Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Cement and Concrete Research, 15, May 3, pp. 411-420, 1985-05-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=7e013027-7c91-473d-9221-e88df1b1fcec
https://publications-cnrc.canada.ca/fra/voir/objet/?id=7e013027-7c91-473d-9221-e88df1b1fcec
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1016/0008-8846(85)90114-0
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Resistance of mortars containing silica fume to attack by a solution
containing chlorides
THI.
N21d
National Research
Conseil national
' 0 0
1292
m
+
Council Canada
de recherches Caada
(3.
2
BLDG
Division of
Division des
- -- -
Building Research
recherches en biitiment
I
Resistance of Mortars
Containing Silica Fume to
Attack
by a Solution
Containing Chlorides
by R. F. Feldman and Huang Cheng-yi
Reprinted from
Cement and Concrete Research
Volume 15, No.
3,
1985
p.
41 1
-
420
DBR Paper No. 1292
Price $1 .OO
NRCC 24628
ANALYZED
- -
R E s d
Des mortiers ayant des rapports eautciment
+
poudre de silice(e/c+s) de 0,45 et 0,60 ont St6 expos6s B une solution
contenant un melange de chlorures de Mg, de Ca et de Na afin
dlCvaluer leur resistance 3 l'attaque. Les mglanges
contenaient 0, 10 et 30% de poudre de silice, et le rapport
sabletliant etait de 2,25. La consistance, la distribution du
diamstre des pores, la teneur en Ca(OH)2 et en eau non
Bvaporable ont 6te mesurees avant et apres exposition B la
solution de sels, L'inclusion de poudre de silice s'est
traduite par une augmentation de la durabiliti? du mortier, notamment dans des specimens ayant un rapport e/c+s de 0,45 et durcis pendant 28 jours. Des specimens de m8me rapport titrant 30% de poudre de silice se sont aussi ri?vGles durables, msme apres sept jours de durcissement. L'hydroxyde de calcium a st6
Blimine des mortiers
a
la suite de l'exposition la solutionde sels.
1
3
IIIIII
4809
I ! ~ ~ / ~ ~ ~
02
d
8
l!i[[[
- - - - - - --__
- --- ---
- ._-CEMENT and CONCRETE RESEARCH. Vol. 1 5 , pp. 411-420, 1985. P r i n t e d i n t h e USA. 0008-8846/,85 $3.00+00. Copyright ( c ) 1985 Pergamon P r e s s , L t d .
RESISTANCE OF MORTARS CONTAINING SILICA FUME TO ATTACK BY A SOLUTION CONTAINING CHLORIDES
R.F. Feldman and Huang C h e n g y i D i v i s i o n of B u i l d i n g Re s e a r c h ,
N a t i o n a l Research Council Canada, Ottawa, K l A OR6
(Communicated by M. Regourd) (Received S e p t . 1 2 , 1984) ABSTRACT
M o r t a r s p r e p a r e d a t waterfcement
+
s i l i c a fume r a t i o s , w/ ( e k s f ) , of 0.45 and 0.60 were exposed t o a s o l u t i o n c o n t a i n i n g a m i x t u r e of Mg-, Ca- and Na- c h l o r i d e s t o i n v e s t i g a t e t h e i r r e s i s t a n c e t o a t t a c k . Mixes c o n t a i n e d 0 , 10 and 30% s i l i c a fume, and sand-binder r a t i o was 2.25. S t i f f n e s s , p o r e s i z e d i s t r i b u t i o n , Ca(0H) c o n t e n t and n o w e v a p o r a b l e w a t e r were measured b e f o r e and a f t e r exposure t o s a l t s o l u t i o n . I n c l u s i o n o f s i l i c a fume r e s u l t e d i n i n c r e a s e d d u r a b i l i t y of m o r t a r , e s p e c i a l l y i n specimens p r e p a r e d a t w/(c+sf) of 0.45 and c u r e d f o r 28 days. Specimens p r e p a r e d a t t h e same w / ( c + s f ) w i t h 30% s i l i c a fume were a l s o d u r a b l e even a f t e r s e v e n days of c u r i n g .Calcium h y d r o x i d e i n m o r t a r s was r e d u c e d t o z e r o by e x p o s u r e t o t h e s a l t s o l u t i o n .
I n t r o d u c t i o n
C o r r o s i o n of c o n c r e t e by s a l t s o l u t i o n s h a s been s t u d i e d f o r many y e a r s ( I ) , most o f t e n u s i n g s u l p h a t e s , s e a w a t e r and magnesium c h l o r i d e (2-6): Although much a t t e n t i o n h a s been d i r e c t e d t o a n i o n s s u c h a s SO,'-, i t i s
recognized t h a t c a t i o n s a r e always i n v o l v e d i n t h e d e t e r i o r a t i o n p r o c e s s . S o l u t i o n s c o n t a i n i n g
big++
a r e p a r t i c u l a r l y a g g r e s s i v e .Mg++
may r e a c t w i t h Ca(OIU2 t o form Mg(OH)2, and i n t h e p r e s e n c e of C1- may form magnesiumo x y c h l o r i d e (Mg2(0H) $1 4H20). Calcium s i l i c a t e h y d r a t e may a l s o be g r a d u a l l y broken down ( 2 ) .
The s o l u b i l i t y of many of t h e s e c o r r o s i o n p r o d u c t s i s enhanced i n t h e p r e s e n c e of sodium and c a l c i u m c h l o r i d e s
(S),
and t h i s adds t o t h e complexity of t h e mechanism of a t t a c k by a mixed s o l u t i o n . A major f a c t o r i s t h el e a c h i n g o u t of r e a c t i o n p r o d u c t s t h a t r e s u l t s i n s o f t e n i n g of t h e specimen. The most e f f e c t i v e a c t i o n a g a i n s t s u c h forms of a t t a c k i s t o u s e c o n c r e t e w i t h t h e reduced p e r m e a b i l i t y o b t a i n e d by a d d i n g b l a s t f u r n a c e s l a g and fly-ash b l e n d s (7-10). Prolonged c u r i n g i s always needed, however, and t h i s p o s e s p r a c t i c a l problems. Because s i l i c a fume i s more r e a c t i v e t h a n f l y - a s h o r s l a g s , i t i s e x p e c t e d t h a t d u r a b l e c o n c r e t e s c a n b e o b t a i n e d w i t h s h o r t e r c u r i n g t i m e s by u s i n g i t a s t h e a d d i t i v e (11-13).
412 Vol. 1 5 , No. 3
I R.F. Feldman and Huang Cheng-yi
I
The o b j e c t of t h e p r e s e n t s t u d y was t o d e t e r m i n e t h e e f f e c t of mixed c h l o r i d e s o l u t i o n s on m o r t a r d i s c s c o n t a i n i n g s i l i c a fume and t o r e l a t e t h e s e p r o p e r t i e s t o p o r e s t r u c t u r e , c a l c i u m h y d r o x i d e , and non-evaporable w a t e r c o n t e n t s .
Experiment a
M a t e r i a l s
Type I cement w i t h a C3A c o n t e n t of 11.82% and s i l i c a fume c o n t a i n i n g 95.2% S i 0 2 , 1.56% c a r b o n , 0.27% K20, 0.10% Na20 ( s u r f a c e a r e a 2 1 000 m2/kg) were used. Ottawa s i l i c a s a n d conforming t o ASTM-C109 was u s e d f o r m o r t a r w i t h a sand-binder r a t i o of 2.25. Cement i n t h e m o r t a r c o n t a i n e d e i t h e r 0 , 1 0 o r 30% s i l i c a fume. Mixes were p r e p a r e d a t w / ( c + s f ) r a t i o s of 0.45 and 0.60. C y l i n d e r s 75 x 160 mm w e r e made from s i x d i f f e r e n t mixes; t h e y w e r e s l i c e d i n t o d i s c s 75 nun i n d i a m e t e r and 6.4 nun t h i c k a f t e r c u r i n g f o r 24 h a t 21°C. C u r i n g was c a r r i e d o u t i n s a t u r a t e d l i m e w a t e r a t 21°C f o r s e v e n and 28 days. Specimens were exposed t o 4% MgC12 s o l u t i o n f o r 150-170 d a y s , f o l l o w e d by e x p o s u r e t o a s o l u t i o n of 27.5% CaC12, 3.9% MgC12, 1.2% NaC1, and 2.1% NaHC03 ( 1 4 ) . The s o l u t i o n was renewed a p p r o x i m a t e l y e v e r y t h i r t y days.
1 A l l specimens exposed t o t h e s a l t s o l u t i o n w i l l b e r e f e r r e d t o a c c o r d i n g t o t h e f o l l o w i n g scheme: w/ (c+s £1 S i l i c a fume, % 0.45 0.60 0
c
a
cH
10Bt
II
0 B10 H 3 0 B30 B30 H P r o p e r t i e s L o a d - d e f l e c t i o n measurements w e r e c a r r i e d o u t i n f l e x u r e ; d i s c s were l o a d e d a t a d e f l e c t i o n r a t e of 0.5 mm/min t o a maximum l o a d of 8 kg; s e v e n d i s c s were used t o o b t a i n t h e v a l u e f o r e a c h specimen. Measurements were made a s a f u n c t i o n of t i m e of e x p o s u r e t o t h e s a l t s o l u t i o n and t h e r e s u l t sf o l l o w i n g e x p o s u r e t o Ca(OH)2 s o l u t i o n were u s e d a s r e f e r e n c e . D e f l e c t i o n was measured on a s a t u r a t e d m o r t a r d i s c l o a d e d a t i t s c e n t e r and s u p p o r t e d a t t h r e e p o i n t s on t h e c i r c u m f e r e n c e ( 1 4 ) . Ca(OH)2 and non-evaporable w a t e r c o n t e n t s w e r e d e t e r m i n e d a s f o l l o w s : TGA of m o r t a r s , by a Cahn B a l a n c e u s i n g a 1000-mg sample; w e i g h t l o s s between a p p r o x i m a t e l y 400 and 600°C and 100 and 1000°C by d e t e r m i n i n g Ca(OH)2 and non-evaporable w a t e r c o n t e n t s , r e s p e c t i v e l y . These v a l u e s were o b t a i n e d b e f o r e and a f t e r e x p o s u r e t o t h e s a l t s o l u t i o n . P o r e s i z e d i s t r i b u t i o n was d e t e r m i n e d a s f o l l o w s : mercury i n t r u s i o n o f specimens was performed up t o a p r e s s u r e of 408 MPa b e f o r e e x p o s u r e , a f t e r s e v e n and a f t e r 28 d a y s of c u r i n g , and a f t e r v a r i o u s p e r i o d s o f e x p o s u r e t o s a l t s o l u t i o n . Samples were d r i e d by vacuum and f i n a l h e a t i n g f o r 8-14 h a t 100°C p r i o r t o t a k i n g t h e s e measurements.
R e s u l t s R e f l e c t i o n measurements
The t i m e dependence of d e f l e c t i o n s i n f l e x u r e a r e p r e s e n t e d i n
Fig. l ( a - c ) . The r e f e r e n c e specimens i n Fig. l ( a ) w e r e measured a f t e r 28 d a y s of i n i t i a l c u r i n g and show a d e c r e a s e i n d e f l e c t i o n f o r a f u r t h e r 1 5 d a y s ; a f t e r t h i s v a l u e s remain r e l a t i v e l y c o n s t a n t a l t h o u g h t h e r e i s some v a r i a t i o n between 100 and 250 d a y s , p o s s i b l y owing t o t h e measurement t e c h n i q u e .
Vol. 15, No. 3
MORTARS, SiO FUME, RESISTANCE, CHLORIDE, ATTACK
2 W / ( C + s f ) S I L I C A F U M E 0.45 0 n 10% f0 - - - -
- -
- - 30% I t ( a ) R E F E R E N C E S P E C I M E N S I N C a ( O H I 2 S O L U T I O N 01
I t 1 I I I I 0 50 100 150 200 250 300 350 400 T I M E , d a y s - ( b ) 7 - D A Y C U R E D S P E C I M E N S - 0 I 1 I I I 1 I 0 50 LOO 150 200 250 300 350 400 T I M E , d a y s FIG. 1 D e f l e c t i o n of m o r t a r specimens i n f l e x u r e d u r i n g exposure t o s a l t s o l u t i o n . ( a ) Reference specimens i n Ca(OH)2 s o l u t i o nTABLE 1
Pore s i z e d i s t r i b u t i o n , p e r c e n t Ca(OH)2, and non-evaporable w a t e r c o n t e n t of m o r t a r s b e f o r e and a f t e r e x p o s u r e t o s a l t s o l u t i o n
---
Age Pore diam ~ ' ( 7 ) ~ ' ( 7 ) - c 9 t 0 ( 7 ) B! (7)-c B t 0 ( 7 ) B: (7)-c c H ( 7 ) c H ( 7 ) - c B Y ~ ( ~ )
BY
(7)-c B Y ~ ( ~ ) BH (7)-c(Days) (nm x 1 0 - 9 -337 d -!37 d -960 d 360 d $13 d 3 j 3 d - f a i l e d
-
f a i l e d-
f a i l e d 97-2.0 97-0.875 0.875-0.175 7 0.175-0.0175 0.0175-0.0029 Tot a 1 Ca(0H) *% (g/g-binder)* Wn% (g/g-binder) ~ ' ( 2 8 ) ~ ' ( 2 8 ) - c Bf0(28) Bf0(28)-c 8 t O ( 2 8 ) Bt0(28)-c cH(28) s ( 2 8 ) - c ~ Y ~ ( 2 8 ) BY0(2 8)- Bt0(28) ~ : ~ ( 2 8)- -319 d -348 d -327 d 339 d-
341 d-
341 d-
f a i l e d 97-2.0 2.0 6.75 2.75 7.50 3.25 3.75 1.50 8.00 1.75 9.00 5.7 5 9.00 97-0.875 2.52 7.04 2.99 8.15 5.53 5.24 1.84 8.84 2.78 9.73 7.55 10.20 0.875-0.175 2.79 1.40 5.35 1.24 0.88 0.69 1.68 2.02 3.47 1.17 1.98 1.2 3 28 0.175-0.0175 7.84 5.91 5.35 2.49 1.33 0.83 10.85 6.58 9.04 3.77 4.13 2.54 0.0175-0.0029 4.04 3.38 7.48 4.84 8.18 5.92 5.81 4.14 7.99 6.36 13.48 6.85 Tot a 1 17.19 17.73 19.24 16.72 15.92 12.68 20.18 21.58 23.28 21.03 27.14 20.82 Ca(0H) 2% ( g / g - b i n d e r ) 15.02 0 5.79 0 0 0 19.14 7.31 10.81 0 0' 0 WnX (g/g-binder) 16.31 17.23 13.53 17.72 11.88 16.67 19.48 20.36 17.60 19.14 15.20 20.99*
g/g-binder = g/g-cement+
s i l i c a fume; Wn = non-evaporable w a t e r ; c = c o r r o d e d ; ( 7 ) = 7 d a y s c u r e d ; (28) = 28 d a y s cured.Vol. 1 5 , No. 3 415 MORTARS, S i 0 2 FUME, RESISTANCE, CHLORIDE, ATTACK
The r e s u l t s f o r seven-day c u r e d specimens p r e s e n t e d i n F i g b l ( b ) a l s o show d e c r e a s e s i n d e f l e c t i o n i n t h e f i r s t t e n days. Specimen B~ shows a s m a l l g r a d u a l i n c r e a s e o v e r 325 days of exposure, w h i l e B I 0 and3:' (which behave s i m i l a r l y ) d i f f e r from a f t e r a b o u t 180 days i n t h a t d e f l e c t i o n s i n c r e a s e markedly. The specimens prepared a t t h e h i g h e r w / ( c + s f ) have, a s expected, a h i g h e r i n i t i a l d e f l e c t i o n ; i n
BY*
and B; d e f l e c t i o n i n c r e a s e d a f t e r about 90 days, i n B y 0 more r a p i d l y , b u t a f t e r 9 1 days b a t h specimensH
;1
f a i l e d , w i t h d e f l e c t i o n s of 0.0725 and 0.0925 mm f o r B and B 30,
r e s p e c t i v e l y . D e f l e c t i o n of C started1 t o i n c r e a s e a f t e r 170 d a y s and
a t t a i n e d 0.0650 mm a f t e r 420 days. A t h i g h e r w / ( c + s f ) r a t i o s t h e s i l i c a fume a d d i t i o n d e c r e a s e s r e s i s t a n c e t o s a l t s o l u t i o n a t t a c k because up t o seven days t h e p o z z o l a n i c r e a c t i o n of t h e s i l i c a fume h a s proceeded o n l y t o a l i m i t e d e x t e n t . A t low w/ ( c + s f ) , however, t h e s i l i c a fume i s b e n e f i c i a l , e s p e c i a l l y
II
f o r specimen B 3 0 , which d i s p l a y s a low i n c r e a s e i n d e f l e c t i o n a f t e r 420 d a y s of exposure.
The r e s u l t s f o r t h e 2 8 d a y c u r e d specimens p r e s e n t e d i n Fig. l ( c ) show no d e c r e a s e i n d e f l e c t i o n i n t h e i n i t i a l p e r i o d of exposure, a s w i t h t h e
a
seven-day c u r e d and r e f e r e n c e samples. Specimen B 3 0 shows a low r a t e of i n c r e a s e o v e r 300 days of exposure, a s does B t O . Specimen C' shows an i n c r e a s e i n d e f l e c t i o n , e s p e c i a l l y a f t e r 160 days of exposure, b u t l e s s t h a n
R
t h e same C ( 7 ) specimen c u r e d f o r o n l y seven days. Specimens p r e p a r e d a t a w / ( c + s f ) of 0.60 a r e t h u s much more r e s i s t a n t t o s a l t s o l u t i o n t h a n t h o s e
H
cured f o r on18 seven days. Specimen B30(28) shows l e s s d e f l e c t i o n t h a n
BY
(28) and C ( 2 8 ) , i n t h a t o r d e r , and a f t e r 380 days of exposure shows a vayue of 0.0435 mm a s compared t o 0.0925 mm f o r specimen 8 t 0 ( 7 ) .FIG. l ( C )
I
I
D e f l e c t i o n of m o r t a rO.l
t
( c ) 2 8 - D A Y CURED SPECIMENS specimens i n f l e x u r e
d u r i n g exposure t o 0 I t I I I I I
J
s a l t s o l u t i o n .o
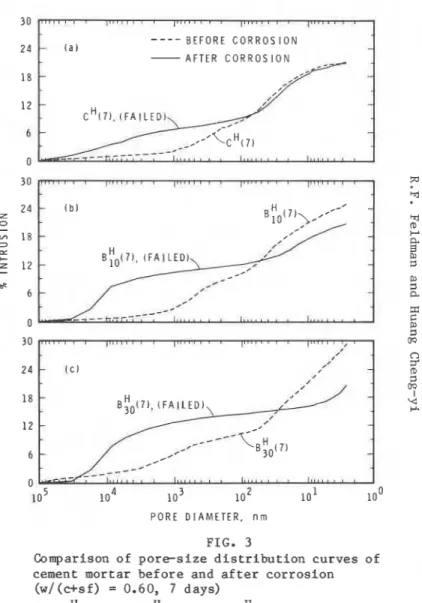
50 loo 150 200 250 300 350 400 2 8 d a y c u r e d T I M E , d a y s specimens Pore s i z e d i s t r i b u t i o n The r e s u l t s f o r p o r e s i z e d i s t r i b u t i o n measurements a r e p r e s e n t e d i n F i g s . 2 t o 5 f o r specimens c u r e d f o r seven days a t w/(c+sf) of 0.4 and 0.6 and f o r t h o s e cured f o r 28 days a t w / ( c + s f ) of 0.4 and 0.6, r e s p e c t i v e l y .Measurements were made b e f o r e and a f t e r exposure t o t h e s a l t s o l u t i o n (Table I ) . The t o t a l i n t r u d e d volume i s l e s s f o r t h e specimens c o n t a i n i n g s i l i c a fume and exposed t o s a l t s o l u t i o n t h a n f o r t h o s e n o t s o exposed; t h e g r e a t e r t h e s i l i c a fume c o n t e n t t h e l e s s t h e i n t r u d e d volume. Specimens w i t h o u t s i l i c a fume, c L ( 7 ) , ~ ' ( 2 8 ) and c H ( 2 8 ) , showed s l i g h t i n c r e a s e i n
-
30 o 30 ^ ! , # 8 . I ~ ' * ~ ~.
* ~ I , , ~ . , , 8 I * , * - - , , C- - - - - B E F O R E C O R R O S I O N- - - -
B E F O R E C O R R O S I O N P 24-
{ a )-
-
2 4 - ( a ) - m AFTER C O R R O S I O N 1 8-
1 8-
! ~ 4 7 7 1 , 3 3 7 d, 12 -30 - , . * t n * I.,..,
-
I - - ~ ~ ~ . m , , ,.
7 I,,,, , 8 . , I-,,,, , 9 8 I... , 8 8 I , , , , , , 8 ,7
r ( b ) r (D
-
+'
30 Z 2 4 - 0-
* 1 8 3 PI 2 4 Z 0 - * 1 8 3 PI-
I " . ' . , r l t r f r ' ,-
' b ' - I-
p O - - .* P, c B y O 1 7 ) , (FAILED),$
Z 1 2 +-
8 f o l 7 ) , 3 3 7 ,d-
z 1 2 - ,-
3 ** c,-
C - P, x I , . . . > . , . I , , . . c-
, z **-e-cBfo( 7)-
I-
__--
I . . , . . , 1 1 I,..,,, 6.
P, 3 0- -
I Y - . ~ . 1 I . ~ -r ~ ~I ~~ 1 *a ~I~~~~~ ~ 1 ~ 3 2 4 - 1 8 1 2-
-
-
l o 5 loz l o 1 looP O R E D I A M E T E R , nm
lo5
l o 3 lozlo1
lo0P O R E D I A M E T E R , n m 4
0
P
FIG. 2 FIG. 3
Comparison of p o r e s i z e d i s t r i b u t i o n c u r v e s of Comparison of p o r e s i z e d i s t r i b u t i o n c u r v e s of F M cement mortar b e f o r e and a f t e r c o r r o s i o n cement mortar b e f o r e and a f t e r c o r r o s i o n
(w/(c+sf) = 0.45, 7 days) (w/(c+sf) = 0.60, 7 days) o 2 ~ ' ( 7 1 , ( b ) ~ : ~ ( 7 ) , ( c ) B ; O ( ~ ) (a) c H ( 7 ) , ( b ) gY0(7), ( c ) B ; O ( ~ ) W ( C )
-
-
~;~(7)>,'-
I 30*-. a r n m . v 3 ..
I , , . , , , . , I , . . . , I . . , , ~ ' . , . - 09,
' n 2 4 - ( C ),
- D- (D I 09 3*
Pa30 - . , . , , , I . , . . , , , . 1 . I . , . . #
-
r,..#...
.
.
3 6 p ~ - . ~.
1 r n 8 8 8 m n I I I ~ a ~ ~ ~ - ~ ~ 2 4 - ( a 1- - - -
B E F O R E C O R R O S I O N-
-
A F T E R C O R R O S I O N- - - -
B E F O R E C O R R O S I O N3
I- 2 4-
( a l-
-
A F T E R C O R R O S I O N I- 18-
1 8 - bl z 12-
0 W 30 -..n88 8.
I I , - ~ . ~ ~ , r m ' t l - m ' " I " . ~ . . ,.
8
- - Z 2 4-
I b ).-
0-
-
E
V1 vl 18 - CIl C w. z 12 --
0 N"3
3 0 - , - - , I I~~~~~~~.
I , ~ ~ . - . * I ~ ~ ~ , - . ~ , 30 s s - . q-
8 1 8 I . , ~ - - ~.
I I - - . . ~ ,-
g
I - V1 I H 2 4 lL I c ) I I - V] I 18 --
C)z
m 24 18 1 2-
C) m P 0E
1 1 1 . , , , , , L , , , , , , 1 1 I . , , , , I , L mlo5 lo3 lo2 l o 1 lo0
P O R E D I A M E T E R , nm P O R E D I A M E T E R , nm
z
2
C)
FIG. 4 FIG. 5 7;:
Comparison of pore-size d i s t r i b u t i o n of cement Comparison of p o r e - s i z e d i s t r i b u t i o n of cement mortar b e f o r e and a f t e r c o r r o s i o n m o r t a r b e f o r e and a f t e r c o r r o s i o n
(w/(c+sf) = 0.45, 7 days) (w/ ( c + s f ) = 0.60, 7 days)
( a ) c R ( 2 8 ) , ( b ) Bf0(28), ( c ) ~ ; ~ ( 2 8 ) (a) c H ( 2 8 ) , (b) BY0(28), ( c ) ~ ! ~ ( 2 8 )
-
-
t c }Vol. 1 5 , No. 3
R.F. Feldman and Huang Cheng-yi
i n t r u d e d volume on e x p o s u r e t o s a l t s o l u t i o n s . In t h e p o r e s i z e r a n g e
(97 + 2) x
lo3
nm, however, t h e i n t r u d e d volume i n c r e a s e s s i g n i f i c a n t l y a f t e r exposure t o s a l t s o l u t i o n . I n c r e a s e i n p o r e volume i n t h e r a n g e(97 + 2) x l o 3 nm, r e s u l t i n g from exposure t o s a l t s o l u t i o n , i s p l o t t e d i n Fig. 6 a g a i n s t i n c r e a s e i n d e f l e c t i o n f o r a l l m o r t a r specimens. Although t h e r e i s some s c a t t e r , t h e d e f l e c t i o n i n c r e a s e s e x p o n e n t i a l l y w i t h i n c r e a s e i n p o r o s i t y i n t h e p o r e r a n g e (97 + 2) x l o 3 nm. The r e l a t i o n i s b e t t e r i f o n l y specimens c o n t a i n i n g s i l i c a fume a r e c o n s i d e r e d . I n c r e a s e s i n p o r o s i t y of o v e r 5% ( i n t h i s r a n g e ) r e s u l t i n l a r g e i n c r e a s e s i n d e f l e c t i o n , and specimens w i t h i n c r e a s e s i n d e f l e c t i o n l e s s t h a n 0.0075 mm (about 30% of o r i g i n a l v a l u e ) a l l c o n t a i n s i l i c a fume.
Ca(OH), and non-evaporable w a t e r c o n t e n t
Values determined b e f o r e and a f t e r e x p o s u r e t o s a l t s o l u t i o n a r e
p r e s e n t e d i n Table I. I n a l l specimens e x c e p t c H ( 2 8 ) , t h e C (OH)2 c o n t e n t
it
i s reduced t o z e r o a s a r e s u l t of s a l t s o l u t i o n exposure. I n C (28) t h e Ca(OH)2 c o n t e n t i s reduced from 19.14 t o 7.31. Three of t h e f o u r specimens c o n t a i n i n g 30% s i l i c a fume had 0% Ca(OH)2 b e f o r e and a f t e r exposure t o s a l ts o l u t i o n , ~ y ~ ( 7 ) b e i n g t h e e x c e p t i o n .
The non-evaporable w a t e r c o n t e n t i n c r e a s e d w i t h e x p o s u r e t o s a l t s o l u t i o n , specimens c o n t a i n i n g s i l i c a fume h a v i n g h i g h e r i n c r e a s e s , t h e g r e a t e r t h e s i l i c a fume c o n t e n t t h e g r e a t e r t h e i n c r e a s e . I n a d d i t i o n , t h i s i n c r e a s e i s greater f o r t h e more porous seven-day c u r e d specimens p r e p a r e d a t
H H
w/(c+sf) of 0.60. Specimens B10(7)-c and B ~ ~ ( ~ ) - C show 23.4 and H
24.03 p e r c e n t / i g n i t e d w e i g h t , r e s p e c t i v e l y . Specimen C (7)-c, however, c o n t a i n s 19.73% w h i l e specimen ~ : ~ ( 2 8 ) , which i s t h e most d u r a b l e , shows an i n c r e a s e i n nox-evagorablc-Jater c o n t e n t f r m 1 1 . 8 8 t o 16.67%; specimen c R ( 2 8 ) i n c r e a s e s from 16.31 t o 17.23%. 0 . 7
-
I I I 1 I N W / ~ C + s f ) = 0 . 4 5 . 7 D A Y S '0 0 . 6 - d W / ( C + s f ) = 0 . 6 0 . 7 D A Y S x W / ( C + sf1 = 0 . 4 5 . 2 8 D A Y S 0 . 5 - W / ( C + s f ) = 0 . 6 0 . 2 8 D A Y S Z 0,
0 . 4 - U w 4 Yz
0 . 3 - Z x C-
; 0 . 2 - c d 4 w = 0 . 1 - 8f0-
0 I I I I I 0 1 2 3 4 5 6 7 8 9 I N C R E A S E I N P O R O S I T Y , v o l u m e per c e n t FIG. 6 Dependence of i n c r e a s e i n f l e x u r a l d e f l e c t i o n on i n c r e a s e i n p o r o s i t y (97 + 2) x l o 3 nm) f o r cement m o r t a r s a f t e r exposure t o s a l t s o l u t i o n s ( ~ 3 6 0 days) DiscussionThe mixed c h l o r i d e s o l u t i o n i n m o r t a r s i n t e r a c t s w i t h cement t o form s e v e r a l compounds, i n c l u d i n g c a l c i u m c h l o r o a l u m i n a t e , magnesium o x y c h l o r i d e , magnesium s i l i c a t e , and magnesium calcium s i l i c a t e ( 2 , 4 ) . These compounds may
r e i n f o r c e , weaken, o r have n o e f f e c t on t h e specimen. The main weakening e f f e c t i s due t o l e a c h i n g of Ca(OH)2 o r decomposition of CSH. It i s p o s s i b l e t h a t t h e r e l a t i o n between i n c r e a s e i n p o r e s between (97 and 2) x 103 nm and weakening i s a consequence of l e a c h i n g o u t of Ca(OH)2 and o t h e r p r o d u c t s .
Vol. 15, No. 3
MORTARS, S i 0 2 FUME, RESISTANCE, CHLORIDE, ATTACK
R e s u l t s show t h a t Ca(OH)2 h a s been t o t a l l y removed from a l l t h e specimens
H
e x c e p t C (28)-c, where a s m a l l amount was unleached.
H H
Specimens B3 ( 7 ) and B10(7) b o t h f a i l e d r a p i d l y , a l t h o u g h t h e former c o n t a i n e d o n l y 4.86% Ca(OH)2. I n b o t h c a s e s , t h e h i g h w/(c+sf) and t h e s h o r t h y d r a t i o n t i m e e n s u r e d t h e i r h i g h p e r m e a b i l i t y , and p r o d u c t s o t h e r t h a n
i
Ca(OHI2 c o u l d be r e a d i l y a t t a c k e d o r leached.! -
R I R
Specimens B30(28), B10(28) and B 3 ( 7 ) c o n t a i n i n g s i l i c a fume and p r e p a r e d I
a t low w/(c+sf) proved t o b e t h e moat g u r a b l e . These a r e f o l l o w e d by
specimens ~ ~ ( 2 8 ) a n d :B (28). It must be concluded t h a t t h e low p e r m e a b i l i t y provided by t h e d i s c o n t f n u o u s s t r u c t u r e of b l e n d s i s t h e major r e a s o n why low
W / C and l o n g e r curlng f o r ~'(28) provided a measure o f p r o t e c t i o n .
The p o r e s i z e d i s t r i b u t i o n of specimen ~ : ~ ( 2 8 ) (Fig. 4 ( c ) ), which was t h e most r e s i s t a n t , shows l i t t l e change o v e r t h e whole p o r e s i z e r a n g e , a l t h o u g h t h e r e i s a d e c r e a s e i n p o r o s i t y of 3.24% and an i n c r e a s e i n non-evaporable w a t e r of 4.79% a s a r e s u l t of exposure t o t h e s a l t s o l u t i o n . These v a l u e s a r e even g r e a t e r f o r t h e more permeable ~ $ ~ ( 7 ) sample w i t h 7.08% f o r p o r o s i t y and 10.5% f o r non-evaporable w a t e r . The r e d u c t i o n i n p o r o s i t y does n o t o c c u r f o r cement m o r t a r s c o n t a i n i n g n o s i l i c a fume and i s lower a t 10% t h a n a t 30% s i l i c a fume a d d i t i o n . It may c o n t r i b u t e t o t h e i n c r e a s e d r e s i s t a n c e t o s a l t a t t a c k w i t h s i l i c a fume a d d i t i o n . The i n c r e a s e i n n o r ' e v a p o r a b l e w a t e r c o n t e n t i s g r e a t e r a l s o a t h i g h e r s i l i c a fume a d d i t i o n s . This s u g g e s t s t h a t r e a c t i o n s o c c u r i n t h e b l e n d s t h a t do n o t o c c u r i n m o r t a r s c o n t a i n i n g cement alone. The r e a c t i o n s may i n v o l v e e x c e s s s i l i c a fume, low c a l c i u m CSH, unhydrated cement and t h e s a l t s o l u t i o n ; a h y d r a t e i s produced.
The a c t i o n of t h e s a l t s o l u t i o n on m o r t a r s i s c l e a r l y t h e r e s u l t o f s e v e r a l r e a c t i o n s : l e a c h i n g of Ca(OH)2, decomposition of CSH, f o r m a t i o n o f calcium c h l o r o a l u m i n a t e , and f o r m a t i o n of p r o d u c t s i n v o l v i n g u n r e a c t e d s i l i c a fume a n d / o r low c a l c i u m CSH and C1- i o n s .
Conclusions
1. S i l i c a fume a d d i t i o n s , u s e of low w / ( c + s f ) r a t i o s , and l o n g e r c u r i n g p e r i o d s markedly i n c r e a s e t h e r e s i s t a n c e of m o r t a r s t o a t t a c k by s a l t s o l u t i o n s .
2. In samples w i t h a s m a l l amount of s i l i c a fume Ca(OH)2 c o n t e n t i n m o r t a r s i s g r e a t l y reduced by s a l t s o l u t i o n exposure.
3. I n c r e a s e i n p o r o s i t y of m o r t a r specimens i n t h e (97 + 2) x l o 3 nm r a n g e a s a r e s u l t of s a l t s o l u t i o n exposure i s f o l l o w e d by r e d u c t i o n i n mechanical s t i f f n e s s .
4. In specimens c o n t a i n i n g s i l i c a fume a r e a c t i o n o c c u r s w i t h s a l t s o l u t i o n , s i l i c a , unhydrated cement and low calcium CSH t h a t r e s u l t ; i n r e d u c t i o n of t o t a l p o r e volume and i n c r e a s e i n non-evaporable w a t e r .
! Acknowledgements
The a u t h o r s w i s h t o t h a n k Gordon W. Chan and R. L a c r o i x f o r t h e i r h e l p i n performing t h e experiments. T h i s paper i s a c o n t r i b u t i o n from t h e D i v i s i o n of B u i l d i n g R e s e a r c h , N a t i o n a l Research Council of Canada, and i s p u b l i s h e d w i t h t h e a p p r o v a l of t h e D i r e c t o r of t h e D i v i s i o n .
References
1. BiczGk, I . , C o n c r e t e C o r r o s i o n a n d c o n c r e t e P r o t e c t i o n , Chemical P u b l i s h i n g Co. I n c . , New York (1967).
2. R i e d e l , W., Zement-Kalk-Gips, 6 , 286-296 (1973).
3. Kalousek, G.L., L.C. P o r t e r , aTd E.J. Benton, Cem. Conc. Res.
2,
78-89 (1972).V o l . 1 5 , N o . 3
F.E. Feldman and Huang Cheng-yi
Regourd, M., M. Hornain, and B. Mortureux, ASTM, STP 691, 253-268 (1978).
Mehta, P.K., A C I , SP-65, 1-20 (1980).
Feldman, R.F., Proc. 5 t h I n t . Symp. Concrete Technol., Monterrey, Mexico, 262-288 (1981).
Bakker, R.F., Conference on A l k a l i - a g g r e g a t e R e a c t i o n i n C o n c r e t e , Cape Town, South A f r i c a (1981).
Feldman, R.F., Annual Meeting of M a t e r i a l s Research S o c i e t y , Boston, 124-133 (1981).
Feldman, R.F., 1 s t I n t e r Conf. Use of Fly-ash, S i l i c a Fume, S l a g and Other Mineral By-Products i n Concrete, A C I , SP 7 9 , V. I , 415-434 (1983). Manmohan, D. and P.K. Mehta, Cement, C o n c r e t e and Aggregates ? ( I ) , 63-67 (1981).
Regourd, M., B . Mortureux, and H. Hornain, 1 s t I n t e r . Conf. Use o f Fly-ash, S i l i c a Fume, S l a g and Other Mineral By-Products i n Concrete,
A C I , SP 79, V. 11, 847-866 (1983).
T r a e t t e b e r g , A . , Proc. 1 s t I n t e r . Conf. on D u r a b i l i t y of B u i l d i n g M a t e r i a l s and Components, ASTM, STP 691, 536-548 (1978).
Feldman, R.F. and Huang Cheng-yi, RILEM Seminar on D u r a b i l i t y of Concrete S t r u c t u r e s u n d e r Normal Outdoor Exposure, Hanover (1 984).
T h i s p a p e r , w h i l e being d i s t r i b u t e d i n r e p r i n t form by t h e D i v i s i o n of B u i l d i n g R e s e a r c h , remains t h e c o p y r i g h t of t h e o r i g i n a l p u b l i s h e r . It s h o u l d n o t be reproduced i n whole o r i n p a r t w i t h o u t t h e p e r m i s s i o n of t h e p u b l i s h e r . A l i s t of a l l p u b l i c a t i o n s a v a i l a b l e from t h e D i v i s i o n may be o b t a i n e d by w r i t i n g t o t h e P u b l i c a t i o n s S e c t i o n . D i v i s i o n of B u i l d i n g R e s e a r c h , N a t i o n a l R e s e a r c h C o u n c i l of C a n a d a . O t t a w a . O n t a r i o , K1A 0R6. Ce document e s t d i s t r i b u S s o u s forme d e t i r € - a - p a r t par l a D i v i s i o n d e s r e c h e r c h e s e n b a t i m e n t . Les d r o i t s d e r e p r o d u c t i o n s o n t t o u t e f o i s l a p r o p r i C t 6 d e l l € d i t e u r o r i g i n a l . Ce document n e p e u t S t r e r e p r o d u i t en t o t a l i t 6 ou en p a r t i e s a n s l e consentement de l 1 € d i t e u r . Une l i s t e d e s p u b l i c a t i o n s d e l a D i v i s i o n p e u t S t r e o b t e n u e en S c r i v a n t 3 l a S e c t i o n d e s p u b l i c a t i o n s . D i v i s i o n d e s r e c h e r c h e s e n b a t i m e n t . C o n s e i l n a t i o n a l de r e c h e r c h e s Canada, Ottawa, O n t a r i o , K1A OR6.