Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Cement and Concrete Research, 17, July 4, pp. 602-612, 1987-07-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=b02484f0-6e36-4ee7-b49a-ae01c2834bf5
https://publications-cnrc.canada.ca/fra/voir/objet/?id=b02484f0-6e36-4ee7-b49a-ae01c2834bf5
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1016/0008-8846(87)90133-5
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Diffusion measurements in cement paste by water replacement using
propan-2-ol
-
Ser
National Research
Conseil national
ITH1
Council Canada
de recherches Canada
N2ld
no.
1481
Institute for
lnstitut de
c. 2
Research in
recherche en
BLDG
Construction
construction
Diffusion Measurements in
Cement Paste by Water
Replacement Using Propan-2-01
by R.F. Feldman
Reprinted from
Cement and Concrete Research
Vol. 17, No. 4, 1987
p. 602
-
61 2
(IRC Paper No. 1481)
Price $3.00
On a mesurt! l e s c h a n g e m e n t s d e l o n g u e u r e t d e p o i d s d e p d t e s d e c i m e n t P o r t l a n d p r s p a r s e s d a n s d e s r a p p o r t s eau-ciment d e 0,3
3
0 , l e t immergges d a n s du propanol-2 e t du methanol. On a d6terminG l a d i f f u s i v i t s e t l e t a u x d e d i f f u s i o n , q u i o n t s t 6 compargs aux p a r a m P t r e s d e s t r u c t u r e d e s p o r e s mesurBs p a r i n t r u s i o n d e m e r c u r e . L e s m e s u r e s d e l o n g u e u r i n d i q u e n t q u e l e m g t h a n o l r 6 a g i t a v e c l e c i m e n t hydrati5 e t n e c o n v i e n t d o n c p a s p o u r m e s u r e r l a d i F E u s i v i t & . P a r c o n t r e , l e propanol-2 e s t r e l a t i v e m e n t i n e r t e e t p r o d u i t d e s v a l e u r s d e d i f f u s i v i t g s i m i l a i r e s 3 c e l l e s o b t e n u e s p o u r l e s i o n s c h l o r u r e d a n s d e s m a t e r i a u x s i m i l a i r e s .
CEMENT and CONCRETE RESEARCH. Vol. 17, pp. 602-612, 1987. P r i n t e d i n t h e USA. 0008-8846/87 $3.00+00. C o p y r i g h t ( c ) 1987 Pergamon J o u r n a l s , L t d .
DIFFUSION MEASUREMENTS I N CEMENT PASTE BY WATER REPLACEMENT USING PROPAN-2-OL
R.F. Feldman
I n s t i t u t e f o r Research i n C o n s t r u c t i o n N a t i o n a l Research Council Canada Ottawa, Ontario, Canada KIA OR6
(Communicated by F.H. W i ttmann) (Received March 20, 1987) ABSTRACT
Length and weight changes were measured when s a t u r a t e d p o r t l a n d cement pastes prepared a t water-cement r a t i o s o f 0.3 t o 1.0 were immersed i n b o t h propan-2-01 and methanol. D i f f u s i v i t i e s and d i f f u s i o n r a t e s were determined, and were c o r r e l a t e d w i t h p o r e s t r u c t u r e parameters, measured by mercury i n t r u s i o n . Length measurements i n d i c a t e t h a t methanol i n t e r a c t s w i t h t h e h y d r a t e d
i
cement and i s t h e r e b y u n s u i t a b l e f o r measuring d i f f u s i v i t i e s .I Propan-2-01 on t h e o t h e r hand i s r e l a t i v e l y i n e r t and produces
I
d i f f u s i v i t y values s i m i l a r t o t h o s e o b t a i n e d f o r c h l o r i d e i o n s i n s i m i l a r m a t e r i a l s .I
Most p h y s i c a l and e n g i n e e r i n g p r o p e r t i e s i n c l u d i n g durabi 1 i t y o f hardened cement pastes and concretes a r e r e l a t e d t o t h e i r m i c r o s t r u c t u r e
( 1 5 ) .
The p h y s i c a l p r o p e r t y most d i r e c t l y r e l a t e d t o d u r a b i l i t y and m i c r o s t r u c t u r e i s p e r m e a b i l i t y . Permeation may occur i n t h e l i q u i d s t a t e , which may i n v o l v e v a r i o u s ions, o r i n t h e gaseous s t a t e . I n many i n s t a n c e s t h e t r a n s p o r t mechanism i n v o l v e s d i f f u s i o n , eg. p e n e t r a t i o n of c h l o r i d e i o n s , and i s r e l a t e d t o pore s t r u c t u r e parameters, as i s p e r m e a b i l i t y (6).S p e c i f i c a t i o n s f o r c o n c r e t e have m o s t l y been based on 28-day
s t r e n g t h s b u t i t i s c l e a r where d u r a b l e h i g h - q u a l i t y c o n c r e t e i s t h e m a j o r concern, permeabi 1 i t y o r d i f f u s i v i t y becomes v e r y i m p o r t a n t .
Several techniques f o r measuring permeabi 1 i t y o r d i f f u s i v i t y have been used (6). However, some o f these techniques may i n v o l v e a p a r t i c u l a r form o f d r y i n g t h a t may unduly damage o r a l t e r t h e s t r u c t u r e of t h e pores, *even t o t h e p o i n t of causing c r a c k i n g (4). I n a d d i t i o n , t h e s p e c i f i c
t e c h n i q u e may n o t be r e l e v a n t t o t h e p a r t i c u l a r form o f m a t e r i a l a t t a c k t h a t t h e c o n c r e t e may be s u b j e c t e d t o .
P a r r o t t (7) showed t h a t several o r g a n i c s o l v e n t s c o u l d r e p l a c e a l a r g e p o r t i o n o f t h e water i n cement p a s t e and suggested t h a t t h e process o f exchange o f water from t h e s a t u r a t e d specimen was a simple p h y s i c a l process o f c o u n t e r d i f f u s i o n . He used methanol t o determine a v a l u e eTi
V o l . 17, No. 4 603 DIFFUSION, LENGTH, WEIGHT CHANGE, SOLVENT REPLACEMENT
t h e t i m e t a k e n t o exchange methanol w i t h h a l f t h e water p r e s e n t i n t h e paste, and used t h i s parameter as a measure o f t h e d i f f u s i v i t y of t h e specimen.
Other workers ( 8 ) measured t h e replacement o f water by methanol from c o n c r e t e and m o r t a r . These a u t h o r s considered t h e i r r e s u l t s
encouraging w i t h r e g a r d t o t h e development o f a t e s t which can i n d i c a t e t h e p o t e n t i a l d u r a b i l i t y o f a specimen. However, several workers (9-11) have suggested t h a t methanol r e a c t s w i t h b o t h Ca(OH)2 and CSH i n h y d r a t e d p a s t e t o form c a l c i u m methoxide o r a m e t h y l a t e d complex. Thus measurements u s i n g methanol may n o t y i e l d r e a l i s t i c values. Propan-2-01 does n o t r e a c t w i t h h y d r a t e d p a s t e and t h u s t h e use o f propan-2-01 as a s o l v e n t f o r d r y i n g s h o u l d be more a p p r o p r i a t e . This paper i n v e s t i g a t e s t h e use of propan-2-01 as a s o l v e n t t o measure t h e i n t r i n s i c d i f f u s i o n c o e f f i c i e n t s o f cement
i
pastes by c o u n t e r - d i f f u s i o n . ExperimentalM a t e r i a l s
Normal Type I p o r t l a n d cement was used. Pastes were prepared a t water/cement r a t i o s o f 0.3, 0.40, 0.5, 0.60, 0.80 and 1.0. A l l samples were cured i n s a t u r a t e d Ca(OH)2 s o l u t i o n f o r more t h a n 1 0 y e a r s except t h e sample prepared a t a water-cement r a t i o o f 0.5, which was cured f o r o n l y 1.4 years.
Reagent grade anhydrous methanol and propan-2-01 were used i n t h i s work.
P r o p e r t i e s
Pore s i z e d i s t r i b u t i o n (Hg p o r o s i m e t r y t o a p r e s s u r e o f 414 MPa) was determined on a1 1 p a s t e p r e p a r a t i o n s . Specimens were d r i e d by soaking pieces o f p a s t e 1.14 mn t h i c k i n anhydrous propan-2-01 f o r 10 days.
Samples were t h e n m a i n t a i n e d i n vacuum f o r a p p r o x i m a t e l y 17 h and t h e n h e a t e d f o r 24 h a t 38OC w h i l e b e i n g m a i n t a i n e d i n vacuum.
Evaporable and non-evaporable w a t e r c o n t e n t s were measured by d e t e r m i n i n g t h e l o s s i n weight between t h e s a t u r a t e d c o n d i t i o n and 100°C, and between 100°C and 1000°C u s i n g a Thermogravimetric Module o f t h e DuPont 1090 system.
Length change was measurzd u s i n g a m o d i f i e d Tuckerman o p t i c a l
s t r a i n gauge. Specimens o f each p a s t e were s l i c e d from t h e i r d i s c s (which had been c u t from c y l i n d e r s by a diamond saw) and were r e c t a n g u l a r ,
a p p r o x i m a t e l y 30 x 10 mm and u s u a l l y 1.14 mm t h i c k . The e f f e c t of t h i c k n e s s on l e n g t h change was a l s o i n v e s t i g a t e d by u s i n g a d d i t i o n a l specimens 2.33 and 3.45 mm t h i c k .
D i f f u s i o n Measurements
S l i c e s o f p a s t e c u t from s a t u r a t e d c y l i n d e r s 30 mm i n d i ameter were used t o measure t h e r a t e o f d i f f u s i o n o f propan-2-01 ( o r methanol) i n t o t h e pores. The s l i c e s were i n t h e form o f d i s c s g e n e r a l l y 1.14 mm t h i c k . The r d i s c s were wiped f r e e o f excess water j u s t b e f o r e weighing and were t h e n
immersed i n anhydrous a l c o h o l . The volume o f t h e a l c o h o l i n t h e
containment vessel (a d e s s i c c a t o r ) was l a r g e (1.5 l i t r e s ) compared t o t h e s i z e o f t h e specimen (25 g ) . Specimen weight was recorded a f t e r s u r f a c e d r y i n g and weighing every few minutes and t h e a l c o h o l was renewed a f t e r 1, 2, 4, 8 and 24 h, and t h e n every 24 h and l e s s f r e q u e n t l y a f t e r 7 days. Some measurements were t a k e n a f t e r 65 days o f immersion. S i m i l a r changes i n t h e i n t a k e o f a l c o h o l were made d u r i n g measurement o f l e n g t h change.
V o l . 17, No. 4
R. F. Feldman
R e s u l t s ( i ) Length Change
The r a t e s of change of l e n g t h o f w a t e r - s a t u r a t e d p a s t e specimens
(when immersed i n propan-2-01) a r e presented i n F i g u r e 1. These specimens
were prepared a t W/C r a t i o o f 0.8, and were 1.14, 2.33 and 3.45 mm t h i c k . The l e n g t h response i s a shrinkage and i t may be observed t h a t t h e r a t e of shrinkage i s g r e a t e s t f o r t h e t h i n n e s t sample a t t h e e a r l i e s t p e r i o d s p r o b a b l y because i t i s s u b j e c t e d t o l e s s e r r e s t r a i n i n g i n f l u e n c e t h a n t h e t h i c k e r specimens. The t h i c k e r specimens a f t e r 500 h o f immersion show l e s s l e n g t h change t h a n t h e sample o f 1.14 mm t h i c k n e s s a f t e r o n l y 6 0 h immersion. T H I C K N E S S 0 1. 1 4 rnv 2 . 3 3 rnm 0 3 . 4 5 rnrn I N M E T H A N O L ' ~ ' ~ ' ~ ' ~ ' ~ ' ~ ' ~ ' ~ ' ~ ' ~ ' ~ i ~ ' 0 4 0 80 120 160 2 0 0 2 4 0 2 8 0 3 7 0 3 6 0 4 0 0 4 4 0 4 8 0 5 2 0 T I M E , h o u r s
FIG.
1Length change versus t i m e on immersion o f water s a t u r a t e d p a s t e specimens i n propan-2-01.
The 1.14 mm t h i c k specimen was removed from t h e propan-1-01 a f t e r
140 h immersion and p l a c e d i n methanol. The l e n g t h change response was a
r e 1 a t i v e l y r a p i d expansion, a p p r o x i m a t e l y 0.11 per cent i n 10 h, compared t o a shrinkage o f 0.057 p e r c e n t i n t h e same time, when t h e water s a t u r a t e d
sample was immersed i n propan-2-01. A f t e r 360 h o f immersion t h e sample
had n o t r e - a t t a i n e d t h e l e n g t h when i n t h e w a t e r - s a t u r a t e d s t a t e , and had expanded t o a t o t a l o f a p p r o x i m a t e l y 0.14 p e r cent.
Samples prepared a t water-cement r a t i o s of 0.3, 0.4, 0.5, 0.6, 0.8
and 1.0 and immersed i n propan-1-01
,
showed shrinkages of 0.21, 0.137,0.173, 0.12, 0.14 and 0.16 p e r c e n t r e s p e c t i v e l y when steady s t a t e was a t t a i n e d a f t e r p e r i o d s o f immersion i n propan-2-01 v a r y i n g from 6 t o 10
days
.
Length change as a f u n c t i o n o f time' o f t h e w a t e r - s a t u r a t e d p a s t e 1 prepared a t water-cement r a t i o o f 0.8 (1.14 mm t h i c k ) on immersion i n
methanol i s presented i n F i g u r e 2. Length change i s p l o t t e d as a f u n c t i o n of t h e square r o o t o f immersion time, and t h e r e s u l t s o f propan-2-01
immersion a r e a l s o i n c l u d e d f o r comparison. The response t o methanol immersion i s an expansion which exceeds 0.10% a f t e r 32.5 h and appears t o be c o n t i n u i n g w h i l e t h e response t o propan-2-01 immersion i s a shrinkage which appears t o t e r m i n a t e a f t e r 100 h.
Vol. 17, No. 4 605 DIFFUSION, LENGTH, WEIGHT CHANGE, SOLVENT REPLACEMENT
( i i ) Weight change on immersion i n methanol o r propan-2-01.
The w a t e r - s a t u r a t e d specimen o f p a s t e l o s e s weight when immersed i n w a t e r - f r e e methanol o r propan-2-01. T h i s i s due t o t h e displacement o f t h e denser water by t h e a l c o h o l
.
A knowledge o f t h e d e n s i t i e s of t h e f l u i d s a l l o w s a c a l c u l a t i o n o f t h e q u a n t i t y o f a l c o h o l (Wt) d i f f u s i n g t h r o u g h t h e pores i n t o t h e specimen. The r e s u l t f o r t h e 1.14 mm t h i c k paste, prepared a t a water-cement r a t i o o f 0.8 i s presented i n F i g u r e 3. These a r e p l o t t e d as Wt/W, versus square r o o t o f time, where W, i s t h e value a t t a i n e d a t a p p r o x i m a t e l y equi 1 i b r i u m c o n d i t i o n s , a f t e r 240 h. The curves a r ea p p r o x i m a t e l y l i n e a r up t o 2.25 h f o r methanol and 6.25 h f o r propan-2-01, and i n d i c a t e d t h a t t h e i n i t i a l processes o f water replacement by t h e a1 coho1 s a r e d i f f u s i o n c o n t r o l l e d . 0 . 1 2 l , , , , , l p l l 1 1 I -
-*--*!*--I-.--i*-=*
0.08--
---
- - - /** se 0 . 04- 0 *'-
W / W I C = O . 8-
U-
0,
Z.'
< o< METHANOL-
I-
"o, 0 PROPAN-2-OL - U-
0-
Z -0.08'
- O - " 3-
- -0.12-
\
-
0-0-0 - o . l b A ' ~ l ~ l ~ ' ~ l l l l l l i l l 0 2 4 6 8 10 12 1 4 16 18 FIG. 2Length change versus ( t i m e ) & on immersion o f water s a t u r a t e d p a s t e specimens i n methanol and propan-2-01 r e s p e c t i v e l y
.
- -
-
METHANOL - O P R O P A N - 2 - O L --
--
1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 n " 7 - 4 6 8 1 0 1 2 1 4 16 1 8 TIME. m s Fig. 3 R e l a t i v e r a t e o f d i f f u s i o n (Wt/W,) o f propan-2-01 and methanol i n t o water- s a t u r a t e d paste.Vol. 17, No. 4 R.F. Feldman
A comparison o f F i g u r e s 2 and 3 shows t h a t when 90 p e r cent of t h e methanol has e n t e r e d t h e specimen, o n l y 0.03 per cent expansion o r l e s s t h a n 30 p e r c e n t o f t h e u l t i m a t e expansion has t a k e n place. On t h e o t h e r hand a t t h e same 1 eve1 o f e n t r y o f propan-2-01 a shrinkage o f 0.095 per c e n t o r 68 p e r c e n t o f t h e u l t i m a t e shrinkage has t a k e n place.
( i i i ) Rate o f d i f f u s i o n o f propan-2-01 on immersion as a f u n c t i o n o f water-cement r a t i o .
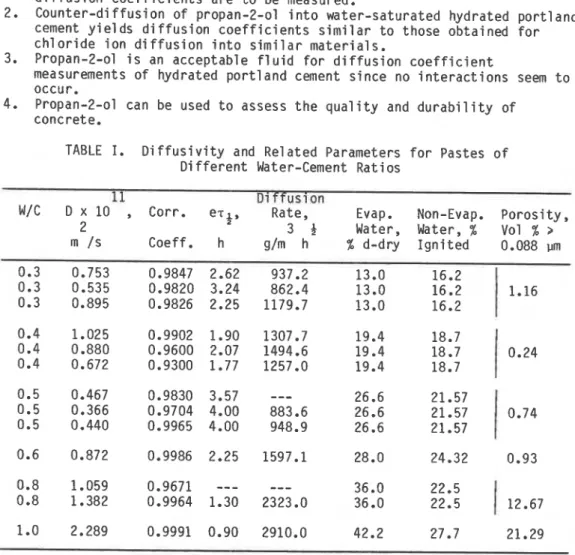
Water s a t u r a t e d p a s t e specimens, 1.14 mm t h i c k were used a t
water-cement r a t i o s o f 0.30, 0.4, 0.5, 0.6, 0.8 and 1.0 t o measure r a t e s o f d i f f u s i o n o f propan-2-01 i n t o t h e specimens. R e s u l t s i n u n i t s o f weight of propan-2-01 e n t e r i n g t h e pores per u n i t volume o f sample as a f u n c t i o n of square r o o t t i m e a r e presented i n F i g u r e 4. R e s u l t s a r e g i v e n f o r t h e FIG. 4 D i f f u s i o n o f propan-2-01 (q/m3)- i n t o water- s a t u r a t e d pastes. versus ( t i m e 1 f as a f u n c t i o n o f water-cement r a t i o . ' I l ' l l l I 1 ' 1 ' 1 ' 1 ' 1 ' 1 ' C 0 . 1 0 . 2 0 . 3 0 . 4 0.5 0.6 0 . 7 0 . 8 0 9 1 . 0 W A T E R I C E M E N T R A T I O FIG.
5
Rate of d i f f u s i o n o f propan-2-01 i n t o water- s a t u r a t e d pastes as a f u n c t i o n o f water- cement r a t i o .
Vol. 17, No. 4 607 DIFFUSION, LENGTH, WEIGHT CHANGE, SOLVENT REPLACEMENT
d i f f e r e n t water-cement r a t i o s a t a c o n s t a n t t h i c k n e s s , and a r e l i n e a r up t o between 65 and 85 per cent o f t h e t o t a l d i f f u s i o n . The slopes o f t h e l i n e a r p o r t i o n s were e s t a b l i s h e d w i t h i n l e s s t h a n one hour. The r e s u l t s f o r t h e sample prepared a t water-cement r a t i o o f 0.5 a r e n o t i n c l u d e d i n t h i s f i g u r e s i n c e t h e y p a r t i a l l y c r o s s over t h e o t h e r curves as can be i n f e r r e d from F i g u r e 5. I n F i g u r e 5, t h e r a t e o f d i f f u s i o n
1)
inu n i t s o f grams o f a l c o h o l per cubic metre o f sample per hour
,
versus water-cement r a t i o . This v a l u e decreases w i t h water-cement r a t i o except-
f o r t h e v a l u e o f t h e specimen prepared a t water-cement r a t i o o f 0.4 whichi s g r e a t e r t h a n t h e v a l u e f o r t h e specimen prepared a t water-cement r a t i o of 0.5 and which i n t u r n i s about equal t o t h e v a l u e o f 0.3. However a t each water-cement r a t i o , t h e r e i s some spread i n t h e r e s u l t s . These
f e a t u r e s i n t h e r e s u l t s a r e probably due t o d i f f i c u l t y i n compaction d u r i n g sample p r e p a r a t i o n a t water-cement r a t i o s o f 0.4 and 0.3. S i m i l a r r e s u l t s were observed i n p l o t s o f d i f f u s i v i t y (m2/s) and rr (h) versus
water-cement r a t i o . rr i s t h e parameter used by s b o t h e r workers and
r e p r e s e n t s t h e t i m e t a k t n f o r h a l f o f t h e t o t a l methanol t o d i f f u s e i n t o t h e specimen. Values o f d i f f u s i v i t y were c a l c u l a t e d from a form o f F i c k ' s Law: ( 6 )
W t / Y , = 1.127
where D i s t h e d i f f u s i v i t y , ni2/s, ' t ' t h e time, s and L (m) i s h a l f t h e t h i c k n e s s o f t h e specimen. Values o f t h e v a r i o u s d i f f u s i o n parameters, c o r r e l a t i o n c o e f f i c i e n t s f o r t h e Wt/W, vs
fi
p l o t s , and values f o r t h e evaporable, non-evaporable water c o n t e n t and p o r o s i t y a t each water-cement r a t i o , a r e presented i n Table I.i v ) R e l a t i o n s h i p between l e n g t h and w i g h t changes due t o immersion i n a1 coho1
.
The p l o t o f t h e l e n g t h change versus weight change due t o immersion of t h e p a s t e prepared a t waterlcement r a t i o o f 0.8 i n t h e a l c o h o l s i s presented i n F i g u r e 6. The f a c t t h a t methanol causes an expansion and propan-2-01, a shrinkage i s immediately c l e a r . However, i n a d d i t i o n t h e i n i t i a l l e n g t h per u n i t weight change response t o methanol immersion i s much l o w e r t h a n f o r propan-2-01; up t o 6 p e r c e n t weight change, t h e l e n g t h
W E I G H T C H A N G E , 70
FIG. 6
Length versus weight changes due t o immersion of w a t e r - s a t u r a t e d p a s t e (waterlcement = 0.8) i n a l c o h o l s .
Vol. 17, No. 4 R.F. Feldman
change f o r propan-2-01 i s t w i c e as g r e a t and a t 8 percent i t i s f o u r times. However, i n t h e f i n a l 0.15 p e r c e n t weight change i n methanol immersion t h e r e i s an expansion o f 0.04 per cent, a l a r g e i n c r e a s e i n slope and r e s u l t i n g i n a t o t a l expansion o f 0.10 p e r cent. T h i s compares t o a t o t a l s h r i n k a g e o f 0.14 per c e n t f o r propan-2-01 immersion. The f i n a l l e n g t h change t o weight change r a t i o (AE/E)/(AW/W) i s unexpectedly h i g h f o r
methanol, 0.27, e l i m i n a t i n g p o s s i b i l i t i e s t h a t a d s o r p t i v e f o r c e s can
,
e x p l a i n t h e expansion due t o methanol immersion (12).
The e f f e c t o f water-cement r a t i o on t h e l e n g t h - w e i g h t change 1
r e l a t i o n s h i p due t o propan-2-01 immersion i s presented i n F i g u r e 7. For
specimens prepared a t water-cement r a t i o s o f 1.0 and 0.8, when 80 p e r cent I
o f t h e weight change has occurred o n l y 25 per cent o f t h e l e n g t h change was recorded. At water-cement r a t i o o f 0.3, however 88 p e r cent o f t h e l e n g t h change has occurred a t t h e above l e v e l o f weight change. I n t e r m e d i a t e t r e n d s were observed f o r water-cement r a t i o s between 0.8 and 0.3.
S H R I N K A G E . % FIG. 7
Length versus weight changes due t o immersion i n propan-2-01 o f water- s a t u r a t e d pastes prepared a t d i f f e r e n t water-cement r a t i 0s.
The model o f a coarse p o r e s t r u c t u r e a t h i g h e r water-cement r a t i o s h a v i ng access t o a f i ner pore s t r u c t u r e adequately expl a i ns these r e s u l t s . The coarse p o r e s t r u c t u r e i s e l i m i n a t e d a t l o w water-cement r a t i o s and most of t h e l e n g t h change occurs when t h e propan-2-01 d i r e c t l y e n t e r s t h e f i n e r pores. Most o f t h e s u r f a c e area o f t h e h y d r a t e d p a s t e i s a s s o c i a t e d w i t h t h e f i n e r pores. High s u r f a c e area m a t e r i a l s a r e a s s o c i a t e d w i t h a
s i g n i f i c a n t amount o f s u r f a c e energy. When a f o r e i g n m a t e r i a l i s adsorbed on t h e surface, an i n t e r a c t i o n occurs, t h e e x t e n t depending on t h e n a t u r e o f t h e adsorbent, ( t h e h y d r a t e d cement) and adsorbate ( t h e a l c o h o l and t h e water). Normally t h e i n t e r a c t i o n r e s u l t s i n a decrease i n s u r f a c e energy and an expansion; however i n t h i s case water i s being removed from t h e s u r f a c e and r e p l a c e d by propan-2-01 which i n t e r a c t s t o a l e s s e r degree t h a n water. The n e t r e s u l t of' t h e rep1 acement o f several mol e c u l a r l a y e r s o f water c l o s e t o t h e s u r f a c e o f t h e h y d r a t e d cement by m o l e c u l a r l a y e r s o f . propan-2-01 should t h u s be a shrinkage.
Vol. 17, No. 4 609 DIFFUSION, LENGTH, WEIGHT CHANGE, SOLVENT REPLACEMENT
P O R E D I A M E T E R . urn
FIG. 8
Pore s i z e d i s t r i b u t i o n o f pastes prepared a t d i f f e r e n t water-cement r a t i o s and d r i e d by propan-2-01 treatment.
( v ) Pore s i z e d i s t r i b u t i o n .
I The pore s i z e d i s t r i b u t i o n s f o r t h e s i x specimens prepared a t t h e
I
d i f f e r e n t water-cement r a t i o s a r e presented i n F i g u r e 8. At h i g h e rpressures, ca. 414 MPa t h e t o t a l volume o f mercury i n t r u d e d decreases w i t h
!
water-cement r a t i o . However t h e volume o f mercury i n t r u d e d i n pores>
;
0.088 pm diameter (880 A), i s r e l a t i v e l y low f o r a l l water-cement r a t i o s except 0.8 and 1.0. Both d u r a b i l i t y and p e r m e a b i l i t y have been shown t o beI
more s t r o n g l y r e l a t e d t o pores o f t h i s s i z e range t h a n t h e t o t a l p o r o s i t yI ( 1 3, 6). The values a r e t a b u l a t e d i n Table I. The p l o t i n F i g u r e 9 f o r
I
d i f f u s i v i t y versus p o r o s i t y>
880 A i l l u s t r a t e s t h e s i m i l a r i t y betweenspecimens prepared a t water-cement r a t i o s o f 0.3, 0.4, 0.5 and 0.6. The
1
spread between p o i n t s w i t h i n a specimen t y p e prepared a t one water-cement r a t i o i s g r e a t e r t h a n t h e d i f f e r e n c e between values f o r specimens preparedP O R O S I T Y . % V O L U M E 2 8 8 0 R D I A M E T E R
F I G . 9
D i f f u s i v i t y o f propan-2-01 i n pastes as a f u n c t i o n o f p o r o s i t y 2 880 A diameter.
Vol. 17, No. 4
R.F. Feldman
a t several water-cement r a t i o s . There may be several reasons f o r t h i s , one b e i n g t h a t t h e r e i s a l a r g e v a r i a t i o n i n p o r e s t r u c t u r e w i t h i n one
water-cement r a t i o formed d u r i n g p r e p a r a t i o n , and t h a t t h e pore s t r u c t u r e measured does n o t necessari 1 y r e p r e s e n t t h e average v a l ue f o r t h e s p e c i f i c sample f o r which t h e d i f f u s i v i t y was measured. Another reason i s t h a t t h e d e t e r m i n a t i o n o f $, which t a k e s l o n g e r p e r i o d s o f t i m e t o measure, might cause e r r o r i n t h e c a l c u l a t i o n o f t h e d i f f u s i v i t y due t o t h e i n c l u s i o n o r e x c l u s i o n o f dead-end o r almost i so ated pores.
1
A p l o t o f d i f f u s i o n r a t e( W t per u n i t volume o f sample per h determined a t constant sample t h i c k n e s s ) versus p o r o s i t y i s presented i n F i g u r e 10. T h i s d i f f u s i o n
parameter, d i f f u s i o n r a t e , i s now f r e e o f t h e i n f l u e n c e o f W,. As can be seen i n F i g u r e 5 t h i s parameter decreases c o n t i n u o u s l y w i t h water-cement r a t i o if t h e v a l u e a t 0.5 i s not considered. Although t h e r e i s an
i n c r e a s e d d i f f e r e n c e between r e s u l t s f o r samples prepared a t water-cement r a t i o s o f 0.6 t o 0.3 on t h e one hand and 0.8 t o 1.0 on t h e o t h e r i n t h e p l o t i n F i g u r e 10, t h e r e i s no c l e a r d i f f e r e n t i a t i o n between t h e lower water-cement r a t i o p r e p a r a t i o n s w i t h r e g a r d t o p o r o s i t y . P O R O S I T Y , % V O L U M E * 8 8 0
W
D I A M E T E R FIG. 10 D i f f u s i o n r a t e o f propan-2-01 i n pastes as a f u n c t i o n o f p o r o s i t y > 800 A diameter. D i s c u s s i o nP r e v i o u s workers (9-11) have discussed t h e p o s s i b i l i t y t h a t
methanol r e a c t s w i t h components o f h y d r a t e d p o r t 1 and cement. Length change measurements c a r r i e d o u t i n t h i s work c o n f i r m these f i n d i n g s . Displacement of water adsorbed on porous s i l i c a g l a s s by methanol produces l i t t l e o r no l e n g t h change, showing t h a t t h e i n t e r a c t i o n o f b o t h water and methanol w i t h porous s i l i c a g l a s s i s s i m i l a r . The t o t a l l e n g t h change measured when t h e g l a s s d r i e d t o zero per cent r e l a t i v e h u m i d i t y i s s a t u r a t e d w i t h water i s 0.30 per cent (13). Thus i n comparison t h e expansion o f a p p r o x i m a t e l y 0.10 p e r c e n t observed when w a t e r - s a t u r a t e d h y d r a t e d p o r t l a n d cement p a s t e i s immersed i n methanol i s r e l a t i v e l y l a r g e . Other evidence i n c l u d i n g a d e t e c t i o n of a c o n s i d e r a b l e d e c l i n e o f Young's modul us f o l 1 owing immersi on i n methanol, a l a r g e shrinkage on d e s o r p t i o n o f methanol immersed specimens ( 1 4 ) and i n c r e a s e s i n s u r f a c e area due t o v a r i o u s t r e a t m e n t s w i t h methanol (15) suggest t h a t methanol p e n e t r a t e s t h e l a y e r e d s t r u c t u r e of t h e c a l c i u m s i l i c a t e s and i n c r e a s e s t h e s e p a r a t i o n o f t h e sheets.
Vol. 17, No. 4 61
1
DIFFUSION, LENGTH, WEIGHT CHANGE, SOLVENT REPLACEMENT
The values f o r t h e d i f f u s i o n c o e f f i c i e n t t a b u l a t e d i n Table I v a r y between a p p r o x i m a t e l y 5 x 1 0 - l 2 t o 2 x 1 0 - l 1 m2/s. T h i s i s i n t h e same rapge as values o b t a i n e d by o t h e r workers f o r i o n s such. as ~a', C1-, I-, Cs d i f f u s i n g t h r o u g h w a t e r - s a t u r a t e d o r d i n a r y p o r t l a n d cement (6). The c o e f f i c i e n t s were determined by b o t h t r a n s i e n t and steady s t a t e methods. Page e t a1 (16) r e p o r t e d a v a l u e o f 4.5 x 1 0 - l 2 m2/s f o r t h e e f f e c t i v e d i f f u s i v i t y o f c h l o r i d e i o n s t h r o u g h o r d i n a r y p o r t l a n d cement paste prepared a t water-cement r a t i o o f 0.5. T h i s i s remarkably s i m i l a r t o t h e v a l u e o b t a i n e d h e r e f o r t h e d i f f u s i o n o f propan-2-01 i n p a s t e prepared a t t h e same water-cement r a t i o .
T h i s t e c h n i q u e shows promise as a r a p i d method o f t e s t i n g f o r t h e q u a l i t y o f concrete. It appears p o s s i b l e t o e v a l u a t e t h i n c o n c r e t e s e c t i o n s i n such areas as cover over r e i n f o r c i n g s t e e l . This method, r e q u i r i n g o n l y simple equipment, i s b e i n g developed f o r i n v e s t i g a t i o n s o f m o r t a r s and concrete.
Conclusions
1. Methanol i n t e r a c t s w i t h h y d r a t e d p o r t l a n d cement by p e n e t r a t i o n o f t h e l a y e r e d s i l i c a t e s t r u c t u r e . It i s t h u s u n s u i t a b l e f o r use when
d i f f u s i o n c o e f f i c i e n t s a r e t o be measured.
2. Counter-di f f u s i on o f propan-2-01 i n t o w a t e r - s a t u r a t e d h y d r a t e d p o r t 1 and cement y i e l d s d i f f u s i o n c o e f f i c i e n t s s i m i l a r t o those o b t a i n e d f o r c h l o r i d e i o n d i f f u s i o n i n t o s i m i l a r m a t e r i a l s .
3. Propan-2-01 i s an a c c e p t a b l e f l u i d f o r d i f f u s i o n c o e f f i c i e n t
measurements o f h y d r a t e d p o r t l a n d cement s i n c e no i n t e r a c t i o n s seem t o occur.
4. Propan-2-01 can be used t o assess t h e q u a l i t y and d u r a b i l i t y o f concrete.
TABLE I. D i f f u s i v i t y and R e l a t e d Parameters f o r Pastes o f D i f f e r e n t Water-Cement R a t i o s
11 - - D i f f u s i o n - -- -
W/C D x 10
,
Corr. e ~ ~ Rate, , Evap. Non-Evap. P o r o s i t y , 2 34
Water, Water, % Vol % 2Vol. 17, No. 4 R.F. Feldman
References
Mehta, P.K., American Concrete I n s t i t u t e J. 74, pp. 440 (1977) Bakker, R.F.M., Proceedings Cape Town C o n f e r z c e , "A1 k a l i-Aggregate
Reaction i n Concrete", pp. 279 (1981)
Feldyan, R.F., M a t e r i a l s Research S o c i e t y Symposium, pp. 124 (1981) Day, R.L., Soshi, R.C., Langan, B.W. and Ward, M.A., Proceedings 7 t h
I n t e r n a t i o n a l Ash U t i l i z a t i o n Symposium, U.S.A., Vol. 2, pp. 811 (1985)
Grube, M. and Lawrence, C.D., Proceedings RILEM Seminar on
" D u r a b i l i t y o f Concrete S t r u c t u r e s under Normal Outdoor Exposure", Hannover, Germany, pp. 68 (1984)
Feldman, R.F., 8 t h I n t e r n a t i o n a l Congress on t h e Chemistry o f Cement, R i o de J a n e i ro, B r a z i l
,
pp. 336 (1986)P a r r o t t , L.J., M a t e r i a l s and S t r u c t u r e s , 17, pp. 131 (1984)
Marcdoigent, S.R.A., Ranc, R.P. and Guyot,~.
,
M a t e r i a l s Science and R e s t o r a t i o n , E d i t i o n Lack und Chemie. F i l d e r s t a d t 4. pp. 51, (1983)Day, R., Canadian Concrete Research, 11, pp. 341 (1981)
Beaudoin, J.J., I n Press (Journal o f M a t e r i a l s and S t r u c t u r e s ) Beaudoin, J.J., I n Press ( I 1 Cemento)
Feldman, R.F., 5 t h I n t e r n a t i o n a l Congress on t h e Chemistry of Cement,
Toleyo, Japan, Vol. 111, pp. 53 (1968)
Feldman, R.F., Canadian Journal of Chemistry,
-
48, pp. 287 (1970)Feldman, R.F., Unpublished
L i t v a n , G.G., Cement Concrete Research, 6, pp. 139 (1976)
Page, C.L., Short, N.R. and El Tarras, AT, Cement Concrete Research
11, pp. 395 (1981)
T h i s p a p e r i s b e i n g d i s t r i b u t e d i n r e p r i n t f o r m by t h e I n s t i t u t e f o r R e s e a r c h i n C o n s t r u c t i o n . A l i s t of b u i l d i n g p r a c t i c e a n d r e s e a r c h p u b l i c a t i o n s a v a i l a b l e from t h e I n s t i t u t e may be o b t a i n e d by w r i t i n g t o t h e P u b l i c a t i o n s S e c t i o n , I n s t i t u t e f o r R e s e a r c h i n C o n s t r u c t i o n , N a t i o n a l R e s e a r c h C o u n c i l o f C a n a d a , O t t a w a , O n t a r i o ,