Long-term discourse outcomes and their relationship to white
matter damage in moderate to severe adulthood traumatic
brain injury
Karine Marcottea,b, Erlan Sancheza,c, Caroline Arboura,d, Simona Maria Brambatie,f, Christophe Bedettie, Sarah Martineaua,b, Maxime Descoteauxg and Nadia Gosselina,f
a Centre de recherche de l’Hôpital du Sacré-Cœur de Montréal, Montréal, Quebec, Canada.
b École d’orthophonie et d’audiologie, Faculté de médecine, Université de Montréal, Montréal, Quebec, Canada.
c Département de neurosciences, Université de Montréal, Montréal, Québec, Canada. d Faculté des sciences infirmières, Université de Montréal, Montréal, Québec, Canada. e Centre de recherche de l’Institut Universitaire de Gériatrie de Montréal, Montréal,
Québec, Canada.
f Département de psychologie, Faculté des arts et Sciences, Université de Montréal, Montréal, Québec, Canada
g Département d’informatique, Université de Sherbrooke, Québec, Canada.
Corresponding author: Karine Marcotte, Ph.D.
Address: École d'orthophonie et d'audiologie, Université de Montréal - Faculté de médecine, C.P. 6128, succursale Centre-Ville, Montréal (Qc), H3C 3J7 Phone number: 514-343-2485
E-mail: karine.marcotte@umontreal.ca
1. INTRODUCTION
Moderate to severe traumatic brain injury (TBI) is the leading cause of death and disability in young adults under 45 years in industrialized countries (Hagen, 1984; Selassie et al., 2008). For those who survive TBI, the consequences can be devastating and can include profound and permanent physical, cognitive, behavioral, and psychosocial impairments. Approximately half of the TBI survivors have significant long-term cognitive impairments in domains such as executive function, memory, information processing speed, and social communication, which can prevent individuals from returning to work (van Velzen, van Bennekom, Edelaar, Sluiter, & Frings-Dresen, 2009). Some studies have indicated that between 80 and 100% of patients with TBI present with communication impairments in the acute phase (Halpern, Darley, & Brown, 1973; Sarno, 1980); however, these communication impairments and their evolution have been less well documented than other TBI-associated cognitive impairments. According to clinical observations and preliminary studies, semantics, pragmatics and discursive abilities are among the most commonly reported communication deficits following TBI, in addition to complex language skills, such as producing discourse (Le, Coelho, Mozeiko, Krueger, & Grafman, 2012; Marini, et al., 2011), irony, or non-literal concepts (Angeleri et al., 2008; Dennis et al., 2015).
In contrast to post-stroke aphasia, which results from primary language function impairments, communication impairments in patients with TBI probably result, at least in
part, from underlying cognitive impairments, including executive functions, judgement, attention, and memory (Togher et al., 2014). Consequently, communication disorders are closely interrelated with cognitive impairments (Togher et al., 2014). The contemporary dual-stream model, which includes the dorsal and ventral streams, was developed to explain language processing (Poeppel, Emmorey, Hickok, & Pylkkänen, 2012; Saur et al., 2008), but not more complex communication abilities such as conversational discourse. These abilities are supported by a more distributed network of brain regions, including the anterior temporal lobes, orbitofrontal cortex, lateral frontopolar cortex, anterior prefrontal cortex, and inferior and superior parietal lobes (Barbey, Colom, & Grafman, 2013). To date, the neural correlates of communication impairments following moderate to severe TBI have not been elucidated. It is essential to determine if post-TBI communication impairments are related to specific language processes and brain structures involved in language processing, or if they are exclusively due to other cognitive impairments such as memory and attention.
TBI often results from heterogeneous neural injuries and diffuse axonal injury is most commonly observed (Nakayama et al., 2006). However, this injury may not be apparent on conventional imaging techniques and thereby, its prognostic value is therefore limited (Meythaler, Peduzzi, Eleftheriou, & Novack, 2001). Diffusion magnetic resonance imaging (dMRI) provides a more objective measure of axonal injury (Betz, Zhuo, Roy, Shanmuganathan, & Gullapalli, 2012) because it can detect the microstructural characteristics of white matter (Johansen-Berg & Behrens, 2014). Therefore, dMRI can provide potential biomarkers of TBI (Huisman et al., 2004).
Tractography is a dMRI analysis that estimates white matter (WM) fiber bundle trajectories and allows the extraction of WM characteristics from the bundle to characterize white matter damage (Jbabdi, Sotiropoulos, Haber, Van Essen, & Behrens, 2015). Until recently, most tractography approaches have been based on diffusion tensor imaging (DTI); however, DTI-based tractography fails to adequately capture complex geometry, such as crossing fibers (Descoteaux, Deriche, Knosche, & Anwander, 2009; Jbabdi & Johansen-Berg, 2011; Maier-Hein et al., 2017). High angular resolution diffusion imaging (HARDI)-based tractography has been developed to overcome some of the limitations of conventional DTI analysis (Descoteaux, 2015; Tournier, Calamante, & Connelly, 2012).
The corpus callosum is the most vulnerable fiber bundle to long-term impairments in chronic TBI (Caeyenberghs et al., 2011; Dinkel et al., 2014); however, some studies have failed to find any damage to the corpus callosum in chronic TBI (Wozniak et al., 2007). Moreover, recent studies have reported positive correlations between reduced fractional anisotropy (FA) in the corpus callosum and a variety of cognitive functions, such as working memory (Palacios et al., 2012) and executive control (Palacios et al., 2011) , in patients with TBI. These results indicate that dMRI is a promising technique for identifying the neural correlates of cognitive deficits in TBI. However, to the best of our knowledge, no studies have investigated the role of WM bundle properties in the persistence of communication impairments in adulthood TBI.
Here, we examined the relationship between white matter damage and persistent cognitive-communication impairments in adult patients with TBI using constrained spherical deconvolution-based tractography. First, we investigated whether changes in tractography-derived properties, which were measured within the arcuate fasciculus, uncinate fasciculus, inferior longitudinal fasciculus, and corpus callosum (genu), were observed in a group of patients with moderate to severe patients chronic TBI compared with healthy aged-matched controls. Second, we investigated the association between damage in language-related fiber bundles and poor communication outcomes following a chronic adulthood TBI, compared with the genu, a less specific-to-language fiber bundle. TBI often result from diffuse axonal injury (Nakayama et al., 2006); therefore, we hypothesized that patients with moderate to severe TBI would exhibit white matter alterations in the corpus callosum and language-related fiber bundles. In addition, we hypothesized that poor communication in adults with moderate-to-severe TBI would be associated with damage in the left arcuate fasciculus, considering its well-documented role language processing. This bundle has largely been associated with speech production (Hickok & Poeppel, 2007; Warren, Wise, & Warren, 2005) and syntactic processing (e.g. Friederici, 2009; Wilson et al., 2011).More recently, the posterior segment of the left arcuate fasciculus has also been associated with communication abilities requiring complex integration (Catani & Bambini, 2014).
2. MATERIALS AND METHODS 2.1. Participants
were not recruited on the basis of communication impairments. Fifteen participants with moderate to severe TBI (11 men, 4 women; mean age, 32.1 ± 14.9 years) and fifteen age-and sex-matched healthy controls (11 men, 4 women; mean age, 30.9 ± 15.4 years) with no history of TBI or concussion participated in the study. TBI participants were recruited according to the following inclusion criteria: (1) one TBI, (2) aged between 18–65 years old and (3) right-handed. Exclusion criteria were as follows: a history of (1) major psychiatric illness, (2) previous neurological injury, (3) intracranial surgery prior to the TBI, (4) alcoholism or drug abuse, (5) learning disabilities, (6) uncorrected hearing or vision deficits, and (7) permanent contraindications for an MRI exam, such as claustrophobia or metallic implants. Control participants were recruited using all the same inclusion and exclusion criteria, except for the TBI inclusion criterion. All participants, except one whose native language was English, were native French speakers.
Patients with TBI had been admitted to a Level I Trauma Center in the last 11–36 months (mean: 21 ± 9.6 months) prior to study initiation. TBI severity was confirmed by a neurosurgeon based on scan readings and the Glasgow Coma Scale (GCS) score obtained upon admission to the intensive care unit. Briefly, GCS scores between 9–12 or ≤8 indicated moderate or severe TBI respectively; however, this classification was re-evaluated by the neurosurgeon based on the patients’ clinical progression during hospitalization. Healthy controls were recruited from a local newspaper advert. Written informed consent was obtained from all participants prior to the experiment. The Ethics Committee of the Centre de recherche de l’Hôpital Sacré-Coeur de Montréal approved
the study. All participants included in the present study were also included in another study on white matter and sleep characteristics (Sanchez et al., 2018).
2.2. Cognitive-communication evaluation
A short cognitive-communication battery was used to assess the communicative skills of all patients with TBI. Cognitively unimpaired controls were not tested on these tasks because of the ceiling effect of these measures. First, a five-minute conversation task (D-MEC) was rated using the conversation scale of the Protocole Montréal
Évaluation de la Communication (MEC) (Joanette, Ska, & Côté, 2004). Semantic
fluency, orthographic fluency, and without constraint fluency were administered from the
MEC to assess lexical access, cognitive flexibility, and inhibition. The indirect speech
acts task from the MEC, consists of short stories that refer to everyday situations while not explicitly stating the intention of the person within the story. This task was used to evaluate non-literal language comprehension. Narrative discourse comprehension from the MEC was included as a measure of complex oral comprehension. A detailed description of the subtests of the MEC used in the present study is presented in Supplementary Material (Supplementary Table 1). A French translation of the LaTrobe questionnaire (Douglas & O’Flaherty et al., 2000) was used as a measure of self-perceived social communication abilities for the patients with TBI patients. Reasoning skills were assessed using the matrices subtest from the Wechsler Adult Intelligence Scale-III (Wechsler, 1997), which has shown to be insensitive to the effects of TBI (Donders, Tulsky, & Zhu, 2001). All cognitive-communication testing was administered and scored according to standard procedures.
2.3. Imaging protocol
Images were acquired at the Functional Neuroimaging Unit of the Centre de
Recherche de l’Institut Universitaire de Gériatrie de Montréal, using a 3 T Trio MRI
Siemens scanner with a standard eight-channel head coil. Participants were placed in the supine position on the MRI table and their heads were padded to reduce movement. A high-resolution structural scan was first obtained using a volumetric three-dimension T1-weighted MPRAGE Turbo Flash multi-echo system. The sequence was acquired following the Massachusetts General Hospital sequence (Boston, Massachusetts, USA): TR = 2530 ms, TE = 4 ms, Echo 1 = 1.64 ms, Echo 2 = 3.50 ms, Echo 3 = 5.36 ms, Echo 4 = 7.22 ms, matrix size = 256 × 256, FOV = 256 × 256, voxel size = 1.0 mm isotropic, flip angle = 7º, and 176 slices in sagittal orientation. Diffusion weighted imaging parameters consisted of 65 noncollinear directions with a b value of 1000 s/mm² with TR = 9500 ms, TE = 93 ms, matrix = 120 X 120 matrix, FOV = 240 mm, slice thickness = 2 mm, voxel size = 2 × 2 × 2 mm in an anterior-posterior (AP) position, as well as two other b = 0 s/mm² images in the AP and posterior-anterior (PA) positions for distortion correction.
2.4. HARDI-based tractography
The HARDI acquisition protocol consists of measuring the diffusion-weighted (DW) signal using a larger number of uniformly distributed DW gradient directions that required for DTI to capture the higher angular contrast of the diffusion signal, which is not adequately modelled by a single diffusion tensor (Tournier et al., 2012). The use of a
constrained spherical deconvolution method to estimate the distribution of fiber orientations directly from high angular resolution DW imaging data improves the robustness of the estimated fiber orientation (Dell’Acqua & Tournier, 2018; Tournier, Calamante, & Connelly, 2007; Tournier, Calamante, Gadian, & Connelly, 2004). Despite persistent limitations in the current state-of-the-art tractography algorithms, DW imaging is the only noninvasive in vivo method that allows the visualization of specific fiber bundle pathways and is adequate for major fasculi (Thomas et al., 2014).
Our preprocessing was conducted using the Toolkit for Analysis in Diffusion MRI (TOAD) pipeline (http://www.unf-montreal.ca/toad). First, the T1-weighted image was segmented and parcellated into anatomical regions with Freesurfer’s recon-all 6.0.0 (http://freesurfer.net) using the CBRAIN platform (Sherif et al., 2014). Next, the DW image was noise-corrected using overcomplete local principal component analysis (PCA) (Manjon et al., 2013). Geometric and eddy-current distortions of DW images were corrected with two b0, and opposite phase encoding (AP and PA) using the FMRIB Diffusion toolbox EDDY (Andersson & Sotiropoulos, 2016) in FSL 5.0.8 (http://www.fmrib.ox.ac.uk/fsl/) (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). DW images were upsampled to 1 mm isotropic resolution using trilinear interpolation (Dyrby et al., 2014; Girard & Descoteaux, 2012; Raffelt et al., 2012; Smith, Tournier, Calamante, & Connelly, 2012; Tournier, Calamante, & Connelly, 2012). Next the segmented and parcellated T1-weighted images were registered to the DW images using FMRIB’s linear registration tool (FLIRT) (Greve & Fischl, 2009; Jenkinson, Bannister, Brady, & Smith, 2002; Jenkinson & Smith, 2001) in FSL. DTI [FA and Radial
Diffusivity (RD)] and HARDI [Number of Fiber Orientation (NuFO)] (Dell’Acqua, Simmons, Williams & Catani, 2013) metrics were reconstructed using Dipy python software (Garyfallidis et al., 2014). Fiber orientation distribution functions (fODFs) were estimated using constrained spherical deconvolution with the single fiber response as an input (Descoteaux et al., 2009; Tournier et al., 2007). A whole-brain tractogram was computed using MRtrix3’s (www.mrtrix.org) probabilistic tractography algorithm with anatomically constrained tracking (Smith et al., 2012). This uses the segmented anatomical image obtained from Freesurfer to limit potential false-negatives (i.e. no-connections) and improve white matter coverage (Girard, Whittingstall, Deriche, & Descoteaux, 2014; Smith et al., 2012). One million streamlines were generated using a 0.5 mm step size.
Each language fiber bundle (left hemisphere arcuate, uncinate, and inferior longitudinal fasciculi), the genu, and splenium of the corpus callosum were reconstructed from the tractogram using the White Matter Query Language (WMQL) (Wassermann et al., 2013, 2016, https://github.com/demianw/tract_querier). WMQL is a user-friendly method that allows for nearly automatic reconstruction of white matter fiber bundles. It consists of defining ‘queries’ or anatomic definitions of the bundles of interest using the anatomic regions from Freesurfer’s Desikan/Killiany atlas. Queries were used to reconstruct tracts, which are presented in the Supplementary Material (Supplementary Table 2). The commands proposed by Wasserman et al. (2013, 2016) were modified to optimize the reconstruction for all bundles, except for the command for the temporo-parietal segment of the arcuate fasciculus, which was modified from Jolles et al. (2016).
A tract-filtering algorithm was applied to remove outlier streamlines from each tract (Côté, Garyfallidis, Larochelle, & Descoteaux, 2015).
After the reconstruction of the white matter bundles, tractometry allows for the extraction of the average tract profile for each diffusion measure of interest (Cousineau et al., 2017). These measures can be used in a complementary way to infer the microstructural abnormalities of white matter (Alexander, Lee, Lazar, & Field, 2007). DTI/HARDI measures were computed along the bundles previously extracted using an automated tractometry pipeline (Cousineau et al., 2017). DTI measures included FA and RD. FA measured orientation coherence or directionality, and RD was defined as the magnitude of water diffusion in the direction perpendicular to the axonal fibers (Song et al., 2003). We choose to use RD over mean and axial diffusivity because changes in RD are relatively consistent across studies; RD typically increases post-TBI, whereas differences in axial diffusivity (Farbota et al., 2012) and FA (Chung et al., 2018) are inconsistent. A growing body of literature supports the hypothesis that RD increases in response to demyelination (Song et al., 2005), which occurs weeks or months post-TBI. Additionally, NuFO indicates the number of distinct fiber orientations in each voxel, which provides valuable information on white matter organization (Dell’Acqua et al., 2013). The volume of each bundle was also extracted using the tractometry pipeline. Following this, we obtained the mean values of FA, RD and NuFO from each bundle for every individual participant
T-tests were applied to measure differences in demographic information (age and level of education) between groups. Mean FA, RD, NuFO, and region volume of the arcuate fasciculus, uncinate fasciculus, inferior longitudinal fasciculus, and genu of the corpus callosum tracts were compared between groups using t-tests. Due to the small sample size, we only selected measures that showed a significant between-group difference for a subsequent Spearman’s correlation analysis with impaired cognitive-communication measures (conversational skills). All statistical analyses were performed using SPSS version 25. Results were deemed significant if p <0.05.
3. RESULTS
3.1. Demographic results
There was no difference in sex between the TBI and healthy control groups (both groups: 11 men and 4 women). In addition, there were no between-group differences between groups in age (t = .039, p = .844) and level of education (t = .014, p = .094). A mean of 21.6 ± 9.3 months had elapsed since the brain injury in our TBI group, which confirmed that participants in the TBI group were in the chronic phase of their recovery. Moreover, there were no between-group differences on the matrices subtest from the Weschler Adult Intelligence Scale- III (t = -.266, p = .792). Detailed demographic results are reported in Table 1.
********************************** Insert Table 1 approximately here **********************************
Table 2 reports the detailed mean raw and calibrated scores of the communicative tasks. The only significant cognitive-communication impairment was observed in conversational skills, as evaluated using the conversation task (D-MEC) in the Protocole
Montréal Evaluation de la communication (Joanette et al., 2004). The mean and standard
deviation (SD) of the D-MEC raw score was 29.93 ± 3.48, with a range between 24 and 34. Calibrated scores were calculated according to age and level of education, which was more representative of performance than the raw score. Sixty percent of patients were <1.5 SD from the mean of their peers, which was considered to be a significant impairment. The D-MEC scores were not correlated with GCS scores in the emergency room (r = .201, p = .510) as well as with the matrices standard score (r = .150, p = .580). Table 3 reports the detailed analysis of impaired communicative behaviors in the D-MEC. Briefly, word finding, inappropriate/unexpected comments were the most observed behaviors along with imprecise expression of ideas, inappropriate topic shifts, monotone voice and a speech rate that was too slow or too fast.
********************************** Insert Table 2 approximately here ********************************** **********************************
Insert Table 3 approximately here **********************************
In the other communication tasks, patients with chronic TBI showed preserved narrative discourse comprehension abilities at one-year post-onset when compared with
healthy controls according to their age and level of education. In addition, they had verbal fluency and indirect speech acts scores within normal limits.
3.3. Group differences in DTI/HARDI metrics
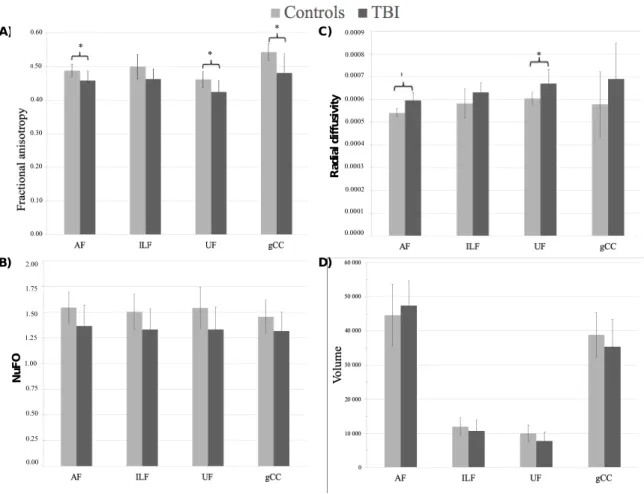
All four bundles were reconstructed in all participants. Figure 1 represents the four bundles of interest from a representative control subject. Tests were conducted using a Bonferroni adjusted alpha levels of .003 per test (.05/16). The TBI group showed a significant reduction in FA in the arcuate (t = -3.508, p = .002) and the uncinate (t = -3.477, p=.002) fasciculi as well as in the genu of the corpus callosum (t = -3.825, p = . 001). They also showed a significant increase in RD in the arcuate (t = 5.27, p < .001) and uncinate fasciculi (t = 3.809, p = .001; Figure 2 and Table 4) when compared with controls (Figure 2 and Table 4).
********************************** Insert Figure 1 approximately here ********************************** **********************************
Insert Figure 2 approximately here ********************************** **********************************
Insert Table 4 approximately here **********************************
We ran non-parametric correlations between the calibrated scores of the D-MEC task and the five measures that showed significant between-group differences. Tests were conducted using a Bonferroni adjusted alpha levels of .01 per test (.05/5). Two non-parametric correlations were found between the calibrated scores in the D-MEC task and the measures extracted in the fiber bundles, one with FA in the left arcuate fasciculus (r= .538, p=.038; see figure 3 A)) and RD in the left arcuate fasciculus (r= -.605, p=.017; see figure 3 B)). However, both correlations did not survive Bonferroni correction. Based on the recently proposed SCALED model (Catani & Bambini, 2014) and our results, a
post hoc analysis was computed with the temporo-parietal segment of arcuate fasciculus.
This segment has been associated with the pragmatic integration and more complex tasks, such as conversational discourse. The results are reported in Supplementary Table 2 and Supplementary Figure 1. We found correlations between the D-MEC task calibrated scores and the FA and RD of the arcuate fasciculus (FA, r = 0.569, p = 0.027; RD, r = −0.544, p = 0.036), but none of them survived Bonferroni correction.
********************************** Insert Figure 3 approximately here ********************************** 4. DISCUSSION
The main aim of this study was to characterize chronic changes in white matter fiber bundles that are known to play a role in persistent communication impairments in patients with moderate to severe TBI. We used recent advances in HARDI (Descoteaux & Poupon, 2012), which allows for a more robust estimation of complex fiber
configurations. The patients with TBI in this study presented with conversational discourse impairments >1 year after TBI. Taken together, and in line with previous studies (Jang, Lee, & Shin, 2016; Kurki et al., 2014; Liegeois et al., 2013), we found persistent damage to major white matter fiber bundles in patients with moderate to severe TBI. Patients with TBI showed significant differences in FA in the arcuate and uncinate fasciculus, which are traditionally associated with language processing, and the genu of the corpus callosum. TBI was also associated with increased RD only in language related bundles, namely the arcuate and the uncinate fasciculi. Furthermore, wethe present results suggest that the cohort of the participants with TBI involved in this study had damage to the left fronto-temporal region, which would explain the persistent long-term
communication impairments and damage to the left arcuate fasciculus.
4.1. Communication outcomes
Conversational discourse impairments have been previously identified using the D-MEC in a Level 1 Trauma Center in 195 adults with different TBI severities (LeBlanc et al., 2014). In that study, the authors reported that patients with the most impaired conversational discourse in the sub-acute phase had a greater chance of being discharged to rehabilitation than patients with better conversational abilities. They showed that word finding difficulties, inappropriate/unexpected comments, and imprecise expression of ideas were the most frequently impaired abilities. discourse task used in the present study could be related to other cognitive skills. Interestingly, several correlations were found between nearly half of the communication behaviors (including word finding, Inappropriate/Unexpected comments, Imprecise expression of ideas, No self-correction
of errors) and various language and cognitive tasks. The other half of the communication behaviors (including monotonic voice, lack of facial expression, misunderstands what is said and the task we used in the present study) did not show significant correlations with any cognitive or language tasks. In the present study, we observed that the same communication behavior impairment was due to various deficits depending on each patient. However, it was not possible in this small sample to correlate more precisely the nature of the deficit with structural lesions. In other words, the observed language production deficits observed in the present study could be related to different cognitive mechanisms depending on each individual.
Nonetheless, the present findings indicate that this subtest is sensitive to communication impairments in the chronic phase of TBI. The communication behaviors that were still impaired at least a year post-injury were the same as the those identified by LeBlanc et al. (2014). Considering that the ability to interact and communicate effectively in everyday social settings is frequently disrupted by moderate to severe TBI, and can interfere with successful rehabilitation (Ganesalingam, Yeates, Sanson, & Anderson, 2007; Janusz, Kirkwood, Yeates, & Taylor, 2002; Muscara, Catroppa, & Anderson, 2008; Warschausky, Cohen, Parker, Levendosky, & Okun, 1997), the systematic assessment of communication abilities is therefore important with this population. Furthermore, communication impairments are recognized as significant factors in determining family interactions and psychosocial, work, and academic reintegration (Dahlberg et al., 2007; Ylvisaker, 1992, 2006), which further that an early identification and management of communication impairments is crucial.
4.2. Anatomical connectivity
Few studies have investigated the relationship between anatomical connectivity using tractography and neuropsychological data, including communication skills in adult patients with TBI. We found diffuse alterations in large-scale fiber bundles, including language related bundles, were found in a group of patients with chronic moderate to severe TBI. Interestingly, alterations in RD were specific to language-related fiber bundles. Axonal degeneration was traditionally thought to be limited to the acute and sub-acute periods (Johnson, Stewart, & Smith, 2013); however, longitudinal evidence has shown that axonal neurodegeneration is also observed years after brain injury (Chen, Johnson, Uryu, Trojanowski, & Smith, 2009). Only a few studies have assessed longitudinal white matter changes associated with moderate to severe TBI recovery. In one such study, FA was reported to be significantly lower and RD was significantly higher in the genu and body of the corpus callosum, and the bilateral corona radiata in the acute phase of recovery compared with control participants in the acute phase of recovery (Dinkel et al., 2014). Interestingly, FA decreased, and RD increased between 2 and 5 years post-TBI, which suggests that major changes are also observed in the chronic phase of severe TBI. Several studies have reported an increased RD months to years’ post-TBI, which may drive the reduced FA (Farbota et al., 2012; Perez et al., 2014). Conversely, increased FA and reduced RD has been found in subacute patients with TBI (Mayer et al., 2010). Converging evidence has supported the idea that elevated RD is a consequence of myelin degradation, which causes reduced water restriction along WM bundles that allows an increased water diffusion perpendicular to axons (Aung, Mar, & Benzinger,
2013). Multimodal studies combining dMRI and measures of fiber functions have provided further evidence that the combination of decreased FA and increased RD are indicative of demyelination (Dennis et al., 2015). It was not possible to determine why RD was specifically increased in the arcuate and uncinate fasciculi in the present study. Farbota et al. (2012) proposed that one potential contributing factor of increased RD is the specific location of their brain injury. Interestingly, approximately 75% of the patients in the present study had initial brain injuries localized, at least partly, in the left fronto-temporal area, which supports the hypothesis of a progressive deterioration of the left arcuate fasciculus which is located in this region. Considering the small number of patients and the heterogeneity of the brain damage observed in this cohort of patients, we were not able to test this hypothesis using a statistical analysis. Another possible explanation is that the fiber bundles would respond differently to the brain injury. However, our results are less consistent with this interpretation, as other studies have reported lower FA and increased RD in the genu of corpus callosum in the chronic phase of TBI (Farbota et al., 2012; Newcombe et al., 2015).
We also found further evidence for the importance of the arcuate fasciculus in discourse processing. The correlational analysis of D-MEC scores showed that conversational abilities decreased with a decrease in FA and an increased RD in the left arcuate fasciculus. The key role of the left arcuate fasciculus in speech production (Hickok & Poeppel, 2007; Warren et al., 2005) and syntactic processing (e.g. Friederici, 2009; Wilson et al., 2011) is well documented. Robust correlations between reduced FA and impaired syntactic comprehension and production have also been reported (Wilson et al., 2011).
Liégeois et al. (2013) also showed that sentence formulation abilities were best predicted by the combined volume of the left arcuate fasciculus and corpus callosum in pediatric patients with mild to severe TBI. However, in the present study, the patients with TBI did not show any significant syntactic or speech impairments during conversation. There is evidence to suggest that the superior longitudinal fasciculus (SLF) is not only involved in syntactic processing, but also semantic processing in addition to its proposed role in syntactic processing (Glasser & Rilling, 2008; Yeatman et al., 2011). In Catani and Bambini’s (2014) SCALED model, it was proposed that the posterior segment of the SLF was more strongly associated with pragmatic integration in the SCALED model. Gauthier et al. (2018) reported that conversational discourse performance in a group of patients with TBI was associated with the left temporal region. Based on these new evidences, we performed a post-hoc exploratory analysis of the temporo-parietal segment of the arcuate fasciculus using the recently proposed query (Jolles et al., 2016). We found that conversational discourse was correlated with the FA and RD of the temporo-parietal segment of the arcuate fasciculus. Higher semantic processing, complex sentence comprehension, and pragmatic skills are important to support efficient conversation. To the best of our knowledge, the relationship between arcuate fasciculus properties and discourse production has not been reported in healthy adults or children. Most studies who investigated the posterior segment of the arcuate fasciculus were assessing reading and mathematics learning in both children and illiterate adults (Jolles et al., 2016). If this was confirmed in a larger sample and with different clinical populations, the role of the posterior segment (temporo-parietal) in conversational discourse would be supported.
Although the corpus callosum is not generally associated with language impairments, this important bundle connects the left and right hemisphere language-related regions and is disrupted following TBI (Caeyenberghs et al., 2011; Dinkel et al., 2014). Recent evidence has indicated that narrative comprehension, which is beyond word-level comprehension, is supported by the anterior temporal lobes (Ferstl, Neumann, Bogler, & von Cramon, 2008; Stowe et al., 1998) and orbitofrontal cortex (Stowe et al., 1998), which integrate information across multiple brain systems. Other studies have reported that the comprehension of inferences depends on a distributed network of frontal and parietal brain regions (Badre & Wagner, 2006; Barbey et al., 2012), and is associated with the integration of working memory and attentional resource allocation (Koechlin, Basso, Pietrini, Panzer, & Grafman, 1999). Taken together, this indicates that the most vulnerable portion of this large bundle, the genu, is important for communication proficiency. However, we did not find a significant correlation between discourse abilities and FA extracted from the genu of the corpus callosum, which supports the idea that the corpus callosum is not a traditionally language-related tract.
Some limitations of our study should be noted. First, the sample sizes of our groups was relatively small and it is therefore difficult to generalize the present results to all patients with moderate to severe TBI. Second, many patients in our facility underwent different types of surgery, such as orthopedic surgery, and as a result were not compatible with a 3 T MRI testing. This exclusion criterion may have resulted in the exclusion of patients with the most severe traumatic injuries. Third, the b-value used in our diffusion sequence was lower than optimal for constraint spherical deconvolution-based
tractography. Nevertheless, the use of constrained spherical deconvolution improves the results which supports its use even with lower b-values (Tournier et al., 2007). Moreover, the use of 64 directions is sufficient to detect the crossing fibers in patients with TBI and other clinical populations, as previously shown (Annen et al., 2016; Mohammadian et al., 2017). Nonetheless, utilizing multi-b-value sequences, such as b = 1000 s/mm², b = 2000 s/mm², b = 3000 s/mm², or b = 1000 s/mm² and b = 3000 s/mm², could help to interpret the differences obtained in the present study by considering other variables, such as intracellular, extracellular, and isotropic volume. Finally, we focused on tracts language-related tracts in the left hemisphere. However, to fully understand the neural basis of communication impairments in TBI, future studies should investigate the fiber bundles in the right hemisphere which might also support language processing. It would also be important to study the relation between chronic grey matter changes and communication outcome.
To the best of our knowledge, this is the first study to demonstrate that patients with moderate to severe TBI have damage to fiber bundles related to language processing. Interestingly, individuals with damage to the left arcuate fasciculus, in particular the posterior segment, were more likely to present with long-term discourse impairments. A better comprehension of these impairments will aid the development of early detection and management protocols. This will provide the neurobiological rationale to develop rehabilitative strategies that enhance the neuroplastic changes occurring in the initial weeks following TBI to optimize functional long-term outcomes.
5. ACKNOWLEDGMENTS
This study was supported by a Réseau de Bio-Imagerie du Québec grant to KM and a Canadian Institutes of Health Research grant to NG. KM, SMB, and NG hold a Career Award from the “Fonds de Recherche du Québec – Santé”. MD thanks the
institutional Research Chair in Neuroinformatics for financial support. The authors would like to thank the participants and their families for their time and commitment in this project.
6. AUTHOR DISCLOSURE STATEMENT No competing financial interests exist.
REFERENCES
Alexander, A. L., Lee, J. E., Lazar, M., & Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics : The Journal of the American Society for
Experimental NeuroTherapeutics. https://doi.org/10.1016/j.nurt.2007.05.011
Andersson, J. L. R., & Sotiropoulos, S. N. (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging.
Neuroimage, 125, 1063–1078. https://doi.org/10.1016/j.neuroimage.2015.10.019
Angeleri, R., Bosco, F. M., Zettin, M., Sacco, K., Colle, L., & Bara, B. G. (2008). Communicative impairment in traumatic brain injury: a complete pragmatic assessment. Brain Lang, 107(3), 229–245.
https://doi.org/10.1016/j.bandl.2008.01.002
Annen, J., Heine, L., Ziegler, E., Frasso, G., Bahri, M., Di Perri, C., … Laureys, S. (2016). Function-structure connectivity in patients with severe brain injury as measured by MRI-DWI and FDG-PET. Hum Brain Mapp, 37(11), 3707–3720. https://doi.org/10.1002/hbm.23269
Aung, W. Y., Mar, S., & Benzinger, T. L. (2013). Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med, 5(5), 427–440.
https://doi.org/10.2217/iim.13.49
Badre, D., & Wagner, A. D. (2006). Computational and neurobiological mechanisms underlying cognitive flexibility. Proc Natl Acad Sci U S A, 103(18), 7186–7191. https://doi.org/10.1073/pnas.0509550103
Barbey, A. K., Colom, R., & Grafman, J. (2013). Architecture of cognitive flexibility revealed by lesion mapping. Neuroimage, 82, 547–554.
https://doi.org/10.1016/j.neuroimage.2013.05.087
Barbey, A. K., Colom, R., Solomon, J., Krueger, F., Forbes, C., & Grafman, J. (2012). An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain, 135(Pt 4), 1154–1164.
https://doi.org/10.1093/brain/aws021
Betz, J., Zhuo, J., Roy, A., Shanmuganathan, K., & Gullapalli, R. P. (2012). Prognostic value of diffusion tensor imaging parameters in severe traumatic brain injury. J
Neurotrauma, 29(7), 1292–1305. https://doi.org/10.1089/neu.2011.2215
Caeyenberghs, K., Leemans, A., Coxon, J., Leunissen, I., Drijkoningen, D., Geurts, M., … Swinnen, S. P. (2011). Bimanual coordination and corpus callosum
microstructure in young adults with traumatic brain injury: a diffusion tensor imaging study. J Neurotrauma, 28(6), 897–913.
https://doi.org/10.1089/neu.2010.1721
Catani, M., & Bambini, V. (2014). A model for Social Communication And Language Evolution and Development (SCALED). Curr Opin Neurobiol, 28, 165–171. https://doi.org/10.1016/j.conb.2014.07.018
Chen, X. H., Johnson, V. E., Uryu, K., Trojanowski, J. Q., & Smith, D. H. (2009). A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol, 19(2), 214–223.
https://doi.org/10.1111/j.1750-3639.2008.00176.x
Chung, S., Fieremans, E., Wang, X., Kucukboyaci, N. E., Morton, C. J., Babb, J., … Lui, Y. W. (2018). White Matter Tract Integrity: An Indicator of Axonal Pathology after
Mild Traumatic Brain Injury. J Neurotrauma, 35(8), 1015–1020. https://doi.org/10.1089/neu.2017.5320
Côté, M., Garyfallidis, E., Larochelle, H., & Descoteaux, M. (2015). Cleaning up the mess: tractography outlier removal using hierarchical QuickBundles clustering. In
Proceedings of ISMRM.
Cousineau, M., Jodoin, P. M., Morency, F. C., Rozanski, V., Grand’Maison, M., Bedell, B. J., & Descoteaux, M. (2017). A test-retest study on Parkinson’s PPMI dataset yields statistically significant white matter fascicles. Neuroimage Clin, 16, 222–233. https://doi.org/10.1016/j.nicl.2017.07.020
Dahlberg, C. A., Cusick, C. P., Hawley, L. A., Newman, J. K., Morey, C. E., Harrison-Felix, C. L., & Whiteneck, G. G. (2007). Treatment efficacy of social
communication skills training after traumatic brain injury: a randomized treatment and deferred treatment controlled trial. Arch Phys Med Rehabil, 88(12), 1561–1573. https://doi.org/10.1016/j.apmr.2007.07.033
Dell’Acqua, F., Simmons, A., Williams, S. C., & Catani, M. (2013). Can spherical deconvolution provide more information than fiber orientations? Hindrance
modulated orientational anisotropy, a true-tract specific index to characterize white matter diffusion. Hum Brain Mapp, 34(10), 2464–2483.
https://doi.org/10.1002/hbm.22080
Dell’Acqua, F., & Tournier, J. D. (2018). Modelling white matter with spherical deconvolution: How and why? NMR in Biomedicine.
https://doi.org/10.1002/nbm.3945
Dennis, E. L., Ellis, M. U., Marion, S. D., Jin, Y., Moran, L., Olsen, A., … Asarnow, R. F. (2015). Callosal Function in Pediatric Traumatic Brain Injury Linked to Disrupted White Matter Integrity. J Neurosci, 35(28), 10202–10211.
https://doi.org/10.1523/JNEUROSCI.1595-15.2015
Descoteaux, M. (2015). High angular resolution diffusion imaging (HARDI). In Wiley
Encyclopedia of Electrical and Electronics Engineering.
https://doi.org/https://doi.org/10.1002/047134608X.W8258le
Descoteaux, M., Deriche, R., Knosche, T. R., & Anwander, A. (2009). Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE
Trans Med Imaging, 28(2), 269–286. https://doi.org/doi:10.1109/TMI.2008.2004424
Descoteaux, M., & Poupon, C. (2012). Diffusion-weighted MRI. In D. Belvic & K. Belvic (Eds.), Comprehension Biomedical Physics. Elsevier.
Dinkel, J., Drier, A., Khalilzadeh, O., Perlbarg, V., Czernecki, V., Gupta, R., … for, N. C. (2014). Long-term white matter changes after severe traumatic brain injury: a 5-year prospective cohort. AJNR Am J Neuroradiol, 35(1), 23–29.
https://doi.org/10.3174/ajnr.A3616
Donders, J., Tulsky, D. S., & Zhu, J. (2001). Criterion validity of new WAIS-III subtest scores after traumatic brain injury. Journal of the International Neuropsychological
Society, 7, 892–898.
Douglas, J. M., & O’Flaherty et al., C. A. (2000). Measuring perception of communicative ability: the development and evaluation of the La Trobe communication questionnaire. Aphasiology, 14(3), 251–268.
NeuroImage, 103, 202–213. https://doi.org/10.1016/j.neuroimage.2014.09.005
Farbota, K. D., Bendlin, B. B., Alexander, A. L., Rowley, H. A., Dempsey, R. J., & Johnson, S. C. (2012). Longitudinal diffusion tensor imaging and
neuropsychological correlates in traumatic brain injury patients. Front Hum
Neurosci, 6, 160. https://doi.org/10.3389/fnhum.2012.00160
Ferstl, E. C., Neumann, J., Bogler, C., & von Cramon, D. Y. (2008). The extended language network: a meta-analysis of neuroimaging studies on text comprehension.
Hum Brain Mapp, 29(5), 581–593. https://doi.org/10.1002/hbm.20422
Friederici, A. D. (2009). Pathways to language: fiber tracts in the human brain. Trends in
Cognitive Sciences, 13(4), 175–181. https://doi.org/10.1016/j.tics.2009.01.001
Ganesalingam, K., Yeates, K. O., Sanson, A., & Anderson, V. (2007). Social problem-solving skills following childhood traumatic brain injury and its association with self-regulation and social and behavioural functioning. J Neuropsychol, 1(Pt 2), 149–170. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19331015
Garyfallidis, E., Brett, M., Amirbekian, B., Rokem, A., van der Walt, S., Descoteaux, M., … Dipy, C. (2014). Dipy, a library for the analysis of diffusion MRI data. Front
Neuroinform, 8, 8. https://doi.org/10.3389/fninf.2014.00008
Gauthier, S., LeBlanc, J., Seresova, A., A., L.-P., Correa, J. A., Alturki, A. Y., … de Guise, E. (2018). Acute prediction of outcome and cognitive-communication impairments following traumatic brain injury: the influence of age, education and site of lesion. Journal of Communication Disorders, in press.
https://doi.org/https://doi.org/10.1016/j.jcomdis.2018.04.003
Girard, G., & Descoteaux, M. (2012). Anatomical tissue probability priors for tractography. In International Conference on Medical Image Computing and
Computer Assisted Intervention (MICCAI’12) - Computational Diffusion MRI Workshop (pp. 174–185). Nice.
Girard, Gabriel, Whittingstall, K., Deriche, R., & Descoteaux, M. (2014). Towards quantitative connectivity analysis: Reducing tractography biases. NeuroImage. https://doi.org/10.1016/j.neuroimage.2014.04.074
Glasser, M. F., & Rilling, J. K. (2008). DTI tractography of the human brain’s language pathways. Cerebral Cortex, 18(11), 2471–2482.
https://doi.org/10.1093/cercor/bhn011
Greve, D., & Fischl, B. (2009). Accurate and robust brain image alignment using boundary-based registration. Neuroimage.
https://doi.org/10.1016/j.neuroimage.2009.06.060
Hagen, C. (1984). Language disorders in head trauma. In A. L. Holland (Ed.), Language
Disorders in Adults (pp. 245–281). San Diego, CA: College Hill Press.
Halpern, H., Darley, F. L., & Brown, J. R. (1973). Differential language and neurologic characteristics in cerebral involvement. J Speech Hear Disord, 38(2), 162–173. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4712947
Hickok, G., & Poeppel, D. (2007). The cortical organization of speech processing.
Nature Reviews Neuroscience, 8(5), 393–402. https://doi.org/10.1038/nrn2113
Huisman, T. A., Schwamm, L. H., Schaefer, P. W., Koroshetz, W. J., Shetty-Alva, N., Ozsunar, Y., … Sorensen, A. G. (2004). Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol,
Jang, S. H., Lee, A. Y., & Shin, S. M. (2016). Injury of the Arcuate Fasciculus in the Dominant Hemisphere in Patients With Mild Traumatic Brain Injury: A
Retrospective Cross-Sectional Study. Medicine (Baltimore), 95(9), e3007. https://doi.org/10.1097/MD.0000000000003007
Janusz, J. A., Kirkwood, M. W., Yeates, K. O., & Taylor, H. G. (2002). Social problem-solving skills in children with traumatic brain injury: long-term outcomes and prediction of social competence. Child Neuropsychol, 8(3), 179–194.
https://doi.org/10.1076/chin.8.3.179.13499
Jbabdi, S., & Johansen-Berg, H. (2011). Tractography: where do we go from here? Brain
Connect, 1(3), 169–183. https://doi.org/10.1089/brain.2011.0033
Jbabdi, S., Sotiropoulos, S. N., Haber, S. N., Van Essen, D. C., & Behrens, T. E. (2015). Measuring macroscopic brain connections in vivo. Nat Neurosci, 18(11), 1546– 1555. https://doi.org/10.1038/nn.4134
Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images.
NeuroImage. https://doi.org/10.1016/S1053-8119(02)91132-8
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W., & Smith, S. M. (2012). FSL. NeuroImage. https://doi.org/10.1016/j.neuroimage.2011.09.015 Jenkinson, M., & Smith, S. (2001). A global optimisation method for robust affine
registration of brain images. Medical Image Analysis. https://doi.org/10.1016/S1361-8415(01)00036-6
Joanette, Y., Ska, B., & Côté, H. (2004). Protocole Montréal d’Évaluation de la
Communication. Isbergues, France: Ortho Édition.
Johansen-Berg, H., & Behrens, T. E. J. (2014). Diffusion MRI (2nd editio). Academic Pess.
Johnson, V. E., Stewart, W., & Smith, D. H. (2013). Axonal pathology in traumatic brain injury. Exp Neurol, 246, 35–43. https://doi.org/10.1016/j.expneurol.2012.01.013 Jolles, D., Wassermann, D., Chokhani, R., Richardson, J., Tenison, C., Bammer, R., …
Menon, V. (2016). Plasticity of left perisylvian white-matter tracts is associated with individual differences in math learning. Brain Struct Funct, 221(3), 1337–1351. https://doi.org/10.1007/s00429-014-0975-6
Koechlin, E., Basso, G., Pietrini, P., Panzer, S., & Grafman, J. (1999). The role of the anterior prefrontal cortex in human cognition. Nature, 399(6732), 148–151. https://doi.org/10.1038/20178
Kurki, T., Himanen, L., Vuorinen, E., Myllyniemi, A., Saarenketo, A. R., Kauko, T., … Tenovuo, O. (2014). Diffusion tensor tractography-based analysis of the cingulum: clinical utility and findings in traumatic brain injury with chronic sequels.
Neuroradiology, 56(10), 833–841. https://doi.org/10.1007/s00234-014-1410-7
Le, K., Coelho, C., Mozeiko, J., Krueger, F., & Grafman, J. (2012). Predicting story goodness performance from cognitive measures following traumatic brain injury.
Am J Speech Lang Pathol, 21(2), S115-25.
https://doi.org/10.1044/1058-0360(2012/11-0114)
LeBlanc, J., de Guise, E., Champoux, M. C., Couturier, C., Lamoureux, J., Marcoux, J., … Feyz, M. (2014). Acute evaluation of conversational discourse skills in traumatic brain injury. Int J Speech Lang Pathol, 16(6), 582–593.
Liegeois, F. J., Mahony, K., Connelly, A., Pigdon, L., Tournier, J. D., & Morgan, A. T. (2013). Pediatric traumatic brain injury: language outcomes and their relationship to the arcuate fasciculus. Brain Lang, 127(3), 388–398.
https://doi.org/10.1016/j.bandl.2013.05.003
Maier-Hein, K. H., Neher, P. F., Houde, J.-C., Côté, M.-A., Garyfallidis, E., Zhong, J., … Descoteaux, M. (2017). The challenge of mapping the human connectome based on diffusion tractography. Nature Communications, 8(1).
https://doi.org/10.1038/s41467-017-01285-x
Manjon, J. V, Coupe, P., Concha, L., Buades, A., Collins, D. L., & Robles, M. (2013). Diffusion weighted image denoising using overcomplete local PCA. PLoS One,
8(9), e73021. https://doi.org/10.1371/journal.pone.0073021
Marini, A., Galetto, V., Zampieri, E., Vorano, L., Zettin, M., & Carlomagno, S. (2011). Narrative language in traumatic brain injury. Neuropsychologia, 49(10), 2904–2910. https://doi.org/10.1016/j.neuropsychologia.2011.06.017
Mayer, A. R., Ling, J., Mannell, M. V, Gasparovic, C., Phillips, J. P., Doezema, D., … Yeo, R. A. (2010). A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology, 74(8), 643–650.
https://doi.org/10.1212/WNL.0b013e3181d0ccdd
Meythaler, J. M., Peduzzi, J. D., Eleftheriou, E., & Novack, T. A. (2001). Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch Phys Med
Rehabil, 82(10), 1461–1471. Retrieved from
http://www.ncbi.nlm.nih.gov/pubmed/11588754
Mohammadian, M., Roine, T., Hirvonen, J., Kurki, T., Ala-Seppala, H., Frantzen, J., … Tenovuo, O. (2017). High angular resolution diffusion-weighted imaging in mild traumatic brain injury. Neuroimage Clin, 13, 174–180.
https://doi.org/10.1016/j.nicl.2016.11.016
Muscara, F., Catroppa, C., & Anderson, V. (2008). Social problem-solving skills as a mediator between executive function and long-term social outcome following paediatric traumatic brain injury. J Neuropsychol, 2(Pt 2), 445–461. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19824165
Nakayama, N., Okumura, A., Shinoda, J., Yasokawa, Y. T., Miwa, K., Yoshimura, S. I., & Iwama, T. (2006). Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry, 77(7), 850–855. https://doi.org/10.1136/jnnp.2005.077875
Newcombe, V. F., Correia, M. M., Ledig, C., Abate, M. G., Outtrim, J. G., Chatfield, D., … Menon, D. K. (2015). Dynamic Changes in White Matter Abnormalities
Correlate With Late Improvement and Deterioration Following TBI: A Diffusion Tensor Imaging Study. Neurorehabil Neural Repair.
https://doi.org/10.1177/1545968315584004
Palacios, E. M., Fernandez-Espejo, D., Junque, C., Sanchez-Carrion, R., Roig, T.,
Tormos, J. M., … Vendrell, P. (2011). Diffusion tensor imaging differences relate to memory deficits in diffuse traumatic brain injury. BMC Neurol, 11, 24.
https://doi.org/10.1186/1471-2377-11-24
Palacios, E. M., Sala-Llonch, R., Junque, C., Roig, T., Tormos, J. M., Bargallo, N., & Vendrell, P. (2012). White matter integrity related to functional working memory networks in traumatic brain injury. Neurology, 78(12), 852–860.
https://doi.org/10.1212/WNL.0b013e31824c465a
Perez, A. M., Adler, J., Kulkarni, N., Strain, J. F., Womack, K. B., Diaz-Arrastia, R., & Marquez de la Plata, C. D. (2014). Longitudinal white matter changes after
traumatic axonal injury. J Neurotrauma, 31(17), 1478–1485. https://doi.org/10.1089/neu.2013.3216
Poeppel, D., Emmorey, K., Hickok, G., & Pylkkänen, L. (2012). Towards a new neurobiology of language. The Journal of Neuroscience, 32(41), 14125–14131. https://doi.org/10.1523/JNEUROSCI.3244-12.2012
Raffelt, D., Tournier, J. D., Rose, S., Ridgway, G. R., Henderson, R., Crozier, S., … Connelly, A. (2012). Apparent Fibre Density : A novel measure for the analysis of diffusion-weighted magnetic resonance images. NeuroImage, 59(4), 3976–3994. https://doi.org/10.1016/j.neuroimage.2011.10.045
Rigon, A., Voss, M. W., Turkstra, L. S., Mutlu, B., & Duff, M. C. (2016). Frontal and Temporal Structural Connectivity Is Associated with Social Communication Impairment Following Traumatic Brain Injury. J Int Neuropsychol Soc, 22(7), 705– 716. https://doi.org/10.1017/S1355617716000539
Sanchez, E., El-Khatib, H., Arbour, C., Bedetti, C., Blais, H., Marcotte, K., … Gosselin, N. (2018). Brain white matter damage and its association with neuronal synchrony during sleep. Brain.
Sarno, M. T. (1980). The nature of verbal impairment after closed head injury. J Nerv
Ment Dis, 168(11), 685–692. Retrieved from
http://www.ncbi.nlm.nih.gov/pubmed/7441232
Saur, D., Kreher, B. W., Schnell, S., Kümmerer, D., Kellmeyer, P., Vry, M.-S., … Weiller, C. (2008). Ventral and dorsal pathways for language. Proceedings of the
National Academy of Sciences of the United States of America, 105(46), 18035–
18040. https://doi.org/10.1073/pnas.0805234105
Selassie, A. W., Zaloshnja, E., Langlois, J. A., Miller, T., Jones, P., & Steiner, C. (2008). Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil, 23(2), 123–131.
https://doi.org/10.1097/01.HTR.0000314531.30401.39
Sherif, T., Rioux, P., Rousseau, M. E., Kassis, N., Beck, N., Adalat, R., … Evans, A. C. (2014). CBRAIN: a web-based, distributed computing platform for collaborative neuroimaging research. Front Neuroinform, 8, 54.
https://doi.org/10.3389/fninf.2014.00054
Smith, R. E., Tournier, J. D., Calamante, F., & Connelly, A. (2012). Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage.
https://doi.org/10.1016/j.neuroimage.2012.06.005
Song, S. K., Sun, S. W., Ju, W. K., Lin, S. J., Cross, A. H., & Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage, 20(3), 1714–1722. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14642481
Song, S. K., Yoshino, J., Le, T. Q., Lin, S. J., Sun, S. W., Cross, A. H., & Armstrong, R. C. (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage, 26(1), 132–140.
Stowe, L. A., Broere, C. A., Paans, A. M., Wijers, A. A., Mulder, G., Vaalburg, W., & Zwarts, F. (1998). Localizing components of a complex task: sentence processing and working memory. Neuroreport, 9(13), 2995–2999. Retrieved from
http://www.ncbi.nlm.nih.gov/pubmed/9804304
Thomas, C., Ye, F. Q., Irfanoglu, M. O., Modi, P., Saleem, K. S., Leopold, D. A., & Pierpaoli, C. (2014). Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci U S A,
111(46), 16574–16579. https://doi.org/10.1073/pnas.1405672111
Togher, L., Wiseman-Hakes, C., Douglas, J., Stergiou-Kita, M., Ponsford, J., Teasell, R., … Panel, I. E. (2014). INCOG recommendations for management of cognition following traumatic brain injury, part IV: cognitive communication. J Head Trauma
Rehabil, 29(4), 353–368. https://doi.org/10.1097/HTR.0000000000000071
Tournier, J. D., Calamante, F., & Connelly, A. (2007). Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. Neuroimage, 35(4), 1459–1472.
https://doi.org/10.1016/j.neuroimage.2007.02.016
Tournier, J. D., Calamante, F., & Connelly, A. (2012). MRtrix: Diffusion tractography in crossing fiber regions. International Journal of Imaging Systems and Technology,
22(1), 53–66. https://doi.org/10.1002/ima.22005
Tournier, J. D., Calamante, F., Gadian, D. G., & Connelly, A. (2004). Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage, 23(3), 1176–1185.
https://doi.org/10.1016/j.neuroimage.2004.07.037
van Velzen, J. M., van Bennekom, C. A. M., Edelaar, M. J. A., Sluiter, J. K., & Frings-Dresen, M. H. W. (2009). How many people return to work after acquired brain injury?: A systematic review. Brain Injury.
https://doi.org/10.1080/02699050902970737
Warren, J. E., Wise, R. J., & Warren, J. D. (2005). Sounds do-able: auditory-motor transformations and the posterior temporal plane. Trends in Neuroscience, 28(12), 636–643. https://doi.org/10.1016/j.tins.2005.09.010
Warschausky, S., Cohen, E. H., Parker, J. G., Levendosky, A. A., & Okun, A. (1997). Social problem-solving skills of children with traumatic brain injury. Pediatr
Rehabil, 1(2), 77–81. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9689242
Wassermann, D., Makris, N., Rathi, Y., Shenton, M., Kikinis, R., Kubicki, M., & Westin, C. F. (2013). On describing human white matter anatomy: the white matter query language. Med Image Comput Comput Assist Interv, 16(Pt 1), 647–654. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24505722
Wassermann, D., Makris, N., Rathi, Y., Shenton, M., Kikinis, R., Kubicki, M., & Westin, C. F. (2016). The white matter query language: a novel approach for describing human white matter anatomy. Brain Structure and Function.
https://doi.org/10.1007/s00429-015-1179-4
Wechsler, D. (1997). WAIS-III administration and scoring manual. San Antonio, TX: The Psychological Corporatione.
Wilson, S. M., Galantucci, S., Tartaglia, M. C., Rising, K., Patterson, D. K., Henry, M. L., … Gorno-Tempini, M. L. (2011). Syntactic processing depends on dorsal language tracts. Neuron, 72(2), 397–403.
https://doi.org/10.1016/j.neuron.2011.09.014
Wozniak, J. R., Krach, L., Ward, E., Mueller, B. A., Muetzel, R., Schnoebelen, S., … Lim, K. O. (2007). Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin
Neuropsychol, 22(5), 555–568. https://doi.org/10.1016/j.acn.2007.03.004
Yeatman, J. D., Dougherty, R. F., Rykhlevskaia, E., Sherbondy, A. J., Deutsch, G. K., Wandell, B. A., & Ben-Shachar, M. (2011). Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. Journal of Cognitive
Neuroscience. https://doi.org/10.1162/jocn_a_00061
Ylvisaker, M. (1992). Communication outcome following traumatic brain injury.
Seminars in Speech and Language, 13, 239–250.
Ylvisaker, M. (2006). Self-coaching: A context-sensitive, person centred approach to social communication after traumatic brain injury. Brain Impairment, 7, 246–258.
Table 1. Demographic informations. TBI participan t Sex Age Years of educatio n Initial Glascow Score
Initial scan findings of injurySeverity
Time post TBI (months ) Type of
TBI Matricessubtest from WAIS-III (SS)
P01 Male 30 11 4 Left fronto-temporal SAH Severe 21 passengerMVA 14
P02 Male 60 10 7T Multiple cerebral contusionsbilaterally
TAI Severe 15
MVA
passenger 10
P03 Male 22 12 3 Left frontal SAH/SDH Severe 16 MVA
passenger 11
P04 Male 19 11 7T Right frontal SDH Left frontal SAH Severe 12 passengerMVA 10
P05 Male 18 12 7T Left temporal and parietal contusions Severe 11 MVA
passenger 13
P06 Female 21 14 9T Left temporal contusion Moderate 36 passengerMVA 6
P07 Female 41 20 7T Right temporal SAH Severe 36 MVAbiker 15
P08 Female 31 19 6
Right frontal SAH Posterior fossa SDH Multiple haemorrhagic contusions bilaterally Oedema
Severe 20 MVAbiker 13
P09 Male 28 13 6
Multiple petechiae in the frontal lobes, thalamus and corpus callosum
TAI
Severe 35 MVA
P10 Male 29 12 n/a Left fronto-parieto-temporalSDH Moderate 34 passengerMVA 15 P11 Female 19 9 10 Frontal SAH bilaterallyOrbito-frontal contusion
Occipital facture Moderate 16 Fall 6
P12 Male 56 9 n/a Right fronto-temporo-pariétal SDH
Right frontal SAH Moderate 15
MVA
passenger 11
P13 Male 18 11 9 Gyrus rectus haemorrhageRight frontal contusion Moderate 12 MVA
pedestrian 14
P14 Male 32 10 11T Right parietal petechiaes Moderate 25 passengerMVA 10
P15 Male 58 9 9T Interpedoncular hemorrhage Moderate 20 MVA
passenger 8 MEAN TBIs (n=15) 11 males, 4 females 32.1 ± 14.9 12.1 ± 3.4 7.3 ± 2.3 -7 moderate , 8 severe 21.6 ± 9.3 -10.8 ± 3.2 Mean Controls (n=15) 11 males, 4 females 30.9 ± 15.4 13.7 ± 1.8 - - - 11.1 ±2.2
Abbreviations: TBI: Traumatic Brain Injury; TAI: traumatic axonal injury; SHA: subarachnoid hemorrhage; SDH: subdural hematomaT: Intubated; MVA:Motor vehicle accident.
Table 2. Cognitive-communication scores of the TBI patient group.
Task Raw score ± SD Calibrated scores ± SD
5 minutes of conversational speech about
work, family or leasures (/34) 29.93 ± 3.48 -3.14 ± 3.04** Narrative discourse - Full story recall (/13) 11.43 ± 1.44 0.74 ± 0.44
Narrative discourse - Comprehension of the
story (/12) 11.14 ± 0.8 0.41 ± 0.67
Free lexical verbal fluency (2min.30sec.) 59.40 ± 16.15 -0.15 ± 0.60
Orthographic verbal fluency (2min.) 21.60 ± 6.65 -0.48 ± 0.64
Semantic verbal fluency (2 min.) 25.73 ± 8.98 -0.49 ± 1.40
Interpretation of indirect speech acts (/40) 33.46 ± 5.97 -0.63 ± 1.94
La Trobe Communication Questionnaire 52.13 ± 11.58 -0.39 ± 1.21
Table 3. Detailed results for the communication behaviours of the D-MEC. Communication behaviours (Score of 0Impaired
or 1) % Preserved(Score of 2) % Word finding/Paraphasias 6 40.0 9 60.0 Inappropriate/Unexpected comments 6 40.0 9 60.0
Imprecise expression of ideas 5 33.3 10 66.7
Inappropriate topic shifts 5 33.3 10 66.7
Monotone voice 5 33.3 10 66.7
Speech rate too slow or too fast 5 33.3 10 66.7
No self-correction of errors 3 20.0 12 80.0
Lack of verbal initiative 3 20.0 12 80.0
Overtalkative 3 20.0 12 80.0
Interrupts the speaker 2 13.3 13 86.7
Frozen facial expression 2 13.1 13 86.7
Repetitiveness 1 6.7 14 93.3
Difficulty understanding what is
being said 1 6.7 14 93.3
Difficulty understanding indirect
language 0 0 15 100
Indifference to humorous comments 0 0 15 100
Loses track of conversation 0 0 15 100
Table 4. Mean and standard diffusion weighted imaging indices for the arcuate fasciculus—temporo-parietal segment for the control and TBI groups, and results of between group comparisons.
FA RD [x 10-4 mm2/sec] NuFO Volume
Controls TBIs Statistics Controls TBIs Statistics Controls TBIs Statistics Controls TBIs Statistics
Left AF .490 ± .018 .458 ± .030 t=-3.51, p=.002* 5.41 ± 0.19 5.96 ± .36 t=5.27., p<.001* 1.54 ± .15 1.36 ± .20 t=.-2.74, p=.011 44626 ± 9005 47342 ±7370 t=.91, p=.370 Left ILF .500 ± .037 .463 ± .031 t=-3.02, p=.005 5.82 ± 0.65 6.31 ± .42 t=2.48, p=.019 1.51 ± .17 1.33 ± .21 t=-2.54, p=.017 11981 ± 2581 10718 ±3139 t=-1.20, p=.239 Left UF .461 ± .022 .424 ± .034 t=-2.69, p=.002* 6.04 ± 0.28 6.70 ± .61 t=3.89, p=.001* 1.54 ± .21 1.33 ± .22 t=-2.69 p=.012 10004 ± 2381 7753 ± 2709 t=-2.42, p=.022 gCC .542 ± .024 .481 ± .056 t=--3.83, p=.001* 5.78 ± 1.42 6.89 ± 1.58 t=-1.86, p=.073 1.46 ± .16 1.32 ± .19 t=-2.21, p=.035 38833 ± 6482 35323 ± 8016 t=-1.31, p=.199
Abbreviations: TBI: Traumatic Brain Injury; AF: Arcuate Fasciculus; UF: Uncinate Fasciculus; gCC: genu of the Corpus Callosum Asterisks denote a significant effect of group on t-test using Bonferroni correction (p<.003)
Figure legend
Figure 1. Representation of the four bundles of interest from a representative control subject. The left arcuate fasciculus is represented in (A), the left inferior longitudinal fasciculus in (B), the uncinate in (C) and the genu of the corpus
callosum in (D).
Figure 2. Means and standard deviations of diffusion weighted imaging indices for each white matter tract for the control and TBI groups, and results of between
C) A) * * * * * * 0.0009 0.0008 0.0007 0.0006 0.0005 0.0004 0.0003 0.0002 0.0001 0.0000 * * * 0.60 0.50 0.10 0.40 0.30 0.20 0.00 2.00 1.75 1.50 1.25 1.00 0.75 0.50 0.25 0.00 B) N uF O R ad ia l d if fu si vi ty D)
Abbreviations: TBI: Traumatic Brain Injury; AF: Arcuate Fasciculus; UF: Uncinate Fasciculus; gCC: genu of the Corpus Callosum
Asterisks denote a significant effect of group on t-test using Bonferroni correction (p<.003)
Figure 3. Anatomical variability in the arcuate fasciculus and its relation to long-term discourse abilities following moderate to severe TBI. The arcuate fasciculus is represented in three patients presenting with different degrees of discourse abilities. These patients are indicated in the significant correlations of the fractional
anisotropy (A) and radial diffusivity (B) of the arcuate fasciculus plotted against the discourse calibrated score.
cut off score Male, 22 years
Supplementary Figure 1. Anatomical variability in the temporo-parietal segment of the arcuate fasciculus and its relation to long-term discourse abilities following moderate-to-severe TBI. The temporo-parietal segment is represented in three patients presenting with different degrees of discourse abilities. These patients are indicated in the represented correlations of the fractional anisotropy (A) and radial diffusivity (B) of the temporo-parietal segment of the arcuate fasciculus plotted against the discourse calibrated score.
Male, 22 years
A)
Male, 58 years Male, 28 years