples of hydrated cement were conditioned to either 0
%
rh. (magnesium perchlorate) or 30%
rh and then mixed for 48 hours in rotating polyethylene bottles with solutions of hexane containing various concentrations of the organic agents. The treated powder was filte- r e d , washed first with pure hexane and then with water, and dried to 30%
rh.Final tests involved hydrated cement powder, which was dried in a vacuum tube at a temperature of 1050 C prior to treatment with the organic agent in hexane solution. Figure 1 shows the experimental apparatus. For silica powder the sample was heated to 1800 C in the above apparatus.
V A C U U M O R G A N I C A D M I X T U R E I N H E X A N E S O L U T I O N F U R N A C E W I T H C O N T R O L L E D T E M P E R A T U R E H Y D R A T E D C E M E N T ( S I L I C A ) P O W D E R
FIG. 1. - Glass tube for the treatment of hydrated cement in vacuum at high temperature.
With hexamethyldisilazane, a 10
%
solution in hex- ane was used and the reaction temperature was 100 C. Two hours' reaction time was allowed with the cement. It was then washed with hexane and with water. Oleic acid was dissolved in hexane (concen- trations 5%
and 50%)
and mixed with the hydratedFIG. 2. - Apparatus for determining the concentration of organic additive.
cement powder for 68 hours in a dry box. Octadecy- lamine was dissolved in heptadecane (concentration 5
%)
and was also mixed with the hydrated cement powder for 68 hours in a dry box. Compacts measu- ring 3.125 cm in diameter and about 1.5 mm thick were made in a steel mould [8].The dry cement (conditioned to 30
%
rh), both treated and untreated, was compacted at a pressure of 1160 kg/cm\ the silica, both treated and untrea- ted, at a pressure of 560 kg/cm3. Although a number of organochlorosilanes, including trimethylchloro- silane, dimethyldichlorosilane, methyltrichlorosilane and ethyltrichlorosilane, were tried in the prelimi- nary experiments, only data for dimethyldichloro- silane a r e given, as representing the group, for comparison with the hexamethyldisilazane.Analyses for the amount of organic material added during treatment involved heating the sample at a temperature of 100 to 8000 C in the presence of a stream of oxygen and analysing the gases for CO, content by absorption in Ba(OH), solution. Excess Ba(OH), was determined by titration with HC1. Figure 2 shows the apparatus.
Samples prepared as compacts were used to mea- sure the adsorption and expansion isotherms. The adsorption isotherm was determined by exposing samples in desiccators to successively different rela- tive humidities and weighing them in a gloved box when equilibrium was established. Stirred solutions of sulphuric acid were used to obtain the desired relative humidity. To determine the expansion isotherm, samples were mounted on modified Tucker- man optical extensometers [9] and conditioned to different relative humidities in cells equipped with optical windows. The apparatus is shown in Figure 3.
I. HOSEK
-
P. 1. SEREDAFIG. 3. - Apparatus for measuring the dimensional changes with changes
in relative humidity.
'r
Microhardness was measured on a Tukon Hardness Tester using the Knoop indenter at 50
%
rh and a stream of C0,- free air was passed over the sample to prevent surface carbonation.RESULTS AND DISCUSSION
Surface reacting agents
-
Organochlorosilanes.In the preliminary work hydrated cement powder at a condition in equilibrium with 30
%
rh was treated with solutions of 1.5, 5 and 20%
dimethyldichloro- silane in hexane. The results of the adsorption and0 2 4 6 8 1 0 1 2 1 4 A D S O R P T I O N , P E R C E N T o H Y D R A T E D C E M E N T + 5 * % M e 2 S i C I 2 H Y D R A T E D C E M E N T + 1 . 5 * % M e 2 S i C I 2 H Y D R A T E D C E M E N T ( " C O N C E N T R A T I O N I N H E X A N E S O L U T I O N FIG. 4.
-
Effect of climethylclichlorosilane treatment.dimensional change measurements on compacts of samples treated as described are shown in figure 4. It may b e seen that treatment with the silane resulted in increased adsorption and a corresponding increase in dimensional change.
There are a number of possible explanations of the undesirable effect of the treatment. At 30
%
rh the hydrated cement contained about 4.5%
free water, which could react with the chlorosilane to form mono- mers, dimers, and polymers not attached to the sur- face and remaining as an inert admixture. This alone, however, should not give such adverse effects. The more likely reason may b e that the HC1, which is a product of the reaction of the chlorosilane with water or - OH groups of the surface, 1s left to react with thehydrated cement in the presence of free water and causes the break-up of the structure of tobermorite and the formation of CaC1,. Analyses of hydrated cement treated in this manner showed a chloride content as high as 2.8
%,
whereas before treatment it showed none.Similar undesirable results were obtained with hydrated cement dried over magnesium perchlorate and treated with a solution of 10
%
trimethylchlo- rosilane in hexane. This was further proof of the undesirable side effects of HC1 released when the reaction of the surface-
OH groups occurs with the organosilane. There is also the possibility that the primary reaction of the chlorosilane with the surface is destructive to the tobermorite structure.The above results showed that to achieve successful surface treatment of hydrated cement the sample shouId b e totally dry and the products of the surface reaction free of HC1. This excludes any of the organo- chlorosilanes.
Surface reacting agents-Organosilazanes.
Hexamethyldisilazane (CH,), SiNHSi(CH,), was selec- ted for treating the dry hydrated cement powder because it does not release any undesirable products
JANVIER-FEVRIER 1968
-
MATERIAUX E T C O N S T R U C T I O N S No 1-
J A N U A R Y - F E B R U A R Y 1968'such as HC1 on reaction with the
-
OH groups of the cement surface. The following reaction should result:-0-H
\
Si-Me /Me \Me hydratedcement
It is evident that hexamethyldisilazane reduces the adsorption of water on hydrated cement by 10 to 20
%
and the corresponding dimensional change by 20 to 30%.
This reduction, although significant, is small compared with what would b e expected if a complete surface coverage by the organosilazane were affected, and the normal surface containing-
OH groups and Ca++ ions would b e replaced by CH, groups.It is not possible to determine how much of the cement surface was covered by the organic material because the area covered by a single molecule is not known. Furthermore, the reaction with - OH groups may not result in close packing of the organic mole- cules, s o that water as single molecules in vapour phase could penetrate to the cement surface where surface
TABLE 1
Organic surface agents used to treat hydrated cement.
Treatment of hydrated cement with hexane solution containing 10
%
of the above agent resulted in the addition to the cement of 2.2%
by weight of the orga- nic component.The effects of this treatment on adsorption and dimensional change of hydrated cement is given in Table 11, along with results for treatment with oleic acid and octadecylamine.
the Ca++ ions would certainly b e exposed and might interact with water to form ions such as CaOH.5H20.
The above explanation seems to b e verified by the results of the treatment of silica powder with the same agent and compared with the results for hydrated cement. Figure 5 shows the effect of the treatment with hexamethyldisilazane on adsorption of water for hydrated cement and for silica. The presence
M. W. 129.1 160.2 282.5 269.5 Organic Compound Dimethyldichlorosilane Hexamethyldisilazane Oleic Acid Octadecylamine TABLE I1 a
Effect of organic surface agents on adsorption in hydrated cement. Chem. Formula (CH,),SiCl, (CH,),SiNHSi(CH,), CH,(CH,),CH = CH(CH,),COOH CH3(CH~)17NH2 TABLE I1 b
Effect of organic surface agents on dimensional changes in hydrated cement. Relative humidity p e r cent 0 15 30 50 75 100
Water adsorption p e r cent
Relative humidity p e r cent 0 15 30 50 75 100 Octadecylamine (5
%
solution) 0.0 2.30 3.30 4.30 5.80 14.9Dimensional changes p e r cent
Oleic acid 25.9
%
0.0 0.68 1.25 1.58 2.65 4.47 Oleic acid 4.1%
0.0 2.63 3.41 4.04 5.22 7.84 - Hydrated cement 0.0 3.27 4.35 5.23 8.25 25.4 Hydrated cement 0.0 0.252 0.334 0.376 0.456 0.732 Hexamethyldisilazane 2.2%
0.0 2.73 3.63 4.51 7.11 26.0 Oleic acid 25.9%
0.0 0.036 0.090 0.122 0.116 0.130 Octadecylamine (5%
solution) 0.0 0.125 0.175 0.220 0.295 0.490 Hexamethyldisilazane 2.2%
0.0 0.200 0.258 0.306 0.370 0.484 Oleic acid 4.1%
0.0 0.304 0.396 0.456 0.556 0.736H Y D R A T E D C E M E N T S I L I C A + + H E X A M E T H Y L - H E X A M E T H Y L D l S I L A Z A N E UNTREATEoD T R E A T E D D l S l L A Z A N o E T R E A T E D 0 15 30 5 0 75 0 15 30 5 0 75 100 R E L A T I V E H U M 1 D I T Y , P E R C E N T
FIG. 5. - Effect on water adsorption of hexnmethyldisilaznne treatment of hydrated cement and silica powder.
of Ca++ ions on the surface of the hydrated cement can account in part for the difference in the above results. The
-
OH groups may b e more easily avai- lable for reaction on the silica surface than on cement, resulting in closer packing of the organic agent after the reaction.Organic surface coating agents- Oleic acid and Octadecylamine.
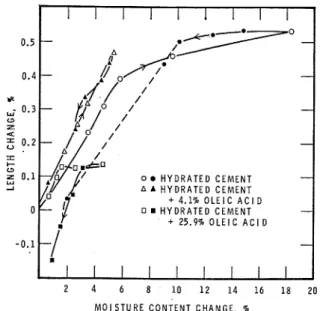
Treatment of the hydrated cement surface with hydrophobic materials such as oleic acid o r octadecy- lamine shows a reduction in adsorption of water, especially in the high end of the relative humidity as shown in Table 11. With oleic acid this reduction in adsorption does not correspond to a reduction in dimensional change unless a high concentration of the material is used. This comparison with samples of hydrated cement may not b e strictly valid because the treated material may have a different structure at particle to particle interface and the expansion may involve a modified mechanism. Figure 6 shows the effect of two concentrations of oleic acid, 4.1
%
2 4 6 8 10 12 14 16 18 20
M O l STURE CONTENT CHANGE, %
FIG. 6.
-
Effect of oleic acid treatment on sorption-dimensional change properties of hydrated cement.J. HOSEK
-
P.J. SEREDAand 25.9
%
by weight of the cement. It is presumed that an effective coverage of the cement is not achie- ved at a concentration of 4.1%
of oleic acid.From data given by Shaw [lo] the area covered by a molecule of oleic acid (vertical positioning) is 34.6 s q
-
and the corresponding theoretical concentration required for monolayer coverage of hydrated cement would b e 28.5%.
It is not likely, however, that a uniform close-packed monolayer of oleic acid would b e deposited on the surface of the hydrated cement. From the results presented in figure 6, it can b e seen that even 25%
oleic acid does not eliminate the sorption and dimensional change completely. The fact that the slopes of the length change to weight change curves a r e the same at the low relative humidity suggests that the surface remaining unco- vered by the oleic acid contributes totally to the dimensional change and that the coverage is not selective if there a r e sites of different specific free energy. It is expected that if the surface was comple- tely covered by oleic acid the dimensional change would b e very small when exposed to changes in water vapour pressure, but the concentration of oleic acid required to achieve this state is in excess of 25%
by weight.Microhardness measurements were used to indicate the effect upon the mechanical properties of the treatment of hydrated cement with oleic acid. Figure 7 shows the reduction of microhardness by a factor of about 2.5 when the concentration of oleic acid was 4.1
%
by weight. The samples having 25.9%
oleic acid were very weak and reliable measurements were not obtained.I I I I I I I I I
-
- T R E A T E D W I T H-
1 0 8 1 0 20 3 0 4 0 5 0 60 P O R O S I T Y , P E R C E N TFIG. 7. - Microhardness as a function of void fraction for treated and untreated hydrated cement compacts.
CONCLUSIONS
This work has demonstrated the usefulness of the compact technique in studying the effect on physi- cal and mechanical properties of organic surface reactive agents o r surface coating agents. All of the organic materials proposed for such use have large molecules, that would not penetrate the hydrated cement gel pore system. There is the possibility, however, that the physical and mechanical properties of the structure a r e altered because the organic
No 1
-
JANUARY-FEBRUARY 1968material finds itself in the bond area between the hydrated particles. This may b e a limitation in the technique.
The reaction of organochlorosilanes with the -OH groups on the cement surface or sorbed water produces HC1, which has a very undesirable side effect and prevents the useful results of such a treat- ment to b e realized. It is also very likely that the primary reaction of the surface with organochloro- silane may cause destruction of the tobermorite gel.
Use of hexamethyldisilazane appears more pro- mising in lowering the surface-free energy of hydra- ted cement, but the apparent large concentration required for significant reduction of dimensional change from sorption of water makes remote its prac- tical use. The above conclusion is equally applicable to the use of oleic acid or octadecylamine.
The results further suggest that small amounts of various surface agents have insignificant effect upon the surface character of the hydrated cement, indicating that most of the surface is playing a part in the sorption and the corresponding dimensional changes as seen by the slopes of these curves. If there are specific high energy sites on the cement surface then these are not selectively screened or have not reacted with the agents used.
It is also quite probable that the surface organic agents do not assume close packing to prevent water molecules from reacting with the cement surface. All the treated samples of hydrated cement exhibited a high degree of hydrophobicity with respect to liquid water, but were not efficient as barriers to the passage of water vapour.
ACKNOWLEDGMENT
The authors acknowledge with thanks the advice and assistance of the staff of Dow Corning Company, especially that of Dr. C. W. Lentz in the use of silicones. The help of Mr. T.F. Doherty in taking the experi- mental observations is gratefully appreciated.
This paper is a contribution from the Division of Building Research, National Research Council of Canada and is published with the approval of the Director of the Division.
REFERENCES
[I] Fox, H. W., P. W. TAYLOR and W. A. ZISMAN. - Polyorganosilanes. Industrial and Engineering Chemistry, 39, (1947), 1401-1409.
[2] TRAPESNIKOV, A. A., ET AL. - Monolayers of
polydimetylsiloxane polymers. DoMady Akademii Nauk SSSR, 160, No. 1, (1965), 174-177.
[3] BABKIN, I. Y. and A. V. KISELEV. - Adsorption and Heats of Adsorption of Various Vapours on a Trimetylsilylated Aerosil Surface. Russian Journal of Physical Chemistry (English Trans.) 36, No. 11, (1962), 1326-1331.
[4] SLINYAKOVA, I. B., M. F. KURKOVA, and I. E. NEI-
MARK. - Adsorption Properties of Mixed Gels of Metylsilanetriol and Silica Acid. Kolloidnyi Zhur- nal, 26, No. 4 (1964), 506-512.
[5] KOLSTOV, S. I.
-
Interaction of Trichlorosilane with Silica Gel. Zhurnal Prikladnoj Khimii, 38, No. 6, (1965), 1384.[6] MOSKVIN, B. M., V. G. BOTROKOV.
-
Beton i Zhelezobeton, No. 2, (1964), 51-56.[7] SEREDA, P. J., R. F. FELDMAN, and E. G. SWEN-
SON. -Effect of Sorbed Water on Some Mechanical
Properties of Hydrated Portland Cement Pastes and Compacts. Highway Research Board, Special Report 90 (1966), 58-73.
[8] SEREDA, P. J. and R. F. FELDMAN.
-
Compacts of Powdered Material as Porous Bodies for Use in Sorption Studies. Journal of Applied Chemistry, 13, No. 4, (1963), 150-158.[9] FELDMAN, R. F., P. J. SEREDA and V. S. RAMA-
CHANDRAN. - A Study of Length Changes of Com- pacts of Portland Cement onExposure to H , O . ~ i g h - way Research Record, No.62 (1964), Highway Rese- arch Board, Washington, D. C., U.S.A., 106-118. [lo] SHAW, D. J. - Introduction to Colloid and Surface