Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Combustion Institute Canadian Section, 2007 Spring Technical Meeting
[Proceedings], 2007
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=315c32df-6626-47fb-a3d5-06db8db40bed https://publications-cnrc.canada.ca/fra/voir/objet/?id=315c32df-6626-47fb-a3d5-06db8db40bed
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Effect of reformate gas enrichment on temperature and NO formation in
strained CH4/air diffusion flames
EFFECT OF REFORMATE GAS ENRICHMENT ON
TEMPERATURE AND NO FORMATION IN STRAINED
CH
4/AIR DIFFUSION FLAMES
Hongsheng Guo, Stuart W. Neill, Gregory J. Smallwood
Institute for Chemical Process and Environmental Technology National Research Council, 1200 Montreal Road, Ottawa, Ontario K1A 0R6
INTRODUCTION
Fuel enrichment is a promising concept for reducing fuel consumption and pollutant emission from combustion systems. Hydrogen has been shown to be an effective enrichment component in many studies. For example, it has been shown that hydrogen enrichment can improve flame stability and thus significantly reduce NOX formation by allowing a combustor to operate at leaner condition [1-3] in
premixed flames. For diffusion flames, hydrogen enrichment can suppress the formation of soot particles [4,5] and shorten ignition delay [6,7].
Relatively, little attention has been paid to the effect of hydrogen enrichment on NOX formation in
diffusion flames. In general, NO, the dominant component of NOX, is mainly formed by the prompt route
in a hydrocarbon diffusion flame. When hydrogen is added, it is expected that the formation of NO by the prompt route can be reduced because of the reduction in radical CH. On the other hand, the addition of hydrogen may modify flame temperature, which in turn may change the formation of NO by the thermal route. Therefore, the net effect of hydrogen enrichment on NOX formation in a hydrocarbon diffusion
flame depends on the relative variations of the thermal and prompt routes. Naha and Aggarwal [8] and our previous study [9] investigated the effect of hydrogen addition on NOX formation in strained
nonpremixed methane and n-heptane flames. It was found that the addition of hydrogen has a minor effect on NOX formation in methane flames and reduces the formation of NOX in n-heptane flames.
However, hydrogen is an energy carrier. It has to be obtained from other hydrocarbon fuels or water. A general method to obtain hydrogen is fuel reforming. The product of fuel reforming, known as reformate gas, contains not only hydrogen, but also carbon monoxide and some other components, depending on the method of reforming. Instead of using hydrogen, it is more practical and economical to directly use reformate gas as an enrichment component. It is of great interest to study the effect of reformate gas enrichment on combustion and pollutant emission. Our study [10] has shown that like hydrogen enrichment, reformate gas enrichment also improves the flame stability and reduces NOX
formation in lean CH4/air premixed flames.
In this paper, we further investigate the effect of reformate gas enrichment on diffusion flames by numerical simulation. The focus will be flame temperature and the formation of NO, the dominant component of NOX. Methane is selected as the hydrocarbon fuel.
NUMERICAL MODEL
The flame configuration studied is an axisymmetric laminar counterflow diffusion flame, with fuel stream issuing from one nozzle and air from another. The simulations assumed the stagnation point flow approximation. The governing equations can be found elsewhere [11]. The calculations were carried out with a code revised from that of Kee et al. [12]. Upwind and center difference schemes were, respectively, used for the convective and diffusion terms in all the governing equations. Adaptive refinement of meshes was done to obtain grid independent results. Radiation heat loss was accounted for by an optically thin model [13]. The potential boundary conditions were used.
The chemical reaction mechanism used is GRI-Mech 3.0 [14], which is an optimized mechanism for methane combustion and has been validated over a wide range of flame conditions. The thermal and transport properties were obtained by using the database of GRI-Mech 3.0 and the algorithms given in [15, 16]. The pressure and the fresh mixture temperature were, respectively, 1 atm and 298 K.
The reformate gas was assumed to be the product of partial oxidation of methane by air via the reaction 2CH4 + O2 + 79
21N2 = 2CO + 4H2 + 79
21N2. Therefore, the volume composition of the reformate
gas is (42CO + 84H2 + 79N2)/205.
RESULTS AND DISCUSSION
In all the studied flames, the fuel stream consists of methane and reformate gas. The fraction of reformate gas is defined asαRG =VRG/(VRG+VCH4), with VRG and VCH4 being, respectively, the volume
flow rates of reformate gas and methane. The quantity a in all the figures represents strain rate. Although we are interested in fuel enrichment combustion, which only requires a small amount of reformate gas addition, the studied fraction of reformate gas covered a range from 0.0 to 1.0 for completeness.
Temperature Variation
Since part of the fuel’s chemical energy is released during reforming, the adiabatic equilibrium temperature of pure reformate gas is lower than that of pure methane at stoichiometric condition. This has been shown to be true for near stoichiometric premixed flames [10]. Therefore, it was expected that the addition of reformate gas to methane would also reduce the temperature for a diffusion flame. However, our simulation does not show such a result, as shown in Fig. 1, where the variations of the maximum flame temperature at three typical strain rates are displayed, as the fraction of added reformate gas changes from 0 to 1.0. The three selected strain rates represent a low, moderate and high value, respectively. The highest one (300 s-1) is close to the strain extinction limit for CH
4/air diffusion flame at
atmosphere pressure and room temperature condition.
It is observed that at a low strain rate (10 s-1), the addition of reformate gas results in a monotonic
increase in the maximum flame temperature, until pure reformate gas condition is reached. When the strain rate is high (300 s-1), the maximum flame temperature first increases, and then decreases, as the
fraction of reformate gas changes from 0 to 1.0. The situation at a moderate strain rate (100 s-1) is
basically similar to that at a high strain rate, except for a slight difference at near pure reformate gas flame condition, at which the maximum temperature rises again with increasing the reformate gas fraction. Being different from the near stoichiometric premixed flames [10], the maximum temperature of pure reformate gas diffusion flame is higher than that of the pure methane at all strain rates.
The above phenomenon is caused by variations in adiabatic flame temperature and fuel Lewis number. As mentioned before, the addition of reformate gas tends to reduces flame temperature due to the reduction in adiabatic equilibrium temperature. However, reformate gas addition also changes the fuel Lewis number, defined as the ratio of the thermal to mass diffusion rate. It is known that non-unity Lewis number of fuel or oxidant affects flame temperature for a diffusion flame [17]. Temperature is increased/decreased with increasing/decreasing Lewis number of either fuel or oxidant. Ultra-low Lewis number sometimes causes the peak temperature to exceed the adiabatic equilibrium flame temperature in a diffusion flame, such as in some hydrogen diffusion flames. The Lewis numbers of fuel in pure methane diffusion flames is close to unity, and hence has negligible effect on flame temperature. When reformate gas is added, fuel becomes a mixture of CH4, H2, CO and N2. The Lewis numbers of CH4, CO and N2 are
close to unity, but that of H2 is significantly lower than unity. As a result, the fuel Lewis number is
reduced to a value lower than unity, which tends to increase flame temperature. If there were not the Lewis number effect, the addition of reformate gas should have caused a monotonic decrease of flame temperature. This is confirmed by the calculations at which the Lewis number was artificially set as unity for all species at strain rate of 100 s-1, as shown in Fig. 1. Flames of other strain rates have similar results.
At a lower strain rate, the effect of Lewis number is stronger than that of the reduction in adiabatic flame temperature. Therefore, the flame temperature monotonically increases with increasing fraction of reformate gas.
The effect of Lewis number becomes weaker with increasing strain rate. At a moderate or high strain rate, the effect of Lewis number is still stronger than that of the adiabatic flame temperature, leading to flame temperature increases with increasing fraction of reformate gas, when the fraction of reformate gas is smaller. However, further increasing the fraction of reformate gas reverses the relative effects of the Lewis number and adiabatic temperature, resulting in the reduction in flame temperature. The slight temperature increase at a moderate strain rate and near pure reformate gas flame condition is due to the Lewis number effect becoming stronger again when a large amount of hydrogen exists in the fuel mixture. However, this slight temperature increase phenomenon does not happen at a high strain rate, since the Lewis number effect is further weakened.
Variation of NO Formation
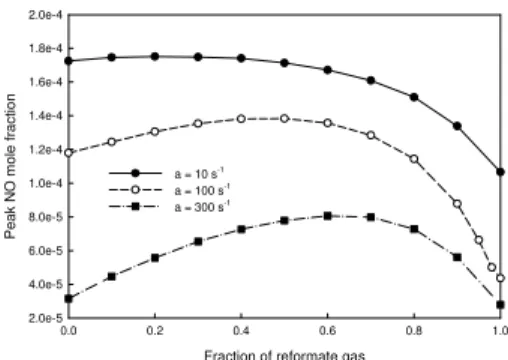
Figures 2 and 3 show the variations of peak NO mole fraction and NO emission index at the three selected strain rates, when reformate gas is added. The NO emission index is defined as the ratio of formed NO (g) to total heat release (J). We observe that at a low strain rate, although flame temperature monotonically increases, as shown in Fig. 1, both peak NO mole fraction and NO emission index first are almost constant and then decrease, when the fraction of reformate gas is increased from 0 to 1.0.
At a moderate or high strain rate, peak NO mole fraction and NO emission index vary in a slightly different way than at a low strain rate. When the fraction of reformate gas is increased from 0 to 1.0, both parameters first increase and then decrease. The critical fraction of reformate gas, at which the two parameters reach the maximum values, varies with strain rate.
The above phenomena are caused by the variation of NO formation mechanism, when reformate gas is added. It is well known that NO generally can be formed by four routes in a hydrocarbon flame, i.e. the thermal, the prompt, the N2O and the NNH intermediate routes. The thermal NO formation route is
comprised of the three reactions: N2 + O = N + NO; N + O2 = NO + O; and N + OH = NO + H; of which
the first one is the initiation reaction that converts molecular nitrogen to NO and atomic nitrogen. The prompt NO in hydrocarbon flames is initiated by the rapid reactions of hydrocarbon radicals with molecular nitrogen, and then the formed atomic nitrogen and species containing elementary nitrogen are converted to NO. The N2O intermediate route is initiated by the reactions: N2O (+M) = N2 + O (+M); N2O
+ H = N2 + OH; N2O + O = N2 + O2; and N2O + OH = N2 + HO2;and then N2O formed is partially
converted to NO. In addition, NO formation can also be initiated by the reactions of molecular nitrogen with other hydrocarbon-free radicals, such as H, OH, H2, to form NNH, and NNH is later convertedto
NO. This last route to form NO is known as the NNH intermediate route.
Figure 4 displays the pathways of NO formation in the pure CH4/air at strain rate of 10 s-1. The
thickness of each line represents the magnitude of the rate and the arrow indicates the direction of the reaction. The paths with rates less than 1.0x10-8 mole/(cm2⋅s) have been neglected. It is observed that
most NO is formed by the reactions HNO (+H, OH) → NO and N (+OH) → NO in the flame. Apparently, the reactions HNO (+H, OH) → NO belong to the prompt route, since species HNO is from the paths resulting from the reaction of molecular nitrogen with radical CH. Although some researchers attributed the reaction N (+ OH) → NO to the thermal NO formation route, it is noted that atomic nitrogen participating in this reaction comes from the paths N2 (+CH)→ HCN → NCO → NH → N, N2
(+CH)→ HCN → NH → N and N2 (+CH)→ N. Therefore, the formation of atomic nitrogen in this
CH4/air flame is initiated by the reaction of molecular nitrogen with radical CH, which is the typical
prompt nitrogen conversion route. This tells us that the method to identify the mechanism of NO formation in a flame should not be based on how NO is finally formed, but on how molecular nitrogen is initially converted to atomic nitrogen or species containing elemental nitrogen. Based on this, we examine the mechanism of NO formation of other flames according to the consumption rates of molecular nitrogen by different routes, rather than the final formation of NO.
Figure 5 shows the variation of molecular nitrogen consumption rates by different routes in flames of strain rates of 10 and 300 s-1, when the fraction of reformate gas is changed. The definition of nitrogen
consumption rate is similar to that for NO emission index. Negative value means nitrogen is consumed (converted to NO or species containing elementary nitrogen), and positive value indicates that nitrogen is formed (NO or species containing elementary nitrogen is converted back to molecular nitrogen). For completeness, the nitrogen consumption rates by the N2O and NNH intermediate routes are also shown,
although their contributions are very small in all the studied flames and do not appear in Fig. 4. The identification method of the nitrogen consumption by different routes can be found elsewhere [3]. It is observed that the prompt route dominates the conversion of nitrogen for the pure CH4/air flame at either
strain rate. The consumption rate of nitrogen by the thermal route is actually slightly positive. It is because a large amount of atomic nitrogen is formed by the reaction N2 + CH = HCN + N, resulting in
that the forward rate of the reaction NO + N = N2 + O exceeds the reverse rate. Differently, the thermal
route contributes most nitrogen conversion in the pure reformate gas flame at both strain rates. The combination of the variations in the nitrogen consumption rates by the thermal and prompt routes can explain most of the phenomena observed in Figs. 2 and 3.
When the strain rate equals 10 s-1, the consumption rates of nitrogen by the thermal and prompt routes
increase and decrease, respectively, leading to that the net (or total) nitrogen consumption rate and NO emission index are almost constant, as the fraction of reformate gas is smaller. The increase and decrease in the nitrogen consumption rates of the thermal and prompt are, respectively, because of the increase in flame temperature and reduction in concentration of radical CH. With the further increase of reformate gas fraction, the consumption rate of nitrogen by the prompt route quickly decreases. On the other hand, although the consumption rate of nitrogen of the thermal route increases due to temperature rise, the increase rate is much smaller than the decrease rate of the prompt rate. Consequently, the net (total) nitrogen consumption rate decreases, as the reformate gas fraction is increased from a moderate value (0.4~0.5) to unity, leading the reduction in peak NO mole fraction and NO emission index.
When strain rate equals 300 s-1, as the fraction of reformate gas first increases from 0.0 to a certain value, the consumption rate of nitrogen by the prompt route quickly increases, while that by the thermal route is almost constant, leading to the quick increase in net nitrogen consumption rate. This results in the increase in peak NO mole fraction and NO emission index. At this stage, the almost constant nitrogen consumption rate of the thermal route is due to the net effect of the temperature increase, which tends to raise the nitrogen consumption rate by the thermal route, and the rise in the nitrogen consumption rate of the prompt route that generates a large amount of atomic nitrogen and thus intensifies the forward rate of the reaction NO + N = N2 + O. The quick increase in the consumption rate of nitrogen by the prompt
route is because at a high strain rate, the combustion of pure methane flame is not complete due to short residence time. When a small amount of reformate gas is added, combustion is intensified and thus the concentration of radical CH is actually increased. The increase in both CH concentration and flame temperature causes the quick increase in nitrogen consumption rate of the prompt route, when the fraction of reformate gas is increased from zero to a moderate vale. The effect of a small amount reformate gas addition on radical CH concentration does not happen at a lower strain rate, since the residence time of reactants in the reaction zone of a lower strain rate flame is long enough to complete the combustion for a CH4/air diffusion flame, and thus the addition of reformate gas only increases flame temperature and
actually reduces the concentration of radical CH. With the fraction of reformate gas being increased to over a certain value, the concentration of radical CH and flame temperature start to decrease, resulting in the reduction in the consumption rate of nitrogen by the prompt route and hence peak NO mole fraction and NO emission. The change in the nitrogen consumption rate of the thermal route is still negligible at this later stage, although flame temperature reduces with increasing fraction of reformate gas. This is because the effect of atomic nitrogen produced from the prompt route on the forward reaction NO + N = N2 + O decreases.
The result at strain rate of 100 s-1 is qualitatively similar to that at strain rate of 300 s-1. Although
there is a slight temperature increase at near pure reformate gas condition, it is not enough to cause the increase in net NO formation rate.
Finally, we should point out that although our simulation show that the addition of a small amount of reformate gas increases the formation of NO in a moderate or higher strain rate flame, this increase is very limited and expected to be controlled by adding some other components, such as EGR. Given the other advantages of reformate gas addition in diffusion flames, like reduced soot formation, there is a definite benefit to employ reformate gas enrichment combustion technology in diffusion flames.
CONCLUSIONS
A detailed numerical study on the effect of reformate gas enrichment on flame temperature and NOX
formation in counterflow CH4/air diffusion flames has been conducted. The result indicates that flame
temperature monotonically increases at a low strain rate, as the fraction of reformate gas is increased. At a moderate strain rate, with increasing enrichment fraction, flame temperature first increases to a maximum value, then decreases, and finally slightly increases again. The variation trend of temperature at a higher strain is qualitatively similar to that at a moderate strain rate, except that the final slight temperature increase does not happen. For a low strain rate flame, both peak NO mole fraction and NO emission index are almost constant or only slightly increase, when the fraction of reformate gas is increased from zero to a small value. With further increase of reformate gas fraction, the peak NO mole fraction and NO emission index decrease. When strain rate is moderate or high, peak NO mole fraction and NO emission index first increase and then decrease, as the fraction of reformate gas is increased from zero to unity. Detailed analyses suggests that the variation of the characteristics in NO formation in strained CH4/air
diffusion flames is caused by the change of flame temperature and NO formation mechanism, when the enrichment fraction and strain rate are changed.
REFERENCES
1. Jackson, G.S., Sai, R., Plaia, J.M., Boggs, C.M., Kiger, K.T., Combust. Flame 132 (2003) 503-511. 2. Ren, J.Y., Qin, W., Egolfopoulos, F.N., Mak, H., Tsotsis, T.T., Chemical Engineering Engineering
Science 56 (2001) 1541-1549.
3. Guo, H., Smallwood, G.J., Liu, F., Ju, Y., Gülder, Ö.L., Proc. Combust. Inst. 30 (2005) 303-311. 4. Gülder, Ö.L., Snelling, D.R., and Sawchuk, R.A., Proc. Combust. Inst. 26 (1996) 2351-2358. 5. Guo, H., Liu, F., Smallwood, G.J., and Gülder, Ö.L., Combust. Flame 145 (2006) 324-338. 6. Ju, Y., and Niioka, T., Combust. Flame 99 (1994) 240-246.
7. Fotache, G.G., Kreutz, T.G., and Law, C.K., Combust. Flame 110 (1997) 429-440. 8. Naha, S., and Aggarwal, S.K., Combus. Flame 139 (2004) 90-105.
9. Guo, H., Neill, S. W., and Smallwood, G. J., Proceedings of 2006 ASME International Mechanical Engineering Congress & Exposition, paper No: IMECE2006-14458, 2006.
10. Guo, H., Smallwood, G.J., Gülder, Ö.L., 2006, Proc. Combust. Inst. 31 (2006) 1197-1204. 11. Giovangigli, V., and Smooke, M.D., 1987, Combust. Sci. Tech. 53 (1987) 23-49.
12. Kee, R.J., Grcar, J.F., Smooke, M.D., and Miller, J.A., Report No. SAND85-8240, Sandia National Laboratories, 1985.
13. Guo, H., Ju, Y., Maruta, K., Niioka, T. and Liu, F., Combust. Flame 109 (1997) 639-646.
14. Gregory P. Smith, David M. Golden, Michael Frenklach, Nigel W. Moriarty, Boris Eiteneer, Mikhail Goldenberg, C. Thomas Bowman, Ronald K. Hanson, Soonho Song, William C. Gardiner, Jr., Vitali V. Lissianski, and Zhiwei Qin http://www.me.berkeley.edu/gri_mech/.
15. Kee., R. J., Warnatz, J., and Miller, J. A., Report No. SAND 83-8209, Sandia National Laboratories, 1983.
16. Kee., R. J., Miller, J. A., and Jefferson, T. H., Report No. SAND 80-8003, Sandia National Laboratories, 1980.
Fraction of reformate gas 0.0 0.2 0.4 0.6 0.8 1.0 M a x imum flame t e mp er at ur e, K 1800 1850 1900 1950 2000 2050 2100 2150 a = 10 s-1 a = 100 s-1 a = 300 s-1 a = 100 s-1, Le = 1.0
Fraction of reformate gas
0.0 0.2 0.4 0.6 0.8 1.0 Pe ak N O m o le fr ac ti on 2.0e-5 4.0e-5 6.0e-5 8.0e-5 1.0e-4 1.2e-4 1.4e-4 1.6e-4 1.8e-4 2.0e-4 a = 10 s-1 a = 100 s-1 a = 300 s-1
Fig. 1 Variation of the maximum temperature. Fig. 2 Variation of peak NO mole fraction.
Fraction of reformate gas
0.0 0.2 0.4 0.6 0.8 1.0 NO emis s ion i ndex , g -NO /J -hea t 1.0e-8 1.5e-8 2.0e-8 2.5e-8 3.0e-8 3.5e-8 4.0e-8 4.5e-8 5.0e-8 a = 10 s-1 a = 100 s-1 a = 300 s-1 N2 HCN HOCN HNCO NH2 NH NCO HNO NO N H2CN HCNO +CH +H,OH +OH +CH2 +HCCO +CH3 +H2O,OH +O +H,OH +O +H +H,OH +H +O +H +H +OH +CH3 ~1.0 ~4.0 ~8.0 Mole/(cm2⋅s)
Fig. 3 Variation of NO emission index. Fig. 4 NO formation pathway in CH4/air
at strain rate of 10 s-1.
a = 10 s-1
Fraction of reformate gas
0.0 0.2 0.4 0.6 0.8 1.0 N2 c o ns u m pt io n ra te , g-N 2 /J -h ea t -5.0e-8 -4.0e-8 -3.0e-8 -2.0e-8 -1.0e-8 0.0 1.0e-8 Total Thermal Prompt NNH route N2O route a = 300 s-1
Fraction of reformate gas
0.0 0.2 0.4 0.6 0.8 1.0 N2 c o ns u m p ti on ra te , g-N2 /J -h e a t -3.0e-8 -2.5e-8 -2.0e-8 -1.5e-8 -1.0e-8 -5.0e-9 0.0 5.0e-9 Total Thermal Prompt NNH route N2O route