Publisher’s version / Version de l'éditeur:
Analytical Biochemistry, 385, pp. 57-64, 2008-10-17
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1016/j.ab.2008.10.014
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Liquid chromatography–mass spectrometry to study chondroitin lyase
action pattern
Zhang, Zhenqing; Park, Youmie; Kemp, Melissa M.; Zhao, Wenjiing; Im,
A-Rang; Shaya, David; Cygler, Miroslaw; Kim, Yeong Shik; Linhardt, Robert J.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=56b32f9b-bc92-4b6b-981a-d88c98585ef0
https://publications-cnrc.canada.ca/fra/voir/objet/?id=56b32f9b-bc92-4b6b-981a-d88c98585ef0
Analytical Biochemistry 385 (2008) 57–64
0003-2697/$ - see front matter © 2008 Else vier Inc. All rights reserved. doi:10.1016/j.ab.2008.10.014
Contents lists available at ScienceDirect
Analytical Biochemistry
j o u r n a l h o m e p a g e : w w w . e l s e v i e r. c o m / l o c a t e / y a b i o
Chon droi tin sul fate (CS)1 is a lin ear, poly dis perse, mi cro
het-er o ge neous, anionic poly sac cha ride hav ing an avhet-er age molec u lar weight of 10,000 to 30,000 Da. Most CS is iso lated from ani mal tis sues, and un sulf at ed chon droi tin is iso lated from cer tain bac-te ria [1,2]. CS con tains disac cha ride repeat ing units com posed of 2-deoxy-2-acet am ido-D-gal act ose (Gal NAc) 1!4 linked to a
uron ic acid such as D-glu cu ronic acid (GlcA) in CS-A and CS-C or L-idu ron ic acid (IdoA) in CS-B (Fig. 1A). Even a sin gle type of
chon-droi tin sul fate, such as CS-A (chon chon-droi tin-4-sul fate), has a range of molec u lar weights and con tains sequence het er o ge ne ity, with minor amounts of 6-sul fated, un sulf at ed, and di sulf at ed disac cha-ride repeat ing units [3]. Bac te rial chon droi tin lyases serve a role in the ini tial micro bial catab o lism of CSs [4] and have found many
appli ca tions as ana lyt i cal tools in car bo hy drate bio chem is try [5,6], for the deter mi na tion of the type of CS pres ent in cells and tis sues
[7–10], for the quan ti ta tive anal y sis of CS [11,12], for the prep a-ra tion of new ther a peu tic agents [1,11,12], and (most recently) in the removal of glial scar CS in the treat ment of spinal cord injury
[13–15].
The increased use of these enzymes, par tic u larly for med i cal appli ca tions, has led to the iso la tion, clon ing, and recombinant expres sion of new bac te rial chon droi tin lyases [16,17]. Thus, a detailed under stand ing of the spec i fic ity of these new chon droi-tin lyases is required, includ ing both their sequence spec i fic ity and action pattern. Ultra vi o let (UV) absor bance [18], vis com e try [19], poly acryl amide gel elec tro pho re sis (PAGE) [19], cap il lary elec-tro pho re sis (CE) [20], thin layer chro ma tog ra phy (TLC) [21], and high-per for mance liquid chro ma tog ra phy (HPLC) [22] have been used to deter mine the action pat terns of chon droi tin lyases. UV and vis com e try are global meth ods to mea sure enzyme action pat-terns and are not able to mon i tor the for ma tion of indi vid ual prod-ucts. In con trast, PAGE, CE, TLC, and HPLC fol low the for ma tion of indi vid ual prod ucts but gen er ally require the use of stan dards to iden tify these prod ucts. Elec tro spray ion i za tion–mass spec trom-e try (ESI–MS) is a soft ion i za tion mtrom-ethod par tic u larly ustrom-e ful to
Liquid chromatography–mass spectrometry to study chondroitin lyase action
pattern
Zhenqing Zhang
a, Youmie Park
a,b, Melissa M. Kemp
a, Wenjing Zhao
a, A-Rang Im
b, David Shaya
c,
Miroslaw Cygler
c,d, Yeong Shik Kim
b, Robert J. Linhardt
a,*a Depart ments of Chem is try and Chem i cal Biol ogy and Chem i cal and Bio log i cal Engi neer ing, Cen ter for Bio tech nol ogy and Inter dis ci plin ary Stud ies, Rens sel aer Poly tech nic Insti tute,
110 8th Street, Troy, NY 12180, USA
b Nat u ral Prod ucts Research Insti tute, Col lege of Phar macy, Seoul National Uni ver sity, Seoul 151-742, Korea c Depart ment of Bio chem is try, McG ill Uni ver sity, Mon treal, Que., Can ada H3G 1Y6
d Bio tech nol ogy Research Insti tute, NRC, Mon treal, Que., Can ada H4P 2R2
a r t i c l e i n f o a b s t r a c t
Article history:
Received 1 August 2008 Available online 17 October 2008
Liquid chro ma tog ra phy–mass spec trom e try was applied to deter mine the action pattern of dif fer ent chon droi tin lyases. Two com mer cial enzymes, chon dro itin ase ABC (Pro teus vul ga ris) and chon dro itin-ase ACII (Arth ro bac ter au res cens), hav ing action pat terns pre vi ously deter mined by vis cos i me try and gel elec tro pho re sis were first exam ined. Next, the action pat terns of recombinant lyases, chon dro itin ase ABC from Bac te roi des theta i ota omi cron (expressed in Esch e richia coli) and chon dro itin ase AC from Fla vo bac te-rium hep ar i num (expressed in its original host), were exam ined. Chon droi tin sul fate A (CS-A, also known as chon droi tin-4-sul fate) was used as the sub strate for these four lyases. Ali quots taken at var i ous time points were ana lyzed. The prod ucts of chon dro itin ase ABC (P. vul ga ris) and chon dro itin ase AC (F. hep-ar i num) con tained unsat u rated oli go sac cha rides of sizes rang ing from disac cha ride to deca sac cha ride, dem on strat ing that both are end o lyt ic enzymes. The prod ucts affor ded by chon dro itin ase ABC (B. theta-i ota omtheta-i cron) and chon dro theta-ittheta-in ase ACII (A. au res cens) con tatheta-ined prtheta-i mar theta-ily unsat u rated dtheta-isac cha rtheta-ide. These two ex o lyt ic enzymes showed dif fer ent minor prod ucts, sug gest ing some sub tle spec i fic ity dif fer ences between the actions of these two ex o lyt ic lyases on chon droi tin sul fate A.
© 2008 Else vier Inc. All rights reserved.
Key words:
Liquid chro ma tog ra phy–mass spec trom e-try, (LC–MS)
Chon droi tin lyase Action pattern
* Cor re spond ing author. Fax: +1 518 276 3405.
E-mail address: lin har@rpi.edu (R.J. Linhardt).
1 Abbre vi a tions used: CS, chon droi tin sul fate; Gal NAc, 2-deoxy-2-acet am ido-D-gal act ose; GlcA, D-glu cu ronic acid; IdoA, L-idu ron ic acid; CS-A, chon droi tin-4-sul fate; UV, ultra vi o let; PAGE, poly acryl amide gel elec tro pho re sis; CE, cap il lary elec tro pho re sis; TLC, thin layer chro ma tog ra phy; HPLC, high-per for mance liquid chro ma tog ra phy; ESI–MS, elec tro spray ion i za tion–mass spec trom e try; LC, liquid chro ma tog ra phy; RPIP, reversed-phase ion pair ing; HA, hya lu ro nan; IPTG, iso pro pyl b-D-1-thio ga lac to py ra no side; SDS, sodium dode cyl sul fate; TBA, tri bu tyl amine; EIC, extracted ion chro mato gram; dp, degree of poly mer i za tion.
58 LC–MS to study chondroitin lyase action pattern / Z. Zhang et al. / Anal. Biochem. 385 (2008) 57–64
mon i tor the for ma tion of CS oli go sac cha rides [23]. Liquid chro-ma tog ra phy (LC)–ESI–MS, rely ing on reversed-phase ion pair ing (RPIP)–HPLC, employs vol a tile ion pair ing reagents to suc cess fully ana lyze gly cos ami no gly can-derived oli go sac cha rides such as hep-a rin, hep hep-a rhep-an sul fhep-ate, he phep-ar o shep-an, hep-and hyhep-a lu ro nhep-an (HA) [24–26]. The sep a ra tion pro files of enzy matic prod ucts and their struc tures or sequences can be deduced by LC–MS.
Chon droi tin lyases orig i nate from var i ous micro bial sources and dis play dif fer ent spec i fic i ties and action pat terns (Fig. 1B) [17]. Chon droi tin lyase ABC (Pro teus vul ga ris, EC 4.2.2.4), a mix ture of ABC lyases I and II, acts on CS-A, CS-B, and CS-C in a pre dom i nately end o lyt ic action pattern [19]. Chon droi tin lyase AC I (Fla vo bac te-rium hep ar i num, EC 4.2.2.5) acts on CS-A and CS-C in a random end o lyt ic action pattern [19]. Chon droi tin lyase AC II (Arth ro bac ter au res cens, EC 4.2.2.5) acts on CS-A and CS-C and dis plays an ex o lyt ic
action pattern [19,20,27–30]. The var i ous chon droi tin ABC lyases dis play pair wise sequence iden tity in the range of 23 to 31% and, there fore, have diverged sub stan tially dur ing evo lu tion. Sim i larly, the sequence iden tity between the two chon dro itin as es AC is also only 23%. Com par i son of struc tures of chon dro itin as es ABC and AC shows clear sim i lar ity of their folds in the sense that the a -heli-cal and b-sheet domains of chon dro itin ase AC have coun ter parts in chon dro itin ase ABC, with the lat ter hav ing an addi tional N-ter-mi nal b-sheet domain. How ever, only the a-heli cal domains show rec og niz able sequence sim i lar ity amount ing to approx i mately 24% iden tity, whereas the sequences of the b-sheet domains diverged beyond rec og ni tion by pro grams such as BLAST.
In this arti cle, the action pattern of com mer cial chon droi tin lyase AC II (A. au res cens) and com mer cial chon droi tin lyase ABC (P. vul ga ris) as well as two recombinant lyases expressed in Esch e richia coli and
Fig. 1. Struc ture of CS and its cleav age by chon droi tin lyase through var i ous action pat terns. (A) The chem i cal struc ture of the major sac cha ride res i dues found, CS-A, CS-B,
and CS-C, where n = 20 to 60. The sym bolic rep re sen ta tion of these struc tures is also shown with h as Gal NAc, as GlcA, and as IdoA. The pre dom i nant sequences of CS-A, CS-B, and CS-C are indi cated. (B) A sin gle typ i cal chain of CS-A is shown with some sequence het er o ge ne ity. The non re duc ing end (nre) and reduc ing end (re) of the chain are indi cated. An ex o lyt ic enzyme cuts the chain at each cleav age site start ing from the nre, whereas a random end o lyt ic enzyme cuts the chain through the random selec-tion of sites. Typ i cal prod uct dis tri bu selec-tion at time points 1 (early reac selec-tion), 2 (inter me di ate reac selec-tion), and 1 (reac tion com ple tion) are shown. The sym bol cor re sponds to unsat u rated uron ic acid.
LC–MS to study chondroitin lyase action pattern / Z. Zhang et al. / Anal. Biochem. 385 (2008) 57–64 59
Fig. 2. EIC of prod ucts digested by chon dro itin ase ABC (P. vul ga ris): (A) 0-min ali quots; (B) 10-min ali quots; (C) 30-min ali quots; (D) 60-min ali quots; (E) 120-min ali quots.
Insets are 5£ inten sity mag ni fi ca tion of 40 to 60 min.
Fig. 3. Mass spec tra of oli go sac cha rides observed in Fig. 2: (A) mass spec trum of dp2; (B) mass spec trum of dp4; (C) mass spec trum of dp6; (D) mass spec trum of dp8; (E)
60 LC–MS to study chondroitin lyase action pattern / Z. Zhang et al. / Anal. Biochem. 385 (2008) 57–64
F. heparinum, chon droi tin lyase ABC (Bac te roi des theta i ota omi cron) and AC (F. hep ar i num), respectively, are exam ined using LC–MS. Mate ri als and meth ods
Mate ri als
CS-A (from bovine tra chea) was pur chased from Cel sus (Cin-cin nati, OH, USA). Chon droi tin lyase ABC (P. vul ga ris) and ACII (A. au res cens) were obtained from Sei kagaku (Tokyo, Japan). Meth ods
Expres sion of chon droi tin lyase ABC from B. theta i ota omi cron and chon droi tin AC lyase from F. hep ar i num
The recombinant chon droi tin lyase ABC (B. theta i ota omi cron) was expressed in E. coli BL21(DE3) and puri fied as described pre-vi ously [31]. Briefly, cells express ing the enzyme were cul tured in Luria broth at 37 °C sup ple mented with 100 lg ml¡1
ampi cil lin, and pro tein expres sion was induced with 1 mM iso pro pyl b -d-1-thio-ga lac to py ra no side (IPTG). Cells were dis rupted by son i ca tion and puri fied using a com bi na tion of DEAE (GE Health care, Pis cat a way, NJ, USA), Ni-NTA (Qiagen, Valen cia, CA, USA), Mono-S HR 10/10 (GE Health care), and Hi Load 16/60 Superdex 200 (GE Health care) col umns. The purity of the eluted frac tions was eval u ated using sodium dode cyl sul fate (SDS)–PAGE, and the spe cific activ ity was deter mined.
The F. hep ar i num chon droi tin AC lyase was expressed and puri-fied as described pre vi ously [32].
Diges tion
CS-A (2 mg/ml) in 1 ml of 50 mM sodium phos phate buffer (pH 7.0) was treated in indi vid ual reac tions with chon droi tin lyases
ABC (P. vul ga ris), ACII (A. au res cens), and AC (F. hep ar i num) (50-l
units) at 37 °C for 3 h with ali quots removed at var i ous time points for anal y sis (0, 10, 30, 60, and 120 min). Chon droi tin lyase ABC (B. theta i ota omi cron) was used in an iden ti cal fash ion except over a 24-h period, with ali quots taken at 0, 1, 5, 12, and 24 h. As soon as each ali quot was removed from an enzy matic reac tion, the enzyme was ther mally inac ti vated by heat ing at 100 °C for 10 min.
CS-A (2 mg/ml) in 0.1 ml of 50 mM sodium phos phate buffer (pH 7.0) was treated in reac tion with both chon droi tin lyases ABC (P. vul ga ris) and ACII (A. au res cens) (50-l units) at 37 °C over night. The enzyme was ther mally inac ti vated by heat ing at 100 °C for 10 min and was removed by cen tri fuge (12,000 g). The super na tant was freeze-dried and ready for disac cha ride anal y sis.
LC–MS
LC–MS anal y ses were per formed on an Ag i lent 1100 LC/MSD instru ment (Ag i lent Tech nol o gies, Wil ming ton, DE, USA) equipped with an ion trap, a binary pump, and a UV detec tor. The col umn used was a 5-lm Ag i lent Zor bax SB-C18 (0.5 £ 250 mm, Ag i lent
Tech nol o gies). Elu ent A was water/ace to ni trile (85:15, v/v), and elu ent B was water/ace to ni trile (35:65, v/v). Both elu ents con tained 12 mM tri bu tyl amine (TBA) and 38 mM NH4OAc with pH adjusted
to 6.5 with HOAc. A gra di ent of 0% B for 15 min, and 0 to 100% B over 85 min was used at a flow rate of 10 ll/min. Another gra di ent of 0% B for 20 min and 0 to 50% B over 25 min was used for disac cha ride anal y sis. Injected sam ples had a vol ume of 8 ll. Mass spec tra were obtained using an Ag i lent 1100 series Clas sic G2445D LC/MSD trap (Ag i lent Tech nol o gies). The elec tro spray inter face was set in neg a-tive ion i za tion mode with the skim mer potential –40.0 V, cap il lary exit at –40.0 V, and a source tem per a ture of 325 °C to obtain max i-mum abun dance of the ions in a full-scan spec trum (150–1500 Da, 10 full scans/s). Nitro gen was used as a dry ing (5 L/min) and neb-u liz ing (20 psi) gas. Extracted ion chro mato grams (EICs) and mass
Fig. 4. EIC of prod ucts digested by chon dro itin ase AC II (A. au res cens): (A) 0-min ali quots; (B) 10-min ali quots; (C) 30-min ali quots; (D) 60-min ali quots; (E) 120-min ali quots.
LC–MS to study chondroitin lyase action pattern / Z. Zhang et al. / Anal. Biochem. 385 (2008) 57–64 61
spec tra were pro cessed using Data Anal y sis 2.0 soft ware (Bru ker Dal ton ics, Bille rica, MA, USA).
Results
Chon droi tin lyase ABC (P. vul ga ris)
The digested CS-A was ana lyzed by LC–MS. Molec u lar weights and MS con firmed the sequence of each sep a rated oli go sac cha ride prod uct. The EICs from dif fer ent diges tion time ali quots of chon-droi tin lyase ABC (P. vul ga ris) are shown in Fig. 2. The ini tial chro-mato gram taken prior to the addi tion of the enzyme (time point 0) showed no peaks cor re spond ing to prod ucts (Fig. 2A). By 10 min, the EIC showed four peaks cor re spond ing to a degree of poly mer-i za tmer-ion (dp) 2, dp4, dp6, and dp8 (see Fig. 2B and inset). The mass spec tra of these peaks (Fig. 3) indi cate clearly that the prod ucts are: mono sulf at ed unsat u rated disac cha ride (dp2), di sulf at ed unsat u rated tet ra sac cha ride (dp4), tri sul fated unsat u rated hexa-sac cha ride (dp6), and tet ra sulf at ed unsat u rated oc ta hexa-sac cha ride (dp8). The molec u lar ion of mono sulf at ed unsat u rated disac cha-ride was observed at m/z 458.0, whereas the di sulf at ed unsat u-rated tet ra sac cha ride showed a dou bly charged molec u lar ion at m/z 458.1, the tri sul fated unsat u rated hexa sac cha ride showed both tri ply charged and dou bly charged molec u lar ions at m/z 458.2 and 687.5, respec tively, and the tet ra sulf at ed unsat u rated oc ta-sac cha ride showed qua dru ply charged and tri ply charged molec-u lar ions at m/z 458.1 and 611.1, respec tively. A tri ply charged ion, cor re spond ing to a tri sul fated unsat u rated oc ta sac cha ride result-ing from frag men ta tion of this highly charged oc ta sac cha ride by cleav age of one of the unsta ble sulfo groups, was also observed in this spec trum. The EIC pre sented in Fig. 2C (inset) cor re sponds to the 30 min diges tion and dis played an addi tional small peak cor-re spond ing to the molec u lar ion of a pen ta sulf at ed unsat u rated deca sac cha ride hav ing charge stages of –5 and –4 at m/z 458.1 and
573.0, respec tively (Fig. 3E). Again, the loss of a sin gle sulfo group through frag men ta tion was observed. The tran sient appear ance of larger oli go sac cha rides such as dp10 (Fig. 2C) and the increase and subsequent decrease in dp8 (Fig. 2B–E) con firm that chon droi-tin lyase ABC (P. vul ga ris) dis plays pri mar ily an end o lyt ic action pattern.
Chon droi tin lyase ACII (A. au res cens)
The EICs of time ali quots of CS-A diges tion with chon droi-tin lyase ACII (A. au res cens) are pre sented in Fig. 4. As expected, this enzyme clearly shows an ex o lyt ic action pattern. The major prod uct of this lyase is the mono sulf at ed unsat u rated disac cha-ride with the peak inten sity increas ing con tin u ously over the time points exam ined (Fig. 4). The mass spec trum of dp2 con-firmed it to be the mono sulf at ed unsat u rated disac cha ride at m/z 458.0 (Fig. 5C). In addi tion, a tiny peak, close to the mono-sulf at ed unsat u rated (dp2) peak, labeled as S-dp2 was observed. This peak cor re sponds to a mono sulf at ed sat u rated disac cha-ride, which affor ded an m/z of 476.1 (Fig. 5B). This peak orig i-nates from the non re duc ing ter mi nal of CS-A and is often not observed by other meth ods because of the low amounts pres ent and its low UV absor bance. Peaks cor re spond ing to non sulf at ed and di sulf at ed unsat u rated disac cha ride could also be observed and are con sis tent with the sequence het er o ge ne ity of the sub-strate. Disac cha ride anal y sis of this CS-A sam ple showed 93% mono sulf at ed disac cha ride, 6% non sulf at ed disac cha ride, and 1% di sulf at ed disac cha ride. These struc tures were con firmed by MS (Fig. 5A and D). The action pattern of chon droi tin lyase ACII (A. au res cens) can eas ily be con firmed by the appear ance of increas-ing amounts of dp2 over time with only very minor sec ond ary prod ucts. The inten si ties of the peaks (non sulf at ed and di sulf-at ed unssulf-at u rsulf-ated disac cha rides) did not increase after 30 min diges tion, sug gest ing that these domains are located close to
Fig. 5. Mass spec tra of oli go sac cha rides observed in Fig. 4: (A) mass spec trum of non sulf at ed unsat u rated dp2; (B) mass spec trum of mono sulf at ed sat u rated dp2; (C) mass
62 LC–MS to study chondroitin lyase action pattern / Z. Zhang et al. / Anal. Biochem. 385 (2008) 57–64
the non re duc ing end of the CS-A chain. The di sulf at ed unsat u-rated tet ra sac cha ride appeared most prom i nently in the early time points and was sub se quently digested. This tet ra sac cha ride appears to be a side prod uct of this ex o lyt ic lyase, which in turn can itself be used as a sub strate.
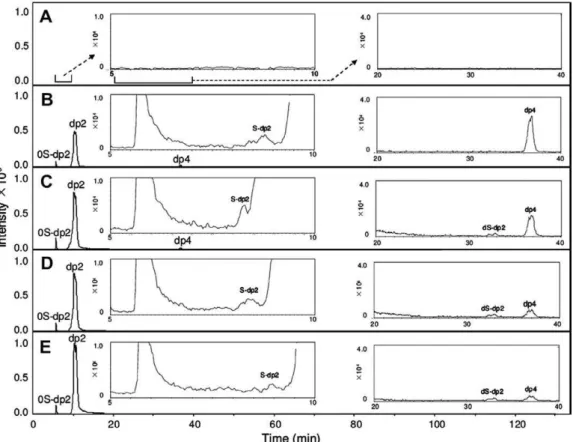
Chon droi tin lyase ABC (B. theta i ota omi cron)
Next, chon droi tin lyase ABC (B. theta i ota omi cron), recom bi-nantly expressed in E. coli, was exam ined. The mono sulf at ed unsat u rated disac cha ride (dp2) was observed as a major peak in the EIC (Fig. 6). The inten sity of this peak increased with diges tion time. In addi tion, a mono sulf at ed sat u rated disac cha ride (S-dp2) and non sulf at ed and di sulf at ed unsat u rated disac cha rides (0S-dp2 and dS-dp2) were also observed in the EIC (Fig. 6), and their struc-tures were deter mined based on molec u lar ions at m/z 476.1, 378.1, and 538.1, respec tively. These results were sim i lar to those observed for chon droi tin lyase ACII (A. au res cens), sug gest ing that recom bi nantly expressed chon droi tin lyase ABC (B. theta i ota omi-cron) is also an ex o lyt ic enzyme. These are the first action pattern data reported for this enzyme. No unsat u rated dp4 was observed among the prod ucts of this lyase, dem on strat ing the pres ence of sub tle dif fer ences between the ex o lyt ic action pat terns of these two lyases.
Chon droi tin lyase AC (F. hep ar i num)
Recombinant chon droi tin lyase AC (F. hep ar i num) expressed in F. heparinum dis plays an end o lyt ic action pattern when act ing on CS-A. Through out the entire course of the reac tion, dp4 was the dom i nant peak in the EIC (Fig. 7). Only when the reac tion was com pleted did dp2 become the major prod uct. Oli go sac cha rides (dp6, dp8, and dp10) appeared tran siently through out the time course of the reac tion.
Dis cus sion
LC–MS has been dem on strated to be a pow er ful method for the anal y sis of prod ucts affor ded through the diges tion of gly cos ami-no gly cans by poly sac cha ride lyases. The prod uct dis tri bu tion can be pro filed through LC, and the prod uct struc ture can be ana lyzed by MS. Based on the prod ucts detected by LC–MS, the action pat-terns of var i ous lyases can be con firmed.
The chon droi tin lyases, inves ti gated in this study, dis played two gen eral action pat terns: end o lyt ic and ex o lyt ic. Chon droi-tin lyase ABC (P. vul ga ris) and recombinant chon droi droi-tin lyase AC (F. hep ar i num) both showed sim i lar end o lyt ic action pat terns (Figs. 2 and 7). In con trast, although both chon droi tin lyase AC (A. au res cens) and recombinant chon droi tin ABC (B. theta i ota omi-cron) are clearly ex o lyt ic, they dis played dis tinc tive dif fer ences (Figs. 4 and 6). In par tic u lar, chon droi tin lyase AC (A. au res cens) showed a tran sient dp4 inter me di ate that was not observed in chon droi tin lyase ABC (B. theta i ota omi cron) diges tion. This sug-gests that chon droi tin lyase AC (A. au res cens) can skip over cleav-able sites only to return and cleave these sites later dur ing the reac tion. Such sub tle dif fer ences in action pat terns are clearly impor tant when using chon droi tin lyases in CS sequenc ing stud-ies and could eas ily be missed when apply ing global anal y sis, such as vis com e try, or more stan dard meth ods of anal y sis for the study of enzyme action pat terns. Fur ther more, the appli ca tion of LC–MS also allows the obser va tion of minor prod ucts aris-ing from the nat u ral sequence micro het er o gene ity of CS, such as un sulf at ed and di sulf at ed sequences and oli go sac cha rides, orig i nat ing from the non re duc ing ter mi nus of the CS chain. In this study, sequence het er o ge ne ity was observed only when using ex o lyt ic enzymes. The mono sulf at ed disac cha ride domain is the most enzy mat i cally sen si tive one [16,21] and is digested first by end o lyt ic enzymes. In con trast, any struc ture pres ent at
Fig. 6. EIC of prod ucts digested by chon dro itin ase ABC (B. theta i ota omi cron): (A) 0-h ali quots; (B) 1-h ali quots; (C) 5-h ali quots; (D) 12-h ali quots; (E) 24-h ali quots. Insets are
LC–MS to study chondroitin lyase action pattern / Z. Zhang et al. / Anal. Biochem. 385 (2008) 57–64 63
the non re duc ing ter mi nus is digested first by ex o lyt ic enzymes. Prod uct struc tures with dif fer ent sul fa tion pat terns have also been observed in pre vi ous stud ies [16,21]. In the cur rent study, we elected to exam ine a sin gle sub strate type, CS-A. It is likely that CS sub strates hav ing dif fer ent sul fa tion pat terns or dif fer-ent domain struc tures will give dif fer ences in prod uct dis tri bu-tion and pos si bly dif fer ences in acbu-tion pat terns. LC–MS offers an excel lent approach for anal y sis because minor sequence het er-o ge ne ities, hav ing a prer-o fer-ound imper-or tance in CS bier-ol er-ogy, can be iden ti fied using this method.
Ref er ences
[1] N. Vol pi, Chon droi tin Sul fate Struc ture, Role, and Phar ma co log i cal Activ ity Advances in Phar ma col ogy, Aca demic Press, San Diego, 2006 pp. 1–10. [2] P.L. DeAn ge lis, N.S. Gunay, T. To ida, W-J. Mao, R.J. Lin hardt, Iden ti fi
ca-tion of the cap su lar poly sac cha rides of type D and F Pas teu rella multo cida as unmod i fied hep a rin and chon droi tin, respec tively, Car bo hy dr. Res. 337 (2002) 1547–1552.
[3] H.E. Bu low, O. Ho bert, The molec u lar diver sity of gly cos ami no gly cans shapes ani mal devel op ment, Annu. Rev. Cell Dev. Biol. 22 (2006) 375–407.
[4] R.J. Lin hardt, P.M. Gal li her, C.L. Coo ney, Poly sac cha ride lyases, Appl. Bio chem. Bio tech nol. 12 (1986) 135–176.
[5] M. Koke tsu, R.J. Lin hardt, Elec tro pho re sis for the anal y sis of acidic oli go sac cha-rides, Anal. Bio chem. 283 (2000) 136–145.
[6] A. Al-Hakim, R.J. Lin hardt, Cap il lary elec tro pho re sis for the anal y sis of chon-droi tin sul fate- and der ma tan sul fate-derived disac cha rides, Anal. Bio chem. 195 (1991) 68–73.
[7] M. Warda, F. Zhang, M. Rad wan, Z. Zhang, N. Kim, Y.N. Kim, R.J. Lin hardt, J. Han, Is human pla centa pro teo gly can remod el ing involved in pre-eclamp sia?, Gly-co Gly-conj. J. 25 (2008) 441–450.
[8] S. Sa kai, J. Onose, H. Na kam ura, H. Toy oda, T. To ida, T. Ima nari, R.J. Lin hardt, Pre treat ment pro ce dure for the mi cro de ter min a tion of chon droi tin sul fate in plasma and urine, Anal. Bio chem. 302 (2002) 169–174.
[9] P. Sin nis, A. Cop pi, T. To ida, H. Toy oda, A. Ki nosh it a-Toy oda, J. Xie, M.M. Kemp, R.J. Lin hardt, Mos quito hep a ran sul fate and its potential role in malaria infec-tion and trans mis sion, J. Biol. Chem. 282 (2007) 25376–25384.
[10] A.V. Nairn, A. Ki nosh it a-Toy oda, H. Toy oda, J. Xie, K. Har ris, S. Dal ton, M. Ku lik, J.M. Pierce, T. To ida, K.W. Mor emen, R.J. Lin hardt, Gly co mics of pro teo gly can
bio syn the sis in murine embry onic stem cell dif fer en ti a tion, J. Pro te ome Res. 6 (2007) 4374–4387.
[11] J-S. Sim, T. To ida, S.Y. Cho, D.W. Choi, S-Y. Chang, R.J. Lin hardt, Y.S. Kim, Quan ti ta tive anal y sis of chon droi tin sul fate in raw mate ri als. Oph thal-mic solu tions, soft cap sules, and liquid prep a ra tions, J. Chro ma togr. B 818 (2005) 133–139.
[12] S. Sa kai, H. Ak iy ama, Y. Sato, Y. Yo shioka, R.J. Lin hardt, Y. Goda, T. Mai tan i, T. To ida, Chon droi tin sul fate intake inhib its the IgE-med i ated aller gic response by down-reg u lat ing Th2 responses in mice, J. Biol. Chem. 281 (2006) 19872– 19880.
[13] J.W. Fawc ett, Over com ing inhi bi tion in the dam aged spinal cord, J. Neu ro-trauma 23 (2006) 371–383.
[14] N.J. Tester, D.R. How land, Chon dro itin ase ABC improves basic and skilled loco mo tion in spinal cord injured cats, Exp. Neu rol. 209 (2008) 483– 496.
[15] J.E. Davies, X. Tang, J.C. Bour nat, S.J. Davies, De co rin pro motes plas min o gen/ plas min expres sion within acute spinal cord inju ries and by adult microg lia in vitro, J. Neu ro trauma 23 (2006) 397–408.
[16] D. Sha ya, B.S. Hahn, N.Y. Park, J.S. Sim, Y.S. Kim, M. Cy gler, Char ac ter iza tion of chon droi tin sul fate lyase ABC from Bac te roi des theta i ota omi cron WAL2926, Bio chem is try 47 (2008) 6650–6661.
[17] R.J. Lin hardt, F.Y. Avci, T. To ida, Y.S. Kim, M. Cy gler, CS lyases: Struc ture, activ ity, and appli ca tions in anal y sis and the treat ment of dis eases, Adv. Phar ma col. 53 (2006) 187–215.
[18] R.J. Lin hardt, J.E. Turn bull, H.M. Wang, D. Log a na than, J.T. Galla gher, Exam i-na tion of the sub strate spec i fic ity of hep a rin and hep a ran sul fate lyases, Bio-chem is try 29 (1990) 2611–2617.
[19] K.A. Jan dik, K. Gu, R.J. Lin hardt, Action pattern of poly sac cha ride lyases on gly-cos ami no gly cans, Gly co bi ol ogy 4 (1994) 289–296.
[20] T. Ya mag at a, H. Sa i to, O. Habu chi, S. Su zuki, Puri fi ca tion and prop er ties of bac-te rial chon dro itin as es and chon drosulfa ta ses, J. Biol. Chem. 243 (1968) 1523– 1535.
[21] Z. Zhang, J. Xie, F. Zhang, R.J. Lin hardt, Thin-layer chro ma tog ra phy for the anal y sis of gly cos ami no gly can oli go sac cha rides, Anal. Bio chem. 371 (2007) 118–120.
[22] K.G. Rice, Y.S. Kim, A.C. Grant, Z.M. Mer chant, R.J. Lin hardt, High-per for mance liquid chro mato graphic sep a ra tion of hep a rin-derived oli go sac cha rides, Anal. Bio chem. 150 (1985) 325–331.
[23] J.B. Fenn, M. Mann, C.K. Meng, S.F. Wong, C.M. White house, Elec tro spray ion i za tion for mass spec trom e try of large bio mol e cules, Sci ence 246 (1989) 64–71.
[24] B. Kub er an, M. Lech, L. Zhang, Z.L. Wu, D.L. Be el er, R.D. Rosen berg, Anal y sis of hep a ran sul fate oli go sac cha rides with ion pair–reverse phase cap il lary high
Fig. 7. EIC of prod ucts digested by chon dro itin ase AC (F. hep ar i num): (A) 0-min ali quots; (B) 10-min ali quots; (C) 30-min ali quots; (D) 60-min ali quots; (E) 120-min ali quots.
64 LC–MS to study chondroitin lyase action pattern / Z. Zhang et al. / Anal. Biochem. 385 (2008) 57–64
per for mance liquid chro ma tog ra phy–mi cro elec tro spray ion i za tion time-of-flight mass spec trom e try, J. Am. Chem. Soc. 124 (2002) 8707–8718.
[25] C. Tha nawi roon, K.G. Rice, T. To ida, R.J. Lin hardt, Liquid chro ma tog ra phy/mass spec trom e try sequenc ing approach for highly sul fated hep a rin-derived oli go-sac cha rides, J. Biol. Chem. 279 (2004) 2608–2615.
[26] Z. Zhang, J. Xie, J. Liu, R.J. Lin hardt, Tan dem MS can dis tin guish hyal u ronic acid from N-acet y lhe par o san, J. Am. Soc. Mass Spec trom. 19 (2008) 82–90. [27] K. Hiy ama, S. Okada, Amino acid com po si tion and phys io chem i cal char ac
ter-iza tion of chon dro itin ase from Arth ro bac ter au res cens, J. Bio chem. 78 (1975) 1183–1190.
[28] W. Hu ang, V.V. Lun in, Y. Li, S. Su zuki, N. Sugi ura, H. Mi yaz on o, M. Cy gler, Crys-tal struc ture of Pro teus vul ga ris chon droi tin sul fate ABC lyase I at 1.9 Å res o lu-tion, J. Mol. Biol. 328 (2003) 623–634.
[29] J. Féthi ère, B. Eggi mann, M. Cy gler, Crys tal struc ture of chon droi tin AC lyase, a rep re sen ta tive of a fam ily of gly cos ami no gly can degrad ing enzymes, J. Mol. Biol. 288 (1999) 635–647.
[30] I. Ca pil a, Y. Wu, D.W. Reth wisch, A. Matte, M. Cy gler, R.J. Lin hardt, Role of argi-nine 292 in the cat a lytic activ ity of chon droi tin AC lyase from Fla vo bac te rium
hep ar i num, Bio chim. Bio phys. Acta 1597 (2002) 260–270.
[31] D. Sha ya, B-S. Hahn, T.M. Bjer kan, W.S. Kim, N.Y. Park, J-S. Sim, Y-S. Kim, M. Cy gler, Com pos ite active site of chon droi tin lyase ABC accept ing both epi mers of uron ic acid, Gly co bi ol ogy 18 (2008) 270–277.
[32] J. Féthière, B.H. Shil ton, Y. Li, M. Al laire, M. Lali berté, B. Eggi mann, M. Cy gler, Crys tal li za tion and preliminary anal y sis of chon dro itin ase AC from Fla vo bac te rium hep ar i num, Acta Crys tal logr. D 54 (1998) 279– 280.