READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Evaluation of concrete admixtures using differential thermal technique
Ramachandran, V. S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=e7a0113e-e9b3-42ac-93fe-656387c148ef
https://publications-cnrc.canada.ca/fra/voir/objet/?id=e7a0113e-e9b3-42ac-93fe-656387c148ef
Ser
1/ Y
2
6/'
THl
National Research Consell national
N21d
I*
Coundl Canada
de
recherche8
Canada
no. 1313
c.
2
1
Division of
Division des
I
Building Research
recherches en biitiment
i
%
Evaluation of Concrete
Admixtures Using Differential
Thermal Technique
by V.S. Ramachandran
ANALYZED
Reprinted from
ACI / RILEM Symposium '85
"Technology of Concrete when Pouolans, Slags and
Chemical Admixtures are used"
Monterrey, Mexico March 10
-
15, 1985
p. 35-52
(DBR Paper No. 1313)
Price $3.00
NRCC 24904
T h i s p a p e r , w h i l e being d i s t r i b u t e d i n r e p r i n t form by t h e D i v i s i o n of B u i l d i n g R e s e a r c h , remains t h e c o p y r i g h t of t h e o r i g i n a l p u b l i s h e r . I t s h o u l d n o t be r e p r o d u c e d i n whole o r i n p a r t w i t h o u t t h e p e r m i s s i o n of t h e p u b l i s h e r . A l i s t of a l l p u b l i c a t i o n s a v a i l a b l e from t h e D i v i s i o n may be o b t a i n e d by w r i t i n g t o t h e P u b l i c a t i o n s S e c t i o n , D i v i s i o n of B u i l d i n g R e s e a r c h . N a t i o n a l R e s e a r c h C o u n c i l of C a n a d a , O t t a w a . O n t a r i o , K1A OR6. Ce document e s t d i s t r i b u 6 s o u s forme de t i r e - 3 - p a r t par l a D i v i s i o n d e s r e c h e r c h e s e n b l t i m e n t . Les d r o i t s d e r e p r o d u c t i o n s o n t t o u t e f o i s l a p r o p r i e t e d e l ' 6 d i t e u r o r i g i n a l . Ce d o c u m e n t n e p e u t S t r e r e p r o d u i t en t o t a l i t 6 ou en p a r t i e s a n s l e consentement de l 1 6 d i t e u r . Une l i s t e d e s p u b l i c a t i o n s de l a D i v i s i o n p e u t B t r e obtenue en Q c r i v a n t
a
l a S e c t i o n d e s p u b l i c a t i o n s . D i v i s i o n d e s r e c h e r c h e s e n b l t i m e n t . C o n s e i l n a t i o n a l d e r e c h e r c h e s Canada. Ottawa, O n t a r i o . K I A OR6."EVALUATION OF CONCRETE ADMIXTURES USING
DIFFERENTIAL THERMAL TECHNIQUE"
By Or. V.S. Ramachandran
,
Reprint of the paper presented by Dr. V . S. Ramachandran in the ACIIRILEM Sympo- sium o n 'Technology o f Concrete when Puzzolans, Slags and Chemical Adm~xturesare used", sponsored by the Autonomous University o f Nuevo Leon, held i n Monterrey, Mexico, March 10-15.1985.
*
Head of the Materials Section Division o f Building Research National Research Council of Canada Ottawa, Canada.Editorial
-
Pacultad de Ingenierfa Civil Universidad Autonora de R u e w LednCiudad Universitarta Monterrey, R.L., X@xico
Evaluation of Concrete
Admixtures
using
Differential Thermal Technique
8v
Ur.V.S.htb8ndrandS y ~ O p s i s : Thennoanalytical methods. especially the differential ther-- 7s; zechnique. are used increarlnqly to evaluate concrete admixtures: tlr
-,.
studyinq the kinetica of hy6ratfon, relative effects of different a d z i x t u r e s , e f f e c t s of variaus factora on the influence of admixtures, ~ceztification, estiaation and cmpositlon of producte, mechanisms,-
c:ysta?linity, etc. E X a ~ p l s s illustrate how one may observe by means=,
:he differential. tnennal nethwl the effects of accelerators, retar- Ssrs, water reducers, superplasticizers and mineral admixtures on-
- . . i r
...-.
a t e 5 pastes formed from cement and cement minerals.ords: Accelerators, admixtures, fly-ash, hydration, retarders.
-
&,"me, slags, superplasticizers, thermal analysis, water redu- cers.Evaluaci6n de Aditivos para Concreto Utilitando
la Tc'cnica del Diferenciai ~ C t m i c o
Por: Dr.
V.S.
~amrchrndrar.-"
SLSCMEN: Se estdn utilizando en una forma incrementada 10s mdtodos
-
ternoanalSticos, especialnente la tecnica del diferencial tgrmico, pa- ra evalnar aditivos para concreto: para el estudio de la cinetica de .-.-,drac;tclbn. efectos relativos de diferentes aditivos, efectos de va- rlos factores en la influencia de aditivos, identificaci6n. estima--
ci6n y co~gosici6n de productos, mecanismos, cristalinidad, etc. Los e;err.glos ilustran c b ~ o pueden obsezvarse por medio del metodo del di- 2-ferezcfal ternico, 10s efectos d e acelerantes, retardantes, reductores
- e aTJa, s~perplastificar~tes y aditivos minerales en las pastas hidra- :ails forxadas de cekerrto y zinerales cementantea.
Palabras clave: Acelerantes, aditlvos, cenira volante, hidratacibn,
-
retardantes, hum0 de sflica, escorias, ~u~erplastificantes, andlisisEvaluation des Adjuvants
du
~ e ' t o n
par une Technique Thermiquc
DiffCrrntielle
Par: Or.
V.S.
RamachandranFGs;?~: On a u t i l i s B d e s methodes thermoanalytiques e t principalement 2 5 :a t e c h r . r q u e t h e r n i q u e d i f f e r e n t i e l l e pour e t u d t e r La cinetique d e
-
a &..A-
...,
r a t a t i o n , l e s e f f e t s r e l a t i f s d e s d i f f e r e n t 8 a d j w a n t s e t l e s-
eff;=ts d e f a c t e u r s variCs slrr I ' & n f l u e n c e d e s a d j u v a n t s a i n s i q u e 1' r l e c t i f r c a t i o n , l ' e s t i m a t i o n et l a caupositaon d e s produits, les me-- =azim:es, l a c r r s t a l l i n l t 9 , etc. On s ' e s t s e r o i d ' e x e m p l e s pour-
-
; I l z s c r ~ r cement o n p e u t o b s e r v e r l e a effets d e s a c c 6 l & r a t e u r s . den r e z a r f a t e u r s d e p r i s e , d e s r e d u c t e u r s d'eau, des s u p e r p l a s t i f i a n t a et15s- a9]n-..antli a i n d r a u w s u r d e s p t t e s hydratges formees d e c i m e n t et 5s :~r.I:a;lx 2 e cinent q r e c e 3 l a mdthode t h e r n i q u e d i f f e r e n t i e l l % .
Y o t s c l 6 s : A c c b l k r a t e u r s , ad;uvar:ts, c e n d r e v o l a n t e h y d r a t a t i o n , r e - t a r d a t e u r s , fun6e d e s i l i c e , l a i t i e r , s u p e r p l a s t i f i a n t s
,
a n a l y s e-
t h e r r n i q u e , r 6 d u c t e u r s d ' e a u .Ramachandran
BIOGRAPHY
Dr. V.S. Ramachandran is the Head of the Materials Section, ,
Division of Building Research, National Research Council of Canada. His main research interests are in the fields of concrete science and technology. He has published five books, 13 chapters and about 120 research papers.
It is estimated that 80a of the concrete produced in Morth hmerica contains one or more admixtures, and they are being used increasingly in many other countries. That there is intense activity in this field is evident from the number of patents, , publications a d conferences devoted to t h u . In spite of
extensive investigation, hwever, the use of admixtures is based pore on art than on science.
Several physical, chemical and mechanical techniques have been applied in studying the role of admixtures in concrete. One that has attained wide recognition in recent years is related to thermal analysis: Differential Thermal Analysis ( M A ) has been - ~ s d with considerable success to study the mechanisme, kinetics and c v s i t i o n involved, and for identification and estimation of the products of hydration. It is simple, reliable, and yields quick results. h i s paper describes several applications of DTA
in the investigation of the role of admixtures such as
accelerators, retarders, water reducers, superplasticizers and mineral admixtures on the hydration of cement and cement minerals.
Differential Thermal Technique
When a substance is gradually heated at a predetermined rate, it may at a particular temperature undergo a thermal change that manifesto itself as an endothermal or exothermal effect. The temperature at which this occurs, the intensity of the effect and its endo- or exothermal nature are characteristics of the
particular substance under study. These thermal changes are caused by reactions such as crystal inversion, dehydration, decosposition, oxidation, reduction, chemical reaction between two or more components, and destruction of lattice. In DTA the temperature difference (termed differential t q r a t u r e ) between the sample and a reference material (such as o-A1203, which does not show any thermal change) is continuously recorded as a function of teaporature of the furnace, reference material, or time. The exothermal peaks are shown as upward peaks and the endothermal peaks as downward ones with respect to the base line. In the differential scanning calorimeter, which yields curves similar to those of DTA, the sample is maintained isothermal to an inert material by a supply of heat to the sample or 'reference naterial. The heat energy required to maintain the isothermal condition is recorded as a function of time or temperature. For most cases report& in this paper a constant araount of sample was use4 and the rate of heating was maintained at 10'C/min.
Thermal Technique
3 9
Tricalcium siticate (C3 S), dicalcim silicate (C2 S ) , and
m. tricalcium aluminate (CIA) were synthesized from pure compounds.
Portland cement type I was cormwrcially obtained and the
admixtures were either pure chemicals or colwrcial samples. The
pastes were prepared by mixing the constituents on specially made
rollers, care being taken to prevent contamination by C02 in the
e atmosphere.
RSSULTS A N 0 DISCUSSION Accelerators
Many substances act as accelerators for concrete: alkali hydroxides, silicates. fluorosilicates, formates, nitrates, thiosulfates, carbonates, chlorides and anines. Of these, CaC12 appears to be the most efficient.
Kinetics--The kinetics of hydration of cament or silicates requires an estimate of the Ca(0H) formed at different times.
Although chemical extraction meth&s can be used. they are known
to be time-consuming and to yield slightly higher values. The
decompsition of Ca(OH) results in an endothermal effect in the
temperature range 4 5 0 - 5 5 0 ~ ~ : its intensity can be used to
determine quantitatively the amount of Ca(OH)2 present in a paste.
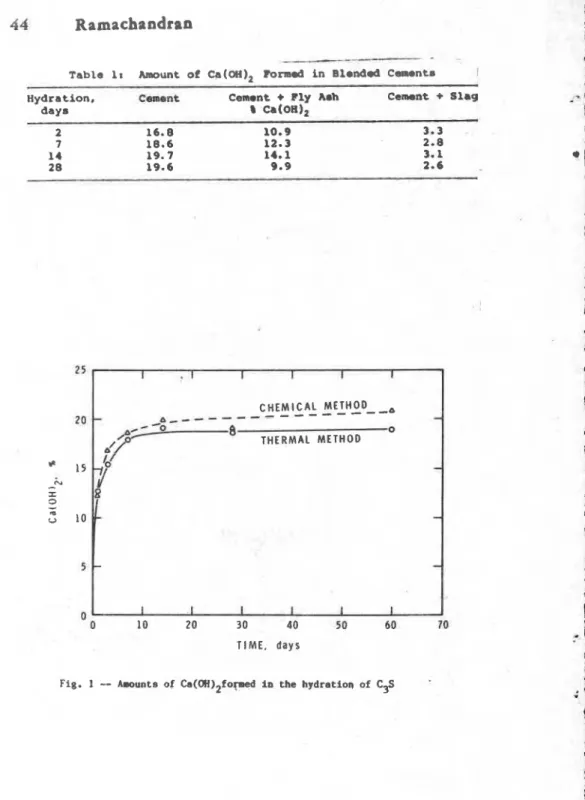
Pigure 1 s h w s that Ca(OH)2 estimated in C 3 S pastes by the thermal
method is comparable to that obtained frcrm chemical analysis (1).
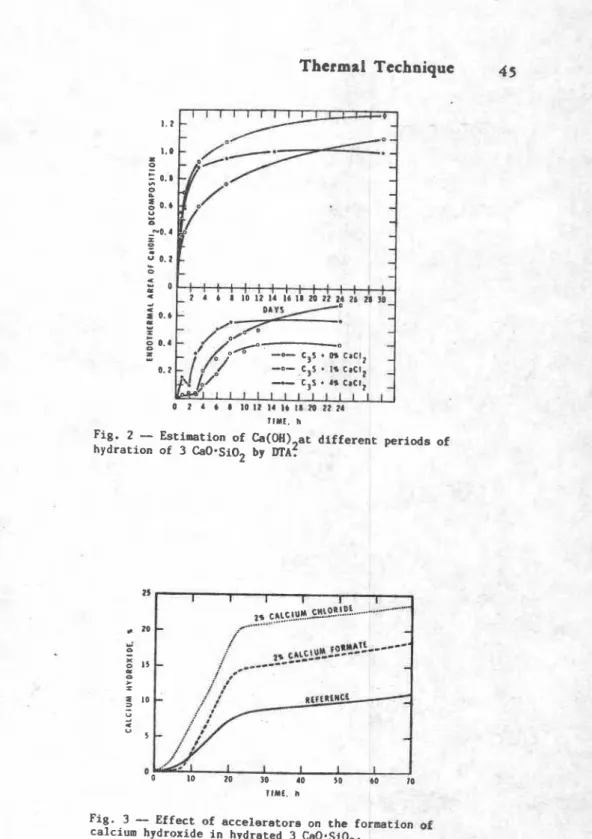
Figure 2 indicate. tlm amount of Ca(W12 iorwd at diffrrent
t i m ~ in the hydration of
Relative accelerating effects--If a new material has to be
assessed for its accelerating action, thermal analysis may be used
to compare its action with respect to other accelerators 1 3 ) .
Pigure 3 compares the relativa a m u n t s of Ca(OIl)2 formed h e n C S
is hydrated in the presence of 2% CaClz or Ca-formate. ~ l t h o u ~ i
Ca-formate is an accelerator, it is not so effective as CaC12.
Factmu influaneing aeccleration--The rate of hydration
&cpmn?la on the c r y a t a l nat-ure, particle size, surface area, water/solid ratio. temperature, etc.. and the influence of these
factors on hydration of cement and cement minerals may be studied
by thermal analysis. In an investigation of the hydration of
cement paste containing 3$% CaCl it vas found that at 24 h lass
-
Ca(OH) was formed at a water/so%id ratio of 0.25 than at one of0.4 (43.
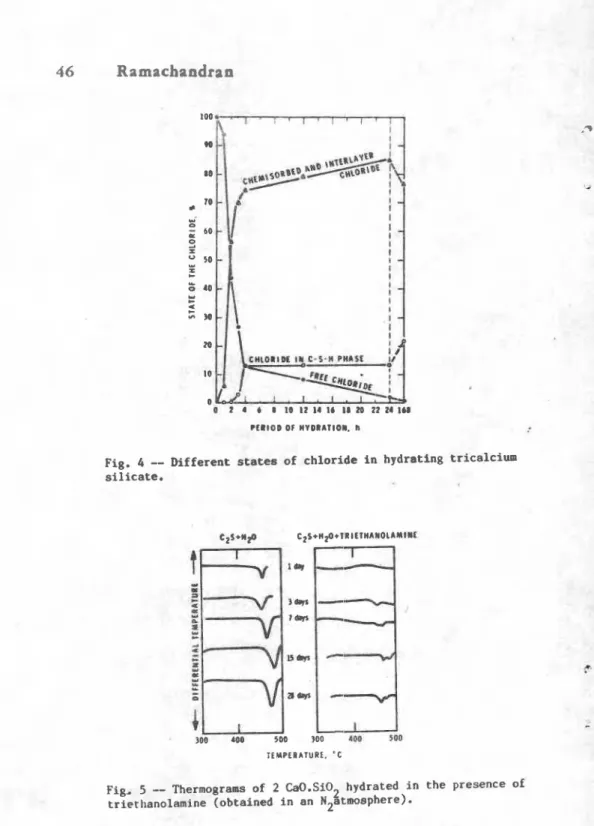
States of chlorida--A knowledge of the state of chloride in cement paste is important in studying the mechanism of hydration and the corrosion potential. In the C3S-CaCt2-H20 systan thermal analysie in conjunction with leaching experiments with alcohol and water indicate that calcium chloride exists in different formma free state, adsorbed on the CjS surface, chemiaorbed on tlie C-8-14
s u r f a c e o r i n t h e i n t e r l a y o r . and mtrongly i n c o r p o r a t e d i n t o t h e l a t t i c e of C-S-8. F i g u r e 4 sh- t h e mmible a t a t e 8 o f c h l o r i d e i n C3 S h y d r a t e d f o r d i f f e r e n t p r i o d m +
C-mition of C44-lturem m y a l t e r t h e compo8ition o f
concluded t h a t tri-1- prarot.8 th. formation o f C-S-8 with a h i g h e r Ca~/Sic+ r r t i o r l a r g e r endothermal p a k 8 balw 300'C and betveen 710 a d 760.C a r e i n d i c a t i v e o f th. e x i s t e n c e o f a h i g h e r CaO/Si% ratio.
C r y s t a l l i n i t y
of
Cat-1 --fa h y d r a t i - C , l and e a o m t m . + M e ia a --rain c o n d i t i o n s n o n - e y m t a l l i n e Ca(OH1 will fom. In thc t r l s t h a n o l ~ i m .t h e en%othsrmal effmct at d-mition o f
CalOH)Z, is of a l a u r i n t m o i t y , i n d i c a t i n q that the m i n e i m a r e t a r d e r o f h y d r a t i o n of
5 s
(Ciq. 5 ) 4 7 ) . In the prmwncm oft r i e t h a n o l a n i n e . dual p k s . m a t i n g i n tha t-rature rango 450-500-C indicate tlu p s m i b i l i t y of f o r a a t i o n of Wth
crystalline and -crpsullirr+ f o m of Ca(m)2-
R e t a r d e r s
Many o r g a n i c and c-i corpoundm. i n c l u d i n g tho80 d e r i v e d a s by-products. c a n be asad
u
retarder.. Unrefined Wa, Ca or QI, s a l t s of l i g n o s u l f m i c rid. their w d i f i c a t i o l u .ad derivativem. hydroxycarboxylic aids aad their 8 a l t m . carbohydratem, and i n o r g a n i c conpoudm M aa @wmphate., f l u o r a t e & oxid-. borax and magnesium salts act a8 retarderm.E drati- rru oC hfltatloa of m ta -k
c a n p o h r m t r r & d t b -retima af tmt.mt.rm. The relative retarding uf varlou* a h l x t u r e s m y b. C o l l a r d by datermining the type d t o f thm hydratad p r d u c t m . h11 aacchacidelr, with tb: c - p t h of t r a h l o n , o t m l y r0-rd thr
s e t of cement. En tk. Wratim of A, a mtrong d o t h a b M 1
errmet observd at 5 5 0 - c at 3 h .1 i 3 i e a t L v . of +hr .table cubic aluminate h y d r a t e ( 5 ) . la the premence o f 1 8 sucr08e. e n d o t h e r w l e f f e c t 8 i n d i c a t i v e of pba888 preHl)t. -80 remultm show t h a t s u c r o s e retards tho convermion o f the l n x a ~ l pham to t h e c u b i c phase. T h a t treblow is 8 weaker r e t a r d e r i m e v i d e n t
from t h e p r e s e n c e o f b t b 1- and cubic ph.808 d e n it i m used.
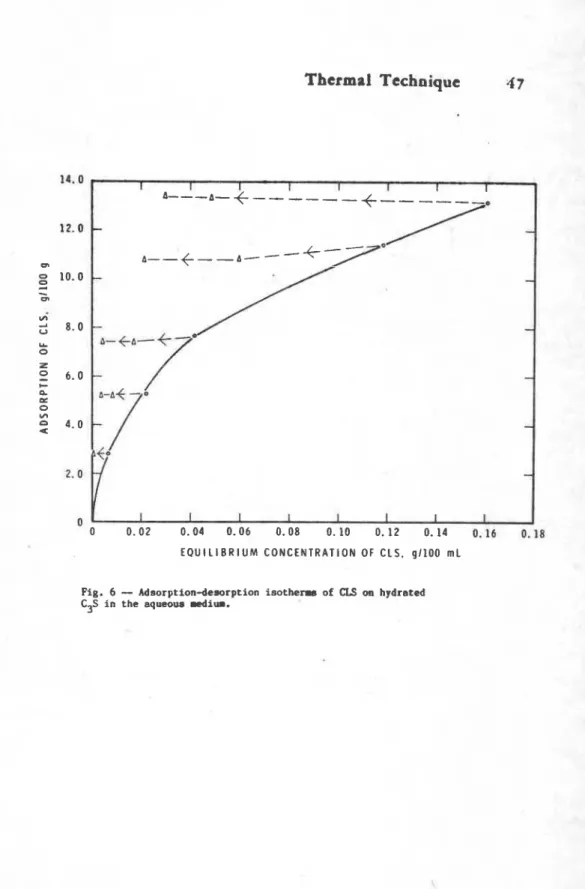
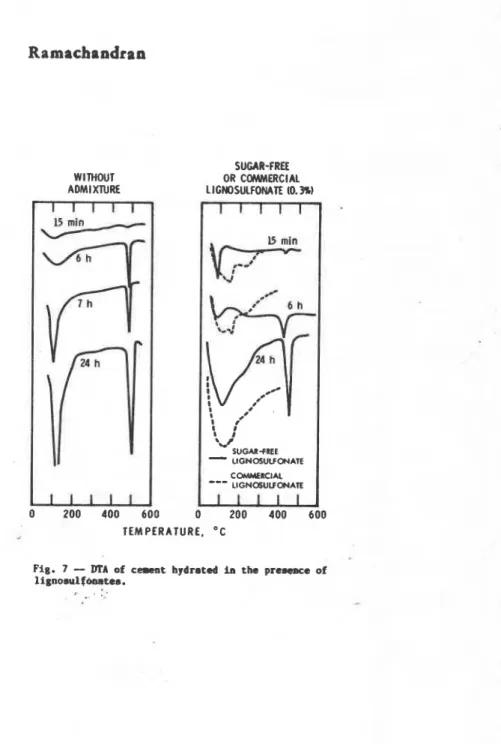
Role of l i p a a m l f a r n r l ' b r e t a r d i q a c t i a r Of c m r c i a l l i g n o 8 u l f o n a t e 8 ( C W J L. attributrd to the p r e n n c e o f sugar.. ~ d s o r ~ t i o n - d e s o r p t h mtadhm of lignowulfonatem i n d i c a t e ,
howover, that they arm frrrr-rribly ad.0- ths hydrating cement components (rig. 6 ) (8). .-sting t h a t p m
l i g n o s u l f o n a t a could u a rmtardmr. v e r i f y t h i n . c u n t 8 h y d r a t s d with and WLt- mUq4r-frm 1 i q n g o u l f ~ t O O Vera
compared. Samplem hydrrtrd i n t h m p r e m n c e of ougar-frae
I l g n o s u l f o n a t e chard of 1-r intoamity f o r C 4 - M ( k l w 1 0 0 * C ) and CatOH), ( 4 3 3 - 4 ~ 0 . ~ 1 (rig. 7 ) ( 3 ) .
Normal Water Reducer.
Thermal Technique
4
1
that reduces the water requirement of concrete for a g i w mconsistency. It is effective with portland cement, portland blaet furnace slag cement, and portland-pozzolan cement. Normal water
"- reducers consist of Ca, Ua or HI$ salts of lignosulfonic acids, hydroxycarboxylic acids and their salts or hydtoxylatod polymer.
Interaction--Ca-lignosulfonat~~ in addition to acting as a water rducing agent, retards tho hydration of G S and C,A
*
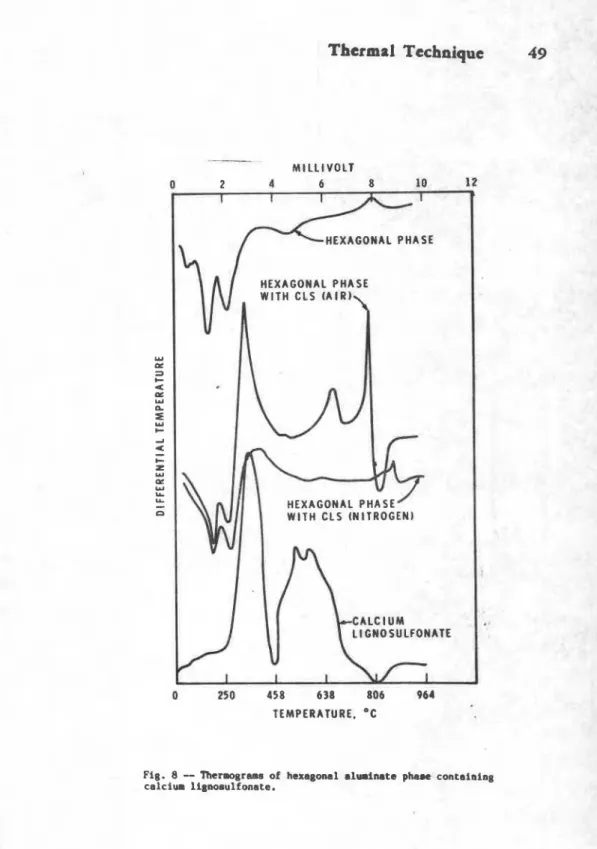
components. T h o - u c h a n i a by which-tho hexagonal phau In tho hydration of C,A is stabilisod by Ca-ligaosulfonato was studiod bysubjecting
tho' lignosulfonatttr&td hougonal p h n to thoma1analysis. The therrogram exhibits an intonso oxothormic poak at 790-C and an ondothoraic poak at 825-C (Pig. 8 ) ( 5 ) . Tho
higher-toaporaturo oxothemic peak at 790-C tin tho caqlox), absent in Ca-lignosulfonate, indicates that in the hexagonal phase the lignosulfonate existing in the intarlayor positions is rwro resistant to oxidation. In tho XRD studios of tho hexagonal pha-so containing 108 lignosulfonato, an incrrso in tho C-axis spacing could bo explainod by the oxistenc?. of lignosulfonatm in tho interlayor positions.
Delayed addition--The addition of lignosulfonato a feu minutes after water comes into contact with cemont rrults in a botter retarding/fluidizing effoct than if it is added along with tho mixing water. Thermal analysis s h o w that tho hydration of C S is cooplotely inhibited
in
the prosonco of 0.5%l\gnosulfonate. Mdition of 58 C3A is sufficient to
pr-•
tho hydration of C3S. The inhibitive actiona~
C,S hydration is p r o pronouncod when ligno8ulfonato is addod to a mixturo of C S+
C 3 hprohydratod for a fow minutes. Those rosult.8 show tb.t &on lignosulfonate is addod a f w minutoe at+.+ ulter caws into contact with cement, the hydratd products adoorb l a r u anounts of admixture and releaso more admixture for retarding .ad fluidizing of the S phase.
Superplasticirers are chaically difforont f r m nornal water reducers and are capable of educing water roquir.unts by about
308. They are classifid in four groups. vir., sulforutd
meladno formaldohydo (SUP), su1formt.d naphthalono for~ldohyde. modifid ligno8uliauto. am3 others including mulfonic r i d ostors, carbohydratos, otc.
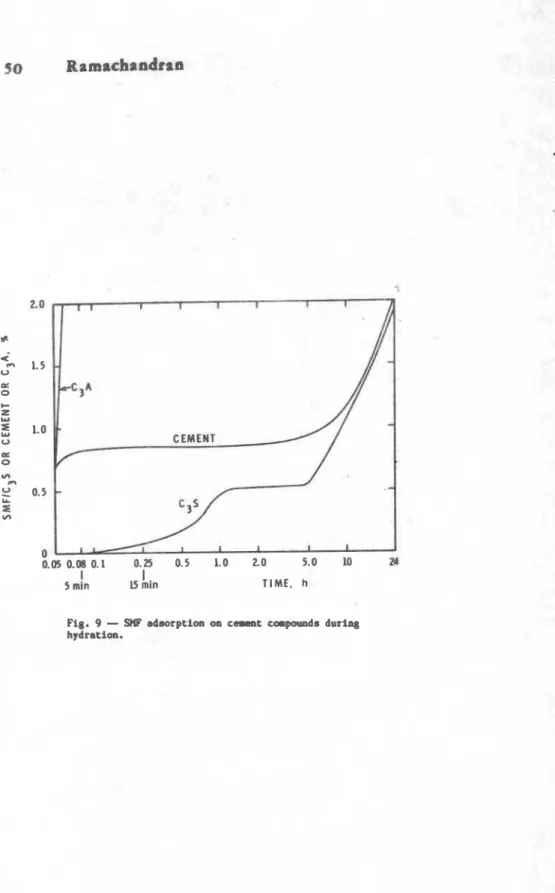
H
dration rate--7b influoncoof
supuplasticimr8 ar th. hydragon of c e w n f and its cwponents Ram been studid by t h e m 1 analysis. Suporplasticir+r (-1 retards the hydration of CIA,% S and c.cpmt but accoloratu tho formation of ottringito f r a CoA
+
gypsum. Thou effect8 arm relatd to the rat. .ad amount ot adsorption of SHP on coaent and cement components (Pig. 9) (9).-
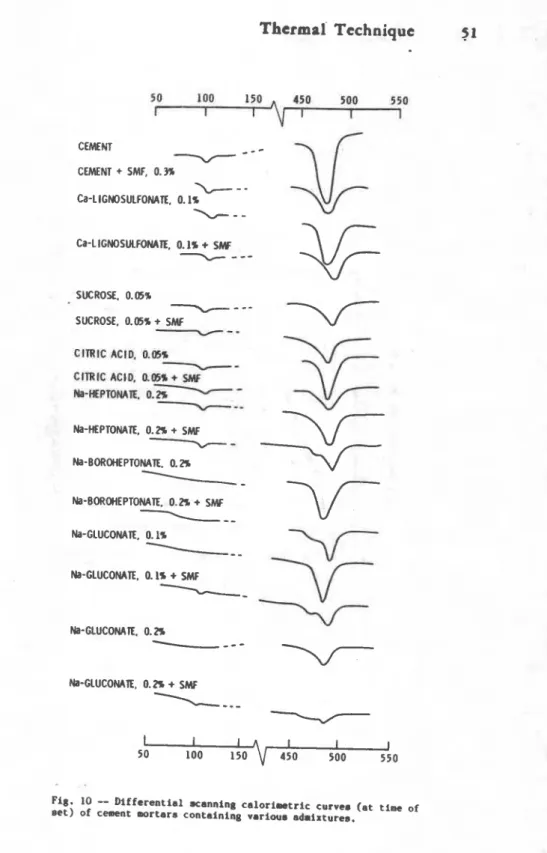
Initial setti --Tho products fornd at the t i r of initial sotting of cements?ontaining various admixtures ifh cmbination with an SMP suporpla~ticizer were examined by thermal analysis. The results shov that both tho referonce nortar and that
; containing the a4mixturo exhibit endothonaal effects in the region of 500.C (Pig. 10) (10). This indicates that at the ti- of
-
initial setting 8- hydration of C3S in the cement has occurrod.Ramachandran
to concrete for economy.%nd to provide desirable properties. Thermal methods are used to study the reactions in cements containing these additions alone and in combination with other admixtures.
PL ash DfA. can bz uu.d to detsratn ths aaaunt
of
Ca(OH)zthat
h i L d
wLth fly amh in fly ash-c.mmn+ r i t t u r r . . Tabls 1corngarom fha amount of C a ( Q n ) formed in t v o bland& csments
containing 35P f l y arh and 70% slaq (71. In th* fLy ash btsnd, lime decreases f r ~ n 14.11 at 14 day. to 9.91 at 28 days. A significant reaction oecurs betwean C a ( O H j z and fly d6h during
this p e r i d . In the alag blends formation of lower m w u n t a of
lime is due ta the presence of only 301 c m s n t in the blend. 'Che
d e c r a r r e i n Ca(Dn)Z frm 3 . 3 to 2.63 I n d i c a t e 8 that # a m l i m reacts with the slag.
Sla .--Hydration sequence in slag cements and the mechanism of a c d i o n of slags has been investigated by DTA. The reactivity of slag is related to the m o u n t and nature of the glassy phase. The DTA ther~aogranu show an exothermal effect beyond 80O.C. The area of the peaks is indicative of the amount of glass in slags.
Bilicm f---Th influence of # i l i a rrac on th. hydration of
eemnt and cement mineraLs m y followed by diifwrential
scanning caloriwtry I l l ) . Silica fm accslera+as the hydration of both 5 S and % A phaees l a tha first few hours. F i g u r e 1l
mhwa the s w u n t clf CalOH)* f o m d at different + i . m m in crmsnt
blend. eontainFng 30t silica f w a or 30# ground silica sand. At
a b u t 8 h E M a,rwunrs of C U [ O H ) ~ f o d in the three samplea are
about the samr, although blends contain only 70l cement. This
indicates that bath ground sand and silica fume accelerate the
hydration of cement.
Carbenstes--S- c m n t u contain small amounts of carbnate, and these i m coatrovaray aa to their effect on cement p r w r t i e s . Thermal eiamination of tha effect o f CaC? m C3S a d cement
hydration has shown that the carlrrrnate haa an accslermting effect.
For exampla, at 7 h tha d e q r a e of hydration of C 5 containing 0
and 15\ C a C V . 5 and 171, respectivelyI and
the
(~o~lm..p~ndinq values at 1 y *.re 3 5 and 1 5 % .References
1. Ramachandran. V.S., Differential Thermal Method of Emtimating Calcium Hydrnride in Calcium Silicate and Cement Pastes, C m . Cbncr. Res.,
2
11979) 677-684.2. Ramachandran. V.S., Kinetics of Hydration of Tticalcium Silicate in Presence of Calcium Chloride by Thermal Methods. Thernochimica Acts,
2
(1971) 41-55.3. Ramachandran, V.S.. Investigation of the Role of Chemical )rdrsixtutes in Cemants
-
A Differential Thermal hpproach, Proc. 7th Int. b n f . T h e m . Analysis, Vol. XI, Wiley, 1982. pp. 1296-1302.4. Ramachandr ln, V. S., and Peldman, R. P., Time-Dependent and Intrinsic Characteristics of Portland Cement Hydrated in the
Presence of Calcium Chloride, I1 Cemento,
71
(1978) 311-322. 5. Ramachandran. V.S., ELucidation of the Role of ChemicalThermal Technique
4 3
idmixtures in Hydratin7:C-~ntn hy D I A Technique, Thermochinica Acta, 1 w 2 ) 343-366.
6. Ranachandran, V.S., Influmnce of Triethanolamine on the
r. Hydration Characteristics o f Triacalcium Silicate, J. Appl.
Chem. Biotechnol.,
52
(1972) 1125-1138.7. Ramachandran. V.S., Emtimation
of
Ca(OHI2 and Ng(OH)-
Implicationm in Cement Chemistry, Israel J. Chom..
&
(1982) ,.
C 240-246.
8. Ramachandran, V.S., Interaction of C a l c i m Lignorulfonate with Tricalcium Silicate, Hydratad fricalcium Silicate and Calcium Hydroxide, Cem. Concr. Rsm.,
2
(1972) 179-194. 9. Ramachandran, V.S., Influence of Superplasticizers on theHydration of Cement. 3rd Xnt. 6 n g r . Polymers in Concrete, Koriyama, Japan, Vol. 11 (1981) 1071-1051.
10. Ramachandran. V.S., Effect of ~ e t a r d e r m / ~ a t e r Reducers on Slump Lass in Superplasticized Concrete, AmLrn. Concr. Inst., Sp. Pub. 68 (1981) 393-407. t
11. Buang Cheng-yi and Peldman, R.P., Hydration Raactions in Portland Cement-Silica Rune Blends. To be published.
This paper is a contribution from the Division of Building Research, National Research Council of Canada, and is published with the approval of the Director of the Divrs~on.
44
Ramachandran
Table 18 Amount of Ca(OH), l b r m d in Blondd CaImont~
-. - -
Hydration, C-nt Cement
+
Fly Aah Cement+
Slagdays
a
c~(;oH), 2 16.8 10.9 3.3 7 18.6 12.3 2.8 14 19.7 14.1 3.1 2 8 19.6 9.9 2.6 0 0 10 20 30 40 50 60 70 TIME, d a y s I 1 I I I 1 CHEMICAL METHOD--,
* &
- - -
- - -
-
-
THERMAL METHOD 0.j.
2-
d-
Thermal Technique
45
Fig. 2
-
Estimation ofCa(OH)
at different periods ofhydration of 3 CaO-SiO by 2
DTA?
Fig. 3
-
Effect of accelerator8 on the formation of46
Ramacbandran
Fig. 4
--
D i f f e r e n t states of c h l o r i d e i n hydrating tricalcirun s i l i c a t e .F i g , 5
-
Thermograias o f 2 Ca0.Si02 hydrated i n t h e presence ofThermal Technique
47
E Q U I L I B R I U M C O N C E N T R A T I O N OF C L S . g l l O O m L Fig. 6
-
Adsorption-desorption isotherms of CLS w hydratedRamachandran
SUGAR-FREE WITHOUT OR COMMERCIAL AOMIXNRE LICNOSULFOMTE 10.3%) 1 1 1 1 1 -I5 mln-
TEMPERATURE. OCFig. 7
-
DTA of e-t hydrated in the prewace oflignosul farrstea.
--M I L L I V O L T
0 2 4 6 8 10 12
0 250 458 638 806 964 T E M P E R A T U R E . O C
Fig. 8
-
Thermgraas of hexagonal alurinate p b a e containing calcium lianosulfonste.I
5 min 15 :in TIME, h
Fig. 9
-
SW adaorptioa oa c m t caparads duriry hydration.Thcrmai Technique
5
1
CEMENT CEMENT + SMF, 0.38 Ca-LIGNOSULFOMTE, 0.1% \ - - Ca-LIGNOSULFONATE, 0.1%-
+
SMF-
--
SUCROSE. 0.05%-
-
--
SUCROSE. 0.05%+ SMF
Y--
ClTRlC ACID, Om.--
CITRIC ACID, 0.m+
5 k l ~ ~ B P T D M E , 0 . a Y---
Na-HEPTOMTE, 0.28+
SMF--
Na-BOROHEPTOMTE. 0.28Fig. 1 0