Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
American Ceramic Society Bulletin, 56, 4, pp. 424-427, 1977-04
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=3b7a02a5-1df3-4a5d-bbac-5f263a4b8c60
https://publications-cnrc.canada.ca/fra/voir/objet/?id=3b7a02a5-1df3-4a5d-bbac-5f263a4b8c60
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Impregnation of magnesium oxychloride cement with sulfur
Ser
TH1
N21d
National Research
Conseil national
c. 2no
'
735
1
+
Council Canada
de
recherches Canada
IMPREGNATION OF MAGNESIUM OXYCHLORIDE
CEMENT WITH SULFUR
by
J. J.
Beaudoin, V.S. Ramachandran and R.F. Feldman
4
Reprinted from
American Ceramic Society Bulletin
Vol.
56,
No. 4, April 1977
p.
424
-
427
BUILDING
RESEARCH
DBR Paper No. 735
Division of Building Research
SO MMAIRE
Une p l t e de ciment dloxychlorure de magndsium a kt6 imprCgn6e de soufre et expoede
h
lleau. La microduret6 et le module d161asticit6 dllchantillons non-impr6gn6s et impr6gnls ont 6t6 d6terminbs2
diff6rents temps dlexposition. Lee dchantillons imprQgn6s manifestaient des p e r t e s de force qui 6taient plus graduelles que celles dee 6chantillons non-impr6gn6s; c e s Cchantillons l o r s-
q u l i l s furent r6-impr6gnds ont montr6 une meilleure durabilitii 3 llexposition subskquente2
lleau. De tous lee bchantillons, c e w initialement expos68h
l l e a u et subs6quement imprdgn6e semblent avoir lea meilleures propridtks m6caniquee. Le module d16lasticitk de l a p t t e imprdgnle d e eoufre peut Btre d6terminb enutili- sant une r'egle simple de mllange. L o r s dlexposition3 lleau, la pPte de ciment dloxychlorure eat convertie
principalement e n Mg (OH)
Reprinted from the American Ceramic Society Bulletin, Vol. 56, No. 4 April 1977 Copyright 1977 by The American Ceramic Society
lmpregnation of
Magnesium Oxychloride
with Sulfur
Cement
Magnesium oxychloride cement paste was
impregnated with sulfur and exposed to wa-
ter. Microhardness and modulus of elasticity
of unimpregnated and impregnated samples
were determined at different times of expo-
sure. Impregnated samples had decreases
in strength which were more gradual than
JAMES J. BEAUDOIN,
those of the unimpregnated samples; these
VANGIPURAM S. RAMACHANDRAN*
' N ~ L Y Z E D
samples, when reimpregnated, showed bet-
and ROLF F. FELDMAN
ter durability to subsequent exposure to wa-
National Research Council of Canada, Ottawa, Ont.
ter. Of all the samples, those initially ex-
posed to water and subsequently impreg-
nated appear to have the best mechanical
M
For
agnesium oxychloride cement, discovered by Sore1 in 1867, has many properties superior to those of portland cement.' example, it has been shown that in situ hydrated oxychloride cement has larger values of modulus of elasticity, microhardness and compressive strength than does portland cement paste for a wide range of p o r o s i t i e ~ . ~ It was also found that when a compacted magnesium oxychloride cement body is leached with water at 8S°C, the residual body contained essentially Mg(OH), and was substan- tially stronger than portland cement paste or gypsum at the same porosity .2
The instability and loss in strength of oxychloride cement paste when exposed to water has prevented its widespread use. Recently, several studies of porous cement systems and their modification by impregnants have indicated that impregnation with sulfur improves the mechanical properties of cement paste cured at room tempera- ture as well as those of autoclaved cement-silica
preparation^.^ The
current surplus of sulfur in western Canada has created interest in its potential use and application in building materials technology. Malhotra has studied sulfur-infiltrated concrete and its potential application to precast concrete units.4 It appears that impregnating oxychloride cement with sulfur could result in a composite in which each component complements the other; oxychloride cement is not resistant to water whereas sulfur has a nonwetting property and is insoluble in water.Magnesium oxychloride cement has good nonflammability characteristics in contrast with sulfur. If magnesium oxychloride products in service are exposed to water, it is expected that a porous surface layer will result which, when impregnated with sulfur, will have increased strength and improved resistance to water penetra- tion. This paper describes the mechanical properties of oxychloride cement impregnated with sulfur and also the stability of the system when exposed to water.
Experimental
Materials
The commercial MgO powder* had the following characteristics: N, surface area, 20 m2/g; active CaO, 1.5%; ignition loss, 4%; fraction passing through 200 mesh, 98%. The material satisfied the requirements of ASTM C 275-61.
An aqueous solution of MgCl, having a specific gravity of 1.18 was prepared by dissolving MgCI,. 6 H 2 0 in a dry form with distil- led water.
The sulfur was reagent grade, containing 3 ppm H2S. Measurements
A helium pycnometer was used to measure the solid volume of
the cement specimens of known geometry. Porosity was determined from the apparent volume and the solid volume. This procedure
properties. Modulus of elasticity of sulfur-
impregnated paste can be determined using
a simple mixing rule. On exposure to water,
oxychloride cement paste converts mainly to
Mg(OH12.
avoids the problem of dissolution that arises when water is used as the displacement medium. Samples were conditioned at 11% rh for each measurement. Application of this technique to other inorganic cement systems is described e l ~ e w h e r e . ~ Residual porosity of sulfur impregnated samples was also determined.
Modulus of elasticity was measured on disks 3.2 cm in diameter and 1.3 mm thick. This procedure involves measuring the deflec- tion of a specimen when it is loaded at its center and supported at three points located on the circumference of a circle 2.5 cm in diameter.s Each value represents an average obtained for three specimens. The same disks used for measuring the modulus of elasticity were conditioned at 50% rh for microhardness determina- tion. Microhardness was measured with a commercial testing ma- chine.' Each microhardness value recorded is an average of three disks; five microhardness measurements were made on each disk. Differential thermograms were obtained using a thermal analysis sytem.3 Twenty milligrams of the material were used for each run. The rate of heating was 20°C/min.
The microstructure of some of the samples was examined on fractured surfaces.
Preparation of Specimens
Cement paste samples were prepared at MgC1,.6H20/Mg0 (solution:solid) ratios of 0.80 and 0.90 by weight. Mixes were cast in 5.1-cm cube molds and cured at 50% rh for 13 months. Disks were cut from cores obtained from the cube samples. The thin disks used for measurements of microhardness and modulus of elasticity reach equilibrium quickly with the surrounding environment. Mod- ulus of elasticity and microhardness were measured after each step given in Table I.
Impregnation
Samples were treated in one of the following ways: 1) immersed in water for 88 days, impregnated, then re-exposed to water (Series I, Table I); or 2) impregnated, immersed in water for 88 days,
Table I. Test Sequence for Measurement of Microhardness and Modulusof Elasticity of Magnesium Oxychloride Samples
Series
3
Test sequenceI 1 Unim~regnated
2 ~ x ~ o i e d ?o water
*Member, the American Ceramic Society. 3 Dried, then impregnated
*Supplied by Basic Chemicals, Cleveland, Ohio. 4 Re-exposed to water
tE. Leitz, Inc., Rockleigh. N.J.; indentor from VickersInstruments, Inc., Woburn, Mass.
$ W o n t 990, W o n t Co., Instrument Products Div.. Sorvall Operations, New-
n
1 town, Conn.§Stereoscan Mark ZA, Cambridge Instrument Co., Inc., Monsey, N.J. 2 3
Unimpregnated, dried and impregnated Exposed to water
Dried, then reimpregnated
4 Re-exposed to water
Y
0 SOLISOLID = 0.80
SOLISOLI D = 0.90
U
Y
111 James J Beaudoin Vangipuram S.
Ramachandran James J. Beaudoin is an as- sociate research officer with the Div. of Building Research, National Research Council in Ottawa, Ont., Canada. Dr. Beaudoin, who holds a B.A.Sc. and an M.A.Sc., earned his Ph.D. in civil engineering from the University of Windsor in 1970. He was then a ~ostdoc-
T I M E I N W A T E R , H
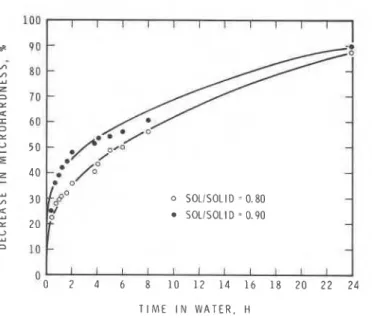
Fig. 1. Microhardness of magnesium oxychloride cement immersed in water at 22°C.
reimpregnated, then re-exposed to water (Series 11, Table I). Prior to impregnation, the samples were maintained at 128OC in vacuum for 24 h. They were then weighed and placed in another vacuum vessel with solid sulfur and evacuated for 1% h. This vessel was then placed in a 128°C bath for a minimum of 24 h. The sulfur melted and the samples became completely immersed in it. The samples were removed and the sulfur was allowed to solidify within the pores. A direct weighing after excess sulfur was removed from the surface with kerosine yielded the quantity of sulfur impreg- nated. . .- L7..:-,;.- p-:: :. .;: ...z:,
....
.. torate fellow at the university 1 . '.'. ..e2.:. .- ., , ...
ig;:.:
of Toronto until he joined NRCin 1972. His research at NRC
hk.37
has concerned the mechanicalRolf F Feldman properties of inorganic cemen-
titious systems.
Vangipuram S. Ramachandran is a senior research offi- cer at the National Research Council. He holds B.Sc., M.Sc. and Ph.D. degrees from Mysore, Banaras and Calcutta Universities, respectively, in India. Dr. Ramachandran joined NRC in 1968. His work involves the chemistry of cementitious materials as related to their physical and mechanical proper-
Results and Discussion
Effect of Water on Unirnpregnated Oxychloride
The percentage decrease in microhardness of unimpregnated oxychloride samples vs time of exposure in water is plotted in Fig. 1. The initial rate of decrease with time is rapid, with a 30 to 40% decrease in the first hour and a 55 to 60% decrease at 8 h. Samples having solution:solid= 0.90 undergo a more rapid decrease than those with solution:solid= 0.80. This is due to higher porosity of the sample formed at a higher solution:solid ratio. It can be expected that compressive strength would also indicate such changes, as rnicrohardness is linearly related to compressive strength for cemen- titious systems . 7
ties.
Rolf F. Feldman is a senior research officer at the National Research Council. He holds B.Sc., M.A.Sc., Dip. Chem. Tech. and D.Sc. degrees. In 1959 Dr. Feldman joined NRC, where his work involves the physical structure of hydrated portland cement paste, its mechanical properties and other factors pertinent to the strength and durability of portland cement concrete.
~iffeiential scanning calorimetric traces reveal that the samples
Fig. 2. Micrographs of magnesium oxychloride cement (A) not exposed to water and (B) immersed in water for 3 days.
Modulus of elasticity Miaohardness
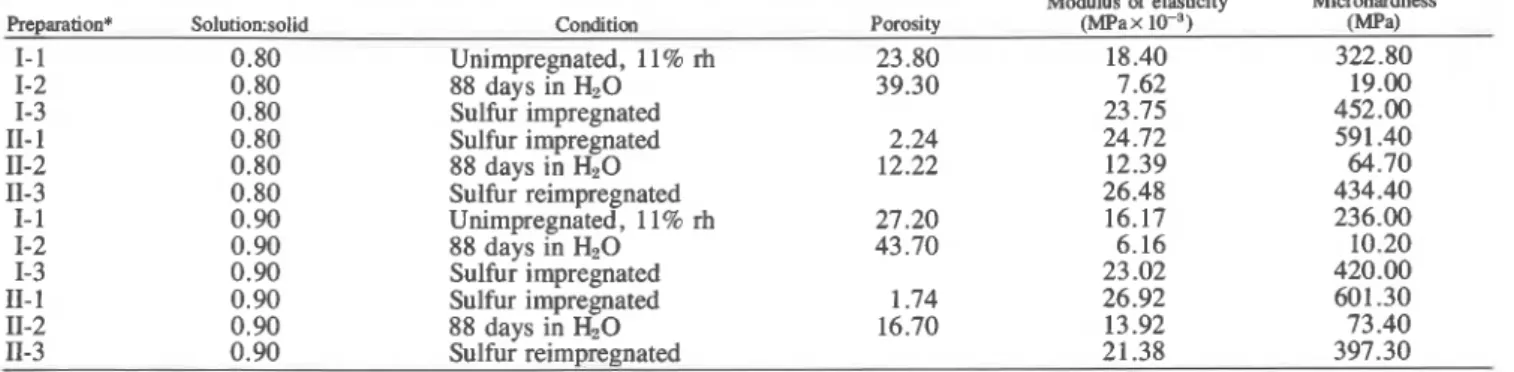
Preparation* So1ution:solid Condition Porosity (MF'ax ( m a )
I- 1 0.80 Unimpregnated, 11% rh 23.80 18.40 322.80 1-2 0.80 88 days in HzO 39.30 7.62 19.00 1-3 0.80 Sulfur impregnated 23.75 452.00 11- 1 0.80 Sulfur impregnated 2.24 24.72 591.40 11-2 0.80 88 days in HzO 12.22 12.39 64.70 11-3 0.80 Sulfur reimpregnated 26.48 434.40 I- 1 0.90 Unimpregnated, 1 1 % rh 27.20 16.17 236.00 1-2 0.90 88 days in HzO 43.70 6.16 10.20 1-3 0.90 Sulfur im~regnated 23.02 420.00 11- 1 0.90 Sulfur imirebated 1.74 26.92 601.30 11-2 0.90 88 days in HzO 16.70 13.92 73.40 11-3 0.90 Sulfur reimpregnated 21.38 397.30
*Series and step Nos. from Table I.
consist essentially of Mg(OH), after 24-h immersion in water and that the dissolution process is nearly complete within three days. Figures 2(A) and 2(B) are SEM's unimpregnated magnesium oxychloride. The sample shown in Fig. 2(A) has not been immersed in water while the sample in Fig. 2(B) has been immersed for three days. The sample which had been immersed in water is much more porous, has a platy morphology and large crystals. The sample which had not been immersed has a nondistinct or amorphous appearance (magnification,
x
lo4), and does not appear to have a &ity morphology unless it is hidden by a finely diiided product. X-ray diffraction results2 indicate that magnesium oxychloride samples contain large amounts of Mg(OH), a i d it appear; possible that the Mg(OH), is coated with a finely divided product. It is also suggested that some recrystallization may have occurred in this sample during immersion in water.Mechanical Properties of Impregnated Magnesium Oxychloride Cement
In Figure 3(A) microhardness of impregnated and unimpregnated oxychloride is plotted against time of exposure in water. The im- pregnated samples have initially higher values of microhardness
SOLISOLID =&so: UNIMPREGNATED
0 SOLISOLID = 0.90: UNIMPREGNATED
1
E' I' EI
5'rl
I .-¤ 1o J O 40 60 8 0 0 1 0 t n 30 1than do the unimpregnated samples. The decrease in microhardness after 88 days in water is less for the initially impregnated samples. The unimpregnated samples have very low values of microhard- ness after 88 days of exposure to water (10.2 and 19.0 MPa). As a result of test conditions 1-3 and 11-3 (Table I), there was a large increase in microhardness. Decreases in microhardness on re- exposure to water were considerably less than those decreases which occurred after initial exposure to water.
In Fig. 3(B) modulus of elasticity of impregnated and unimpregnated oxychloride is plotted against time of exposure in water. The impregnated samples have an initially higher modulus value than the unimpregnated ones and the decrease in modulus after 88 days in water is less for the impregnated samples. After completion of steps 1-3 and 11-3 (Table I), values of modulus of elasticity were larger. On re-exposure to water, step 11-3 samples had modulus values which decreased to the values obtained after 88 days initial exposure. However, step 1-3 samples had modulus values (obtained after a 28-day re-exposure to water) significantly higher than those measured after initial exposure to water (step 1-2).
Porosity, modulus of elasticity and microhardness data are tabu- lated in Table 11. The relative decrease in modulus of elasticity of
0 SOLISOLID = 0.90: IMPREGNATED SOLISOLID = 0.80; UNIMPREGNATED
0 SOLISOLID = 0.90: UNIMPREGNATED ,
T I M E I N W A T E R . D A Y S T I M E I N W A T E R . D A Y S
Fig. 3. Effect of sulfur impregnation on durability of magnesium oxychloride cement paste in t e n s of time in water at 22°C vs (A) microhardness and (B) modulus of elasticity.
fable Ill. Comparison of Measured and Calculated Values of Modulus of Elasticity for Various Preparations of Magnesium Oxychloride Cement
Measured E, E ~ = I I ( $ + ~ )
Preparation* Sample description Solution:solid ( x MPa)
( X MPa) 11- 1 Unimpregnated paste-imp. 0.80 24.72 30.50 11- 1 Unimpregnated paste-imp. 0.90 26.92 29.80 1-3 Unimp. paste 88 days in H 2 0 and impregnated 0.80 23.75 24.00 1-3 Unimp. paste 88 days in HzO and impregnated 0.90 23.02 22.90 11-3 I m p 3 8 8 days H 2 0 +reimpreg. 0.80 26.48 22.90 11-3 I m p 9 8 8 days HzO -reimpreg. 0.90 21.38 23.80
*Series and step Nos. from Table 1.
the unimpregnated oxychloride cement which has been soaked in water is smaller than the relative decrcdse in microhardness under the same condition. It has been &served previously for cement- silica systems that as a result of impregnation with sulfur, hardness values increased up to 500% and modulus of elasticity values increased only up to 300% .3
Soaking oxychloride cement in water is a dissolution process which creates pores. When those pores are filled with sulfur, sites of potential stress concentration at points of contact between particles are modified in a way similar to those of the portland cement-silica system, and a larger relative increase is to be expected for micro- hardness than for modulus of e l a s t i ~ i t y . ~ Also the linear relation between modulus of elasticity and microhardness for magnesium oxychloride cement2 shows that small changes in modulus of elas- ticity are associated with large changes in microhardness.
Previous work on mechanical properties of several portland cement-silica systems impregnated with sulfur showed that a simple mixture rule could be used to estimate the elastic modulus of a two-phase composite consisting of a cementitious matrix with sul- fur as an i m ~ r e g n a n t . ~ It was considered useful to ascertain whether this mixing rule would enable prediction of the elastic modulus of the oxychloride-sulfur system.
The following equation (based on Reuss' model) was used to estimate the modulus of elasticity of the oxychloride-sulfur system and was found to be in good agreement with the experimental results in Table 111:
where V, and V2 are the volume fractions of matrix and impregnant, respectively (the volume fraction of the impregnant is approxi- mately equal to the total porosity), and E,, and E,,, the zero porosity values of modulus of elasticity for matrix and impregnant materials. E,, was taken as 4 5 x lo3 MPa (Ref. 2) and E,, was taken as 1 3 . 9 ~ lo3 MPa (Ref. 9). Implicit in the use of Eq. (1) is that an adequate bond exists between the sulfur impregnant and the oxychloride matrix
A previous study3 found that impregnation of cement paste did not prevent reentry of water vapor and subsequent expansion re- sulted in the destruction of some preparations; a room temperature hydrated portland cement paste sample disintegrated after 12 to 15 h; a sulfur/porous-glass composite expanded rapidly and broke into small pieces after = 2 h exposure to water vapor.
In the oxychloride-sulfur system, the samples remained intact after exposure of 88 days. Upon reimpregnation and subsequent exposure to water for 28 days, the samples remained in good condition. This suggests the possibility that differences in pore size and structure between the water-soaked oxychloride-sulfur system and the portland cement paste-sulfur system may account for their different behavior in water; the surface area of magnesium oxy- chloride cement exposed to water is much less than that of portland
cement paste. It appears that sulfur has a potential use in overcom- ing the instability of oxychloride cement in the presence of water.
Conclusions
1) Impregnation of magnesium oxychloride cement with sulfur increases microhardness and modulus of elasticity. The decrease in these mechanical properties on exposure to water is less for impreg- nated oxychloride than for unimpregnated oxychloride.
2) Impregnation with sulfur of oxychloride cement which has been immersed in water or reimpregnation of initially impregnated oxychloride cement which has been exposed to water increases microhardness and modulus of elasticity. Durability of the compos- ite on subsequent exposure to water is greatly improved.
3) The modulus of elasticity of an oxychloride system which has been exposed to water and then impregnated with sulfur can be determined using an equation derived from Reuss' model for a two-phase composite.
4) The change in modulus of elasticity and microhardness as a result of water reentering the oxychloride-sulfur system is much less than the change in those properties when water reenters the cement paste-sulfur system.
5) A durable oxychloride cement-sulfur composite may be ob- tained by treating the oxychloride cement with hot water followed by impregnation: Impregnation after treatment in water appears to be superior to impregnation followed by treatment in water and reimpregnation.
Acknowledgments
The authors thank G. Aarts, J. Wood and G. Polomark for their assistance with the experimental work. This paper is a contribution from the Division of Building Research, National Research Council of Canada and is published with the approval of the director of the Division.
References
'S. Sorel, "On a New Magnesium Cement." Compr. Rend., 65, 102-04 (1867).
I. J. Beaudoin and V. S. Ramachandran, "Strength Development in Magnesium Oxychloride and Other Cements," Cem. Concr. Res., 5 [6] 617-30 (1975).
'
R. F. Feldman and I. I. Beaudoin, "Structure and Properties of Porous Cement Systems and Their Modification by Impregnants," presented at the Conference on Hydraulic Cement Pastes. 'Iheir Structure and Pmoerties. Universitv of Sheffield. ~lieftield, England, ~ ~ r i 1 . 8 - 9 , 1976.'
V . M. Malhotra, "Development of Sulfur-InfiltratedHigh-Strength Concrete," I .Am. Concr. Imt., 7 2 191 466-73 (1975).
R. F. Feldman. "Density and Porosity Studies of Hydrated Portland Cement,"
Cem. Technol., 3 [l] 5-14 (1972).
"P. J. Sereda, R. F. FeldmanandE. G . Swenson, "Effect of Sorbed Water on Some ..
- - .
Mechanical Roperties of Hydrated Portland Cement Pastes and Compacts;' Highw.
Res. Board, Spec. Rept. No. 90, pp. 58-73 (1%6).
'
3. J. Beaudoinand R. F. Feldman, "Mechanical Roperties of Autoclaved Calcium Silicate Systems," Cem. Concr. Res., 5 [2] 103- 18 (1975).R. F. Feldmanand J. J. Beaudoin, "Studies of Composites Made by Impregnation of Porous Bodies: I, Sulfur Impregnant in Portland Cement Systems," ibid., in press.