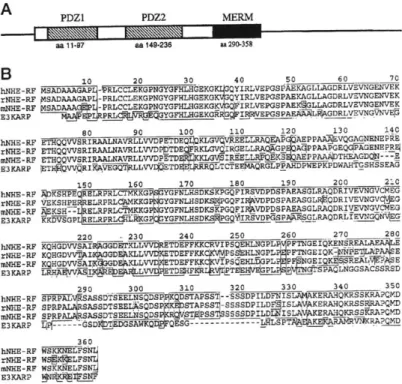
ANALYSIS OF INTERACTIONS BETWEEN RADIXIN AND ITS
LIGANDS NHE-RF AND LAYILIN
BY
ETCHELL ANN CORDERO
B.S., University of California, Berkeley
1991
Submitted to the Department of Biology in partial fulfillment of the requirements for the degree of
Doctor of Philosophy in Biology at the
Massachusetts Institute of Technology February 1999
@1999 Massachusetts Institute of Technology All rights reserved
Signature of Author
Department of Biology Certified by
-- - ----- -- ----- -- --- - - - - - - - - - -- - - -Thesis Ad isor Professor Frank Solomon Accepted by
Chairman of the Graduate Comn tte
SSACHUSETTS INSTITUTE
ANALYSIS OF INTERACTIONS BETWEEN RADIXIN AND ITS
LIGANDS NHE-RF AND LAYILIN
BY
ETCHELL ANN CORDERO
Submitted to the Department of Biology on February 1, 1999 in partial fulfillment of the requirements for the degree of
Doctor of Philosophy in Biology
ABSTRACT
ERM proteins (ezrin, radixin, moesin) are cytoskeletal-membrane linkers. The regulation of the interactions between ERM proteins and their ligands may
modulate the organization of cortical structures in the cell. Previously we identified NHE-RF as a ligand for merlin and ERM proteins (Murthy 1998). In this paper, we characterize the interaction between NHE-RF and radixin as well as identify a novel ligand for radixin - layilin. Using affinity chromatography binding assays, we show
that layilin and NHE-RF bind directly to radixin at its amino-terminal domain. Our results show that an interdomain interaction between the amino- and carboxy-terminal domains of radixin inhibits the ability of the ligands, NHE-RF and layilin, to bind to the amino-terminal domain of radixin. Furthermore, we show that the conformation of the amino-terminal domain changes in the presence of PIP. In the presence of PIP, this interdomain interaction is inhibited and ligand binding to full-length radixin is enhanced. Taken together, our results give a mechanistic rationale for the regulation of the interaction between ERM proteins and their ligands by phospholipids.
Thesis Advisor: Dr. Frank Solomon Title: Professor of Biology
Acknowledgements
To Shau Neen - I couldn't have done this without you. You had dreams and
visions for me that I never considered for myself. You knew I had the abilities to get a Ph.D. before I knew it was an option. Thank you for being my colleague and best
friend.
To Frank - You are my mentor, my therapist and my advisor for the rest of my life. Thank you for being confident in my abilities and teaching me what is important in life and in science.
To my collaborators Charo Gonzalez-Agosti, Vijaya Ramesh, and Mark Borowsky. Thank you for sharing your reagents and your ideas with me. You helped me bring my work to a higher level and it was a joy working with such nice people.
To my thesis committee members Tyler Jacks, Richard Hynes, Frank Gertler and Vijaya Ramesh - I appreciate the guidance you have given me to make my thesis
stronger. I also thank you for making my thesis defense a stimulating and exciting experience and I regret not taking advantage of these meetings earlier in my career. To Jim - You are "a ray of sunshine in my life" and my favorite baymate. Thanks for putting up with all my moody days and for knowing when and when not to be a bully. Now you don't have to worry about me taking up more than my half of the
bay - enjoy!
To Letty - You are my girlfriend. Thank you for filling my days in lab with (neverending) stories, hugs, love and smiles.
To Alice Rushforth and Sylvia Sanders - Thank you for showing me that it's possible to be a good mother and scientist at the same time.
To the rest of the Solomon Lab - Margaret Magendantz, Kate Compton, Adelle Smith, and Will Chen - thank you for your helpful discussions and advice at group meetings. I wish the best for you all.
To the rest of the people in my life who gave me something to do besides
benchwork: Thanks to all my friends who made my life outside of lab memorable and fun. Thanks to my ultimate frisbee buddies who gave me a sport to play and memories of success that I'll keep with me for the rest of my life. Finally, thanks to my family who unconditionally supported everything I did.
yeah!
Table of Contents
Title Page 1 Abstract 2 Acknowledgements 3 Table of Contents 4 List of Figures 6 CHAPTER 1: 7 Introduction 8 Thesis overview 29 Bibliography 30 CHAPTER 2:Intra- and inter-domain interactions 36
Introduction 37
Materials and Methods 40
Results 47
Discussion 72
Bibliography 78
CHAPTER 3:
Radixin and NHE-RF interactions 80
Introduction 81
Materials and Methods 85
Results 88
Discussion 108
Bibliography 114
CHAPTER 4:
Radixin and layilin interactions 117
Introduction 118
Materials and Methods 120
Results 123
Discussion 134
CHAPTER 5:
Analysis of Merlin 139
Introduction 140
Materials and Methods 143
Results 145
Discussion 149
Bibliography 153
CHAPTER 6: 156
Model and conclusions 157
Bibliography 167 CHAPTER 7: 170 Future Prospects 171 CHAPTER 8: 175 Appendix - Publications 176 5
List of Figures
Figure Figure Figure 1-1 1-2 2-1 Figure 2-2 Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure 2-3 2-4 2-5 2-6 2-7 2-8ERM family of proteins
Current model of ERM regulation
Detecting an amino- and carboxy-terminal domain interaction
Phospholipids bind to radixin at its amino-terminal domain
Phospholipids regulate the interdomain interaction Cross-linkers capture an intra-molecular interaction Formation of the faster band is dependent on an
intact cross-linker and the conformation of the protein
Cross-linker kinetics
Cross-linking domains of ERM proteins Phospholipids disrupt the formation of the intramolecular interaction
Specific phospholipids disrupt cross-linking
Intra- and inter-domain interactions of radixin are regulated by phospholipids
Immunofluorescence of NHE-RF and ERM proteins NHE-RF is a ligand for radixin
NHE-RF binds to radixin in a concentration dependent manner
Binding between NHE-RF and the amino-terminal domain of radixin is saturable
Radixin binds to NHE-RF on glutathione beads An interdomain interaction between the amino- and carboxy-terminal domains of radixin block NHE-RF binding to radixin 2-9 2-10 3-1 3-2 3-3 Figure 3-4 Figure 3-5 Figure 3-6
Figure 3-7 Figure 3-8 Figure 3-9 Figure 4-1 Figure Figure Figure 4-2 4-3 4-4 Figure 4-5 Figure 5-1 Figure 6-1
Phospholipids enhance NHE-RF binding to full length radixin
Cross-linking radixin does not affect ligand binding or domain competition
Model for phospholipid regulation of radixin binding to NHE-RF
Layilin and ERM proteins partially co-localize at the ruffling edges
Layilin is a ligand for radixin
An interdomain interaction blocks layilin binding Phospholipids enhance layilin binding to full length radixin
Model for phospholipid regulation of radixin binding to layilin
Merlin forms an intramolecular interaction which can be captured by cross-linkers
Model for ERM regulation
CHAPTER 1:
INTRODUCTION
The cortical cytoskeleton participates in crucial aspects of cell motility, morphological differentiation and organization of the plasma membrane.
Understanding the molecular basis for those functions remains a central question for cell biologists. It seems likely that specific molecules are required to localize differentially in the membrane, where they mediate specific organizations of cytoskeletal and membrane components.
Among the cortical cytoskeletal components, the localization patterns of ERM proteins (ezrin, radixin, and moesin) are particularly intriguing. Ezrin, radixin and moesin - the ERM proteins, closely related by primary sequence - localize to a wide variety of cortical structures associated with microfilaments. This pattern suggests that they may participate in the establishment and maintenance of differentiated morphology. The discovery of a fourth, somewhat less closely related polypeptide, merlin, as the product of a tumor suppressor gene greatly increased interest in this family of proteins.
IDENTIFICATION OF EZRIN, RADIXIN AND MOESIN
Ezrin was first identified by Bretscher (Bretscher, 1983) in the course of a systematic analysis of the intestinal microvillar cytoskeleton, an organelle that has been a valuable tool for understanding actin-membrane interactions. A
collaboration between groups demonstrated that ezrin was identical to a previously identified endogenous substrate for tyrosine kinase activity (Gould et al., 1986). Biochemical searches for actin- and membrane-associated proteins led to the identification of moesin (Lankes et al., 1988) and radixin (Tsao et al., 1990). The
Introduction
three proteins were identified as a family on the basis, first, of their sequence similarities (between 72% and 80% identity for the pairwise comparisons of the three proteins). Subsequent experiments revealed that they share many other properties. The ERM proteins are most related in their amino-terminal halves (between 85 and 89% identity), which in turn have significant sequence homology to the amino-terminal domain of band 4.1 (Figure 1-1). The carboxy-terminal domain varies among the three proteins, although they may all specify binding sites for ligands including actin (see below). Whether this diversity has any functional
consequences is not established, but it has permitted generation of antibodies specific for each of the polypeptides.
Other proteins have also been identified as members of the Band 4.1 superfamily by sequence homology, and so are related to ERM proteins. EM10, a protein identified as a surface antigen on the tapeworm that causes echinocosis, is
55% identical to ezrin in their amino-terminal domains (Frosch et al., 1991). A
series of protein tyrosine phosphatases have also been identified because of their homology to the N-terminal domain of Band 4.1 and ERM proteins (Yang and Tonks, 1991; L'Abbe et al., 1994; Sawada et al., 1994; Higashitsuji et al., 1995). The most provocative sequence homology is to merlin, the NF2 gene product (see below). What is not clear is what common elements of structure and function this family represents.
ERM PROTEINS LOCALIZE TO SPECIALIZATIONS OF THE CELL CORTEX
Although the immunofluorescence localizations and relative abundance of each of the proteins may vary among cell types (Berryman, 1993), at least two of the proteins are detectable in nearly every cell type tested. There also is no compelling evidence demonstrating segregation of individual ERM proteins within the same cell. Instead, there are several examples of two or more proteins co-localizing precisely. These descriptive experiments support the notion that different ERM proteins may have overlapping, if not identical, functions. They also permit the discussion of ERM localization without distinguishing among the family members.
The localized fraction of cellular ERM proteins is cortical, in particular to domains of the cortex juxtaposed to F-actin structures: ruffling edges, blebs, microspikes, and neuronal growth cones (Bretscher, 1983; Gould et al., 1986;
Pakkanen et al., 1987; Pakkanen, 1988; Goslin et al., 1989; Birgbauer, 1991; Berryman,
1993; Franck, 1993; Gonzalez et al., 1996). ERM proteins are also prominent in
non-motile regions such as intestinal microvilli (Bretscher, 1983; Gould et al., 1986; Pakkanen et al., 1987; Pakkanen, 1988; Berryman, 1993). In mitotic cells, ERM proteins are greatly enriched at the cleavage furrow (Sato et al., 1991; Franck, 1993; Henry et al., 1995).), adherens junctions (Tucker et al., 1989) and the marginal band of nucleated erythrocytes (Birgbauer and Solomon, 1989). The localization of ERM proteins, then may represent a common structural feature - for example, specialized interactions between cortex and cytoskeleton - rather than a function dedicated to motility. And while ERM proteins can co-localize precisely with certain cellular actin structures, they are conspicuously absent from others, in particular the
Introduction
prominent cytoplasmic actin fibers, such as stress fibers. Even in organelles where both ERM and F-actin are enriched, such as the growth cone, their patterns overlap rather than coincide (Goslin et al., 1989). In this sense, ERM proteins do not behave as do simple F-actin associated proteins. These restrictions on ERM localization - to a subset of both cortical structures and F-actin - are evidence of in vivo mechanisms that regulate crucial interactions. Analysis of possible regulatory mechanisms is a major focus of the field.
INTERACTIONS IN CIS AND IN TRANS:
ERM PROTEINS AS BIFUNCTIONAL CONNECTORS
A series of in vitro and in vivo assays detect two sorts of non-covalent
interactions for ERM proteins: intermolecular binding of other species; and
interdomain binding that could either mediate self-association to form oligomers or act in an intramolecular fashion to stabilize conformations of individual molecules. Results from several laboratories suggest that these two types of interactions may be mutually exclusive. Perhaps the sites necessary for binding of other species are occluded when the domains of ERM proteins are able to interact with one another. This reciprocal relationship could provide the molecular mechanism for regulation of ERM interactions and localization. As discussed below, this model explains some but not all of the relevant experimental data.
Heterologous ligands: Given their localization in actin-rich structures, it is
possible that ERM proteins bind directly to F-actin. Different assays, using different isoforms of actin and different sources of ERM proteins, produce conflicting binding
results (Turunen et al., 1994; Pestonjamasp et al., 1995; Shuster and Herman, 1995; Yao et al., 1995; Yao et al., 1996). Attempts using standard actin pelleting assays to detect an interaction between actin and full-length ERM proteins have been unsuccessful (Bretscher, 1986), Cordero and Solomon, unpublished observations). In the experiments which succeeded in detecting an interaction between actin and ERM proteins, the ERM proteins are denatured (Pestonjamasp et al., 1995) or ERM fragments are used rather than the full-length polypeptide (Turunen et al., 1994). Those assays delineate the sequences in the carboxy-terminus necessary for this interaction (Turunen et al., 1994). Others have argued that native ezrin interacts specifically with the
P-actin
isoform and not with skeletal actin (Shuster andHerman, 1995; Yao et al., 1995; Roy et al., 1997). In at least one of those cases (Shuster and Herman, 1995), this unusual specificity appears to depend upon the presence of other proteins, which could be considered as consistent with the requirement that the association with F-actin is not a simple binding reaction but rather one that must be regulated. Thus, it is unclear whether the specificity they detect is a
property of ERM proteins or other proteins associating with ERM proteins. Using a microtiter binding assay similar to ELISA tests, Roy et al (1997) have identified an actin binding site present in the amino-terminus of ezrin which has not been
detected in any previous assay. One explanation for this discrepancy may be that the state of the protein, specifically the presence or absence of a protein tag, affects the conformation of the amino-terminal domain. The authors suggest that a free
terminus is crucial for unmasking the actin-binding properties at the
amino-Introduction
terminus. The details of the ERM protein interaction with actin are yet to be resolved.
The full-length molecule must have ligands other than F-actin. For example, nearly wild type amounts of radixin expressed in stable transfectants not only
localize normally, but displace endogenous moesin from all its normal loci (Henry et al., 1995). This result suggests that the cell contains a saturable element required for proper localization of ERM proteins. To identify such ligands, Tsukita and colleagues (Tsukita et al., 1994) analyzed the proteins that co-immunoprecipitate with anti-ERM antibodies from cultured cells. They showed that a fraction of the cellular CD44, a cell surface glycoprotein expressed in a wide variety of cells,
associates with ERM proteins both by co-precipitation and by co-localization at the level of light microscopy. The domains of co-localization include surface microvilli, cell-cell adhesion sites, and the cleavage furrow. Significantly, there appear to be significant domains of at least CD44 staining that do not co-localize with ERM, suggesting again that the presence of this ligand is not sufficient to specify ERM localization. Several other membrane-associated proteins, such as CD43 , ICAM-1
and ICAM-2 (Heiska et al., 1998; Serrador et al., 1998; Yonemura et al., 1998) have also been identified as binding partners for ERM proteins. Ezrin was identified as a ligand for ICAM-1 after placenta lysate was incubated with a matrix containing
ICAM-1, and one of the most prominent bands eluting from the affinity column
was ezrin (Heiska et al., 1998). In addition, a peptide encompassing the cytoplasmic region of ICAM-2 coupled to Sepharose beads interacted with ezrin (Heiska et al.,
acid motif which was found to be necessary and sufficient for ERM protein binding (Yonemura et al., 1998). Using deletion constructs in in vitro binding studies, these authors mapped the ERM binding site to the first 20 amino acids of the cytoplasmic domain of CD43 and CD44. These amino acids were also important for the correct co-localization and immunoprecipitation of CD44 with ERM proteins. Lastly, by site-directed mutagenesis the authors identified the positively charged amino acid clusters in these three integral membrane proteins as necessary for their interaction with ERM proteins (Yonemura et al., 1998).
Several ligands integrate ERM proteins into the Rho signal transduction pathway. The Rho-GDP dissociation inhibitor, Rho-GDI, a regulator of the GTPase activity for Rho, immunoprecipitates with anti-ERM protein antibodies from cultured cells (Hirao et al., 1996). Another protein in the Rho signal transduction pathway, the myosin binding subunit (MBS) of myosin phosphatase, co-localizes with moesin to Rho induced ruffling edges in MDCK cells. By
co-immunoprecipitation experiments, moesin and MBS interact with one another and binding studies showed a direct interaction between MBS and the N-terminal
domains of moesin and ezrin (Fukata et al., 1998). Furthermore, the interaction between ERM proteins and the pelletable fraction from cell culture lysates increases in the presence of GTPyS and decreases after the addition of C3 toxin, a specific inhibitor of Rho (Heiska et al., 1996). These data suggest that ERM proteins may be recruited to the plasma membrane and its interactions may be regulated in a Rho dependent manner.
Introduction
The effect of phosphorylating ERM proteins and other possible methods of regulating intermolecular interactions will be discussed later in this chapter.
Interdomain interactions: The amino- and carboxy-terminal halves of the
molecule not only bind to other ligands, but to one another as well. Such an interaction was first suggested by the demonstration that a significant fraction of ezrin and moesin from cultured cells could be detected in complexes that contain both proteins (Gary and Bretscher, 1993). By gel overlay assays, purified ezrin and moesin bind to one another, suggesting that the interaction may be direct and binary. Mapping the sequences involved in these associations identifies two domains, in the two extremes of the molecule (Gary and Bretscher, 1995). A direct interaction between the domains is detectable by affinity co-electrophoresis; the data are consistent with a 1:1 complex of the amino- and carboxy-terminal halves of the protein, with a dissociation constant Kd = -4x1O-8M (Magendantz et al., 1995).
Significantly, many of the binding studies described above between ERM proteins and their ligands suggest that the relevant binding site which is embedded in the separate domains is not functional in full-length molecules except upon denaturation. Several ligands such as actin, CD44, band 4.1 and NHE-RF/EBP50 bind to a separate domain of ERM proteins more efficiently than to the full-length proteins (Tsukita et al., 1994; Magendantz et al., 1995; Pestonjamasp et al., 1995; Reczek et al., 1997; Murthy et al., 1998). These data suggest that interdomain
view are borne out by the properties of ERM polypeptides ectopically expressed in cultured cells (see below).
These results support a model in which the amino-terminus interacts with some membrane-associated protein at the same time as the carboxy-terminus acts with a cytoskeletal protein (Figure 1-2). One could imagine that various regulators could interact with ERM proteins to open up the molecule and make both sites accessible; or that the appropriate juxtaposition of the two ligands competes
successfully with the intramolecular binding reaction. Either method would limit the interaction of ERM proteins to only a specific region of the cell. The following section discusses the consequence of misregulated interactions.
DISSECTING ERM FUNCTIONS
Disruption of protein expression. Studying function of ERM proteins in tissue
culture cells by interference experiments is complicated by the likelihood that the three distinct gene products have overlapping roles. Takeuchi et al. (1994)
performed a series of systematic antisense oligonucleotide experiments which demonstrated a function of the three proteins together. Oligonucleotides designed to interfere specifically with expression of each of the ERM transcripts dramatically decreased the level of that protein. For cells attached to substrata, depletion of one ERM protein, or any pair of ERM proteins, had no effect on cell morphology. Loss of all three ERM proteins did have an effect: after 2 days, the cells rounded up and eventually detached. The result suggests that ERM proteins have a direct or indirect effect on maintaining adhesion to solid substrata. That the phenotype is manifested
Introduction
only after a long time may mean that the structures containing ERM proteins in these cells must be relatively stable. Another assay may distinguish among the three proteins. Cells treated with the antisense oligonucleotide directed to moesin can re-attach after trypsinization, but those depleted of ezrin or radixin, respectively, can do so only partially or not at all. Similarly, the effect of antisense
oligonucleotides on the surface morphology of thymoma cells in suspension is obvious when all three proteins are depleted; disrupted singly, only loss of moesin has a detectable effect. These experiments may signify that each of the proteins has
a different function. But the data fit equally well with a quantitative rather than qualitative difference among the three proteins: that the severity of the phenotype may increase with the relative abundance of the particular polypeptide.
Consequences of over-expressing ERM domains. As discussed in the previous
section, in vitro experiments detect an actin binding site in the carboxy-terminal domain conserved in the ERM proteins, binding sites for membrane-associated proteins at the amino-terminal domain and an interaction between the carboxy- and the amino-terminal domains. Expression of the domains in transfected cells
partially confirm and extend these results.
At moderate levels of expression comparable to that of the endogenous protein, the carboxy-terminal domain localizes to the same cortical structures as transfected full-length protein as well as endogenous ERM proteins, except one - the cleavage furrow (Algrain et al., 1993; Henry et al., 1995). Like the full-length protein, the carboxy-terminal domain displaces endogenous ERM proteins. Unlike the
full-length protein, the carboxy-terminal domain localizes to cytoplasmic actin structures in the cell such as stress fibers. At high levels, the carboxy-terminal domain induces the formation of aberrant cortical projections filled with F-actin (Henry et al., 1995; Martin et al., 1995). These cells also fail to undergo cytokinesis; therefore, high levels of the carboxy-terminus can disrupt this function without localizing to the cleavage furrow, perhaps by sequestering some other essential component (Henry et al., 1995).
These results suggest that the C-terminal domain contains enough
information to localize it to many cortical structures, by a mechanism that competes with endogenous ERM proteins. The properties of the transfected cells demonstrate that the carboxy-terminal sequence specifies a binding site sufficient to localize it to the cortex and apparently supplant the endogenous protein. These properties are not readily explicable if the carboxy-terminus can bind only to the amino-terminus. It is important to note that ectopic expression of other actin binding proteins
typically has quite different phenotypes than those observed for this ERM fragment. Among many examples, gelsolin at high levels causes disruption of the major F-actin filaments (Finidori et al., 1992). In contrast, transfected full-length villin induces projections like those seen with the carboxy-terminus, but not full-length, ERM proteins (Friederich et al., 1989).
The consequences of expressing the full-length protein or the amino-terminal domain are quite different from one another. For example, the amino-terminal half of the protein at moderate levels of expression does not show appropriate
localization. When significantly over-expressed, it does localize to all the structures
Introduction
that normally contain ERM proteins - including the cleavage furrow (Henry et al.,
1995). These results suggest that there are cortical binding sites in this domain
-perhaps even one specific for the cleavage furrow. And the full-length protein behaves indistinguishably from the endogenous protein, even when substantially over-expressed. Therefore, the deleterious interactions that mediate the phenotypes of carboxy-terminal over-expression are suppressed in cis (Henry et al., 1995; Martin et al., 1995) - and, in one study, even in trans, when the two fragments are
co-overexpressed (Martin et al., 1995). These results strengthen the notion that the intermolecular interactions of ERM proteins with other proteins are regulated by virtue of being mutually exclusive with intramolecular interactions.
POTENTIAL REGULATORS OF ERM INTERACTIONS
The mechanism by which ERM proteins are restricted to domains of the cortical cytoskeleton is likely to be a crucial aspect of function. Perhaps binding is determined by the physical proximity of two ligands, juxtaposed so that they can compete effectively with postulated interdomain interactions. Alternatively, the intermolecular interactions could be regulated by covalent modification, or by binding of some small molecular ligand.
ERM proteins are phosphorylated in many cells types on tyrosine as well as threonine and serine residues (Gould et al., 1986; Bretscher, 1989; Egerton, 1992; Tsukita and Yonemura, 1997). After EGF stimulation of A431 cells, ERM proteins are phosphorylated within 30 seconds and dephosphorylated after 20 minutes (Bretscher, 1989). This fast and transient time course parallels the formation and
disappearances of cortical structures after EGF stimulation (Bretscher, 1989). Recently, several experiments suggest that ERM proteins may be part of the Rho signal transduction pathway. In LPA-stimulated cells, ERM proteins are
phosphorylated in a Rho-kinase dependent manner and ERM proteins relocalize into cortical protrusions (Shaw et al., 1998). In vitro binding studies showed that the phosphorylation state of ERM proteins affects their binding properties. In a gel overlay assay, the N-terminal domain of radixin- bound to the unphosphorylated form of the C-terminal domain of radixin, but not to the phosphorylated form (Matsui et al., 1998). The phosphorylation state of the C-terminal domain did not affect its ability to bind actin in a pelleting assay (Matsui et al., 1998). These
experiments suggest that the phosphorylation of ERM proteins may regulate their intermolecular interactions by decreasing the competing interdomain interactions. But the fact that the full-length protein is inefficiently phosphorylated and the ERM proteins are phosphorylated and dephosphorylated within two minutes (Matsui et al., 1998) suggests that this method of regulation may be only one of many factors necessary to regulate ERM protein binding to their ligands.
The interaction between ERM proteins and their ligands may also be regulated by binding to small molecules. ERM proteins interact specifically with phosphoinositides as assayed by pelleting with phospholipid micelles (Niggli et al.,
1995) and by their elution profile in the presence of PL through a column containing
Superose 12R gel (Hirao et al., 1996). Furthermore, the presence of phospholipids affects the binding of several ERM proteins to their ligands in vitro. At
physiological conditions, CD44 binds only weakly to ezrin, radixin and moesin. The
Introduction
presence of phosphatidyl inositol (PI), phosphatidyl inositol-4-phosphate (PIP) and phosphatidyl inositol 4, 5 bisphosphate (PIP2) enhanced the binding of CD44 and ERM proteins (Hirao et al., 1996). Phosphoinositides also enhanced ezrin binding to
ICAM-2 and induced the interaction between ezrin and ICAM-1 (Heiska et al., 1998).
That phospholipids have a role in regulating protein-protein interactions has been previously documented with other cytoskeletal proteins, but the exact mechanism
by which phospholipids work is not yet known. Understanding how ERM proteins
are recruited to specific regions of the cell will increase our understanding of their role in the cell.
IDENTIFICATION OF MERLIN
A detailed function for any member of the ERM family still remains
unknown. However the identification of merlin, raises the possibility that aspects of ERM function may be involved in cell growth control. Mutations in merlin are implicated for neurofibromatosis type 2, a human disease causing the formation of multiple nervous system tumors, especially schwannomas. A first crucial question is whether or not merlin is indeed a member of the ERM family. That is, does merlin behave like an ERM protein in those assays that are available? Transfected merlin in COS cells gives a punctate pattern, and general staining of the membrane (den Bakker et al., 1995). More apposite is the identification of endogenous merlin in both fibroblast and meningioma cells in motile domains of the cortex (Gonzalez et al., 1996). Despite this similarity to ERM proteins, the anti-merlin staining in meningioma cells is clearly distinguishable from that of anti-ezrin or anti-moesin.
In Drosophila, the merlin homolog is in quite different localizations than is the moesin homolog, providing a system for genetic tests of functions of both proteins (McCartney and Fehon, 1996). These striking differences between merlin and ERM proteins may mean that properties of merlin overlap but are not coincident with those of other ERM proteins. Obviously, there are further assays that could assess parallels of properties: does ectopic expression of merlin domains have phenotypes?
do those domains bind to one another, or to other ERM ligands? Finally, the intriguing finding that transfection of merlin can suppress some phenotypes of transformed cells (Tikoo et al., 1994; Sherman et al., 1997) suggests an assay for ERM
function as well, and the possibility of identifying ERM and merlin sequences that are essential for certain properties.
ERM PROTEIN HOMOLOGS IN OTHER ORGANISMS
The possibilities for understanding the relationship between ERM structure and function are enhanced by the identification of homologs in several invertebrate species. D. melanogaster expresses both moesin and merlin proteins (Edwards, 1994; McCartney and Fehon, 1996). These two proteins are differentially distributed in the developing fly; moesin distributes to the plasma membrane, while merlin was seen in punctate structures at the plasma membrane and cytoplasm. Analysis of mutant phenotypes suggested that merlin is involved in endocytic processes by studies in cultured cells. Sea urchins express a moesin homolog and both localization data and drug studies suggested that it interacts with actin (Bachman and McClay, 1995). There is also evidence for homologs in Schistosorna japonicum (Kurtis et al., 1997)
Introduction
and in C. elegans (Genbank, accession: U10414). An ERM-homolog, called EM10, was identified in the tapeworm Echinococcus multilocularis (Frosch et al., 1991). EM10 is a highly immunogenic protein, and is the dominant antigen associated with the parasitic disease alveolar echinococcosis. The sequence of EM10 is 46.9% identical to that of human ezrin. As for other homologs, the EM10 homology is more pronounced in the amino-terminal half of the protein. To learn more about EM10 and ERM function, we have compared the properties of these proteins when they are ectopically expressed in cultured cells. In particular, following on studies of ERM proteins, we analyzed the localization and phenotypic consequences of
expressing full-length EM10 and its amino- and carboxy-terminal halves. The results demonstrate that EM10 sequences behave similarly but not identically to those of ERM-proteins (Hubert, Cordero et al., in press). By studying the role of ERM protein homologs in other organisms, which may be genetically tractable, we may uncover the role for ERM proteins in animal cells.
OTHER CYTOSKELETAL PROTEINS USE SIMILAR MODES FOR REGULATING THEIR INTERACTIONS WITH LIGAND
The generally-accepted model of regulation for ERM proteins (Figure 1-2) in which a reciprocal relationship between interdomain binding and intermolecular binding is not novel for ERM proteins. A similar model was proposed earlier for vinculin and its binding to talin and actin (Johnson and Craig, 1995). In that case, the interaction between the carboxy-terminal domain and F-actin is blocked by the presence of the amino-terminal domain polypeptide. The actin binding activity is
not detected with full-length vinculin. This same binding property is detected between vinculin and talin - the vinculin head binds to talin whereas the
full-length protein does not. An interdomain interaction has been proposed for this effect (Johnson and Craig, 1995). This interdomain interaction identified for vinculin is disrupted by phospholipids which allows vinculin to bind to talin (Johnson and Craig, 1994; Gilmore and Burridge, 1996).
Another cytoskeletal protein which uses these same method for regulation is band 4.1 and its interaction with glycophorin (Anderson and Marchesi, 1985). The binding between these two proteins increases in the presence of a
polyphosphoinositide cofactor. Thus the regulation of intermolecular interactions
by phospholipids occurs with other cytoskeletal proteins and their ligands.
Introduction
Figure 1-1: ERM family of proteins
Radixin is a member of the ERM (ezrin, radixin, moesin) family of proteins. The amino-terminal domains are highly homologous. The ERM family is part of the Band 4.1 superfamily which includes the proteins Band 4.1, talin, a homologous protein found in echinococcus - EM10 and the product of a tumor suppressor gene - merlin.
The Band 4.1
ERM Family
Ezrin
Radixin
Moesin
EM-10
Talin
Band 4.1
Superfamily
35%Merlin
62% NC
84% U 83%/ 58% .. ... ...Figure 1-2: Current model of ERM regulation
The current model in the field is that the full-length ERM protein forms an
interdomain interaction which blocks the binding sites present on the
individual domains. It isn't until a modification occurs that the ERM protein changes to the "open" conformation and makes the binding sites available to their ligands.
-
Cortical
Proteins
-Actin
ERM proteins
THESIS OVERVIEW
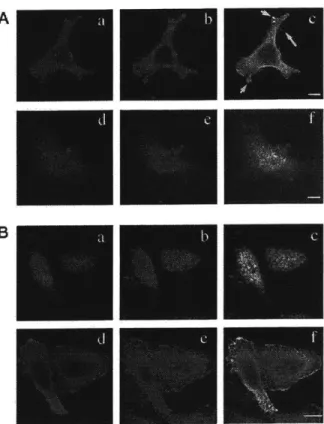
In my thesis, I use two proteins, NHE-RF and layilin, to study the regulation of ERM protein binding to its ligands. NHE-RF interacts with ezrin, radixin and moesin in affinity chromatography binding assays (Murthy et al., 1998; Reczek and Bretscher, 1998). NHE-RF also binds to a protein homologous to ERM proteins, merlin which is the product of the tumor suppresser gene, NF2 (Murthy et al., 1998). In HeLa cells, NHE-RF co-aligns with moesin at the microvilli, filopodia and
ruffling edges (Murthy et al., 1998). NHE-RF was originally identified as a regulatory co-factor necessary for protein kinase A inhibition of a Na+/H+
exchanger (NHE) (Weinman et al., 1995). A second ligand I study for my thesis is layilin. Layilin is a novel transmembrane protein which binds talin and has homology to C-type lectins. It localizes to ruffling edges in NIL8 and CHO cells
(Borowsky and Hynes, 1998).
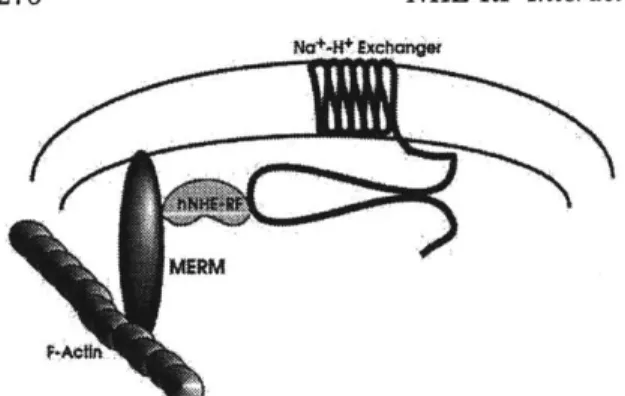
My work shows that layilin and NHE-RF are direct ligands for radixin and
their binding sites map to the amino-terminal domain of radixin. Ligand binding to the amino-terminal domain is decreased by the presence of the C-terminal domain of radixin in cis as well as in trans. The interaction between radixin and its ligands can be enhanced by phosphatidyl-inositol 4 phosphate (PIP). We show that PIP changes the conformation of radixin at the amino-terminal domain and decreases the amino- and carboxy- interdomain interaction. By releasing this interdomain
interaction, phosphoinositides can regulate the interaction between radixin and its ligands.
BIBLIOGRAPHY
Algrain, M., Turunen, 0., Vaheri, A., Louvard, D., and Arpin, M. (1993). Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membran-cytoskeletal linker.
J.
Cell Biol. 120, 129-139.Anderson, R. A., and Marchesi, V. T. (1985). Regulation of the association of
membrane skeletal protein 4.1 with glycophorin by a polyphosphoinositide. Nature
318, 295-298.
Bachman, E. S., and McClay, D. R. (1995). Characterization of moesin in the sea urchin Lytechinus variegatus: redistribution to the plasma membrane following fertilization is inhibited by cytochalasin B.
J
Cell Sci 108, 161-71.Berryman, M., Zsofia Franck and Anthony Bretscher (1993). Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. Journal of Cell Science 105, 1025-1043.
Birgbauer, E., and Solomon, F. (1989). A marginal band-associated protein has properties of both microtubule- and microfilament-associated proteins. Journal of Cell Biology 109, 1609-1620.
Birgbauer, E. C. (1991). Cytoskeletal interactions of ezrin in differentiated cells: M.I.T.).
Borowsky, M. L., and Hynes, R. 0. (1998). Layilin, a novel talin-binding
transmembrane protein homologous with C- type lectins, is localized in membrane ruffles.
J
Cell Biol 143, 429-42.Bretscher, A. (1983). Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells.
J.
of Cell Biology 97, 425-532.Bretscher, A. (1986). Purification of the intestinal microvillus cytoskeletal proteins villin, fimbrin, and ezrin. Meth. Enzymology 134, 24-37.
Bretscher, A. (1989). Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes Induced in A-431 cells by epidermal growth factor. Journal of Cell Biology 108, 921-930.
den Bakker, M., Riegman, P., Hekman, R., Boersma, W., Janssen, P., van der Kwast, T., and Zwarthoff, E. (1995). The product of the NF2 tumour suppressor gene
localizes near the plasma membrane and is highly expressed in muscle cells. Oncogene 10, 756-763.
Introduction
Edwards, K. A., Ruth A. Montague, Scott Shepard, Bruce A. Edgar, Raymond L. Erikson and Daniel P. Kiehart (1994). Identification of Drosophila cytoskeletal proteins by induction of abnormal cell shape in fission yeast. Proc. Natl. Acad. Sci.
USA 91, 4589-4593.
Egerton, M., Wilson H. Burgess, Douglas Chen, Brian
J.
Druker, Anthony Bretscher and Lawrence E. Samelson (1992). Identification of Ezrin as an 81-kDa Tyrosine-Phosphorylated Protein in T Cells. Journal of Immunology 149, 1847-1852.Finidori,
J.,
Friederich, E., Kwiatkowki, D., and Louvard, D. (1992). In vivo analysis of functional domains from villin and gelsolin. Jour. Cell Biol. 116, 1145-1155. Franck, Z., Ronald Gary and Anthony Bretscher (1993). Moesin, like ezrin,colocalizes with actin in the cortical cytoskeleton in cultured cells, but its expression is more variable. Journal of Cell Science 105, 219-231.
Friederich, Huet, Arpin, and Louvard (1989). Villin induces microvilli growth and actin redistribution in transfected fibroblasts. cell 59, 461-475.
Frosch, P. M., Frosch, M., Pfister, T., Schaad, V., and Bitter-Suermann, D. (1991). Cloning and characterisation of an immunodominant major surface antigen of Echinococcus multilocularis. Mol Biochem Parasitol 48, 121-130.
Fukata, Y., Kimura, K., Oshiro, N., Saya, H., Matsuura, Y., and Kaibuchi, K. (1998). Association of the myosin-binding subunit of myosin phosphatase and moesin: dual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase.
J
Cell Biol 141, 409-18.Gary, R., and Bretscher, A. (1993). Heterotypic and homotypic associations between ezrin and moesin, two putative membrane-cytoskeletal linking proteins. Proc. Natl. Acad. Sci. USA 90, 10846-10850.
Gary, R., and Bretscher, A. (1995). Ezrin self-association involves binding of an N-terminal domain to a normally masked C-N-terminal domain that includes the F-actin binding site. Mol. Biol. Cell 6, 1061-1075.
Gilmore, A. P., and Burridge, K. (1996). Regulation of vinculin binding to talin and actin by phosphatidyl-inositiol-4-5- bisphosphate. Nature 381, 531-535.
Gonzalez, -. A., C., Xu, L., Pinney, D., Beauchamp, R., Hobbs, W., Gusella,
J.,
andRamesh, V. (1996). The merlin tumor suppressor localizes preferentially in membrane ruffles. Oncogene 13, 1239-1247.
Goslin, K., Birgbauer, E., Banker, G., and Solomon, F. (1989). The role of cytoskeleton in organizing growth cones: a microfilament-associated growth cone component depends upon microtubules for its localization.
J.
Cell Biol. 109, 1621-1631.Gould, K. L., Bretcher, A., Cooper,
J.
A., and Hunter, T. (1986). The protein-tyrosine kinase substrate, p81, is homologous to a chicken microvillar core protein. JCB 102,660-669.
Heiska, L., Alfthan, K., Gronholm, M., Vilja, P., Vaheri, A., and Carpen, 0. (1998). Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and
ICAM-2). Regulation by phosphatidylinositol 4, 5- bisphosphate.
J
Biol Chem 273, 21893-900.Heiska, L., Kantor, C., Parr, T., Critchley, D. R., Vilja, P., Gahmberg, C. G., and Carpen, 0. (1996). Binding of the cytoplasmic domain of intercellular adhesion molecule-2 (ICAM-2) to alpha-actinin.
J
Biol Chem 271, 26214-9.Henry, M., Gonzalez-Agosti, C., and Solomon, F. (1995). Molecular dissection of radixin: Distinct and interdependent functions of the amino- and carboxy-terminal domains. Journal of Cell Biology 129, 1007-1022.
Higashitsuji, H., Arii, S., Furutani, M., Imamura, M., Kaneko, Y., Takenawa,
J.,
Nakayama, H., and Fujita,J.
(1995). Enhanced expression of multiple proteintyrosine phosphatases in the
regenerating mouse liver: isolation of PTP-RL10, a novel cytoplasmic-type
phosphatase with sequence homology to cytoskeletal protein 4.1. Oncogene 10, 407-414.
Hirao, M., Sato, H., Kondo, T., Yonemura, S., Monden, M., Sasaki, T., Takai, Y., Tsukita, S., and Stukit, S. (1996). Regulation mechanism of ERM
(ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. Jour. Cell Biol. 135, 37-51.
Johnson, R. P., and Craig, S. W. (1994). An intramolecular association between the head and tail domains of vinculin modulates talin binding. Journal of Biological Chemistry 269, 12611-12619.
Johnson, R. P., and Craig, S. W. (1995). F-actin binding site masked by the
intramolecular association of vinculin head and tail domains. Nature 373, 261-264. Kurtis,
J.
D., Ramirez, B. L., Wiest, P. M., Dong, K. L., El-Meanawy, A., Petzke, M. M.,Johnson, J. H., Edmison,
J.,
Maier, R. A., Jr., and Olds, G. R. (1997). Identification and molecular cloning of a 67-kilodalton protein in Schistosoma japonicumhomologous to a family of actin-binding proteins. Infect Immun 65, 344-7. L'Abbe, D., Banville, D., Tong, Y., Stocco, R., Masson, S., Ma, S., Fantus, G., and Shen, S.-H. (1994). Identification of a novel protein tyrosine phosphatase with
Introduction
sequence homology to the cytoskeleltal proteins of the band 4.1 family. FEBS Letters
356, 351-356.
Lankes, W., Griesmacher, A., Grunwald,
J.,
Schwartz-Albietz, R., and Keller, R.(1988). A heparin-binding protein involved in inhibition of smooth-muscle cell
proliferation. Biochem.
J.
251, 831-842.Magendantz, M., Henry, M., Lander, A., and Solomon, F. (1995). Inter-domain interactions of radixin in vitro. Jour. Biol. Chem. 270, 25324-25327.
Martin, M., Andreoli, C., Sahuquet, A., Montcourrier, P., Algrain, M., and Mangeat, P. (1995). Ezrin NH2-terminal domain inhibits the cell extension activity of the COOH-terminal domain. Jour. Cell Biol. 128, 1081-1093.
Matsui, T., Maeda, M., Doi, Y., Yonemura, S., Amano, M., Kaibuchi, K., and Tsukita,
S. (1998). Rho-kinase phosphorylates COOH-terminal threonines of
ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association.
J
Cell Biol 140, 647-57.McCartney, B., and Fehon, R. (1996). Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophila homologues of moesin and the neurofibromatosis 2 tumor supressor, merlin. Jour. Cell Biol. 133, 843-852. Murthy, A., Gonzalez-Agosti, C., Cordero, E., Pinney, D., Candia, C., Solomon, F., Gusella,
J.,
and Ramesh, V. (1998). NHE-RF, a regulatory cofactor for Na(+)-H+ exchange, is a common interactor for merlin and ERM (MERM) proteins.J
Biol Chem 273, 1273-6.Niggli, V., Andreoli, C., Roy, C., and Mangeat, P. (1995). Identification of a
phosphatidylinositiol-4,5-bisphosphate-binding domainin the N-terminal region of ezrin. FEBS Letters 376, 172-176.
Pakkanen, R. (1988). Immunofluorescent and immunochemical evidence for the expression of cytovillin in the microvilli of a wide range of cultured human cells.
J.
Cell. Biochem. 38, 65-75.Pakkanen, R., Hedman, K., Turunen, 0., Wahlstrom, T., and Vaheri, A. (1987). Microvillus-specific Mr 75,000 plasma membrane protein of human
choriocarcinoma cells.
J.
of Histochem. Cytochem. 35, 809-816.Pestonjamasp, K., Amieva, M., Strassel, C., Nauseef, W., Furthmayr, H., and Luna,
E. (1995). Moesin, ezrin and p205 are actin-binding proteins associated with
Reczek, D., Berryman, M., and Bretscher, A. (1997). Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family.
J
Cell Biol 139, 169-79.Reczek, D., and Bretscher, A. (1998). The carboxyl-terminal region of EBP50 binds to a site in the amino- terminal domain of ezrin that is masked in the dormant
molecule.
J
Biol Chem 273, 18452-8.Roy, C., Martin, M., and Mangeat, P. (1997). A dual involvement of the amino-terminal domain of ezrin in F- and G- actin binding.
J
Biol Chem 272, 20088-95. Sato, N., Yonemura, S., Obinata, T., Tsukita, S., and Tsukita, S. (1991). Radixin, a barbed-end-capping actin-modulating protein, is concnetrated at the cleavage furrow during cytokinesis. Journal of Cell Biology 113, 321-330.Sawada, M., Ogata, M., Fujino, Y., and Hamaoka, T. (1994). cDNA cloning of a novel protein tyrosine phosphatase with homology to cytoskeletal protein 4.1 and its expression in T-lineage cells. BBRC 203, 479-484.
Serrador,
J.
M., Nieto, M., Alonso-Lebrero,J.
L., del Pozo, M. A., Calvo,J.,
Furthmayr, H., Schwartz-Albiez, R., Lozano, F., Gonzalez-Amaro, R., Sanchez-Mateos, P., and Sanchez-Madrid, F. (1998). CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood 91, 4632-44.Shaw, R.
J.,
Henry, M., Solomon, F., and Jacks, T. (1998). RhoA-dependentphosphorylation and relocalization of ERM proteins into apical membrane/actin protrusions in fibroblasts. Mol Biol Cell 9, 403-19.
Sherman, L., Xu, H. M., Geist, R. T., Saporito-Irwin, S., Howells, N., Ponta, H., Herrlich, P., and Gutmann, D. H. (1997). Interdomain binding mediates tumor growth suppression by the NF2 gene product. Oncogene 15, 2505-9.
Shuster, C., and Herman, I. (1995). Indirect association of ezrin with F-actin: isoform specificity and calcium sensitivity. Jour. Cell. Biol. 128, 837-848.
Tikoo, A., Varga, M., Ramesh, V., Gusella,
J.,
and Maruta, H. (1994). An anti-ras function of neurofibromatosis type 2 gene product (NF2/Merlin). Jour. Biol. Chem. 269, 23387-23390.Tsao, H., Aletta,
J.
M., and Greene, L. A. (1990). Nerve growth factor and fibroblast growth factor selectively activate a protein kinase that phosphorylates highmolecular weight microtubule-associated proteins. Detection, partial purification, and characterization in PC12 cells.
J
Biol Chem 265, 15471-80.Introduction
Tsukita, S., Oishi, K., Sato, N., Sagara,
J.,
Kawai, A., and Tsukita, S. (1994). ERM family members as molecular linkers between the cell surface glycoprotein CD44and actin-based cytoskeletons. Journal of Cell Biology 126, 391-401.
Tsukita, S., and Yonemura, S. (1997). ERM proteins: head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem Sci 22, 53-8.
Tucker, R. P., Garner, C. C., and Matus, A. (1989). In Situ localization of microtubule-associated protein mRNA in the developing and adult rat brain. Neuron 2,
1245-1256.
Turunen, 0., Wahlstrom, T., and Vaheri, A. (1994). Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. Journal of Cell Biology 126, 1445-1453.
Weinman, E.
J.,
Steplock, D., Wang, Y., and Shenolikar, S. (1995). Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brushborder membrane Na(+)-H+ exchanger.
J
Clin Invest 95, 2143-9.Yang,
Q.,
and Tonks, N. K. (1991). Isolation of a cDNA clone encoding a human protein-tyrosine phosphatase with homology to the cytoskeletal-associated proteins band 4.1, ezrin and talin. Proc. Natl. Acad. Sci. USA 88, 5949-5953.Yao, X., Chaponnier, C., Gabbiani, G., and Forte,
J.
G. (1995). Polarized distribution ofactin isoforms in gastric parietal cells. Mol Biol Cell 6, 541-57.
Yao, X., Cheng, L., and Forte,
J.
(1996). Biochemical characterization of ezrin-actininteraction. Jour. Biol. Chem. 271, 7224-7229.
Yonemura, S., Hirao, M., Doi, Y., Takahashi, N., Kondo, T., and Tsukita, S. (1998). Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2.
J
CHAPTER 2:
The interaction between the amino and carboxy-terminal domains of ERM proteins is well documented. By gel overlay assays, purified ezrin and moesin bind
to one another, and the sequence involved in the association was mapped to the two domains in the extreme domains of the molecule (Gary and Bretscher, 1995). Furthermore, for many assays, the activities which are seen with fragments of ERM proteins are diminished when the assays are done with full-length proteins. In
vitro experiments show that several ligands such as actin, CD44, band 4.1 and
NHE-RF/EBP50 bind to a separate domain of ERM proteins more efficiently than to the full-length proteins suggesting an inhibitory effect of the complementary domain (Tsukita et al., 1994; Magendantz et al., 1995; Pestonjamasp et al., 1995; Murthy et al.,
1998; Reczek and Bretscher, 1998). This difference between full-length and fragment
activities is also seen in in vivo experiments. Unlike the full-length ERM protein, the exogenously expressed carboxy-terminus localizes to stress fibers (Algrain et al.,
1993), and upon overexpression induces the formation of aberrant actin-rich cortical
extensions in animal cells (Algrain et al., 1993; Henry et al., 1995). These data could be explained by a direct interaction between the amino- and carboxy-terminal
domains. In fact an interaction between the domains is detectable by affinity co-electrophoresis; the data are consistent with a 1:1 complex of the amino- and carboxy-terminal halves of the protein with a Kd of ~4x1O-8M (Magendantz et al.,
1995). In the full-length proteins, this interdomain interaction could mask the
Since the interdomain interaction may inhibit the binding between ERM proteins and their ligands, the regulation of this interdomain interaction can modulate the interaction between radixin and its ligands. The effect of
phosphorylating the carboxy-terminal domain of radixin was shown to decrease its ability to bind to the amino-terminal domain of radixin, but it did not affect its ability to bind to actin (Matsui et al., 1998). In this case the release of the
interdomain interaction by a phosphorylation event may increase the interaction between radixin and actin. Another possible method of regulating these
interactions is by binding to small molecule regulators. The presence of
phospholipids affects the binding of several ERM proteins to their ligands in vitro. CD44 does not bind to full-length ezrin at physiological conditions except in the presence of PIP2 (Hirao et al., 1996). PIP2 also increases the affinity between ezrin and ICAM-1 and ICAM-2 (Heiska et al., 1998). Although binding sites for specific phospholipids have been identified at the amino-terminal domain of ezrin (Niggli et al., 1995), the exact mechanism for how these phospholipids affect the protein-protein interactions is not known.
In this chapter, I describe the interdomain interactions between the amino and carboxy-terminal domains of radixin. I show that this interdomain interaction can be disrupted by the presence of PIP. The interdomain interaction and its
modulation by phospholipids may represent a regulated change in the tertiary structure of the protein. A specific conformation of radixin can be captured by cross-linkers. Cross-linking reagents capture an intramolecular interaction at the amino-terminal domain of radixin and this intramolecular interaction is disrupted by the
Domain interactions
presence of specific phospholipids. PIP changes the conformation of the amino-terminal domain and decreases the interdomain interaction. As I will describe later, the loss of the interdomain interaction increases ligand binding to full-length
MATERIALS AND METHODS
Antibodies
To detect radixin proteins by immunoblotting, we used polyclonal antibodies specific for the amino-terminal domain of ERM proteins (220) or a carboxy-terminal
sequence specific for radixin (457-3) (Winckler, 1994).
Recombinant Proteins
His6-tagged versions of murine radixin constructs were expressed and purified as previously described for chicken radixin (Magendantz et al., 1995). The His6-tagged radixin constructs we used were His6-tagged full-length radixin
(HisRadFL, residues 1-583, strain EC74), the amino-terminal domain of radixin (HisRadN, residues 1-318, strain EC75), and the carboxy-terminal domain of radixin (HisRadC, residues 319-583, strain EC76). To purify the protein, a saturated culture was diluted 1:10 in 500 ml of LB with 100gg/ml ampicillin and grown for 2 hours at
37*C. We induced protein expression by adding
isopropyl-p-D-thiogalactopyranoside (IPTG) to a final concentration of 1mM and grew for an additional 3-4 hours. The cells were collected by centrifugation in a Beckman JA-14 rotor at 5000rpm for 20 minutes. We froze the cell pellets in a dry ice/ethanol solution and stored them at -85'C and quickly thawed the pellets in 10 ml lysis buffer (50mM NaH2PO4/NaHPO 4, pH 8.0, 300mM NaCl; 20mM imidazole, pH 8.0;
1mM pefabloc, 1mM leupeptin, 1mM pepstatin, 0.007 TIU/ml aprotinin), lysed the
cells by sonication, and collected the high speed supernatant by centrifugation at
15000 rpm in a Beckman JA-20 rotor for 20 minutes. The lysate was supplemented
with 10mM f-mercaptoethanol then incubated with 3.0 ml Ni-NTA Sepharose CL-6B resin (Qiagen) which had been pre-equilibrated with Buffer I (300mM NaCl, 50mM NaH2PO4/NaHPO 4, 20mM imidazole, pH 8.0) for 20 minutes at 4'C. The beads were washed twice with Buffer V (40mM imidazole buffer, 300mM NaCl, 50mM NaH2PO4/NaHPO 4, pH 8.0, 10% glycerol) and once with Buffer VI (40mM imidazole buffer, 300mM NaCl, 50mM NaH2PO4/NaHPO4, pH 8.0). We loaded the beads into a 5ml column and eluted the protein fractions with Buffer VII (250mp. imidazole, 300mM NaCl, 50mM NaPhosphate pH8.0). The fractions containing the highest concentrations of proteins were pooled.
All purified proteins were dialyzed against PBS using rotating Pierce
Slide-a-lyzers for four hours changing the buffer every hour. We added a protease inhibitor cocktail (1mM pefabloc, 1mM leupeptin, 1mM pepstatin, 0.007 TIU/ml aprotinin and 1mM phenylmethylsulfonyl fluoride). The protein preparations were separated into small aliquots, quickly frozen in liquid nitrogen and stored at -40'C.
Immediately before each experiment, protein aliquots were quickly thawed and held on ice until used.
We created a GST tagged version of the carboxy-terminal domain of radixin (GSTRadC) using a previously described plasmid containing the carboxy-terminal domain of radixin behind an inducible promoter - pUHD-HAC-RADC (Henry et al., 1995). The plasmid pUHD-HAC-RADC, was digested at its XhoI sites (New England
the protein (Henry et al., 1995). The radixin insert was separated from the pUHD vector on an agarose gel and purified using a Qiaex gel extraction kit (Qiagen, Chatsworth, CA). This insert was ligated into a unique XhoI site within the polylinker of pGEX-5X-1 (Pharmacia) which keeps the coding sequence in frame.
Escherichia coli DH5aF'IQ containing the correct insert orientation was identified by
diagnostic restriction enzyme digests (EC88).
GST fusions of the carboxy-terminal domain of radixin (GSTRadC, residues
319-583) were expressed in Escherichia coli DH5cL and purified using glutathione
beads. A saturated culture was diluted 1:30 into 450ml LB medium containing 50 jg/ml ampicillin. We cultured the cells at 37'C until an OD600 reading of a sample of the culture reached between 0.45-0.60. Protein expression was induced by incubating the cells with 0.05mM isopropyl-1--D-thiogalactopyranoside (IPTG) for two hours. The cells were harvested by centrifugation 5000 rpm in a Beckman JA-14 rotor at 4'C for 20 min. We lysed the cells by freeze/thawing and removed the insoluble fraction by centrifugation (16000 rpm in a Beckman JA-20 rotor at 4'C). The high speed supernatant was incubated with glutathione Sepharose 4B beads (Pharmacia) with 0.2% Tween-20 for 1 hour at 4'C. The beads were washed with PBS/0.2% Tween-20 and eluted the proteins with glutathione elution buffer (5mM glutathione, 50mM Tris pH 8.0, 0.2% Tween-20). We dialyzed and stored these proteins as stated for the His6 tagged radixin proteins.
Domain interactions
Phospholipids
The phospholipids used in this study were: PIP2 (Phosphatidylinositol
4,5-bisphosphate disodium salt); PS (O-(3-sn-Phosphatidyl)-L-serine sodium salt); IP3 (D-myo-inositol-1,4,5-triphosphate penta-potassium salt); PIP (Phosphatidylinositol 4-phosphate sodium salt); OAG (1-oleolyl-2-acetyl-rac-glycerol); and PC (Phosphatidyl
choline). All phospholipids were purchased from Sigma. The phospholipids were resuspended in water to a final concentration of 1mg/ml, then sonicated for 5
minutes; the stock solutions were frozen in small aliquots using liquid nitrogen and stored at -40'C. The solutions of PC and OAG did not completely clarify. The
phospholipids were sonicated for 30s - 1min immediately before use in the cross-linking assays.
Cross-linking reagents
The cross-linking reagents and abbreviations used in this study were: BS3 (Bis[sulfosuccinimidyl]suberate), DTSSP
(3,3'-Dithiobis[sulfosuccinimidyl-propionate]); DSP (Dithiobis[succinimidyl-propionatel); BSOCOES (Bis[2-(succinimidoxycarbonyloxy)ethylsulfone); EGS (ethylene
glycolbis-[succinimidylsuccinate]); DST (Disuccinimidyl tartarate); DTBP (Dimethyl 3,3'-dithiobispropionimidate 2HCl); and EDC (1-Ethyl-3[3-dimethylaminopropyl]-carbodiimide Hydrochloride). All were purchased from Pierce. We prepared fresh 10x stock solutions in DMSO immediately before use.