Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Paper (National Research Council of Canada. Division of Building Research); no.
DBR-P-711, 1977
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=149a0396-b483-4384-a153-06f2313b2abe https://publications-cnrc.canada.ca/fra/voir/objet/?id=149a0396-b483-4384-a153-06f2313b2abe
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001670
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Evaluating toxicity of decomposition products from analytical data
Ser
TH1
N21d
no.
711
National Research
Conseil national
c, 2
I
*
Council Canada
de recherches Canada
EVALUATING TOXICITY OF DECOMPOSITION
PRODUCTS FROM ANALYTICAL DATA
by K.
Sumi and Y. TsuchiyaReprinted from Fire Retardants
Proceedings of 1976 International Symposium on Flammability and Fire Retardants
p. 241 248
DBR Paper No.
711
Division of Building Research
t
SO MMAIRE
On p r g s e n t e l a r e c h e r c h e s u r l a toxicit6 du f e u e n t r e p r i s e p a r l a S e c t i o n d'Ctude du feu d e Conseil national de r e c h e r c h e s du Canada. L e t r a v a i l jusqul'a prCsent a 6th o r i e n t 6 v e r s l l a n a l y s e q u a n t i t a t i v e d e s p r o d u i t s de dCcomposition e t 1'6valuation s u b - s e q u e n t e de l a toxicitC. L e concept d e toxicit6 d u feu p r o p o s 6 p a r l e s a u t e u r s donne d e s indications d e l ' i m p o r t a n c e r e l a t i v e
des esp'eces toxiques p r o d u i t e s p a r un m a t e r i a u donnC, e t l a p r o p e n s i o n d e s m a t 6 r i a u x 'a g6nCrer d e s p r o d u i t s d e dCcompo- s i t i o n toxique.
I
-
III
3
IIIII]IIII[I~~
8 9 002II~[[II
88111 1~11
811/11
'
-EVALUATING TOXICITY OF DECOMPOSITION PRODUCTS FROM ANALYTICAL DATA
K. Sumi and Y. Tsuchiya
National Research Council of Canada Ottawa, Ontario K I A OR6
ABSTRACT
Research on fire toxicity undertaken by the Fire Research Section of the National Research Council of Canada is presented. Work to date has been directed towards quantitative analysis of decomposition products and subsequent assessment of toxicity. The fire toxicity concept proposed by the authors provides indications of the relative importance of toxic species produced from a given material, and the propensity of materials for generating toxic decompo- sition products.
Toxic gases and vapours produced a t fires are responsible for a large number of deaths in building fires. Statistics on fires obtained from a number of countries reveal that more people die from inhalation of decomposition products than from burns [ I , 2,
31.
Today it i s generally accepted that this is the major cause of fire deaths. The concern over the danger of inhaling decomposition (combustion and pyrolysis) products has increased in recent years due primar- ily to the development and increasing use of synthetic materials, both as building materials and furnishings. Some of these new materials release decomposition products very rapidly, and some generate products that are more toxic than those produced by traditional materials such -as wood and paper. The question of whether the steady increase in the use of new materials is likely to result in an increase in the life hazard for the occupants of buildings in the event of fire is of paramount importance.
The potential fire hazard of new materials has been recognized for many years. The concern over this problem is reflected in regulations applied in efforts to reduce fire losses. The hazard includes flammability, generation of dense smoke and release of toxic gases and vapours. The present regulations apply primarily to flammability; most of the regulations are intended t o restrict materials that are easy to ignite or tend to contribute to rapid spread of flame. One of the ways in which industry has responded to this demand is by the development of fire retardants. Since materials formulated to resist ignition or rapid flame spread often produce more smoke and, in some cases, more harmful decomposition products than untreated mate- rials, concern with the problems of smoke and fire toxicity have become even greater. Some Reprinted from FIRE RETARDARTS: Proceedings of 1976 International Symposium on Flammability and Fire Retardants
regulations on smoke developed ratings of materials have been introduced in building regula- tions in Canada [4] ; regulations on fire toxicity of materials are yet to be promulgated in this country.
In recent years the toxicity of decomposition products has received a great deal of attention from fire scientists and engineers throughout the world. Laboratory studies on this subject can be classified into two main types. One involves the biological response of laboratory animals to fire gases or artificial mixtures of components found in fire gases, the other involves chemical analysis of components found in fire gases. Both approaches are needed to elucidate various aspects of the problem.
Animal experiments provide information on physiological and toxicological effects of harm- I ful gases and vapours. They also offer a means to screen out materials with a high propensity
for generating harmful decomposition products or to evaluate materials on this basis. Chemical analysis of decomposition products provides a method of identifying the species primarily responsible for toxic effects.
The purpose of this paper is to describe some of the efforts made by the Fire Research I I Section of the Division of Building Research towards a better understanding of fire toxicity. ! The work to date has been confined to analysis of decomposition products and assessment of
experimental data using a fire toxicity concept (originally called toxicity index concept). \ Bio-assay experiments with animals have not been undertaken.
1
ANALYSIS OF DECOMPOSITION PRODUCTS
Quantitative analyses of decomposition products have been conducted by many investi-
I
gators over the years to determine the harmful gases that could be produced from various II
materials. Detailed analysis of these products has been difficult because, in general, polymersdecompose into many compounds. The analysis of pyrolysis products of cellulose has revealed
the presence of some 175 organic compounds, of which about 150 have been identified [51.
i
Some 75 organic compounds have been detected in the pyrolysis products of PVC[GI.
Theidentification and quantitative analysis of products generated by these materials can be ex- tended further by using more sensitive methods of analysis. Because of the large number of possible components, the researcher must arbitrarily decide how detailed the analysis should be. For practical purposes, it i s not always necessary to obtain comprehensive data on thermal decomposition products. Testing for a few components that are known or suspected to be
1
significant from the viewpoint of inhalation toxicity often gives useful information. This wasthe approach taken by the present authors in developing data on CO and HCI generated by combustion and pyrolysis of PVC [7] and CO and HCN generated by materials containing nitrogen [8]
.
Recent advances in analytical techniques, particularly in gas chromatography and mass spectrometry, have increased the capability of chemists to analyze decomposition products. This has resulted in improvements in developing data on decomposition products, although the work continues to be time consuming. The information obtained is useful to polymer chemists in the synthesis of new materials (with reduced toxic gas producing potential), to the medical profession in the treatment of casualties, and to those responsible for promulgating regulations on fire safety.
The increasing use of new synthetic materials makes it imperative that researchers be aware of those that may increase the toxic hazard a t fires. Because the formation of decomposition products depends on the environmental conditions of the fire, data for a wide range of
temperature and oxygen supply conditions are required. Even after these data are obtained it is s t i l l difficult to assess the toxic hazard created by decomposition products.
FIRE TOXICITY CONCEPT
The authors of this paper proposed a fire toxicity concept for assessing the toxicity of decomposition products generated by various materials
[7,
91.
The assessment considers the amounts of harmful products released and the known toxicities of the individual components. It indicates: (a) the relative significance of individual decomposition products t o the toxicity of a mixture; and (b) propensity of various materials for generating toxic decomposition products. The former is the primary function of the concept, the latter, which is of greater practical significance, has a limitation that should be understood by those using the concept. The development of the assessment method is as follows: Toxicity, t, due to a gaseous or volatile compound is assumed to be proportional to clc, wherec = concentration of a gas or vapour, and
c, = concentration of the same compound considered fatal (or dangerous) to man
in 30 minutes.
This is the maximum order of time.available for the fire service t o effect rescue before a fire spreads throughout a building.
The toxic gas generating characteristic of a material, called "toxicity factor" in this paper, is defined as
where T = toxicity factor
t = toxicity of gas or vapour
V = volume into which the products are dissipated
W = weight of original material
ce = experimentally determined concentration of volatile or gaseous product cf = concentration of the same product that is dangerous to man
in 30 minutes.
When c, and cf are both expressed in volume t o volume ratio,
where v is the volume at S.T.P. of toxic gas produced. Then T = v/cfW is an expression for the toxicity factor of a single component based on experimental data. Fire toxicity of a material is obtained by assuming that the toxic effect due to individual components is additive.
Toxicity factor depends on the experimentally determined concentration of a decomposi- tion product and c, of the same compound. Some c, values that the authors have used in determining toxicity factors are given in Table 1.
Table 1. cf Values. I ) c c o ~ ~ ~ l , o s i t i o n c . - 1)angcrous c o n c e n t r a t i o n f o r f p r o d u c t a 30- m i n u t e e x p o s u r e (ppm) IlCN 135 CO 4000 co2 7 0 , 0 0 0 - 1 0 0 , 0 0 0 llCR 1000
The concentration of HCN that is fatal to man in 30 minutes is reported to be 135 ppm
[ l o ] while that of CO that is fatal in exposures of less than one hour is reported to be 4000
ppm
[ I
11. These examples show that HCN is thirty times as toxic as CO on a volume basis. Such information is taken into account in the determination of the toxicity factors.The fire toxicity of a material can be assessed from the sum of the toxicity factors of the main toxic components. Because Z T, the sum of toxicity factors of a material, depends on experimental conditions used to obtain the data, a ranking of materials would depend on the conditions selected. The authors decided to rank materials on the basis of maximum 2 T determined from experimental data. The maximum C T of a material gives an indication of the potential hazard of that material in generating toxic gases and vapours.
STANDARDIZED METHOD OF DECOMPOSITION
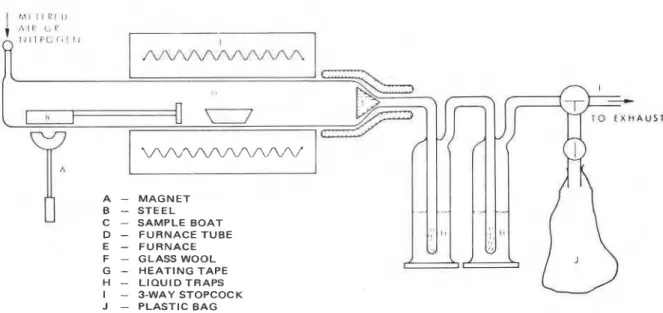
The need for a standardized method of decomposition becomes apparent when one wishes to embark on a systematic study to assess toxicity following quantitative analysis of decompo- sition products of a wide variety of organic materials. Because environmental conditions in a fire atmosphere vary over wide ranges of temperature and oxygen concentration, and these variations influence the generation of decomposition products, the method of decomposition used in the laboratory should allow these conditions to be varied. A new flow method of thermal decomposition was developed. The experimental set-up is shown in Figure 1. Samples were decomposed in a large, horizontal quartz tube through which air or inert gas was metered. The temperature of decomposition was adjustable up to 800°C.
The sample is weighed in a quartz boat and moved to the hot zone of the furnace by a magnetic sample insertion device. Glass wool is placed at the outlet end of the furnace tube to trap solid particles, and a heating tape is wound around the same section of the furnace tube to prevent condensation of volatile products. Sample weight, composition of the atmosphere, flow rate of the atmosphere and decomposition temperature can all be varied. HCN i s collected in liquid traps containing NaOH and then analyzed using a specific ion electrode for cyanide ion or by titration using a modified Liebig method. The specific ion method is convenient for
HCN analysis but precautions are necessary for accurate determination. In addition, possible
Figure 1. Thermal decomposition apparatus.
interference due t o the presence of other components in the mixture must be checked. The modified Liebig method was suitable for this purpose because it provided a good alternative for determining HCN. HCI is also trapped in alkali solution and i t s concentration determined with the specific ion meter. CO, CO, and other gases are collected in a plastic bag and analyzed using gas chromatography. In some cases, the condensable products are collected in a cold trap and analyzed.
RESULTS
The maximum Z T of various materials determined from analytical data obtained using the new flow method of decomposition i s presented in Table 2.
Table 2. Maximum Z T (Sum of Toxicity Factors) of Materials.
,
-
M ; l t c r i a l Maxi Z mum 'I' I ' o l y s t y r c n c 19 20 I ' o l y e t l i y l c n c 21 20 P o l y c s t c r f a b r i c 24 30 P h e n o l i c r e s i n 5 22 30 IVood ( w l i i t c p i n c ) 47 50 C o t t o n 59 2 60 1°K 12 1 543 360 Wool 14 1 5 75 390 A US 10 1 2b7 2 80 l l r c t h a n e ( r i g i d ) 14 I 273 290 Ny l o n - 6 17 I 9 31 950 P o l y a c r y l o n i t r i l c 7 1 1201 1210Among the materials tested, polystyrene, polyethylene and polyester (fabric) seem to have the lowest potential for generating harmful decomposition products. CO is believed t o be the
primary toxicant. Phenolic resin also exhibited a low potential from a consideration of the
toxicity due to phenols, CO and C02 [ 121
.
When wood or cotton is decomposed by heat, a large variety of combustible vapours is
produced along w ~ t h carbon, GO,
CO,
and water vapour. Some pyrolysis products act asirritants t o the eyes and respiratory tract. Because large amounts of CO and C02 are produced in comparison with other harmful compounds, the toxicity due t o these two gases only were
considered. The maximum
Z
T of white pine and cotton was a little higher than that of thefirst four materials.
When PVC is heated to temperatures above 250°C, it decomposes and produces HCI. The removal of HCI leaves a residue having a polyene structure which breaks down further t o yield
benzene and other aromatics. When PVC is heated in an oxidizing atmosphere, some of the
volatile components burn t o produce oxidation products such as CO, COP and H, 0. The yield
of HCI was approximately equal to the theoretical amount expected from complete conversion
of CI atoms t o HCI. In an earlier study
[7],
the authors investigated the presence of otherchlorine-containing compounds such as phosgene and chlorine gas. The concentrations of these I
compounds were below the detectable limits of measurements. The fire toxicity data of PVC indicated that the toxicity of the decomposition products was due primarily t o HCI. The
additional effect of CO was very small. The maximum
Z
T of PVC was substantially higherI
than that of the preceding materials.
1
Materials containing nitrogen were assessed after determining HCN, CO and CO,. Poly- I
acrylonitrile and nylon had the highest 2 T. Toxicity was primarily due t o HCN which in-
creased with temperature. The maximum 2 T of nitrogen-containing materials may be even higher than those reported if higher temperatures were employed and if other toxic com- ponents having C and N atoms were also considered in the evaluation.
The specific ion electrode was not suitable for determining HCN following decomposition
of wool. There was evidence of interference which i s attributed t o the presence of sulfide ions
in the decomposition products. The modified Liebig method, which gave results in agreement with the specific ion electrode method in the determination of HCN from the other nitrogen- containing compounds, was used for experiments with wool.
DISCUSSION
The fire toxicity concept gives indications of (a) the relative significance of individual decomposition products to the toxicity of a mixture, and (b) the propensity of various materials for generating toxic decomposition products. The former is the primary role of the
concept. The latter, which has greater practical significance, is of benefit when used with the
understanding that it i s not possible t o consider all the toxic products.
Interaction Effects
I
The fire toxicity of a material is determined by assuming that the toxic effects of individual components in a mixture are additive. There has been a great deal of speculation concerning possible interaction or synergism resulting from the combined effect of the components.
Synergism, as used in this paper, refers to a situation where the toxicity of a mixture is
Although there i s very little evidence of synergism resulting from inhalation of fire gases, the possibility of this phenomenon occurring cannot be ruled out. Animal experiments provide the only method to determine if a given mixture of toxic gases is synergistic, additive or antago- nistic. If synergism is found and its magnitude determined for specific combinations of toxic gases, the fire toxicity data can be modified accordingly.
People engaged in bio-assay experiments have expressed concern with the effect of partic- ulates on inhalation toxicity E13, 141. The effect of particulates can be taken into account in the toxicity calculations when data on acute inhalation toxicity become available and inter- action effects are quantified.
Correlation with Animal Data
The reliability of maximum 2 T data as an indicator of the propensity of materials for generating toxic decomposition products should be examined by testing against animal exposure data. The best way to undertake such an examination is by duplicating the experi- mental conditions for obtaining maximum Z T data and exposing animals to the decomposi- tion products. Unfortunately, the authors do not have facilities to conduct such a study. A
recent paper by Sakai and co-workers [ 151 using an experimental set-up that has a number of similar features gives some relevant information. They conducted combustion experiments with five different fabrics and determined the amounts of materials required to kill 5 0 percent of the mice exposed to the products. Their findings, in decreasing order of toxicity, were: polyacrylonitrile, nylon, wool, cotton and polyester, the last two being about equal in toxicity. The results of their experiments are in agreement with the maximum Z T data obtained in this study on five similar mate'rials.
Rate of Generation of Toxic Products
The present fire toxicity concept considers the toxic hazard created by burning a unit weight of material under various experimental conditions. The data could be made more meaningful if the rate of generation of toxic gases was also considered because the hazard due to generation of fire gases is increased with increased rate of burning. It is not possible at the present time to incorporate the rate of production of toxic gases in the toxicity equations because of lack of knowledge of the rates of combustion and pyrolysis of solid materials. Data on burning characteristics of a wide range of materials are required for further development of the fire toxicity concept.
Regulations on Fire Toxicity of Materials
The possible use of the fire toxicity concept is frequently brought up by members of committees engaged in writing codes and standards as a method of regulating materials having a high propensity for generating toxic decomposition products. Assessment of materials on the basis of the fire toxicity concept, however, cannot consider all the harmful components found in a fire environment. The control of materials would have to be based on a limited number of toxic compounds that are known from experience to be significant. This approach cannot avoid the possibility of missing toxic components that may be even more important than those considered in the assessment. Bio-assay experiments do not have this disadvantage. A standard method of evaluating fire toxicity of materials using laboratory animals i s therefore needed. Research by a number of organizations is now being directed towards this objective.
The findings from chemical analysis and subsequent assessment of fire toxicity undertaken by NRC's Fire Research Section and elsewhere have been used in France to promulgate a new national standard [16]
.
The regulations apply to interior furnishings for establishments open to the public. The furnishings include decorations, drapes and curtains, and fixed furniture and materials used to cover floors, walls and ceilings. The regulations specify that the total amount of nitrogen and chlorine that may be released into a given space as HCN or HCI shall not exceed 5 and 25 grams per cubic metre of that space, respectively. The manufacturer is responsible for providing information on the nitrogen and chlorine content of materials.CONCLUDING STATEMENT
Some studies conducted by NRC's Fire Research Section towards a better understanding of fire toxicity have been described. The work to date has been confined to analysis of decompo- sition products followed by assessment of experimental data using a mathematical approach. Bio-assay experiments have not been undertaken.
The fire toxicity concept indicates, (a) the relative significance of toxic components in the decomposition products, and (b) the relative propensity of materials t o release toxic products. The information obtained by the assessment of toxicity from analytical data complements that from other sources and contributes to a better understanding of combustion toxicology.
REFERENCES
1 . United Kingdom Fire and Loss Statistics 1971, Her Majesty's Stationery Office, London (1973). 2. The Single Fatality Fire, Fire Journal, 63, 34 (1969).
3. K. Kishitani, J. Faculty of Engineering, Univ. of Tokyo (B),31, No. 1 , l(1971).
4. National Building Code of Canada 1975, National Research Council of Canada, NRCC 13982, p. 105. 5. H. W. Emmons, Scientific American, 231, 21(1974).
6. W. D. Woolley, Br. Polym. J.3, 186(1971).
7. Y. Tsuchiya and K. Sumi, J. App. Chem. 17, 364(1967). 8. K. Sumi and Y. Tsuchiya, J. Fire and Flammability 4, 15(1973). 9. Y. Tsuchiya and K. Sumi, J. Fire and Flammability 3,46(1972).
10. Documentation of Threshold Limit Values, American Conference of Governmental Industrial Hygienists, p. 130, 1966.
1 1 . Y. Henderson and H. W. Haggard, Noxious Gases and Principles of Respiration Influencing their Action, The Chemical Catalog Co., Inc., New York, 212 p. (1927).
12. Y. Tsuchiya and K. Sumi, Toxicity of Decomposition Products-Phenolic Resin, t o be published.
13. 1. N. Einhorn, Private communication, September 1975. 14. K. Yamamoto, Zeitschrift fur Rechtmedizin 76, 1 1 (1975).
15. T. Sakai, H. Tsukamoto, and M. Ohashi, Medicine and Biology (Japanese) 90(3), 1 1 l(1975). 16. Flammability News Bulletin, Inc. 4 ( 3 ) , p. 20 (Nov.-Dec. 1975).
This publication is being distributed by the Division of Building R e e e a r c h of the National R e s e a r c h Council of Canada. I t shouldnot be reproduced in whole o r in p a r t without p e r m i s s i o n of the original publimher. The Di- vision would b e glad to b e of a s s i s t a n c e in obtaining such permission.
Publications of the Division m a y b e obtained by m a i l - ing the a p p r o p r i a t e r e m i t t a n c e ( a Bank, E x p r e s s , o r P o e t Office Money Order, o r a cheque, m a d e payable t o the R e c e i v e r General'of Canada, c r e d i t NRC) t o the NationalReeearch Council of Canada, Ottawa. KIA OR6. Stamps a r e not acceptable.
A l i s t of allpublications of the Division i e available and m a y be.obtained f r o m the Publications Section, Division of Building Research, National R e s e a r c h Council of Canada, Ottawa. KIA OR6.