READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Hydration reactions in cement containing condensed silica fume
Ramachandran, V. S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=d8d0ff33-43ca-4b8f-8a97-8c70130e4763 https://publications-cnrc.canada.ca/fra/voir/objet/?id=d8d0ff33-43ca-4b8f-8a97-8c70130e4763
Ser L l L *
~ 2 l d Natlonal Research Consell natlonal
no. 1493
'1
Coundl Canada de recherchm Canada c. 2BLDG Institute for lnstitut de
Research in recherche en
.- Construction construction
Hydra tion Reactions in Cement Con
tainlng
Condensed Silica Fume
by V.S. Ramachandran
Presented
International Workshop on
Condensed Silica Fume in Concrete Montreal, May 1987
(IRC Paper No. 1493)
Price $3.00 NRCC 28504 NRC
-
C I S f I L I B R A R Y B I B L I O T H Z Q U EI R C
CNRC-
lClSTABSTRACT
This paper assesses the influence of silica fume on the hydration characteristics of
tricalcium silicate (a major component of cement) and cement.
Ce document d6crit l'influence de la silice fine
surl'hydratation du silicate tricalcique (un
important composant du ciment) et
du ciment.
HYDRATION REACTIONS IN CEMENT CONTAINING CONDENSED SILICA FUME V.S. Ramachandran, Building Materials Section, Institute for Research in
Construction, National Research Council of Canada, OTTAWA, CANADA
1. INTRODUCTION
In concrete technology a major effort has been directed to research on the utilization of wastes and by-products such as fly ash and slags as additives or replacements of cement in concrete. Many other wastes and by-products have been examined. For example, burnt rice husk, containing 80-952 silica and having a surface area of 50-60 x lo3 m2/kg, is reported to produce mortars of compressive strength about 40% higher than the reference mortar containing no additive. l Similarly, condensed silica fume, a
by-product of the metallic silicon or ferrosilicon industries (with 90-95% Si02 and surface area of 20-25 x 10 m2/kg), has a large potential for use in concrete. It acts not only as a pozzolan in cement but also as an
accelerator of the hydration reaction. It influences the composition of the silicate hydrate, the binding material in concrete, the microstructure of the cement paste, and interfacial characteristics of the cement paste and aggregate.
The physical, mechanical and durability characteristics of concrete are largely determined by the hydration properties of cement paste. This paper assesses the influence of silica fume on the hydration characteristics of tricalcium silicate (a major component of cement) and cement.
2. TRICALCIUM SILICATE 2.1 Hydration
Tricalcium silicate is a major component of cement, and its rate of hydration will determine the setting characteristics, strength and
microstructural development in cement pastes. The rate of hydration may be followed by determining calcium hydroxide, non-evaporable water content, heat development, or disappearance of the C3S phase. In the presence of silica fume, however, the amount of does not give accurate results on rate of hydration. It is known that part of the Ca(OH)2 reacts with Si02. At higher SiO additions the reaction products may contain almost no Ca(OH)2, although hyiration may have advanced to a considerable degree.
The rate of disappearance of the silicate phase can be used as a measure of the influence of Si02 fume on hydration. Rate of hydration of silicate depends on the amount of SiO
,
the impurities contained in it, and its surface area. Whereas quartz of ?ow surface area has practically no effect on hydration, Si02 in the form of high surface area aerosil(200 x 103 m2/kg) accelerates the hydration of tricalcium silicate.2 Even the so-called inert calcium carbonate of surface area 6 x lo3 m2/kg is known to accelerate hydration of tricalcium silicate.
The relative acceleration effects of different percentages of Si02 fume may be followed by applying conduction calorimetry, which measures heat developed during hydration. With additions of 0, 6, 14, 21, 28 and 34% Si02
of surface area 200 x
lo3
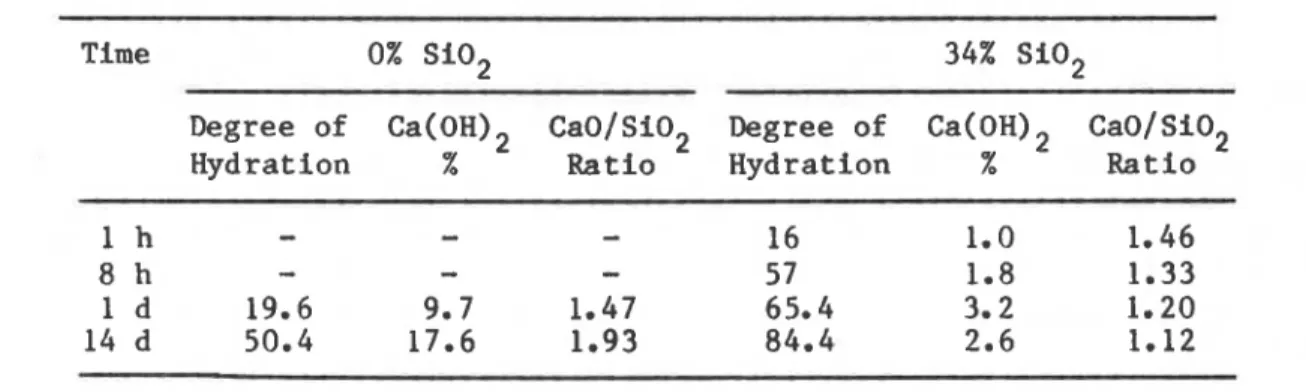
m2/kg there was evidence, even at 2 h, ofsignificant heat development with increased additions of silica fume.4 This investigation also indicates another peak, suggesting interaction between silica and Ca(OH)2 released during hydration. Table 1 shows the influence of Si02 on the degree of hydration and amount of Ca(OH)2 formed at different times4: the degree of hydration increases as the amount of added SiO
increases. It is also evident that at later ages the amount of c ~ ( o H ~ ~ formed in the presence of Si02 is almost nil compared to that of the
reference material containing no addition.
TABLE I. Influence of High Surface Area Si02 on the Hydration of Tricalcium Silicate
Time 0% Si02 34% Si02
- - - - - . .
Degree of Ca(OH)2 CaO/Si02 Degree of Ca(OH)2 CaO/Si02
Hydration % Ratio Hydration % Ratio
A survey of the influence of various types of silica fume on the
hydration of tricalcium silicate and cement suggests variability of results. This is to he expected because of the variability in the types of silica fume,'tricalcium silicate, waterlsolid ratio and procedures adopted by different investigators.
2.2 Hydration mechanism
Several theories have been proposed to explain the accelerating action of Si02 fume. The mechanism involved in the action of water on the
tricalcium silicate itself has not been resolved and it becomes more complex when an additive is added to the silicate. In one theory5 the existence of a quasi-stationary supersaturated layer of calcium ions around tricalcium silicate is envisaged. The diffusion of calcium ions through this layer depends on concentration within the layer and that in the bulk solution. Addition of silica fume promotes a low CaO/Si02 ratio Ca-silicate hydrate product and decreased Ca ion concentration in the bulk solution. The quasi-stationary supersaturated layer is affected, thus promoting
acceleration of the silicate phase. Nucleation effects are not envisaged. According to another theory, the acceleration effect is explained by the formation of a more permeable layer of calcium silicate hydrate around the silicate phase. 2 Ogawa et ale6 have proposed that the silica surface acts as a preferential site for precipitation of the products and therefore promotes acceleration of hydration. Wu and young4 suggest that the usual barrier of calcium silicate hydrate around the tricalcitim silicate is not Eormed when high surface area silica is added but forms, rather, on the silica.
Ramachandran and chung-mei, working on the acceleration effect of CaC03 on the hydration of tricalcium silicate, concluded that the nucleating
effect of CaC03 and modification of the calcium silicate hydrate layer over the hydrating silicate phase are responsible for the action of CaC03.
Analogous effects may occur when silica fume is added to tricalcium silicate.
2.3 CaO/Si02 ratio of product
In the hydration of tricalcium silicate the products are calcium silicate hydrate and Ca(OH)2. The CaO/Si02 ratio of the hydrate is about 1.5
-
1.6. Three kinds of calcium silicate hydrate seem to form in the presence of silica fume.4 One forms directly from the hydration of tricalcium silicate. The second type, which has a lower CaO/SiO ratio, forms by the reaction of Si02 with Ca(OH)2 (formed during hydratfon). The third forms from the reaction between SiO and calcium silicate hydrate. The overall ratio of Ca0/SiO is decrease8 with respect to that formed in the paste without Si02 fume P~able 1).Microanalytical techniques have been employed6 to determine the
CaO/Si02 ratio from the edge of a C3S particle through to the silica fume in a paste. In the absence of Si02, the hydrate product (at three days)
existing between two tricalcium silicate particles has a CaO/Si02 ratio of 2
-
2.7. In the presence of Si02, the hydrate close to the tricalcium silicate particle has a Ca0/Si02 ratio of about 2.5, although it is less than 1.5 o n the surface of the Si02 particle. These observations indicate that the interaction between SiOp and Ca(OH)2 or calcium silicate hydrate depends on the distance between the silicate and silica particles.2.4 Polymerization
It is known that a hydrated calcium silicate is in a polymerized state. In a fully hydrated cement paste, silica exists as monomer, dimer, linear trimer and polymer. The degree of polymerization of calcium silicate hydrate is modified in the presence of Si02 fume.7 The polymer content increases from 20 to 40% at 20 to 100% hydration in a mixture containing 20%
silica fume. In the presence of 34% Si02 fume content, polymerization achieves a level of 80% when hydration reaches 95%. The higher polymer content may be related to the reaction of Si02 with C-S-H. The implication of polymerization on the physical and mechanical properties has yet to be ascertained.
I
3. PORTLANI) CEMENTI
3.1 HydrationThe kinetics of hydration of cement may be followed, as described for the calcium silicates, by estimating Ca(08l2, non-evaporable water,
consumption of SiO and heat evolution curves. In the presence of St0 fume and after an fnitial increase in Ca(OH)*, it decreases in all samples
containing silica fume.l At higher dosages of Si02 fume there is a large decrease in Ca(OH)2, until at 40% Si02 no Ca(0H) could be detected. These results confirm that silica fume reacts with ~ a ( 6 ~ ) ~ formed during
Non-evaporable water content, which includes all water combined chemically with Ca(OH)2, the silicate and aluminate phases offers another method of following the influence of Si02 fume on the hydration of cement.
It is generally observed that the non-evaporable water content at early ages of hydration is higher in pastes containing silica fume but later decreases with respect to the reference cement containing no silica fumeO9
The conduction calorimetric curve of cement in the presence of Si02 fume is similar to that of the tricalcium silicate system. Pdditton of Si02 fume to cement increases the heat effect attributed to tricalcium silicate and a second peak occurs, suggesting Ca(0H) -silica fume interaction. The results do not indicate whether hydration o$ the aluminate phase is
af fected.
The hydration of cement has also been followed by estimating the consumption of Si02 fume.8 In cement mixtures containing 10, 20 and 40% Si02, about 60, 52 and 43% SiO is consumed at 28 days. It may be concluded that silica fume reacts not only with Ca ions of the silicate hydrate but also with the Ca(OH)2 derived from hydration.
3.2 CaO/Si02 ratio of calcium silicate hydrate
In the hydration of cement in the presence of silica fume, variation would be expected in the overall CaO/Si02 ratio in the product because of the interaction of silica fume with Ca(0H) and calcium silicate hydrate. The CaO/Si02 ratio varies between 0.9 and f . 3 . In a normal cement
containing no additive this ratio is 1.4
-
1.6. 3.3 Pore solution analysisAnalysis of the pore solution in cement paste containing silica fume is important in investigating the mechanism involved and in assessing the
potential for corrosion and alkali-aggregate reaction in concrete. In determining the concentration of ~ a + , ,'K ca2+, OH- and SO:- in cement pastes containing silica fume, it has been reported that the concentration of cations and anions is substantially decreased. lo The possibility of higher absorption capacity of low calcium silicate hydrate and precipitation mechanisms has been suggested to explain this
observation.
Addition of silica fume decreases the pH of the solution in the cement- water system. The plain cement paste has a pH of 13.9.1° At 10
-
20% Si02 fume this value decreases, but the pH is still in excess of 12.5. The passivity of steel is maintained if the pH > 11.5 and hence addition of silica fume should not encourage corrosion of steel in concrete.In the presence of chloride ions the potential for corrosion of steel is increased. Addition of silica fume seems to promote a greater number of unbound chloride ions than in plain cement pastes.1° As this is
undesirable, further work should be carried out to confirm the results and to determine how they are applicable to reinforced concrete.
4. SILICA FUME AS A POZZOLAN
4.1 Cement
-
silica fume-
H20 systemIt has been stated that in the hydration of cement Ca(OH)* reacts with
silica fume. It has also been reported that silica fume has a high
pozzolanic activity since all Ca(OH)2 formed in the hydration of cement is
consumed at 28 days.11 Results have varied, but this can be explained by
differences in the types and amounts of silica fume, waterlsolid ratios and
the techniques adopted for estimating Ca(OH)2. The high pozzolanic activity
of silica fume allows it to be used in blended cements.
4.2 Ca(OH)2
-
Silica fume-
water systemIn standard specifications the pozzolanic activity index of pozzolan with lime is determined with respect to strength development. The minimum strength requirement at seven days is 5.5 MPa according to the ASTM method.
The 1:l silica f~e:ca(OH)~ mixture shows a compressive strength of 9.3 MPa
at seven days, demonstrating that silica fume is an efficient pozzolan. Calorimetric investigations indicate that in the presence of water the
Ca(OH)2-silica fume mixture develops a large amount of heat, even within a
few minutes. 'It has also been observed that the rate of reaction depends on the surface area of the Si02 fume, and that the reaction is enhanced further at higher temperatures. At ordinary temperatures the reaction product of
the Ca(OH)2-silica fume mixture consists of C-S-H (I) ,2 a calcium silicate
hydrate product in which the CaO:Si02 ratio is between 0.8 and 1.5. At
higher temperatures of curing, crystalline calcium silicate hydrates are formed.
4.3 Mechanism
In the mechanism proposed by Grutzeck et al.l2 for Ca(OH)2-Si02 fume reaction, Si02 dissolves in the initial stages and forms an amorphous silica-rich Ca-poor gel on the surface of the silica fume. This coating
dissolves with time and the silica fume reacts with Ca(OH)2 to form calcium
REFERENCES
Ramachandran, V.S., (Ed.), Concrete Admixtures Handbook, Noyes P u b l i c a t i o n s , U.S.A., 626 pp., 1984.
S t e i n , H.N., and J.M. S t e v e l s , J. App. Chem., l4, 338-346, 1964.
Ramachandran, V.S., and Zhang Chun-mei, I1 Cemento, 83 129-152, 1986. Wu, Z.Q., and J.F. Young, J. Mats. Sci.,
E,
3 4 7 7 - 3 4 ~ : 1984.Kurdowski, W., and W. Nocum-Wczelik, Cem. Concr. Res.,
-
13, 341-348, 1983.Ogawa, K., H. Uchikawa, K. Takemoto and I. Yusui, Cern. Concr. Res.,
10,
683-696, 1980.Tamas, F.D., A.K. S a r k a r and D.M. Roy, " H y d r a u l i c Cement P a s t e s : T h e i r S t r u c t u r e and P r o p e r t i e s , " Cem. Concr. Assn., Slough, U.K., pp. 55, 1976.
Ono, K., K. Asaga and M. Daimon ( P r i v a t e communication).
Chengyi, H., and R.F. Feldman, Cem. Concr. Res., l5, 585-592, 1985. Page, C.L., and D. Vennesland, Mats. Sc., Constru. No. 91, 19-25, 1983.
Buck, A.D., and J.P. Burkes, " C h a r a c t e r i z a t i o n and R e a c t i v i t y of S i l i c a Fume," Proc. 3rd I n t l . Conf. Cement Microscopy, pp. 279-285, Houston, U.S.A., March 1981.
Grutzek, M.W., S. Atkinson and D.M. Roy, "Mechanism of H y d r a t i o n of Condensed S i l i c a Fume i n Calcium Hydroxide S o l u t i o n , " Proc. 1st Conf. Use of F l y Ash, S i l i c a Fume, S l a g and Other M i n e r a l By-Products i n C o n c r e t e , Montebello, Canada, Vol. 2, p. 665-676, 1983.
T h i s p a p e r i s b e i n g d i s t r i b u t e d i n r e p r i n t f o r m by t h e I n s t i t u t e f o r R e s e a r c h i n C o n s t r u c t i o n . A l i s t of b u i l d i n g p r a c t i c e and r e s e a r c h p u b l i c a t i o n s a v a i l a b l e from t h e I n s t i t u t e may be o b t a i n e d by w r i t i n g t o t h e P u b l i c a t i o n s S e c t i o n , I n s t i t u t e f o r R e s e a r c h i n C o n s t r u c t i o n , N a t i o n a l R e s e a r c h C o u n c i l o f C a n a d a , Ottawa, O n t a r i o , K1A 0R6. Ce document e s t d i s t r i b u e s o u s .forme d e t i r 6 - 2 - p a r t p a r 1' I n s t i t u t de r e c h e r c h e e n c o n s t r u c t i o n . On p e u t o b t e n i r une l i s t e d e s p u b l i c a t i o n s d e 1 ' I n s t i t u t p o r t a n t s u r l e s t e c h n i q u e s ou l e s r e c h e r c h e s e n m a t i z r e d e b a t i m e n t e n B c r i v a n t 3 l a S e c t i o n d e s p u b l i c a t i o n s , I n s t i t u t d e r e c h e r c h e e n c o n s t r u c t i o n , C o n s e i l n a t i o n a l d e r e c h e r c h e s du Canada, Ottawa ( O n t a r i o ) , KlA 0R6.