Publisher’s version / Version de l'éditeur:
Canadian Metallurgical Quarterly, 44, 1, pp. 33-40, 2005-01-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
The Role of Coupling Agent in the Formation of Polypropylene
Nanocomposites
Lei, S. G.; Ton-That, M. T.; Hoa, S. V.; Cole, K. C.; Pesneau, I.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=294dd7cf-8efe-46d4-af05-5ec881e284a6 https://publications-cnrc.canada.ca/fra/voir/objet/?id=294dd7cf-8efe-46d4-af05-5ec881e284a6
THE ROLE OF COUPLING AGENTS IN THE FORMATION OF
POLYPROPYLENE NANOCOMPOSITES
S.G. LEI2, M.-T. TON-THAT1, S.V. HOA2, K.C. COLE1 and I. PESNEAU1 1National Research Council Canada, Industrial Materials Institute, Boucherville, Quebec, Canada J4B 6Y4
2Department of Mechanical Engineering, Concordia University, Montreal, Quebec, Canada H3G 1M8
(Received in revised form August, 2004)
Abstract — The effect of the functionalization of polypropylene by different chemicals on the fabrica-tion and properties of the resulting nanocomposites was studied. Different nanocomposites were prepared using different functionalized polypropylenes (PP) as coupling agents. In order to have better control of the interaction between the functional groups and the clay, samples were mixed in a Brabender plasti-corder. The nanocomposite properties were then investigated. Different analysis techniques were used to characterize the dispersion and the properties of the nanocomposites including scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction analysis, dynamic mechanical analysis (DMA) and a conventional flexural test.
Résumé — On a étudié l’effet de la fonctionnalisation du propylène par différents produits chimiques sur la fabrication et les propriétés des nanocomposites qui en résultent. On a préparé divers nanocomposites en utilisant différents polypropylènes (PP) fonctionnalisés comme agents d’accrochage. Afin d’avoir un meilleur contrôle de l’interaction entre les groupes fonctionnels et l’argile, on a mélangé les échantillons dans un “plasticorder” Brabender. On a ensuite évalué les propriétés des nanocomposites. On a utilisé diverses techniques d’analyse pour caractériser la dispersion et les propriétés des nanocomposites, incluant la microscopie électronique à balayage (SEM), la microscopie électronique à transmission (TEM), l’analyse de la diffraction des rayons x, l’analyse mécanique dynamique (DMA) et un essai conventionnel de pliage.
INTRODUCTION
Polymer nanocomposites form a new class of composite materials derived from nano-particles with at least one dimension in the nanometer range which are dispersed in the polymer matrix at a relatively low loading (often under 6% by weight). Since the nano-particles (such as nan-oclays, nanofibers and carbon nanotubes) are so small and their aspect ratios (largest dimension/smallest dimension) are very high, even at such low loadings certain polymer properties can be greatly improved without the detrimental impact on density, brittleness, transparency and process-ability associated with conventional reinforcements like talc or glass. In general, nanoparticles can significantly improve the stiffness, heat deflection temperature (HDT), dimensional stability, gas barrier properties, electrical con-ductivity and flame retardancy of the polymer matrix [1-2]. Among polymer nanocomposites, those based on polypropylene (PP) and nanoclay have attracted consider-able interest [3-13] because PP falls in the most widely
used and fastest growing class of thermoplastics, while nanoclay is one of the most widely accepted and effective nanoreinforcements. However, scientists and engineers are faced with several challenges. Nanoclay is naturally hydrophilic, whereas PP has no polar groups in its back-bone and is one of the most hydrophobic polymers. The result is usually a low level of dispersion of the clay platelets in the PP matrix and a poor interface between the clay surface and the PP matrix. This limits the advantages of incorporation of the nanoclay into the polymer matrix. Attempts to resolve these problems involve modification of the nanoclay surface and the matrix. Several types of organo-clay are currently commercially available [14,15]. In general, the main difference among them concerns the organic modifiers bound to the nanoclay surface which are used to improve the compatibility of nanoclay with the matrix. Alternatively, the inclusion of a coupling agent is a popular way to modify the matrix because it is simple, eco-nomical and can be done with conventional mixing equip-ment. The coupling agent plays a crucial role in dispersing the clay in the polymer matrix and in producing a strong © Canadian Institute of Mining, Metallurgy and Petroleum Published by Canadian Institute of Mining, Metallurgy and Petroleum Printed in Canada. All rights reserved
S.G. LEI, M.-T. TON-THAT, S.V. HOA, K.C. COLE and I. PESNEAU
interface between the two. Since it must be compatible with the matrix and at the same time show affinity for the clay surface, the coupling agent should possess a main PP chain together with functional groups such as maleic anhy-dride (MA), hydroxyl (OH), amine (NH2), or ammonium (NH3+) that can provide good interaction with the clay sur-face. Among these, MA grafted PP (PP-g-MA) is the most popular one for both conventional composites and nanocomposites. It is believed that hydrogen bonding between the hydroxyl groups of the silicates and the MA groups of the PP-g-MA accounts for the improvement of the interaction between the coupling agent and the clay sur-face [3]. On the other hand, amines and amine cations may also provide effective interaction with the clay surface since they are often used to treat it. These types of coupling agents are not readily commercially available and their use in nanocomposites has not been fully explored.
The objective of the work described here is to study the effect of the coupling agent chemistry on the fabrication and thus the morphology and properties of PP nanocom-posites. This paper reports some preliminary results on the effect of different types of coupling agents, with different functional groups on the fabrication and mechanical prop-erties of PP nanocomposites.
EXPERIMENTAL Materials
The polypropylene (PP) used in this study was PP6100SM, a general purpose injection grade from Montell.
Two different types of commercial coupling agent were used, Polybond 3150 (designated CA1) and Epolene 3015 (designated CA2). Both are based on PP-g-MA. Purified CA2, designated as CA2P, was prepared by dissolving CA2 in hot toluene, followed by precipitation with acetone. CA3 is an amine terminated (NH2-t) coupling agent which was prepared by reacting Polybond 3150 and Jeffamine T-403 (1.5 wt%) in a Brabender mixer at 180 °C
for 15 minutes [16]. CA4 was produced from CA3 by immersing the CA3 thin film in HCl solution (20 wt%) at 80 °C for 12 hours; the amine functional group of CA3 is transformed to onium ion (NH3+) in CA4. Table I gives more detailed information on the coupling agents. Cloisite 15A nanoclay was obtained from Southern Clay Products Incorporated and its technical data [15] are given in Table II.
Nanocomposite Preparation
The blends and composites were prepared by mixing them in a C.W. Brabender PL2000 plasticorder. The mixing tem-perature was kept at 180 °C in order to ensure good vis-cosity for the mixing while at the same time minimizing degradation. The rotation speed was set at 60 rpm. After all the ingredients were introduced into the Brabender plasti-corder, melt mixing was continued for an additional period of 5 minutes. The total weight of material per batch was 40 g which gives a suitable volume for the Brabender mixer. In all the nanocomposite samples, the concentration of nan-oclay was kept at 3 wt% and the level of coupling agent was 6 wt% when present.
PP and its blends with coupling agents were also processed under the same conditions for comparison pur-poses. Specimens for testing were prepared in a Model M Carver laboratory press under a pressure of 40,000 psi with a temperature of 180 °C for both upper and lower platens.
Characterization
Data concerning the rheological behaviour during mixing were collected directly from the mixer. To evaluate the dispersion of the nanoclay in the polymer matrix, X-ray diffraction analysis was carried out using a Philips PW1710 X-ray diffractometer. The generator power was 40 kV and 20 mA. The instrument uses radiation from a copper target tube (CuKa radiation, l = 1.54250 Å) with the 2q scan ranging from 1° to 10°. The scanning speed was 1°/min. To ensure accuracy, the measurement was 34
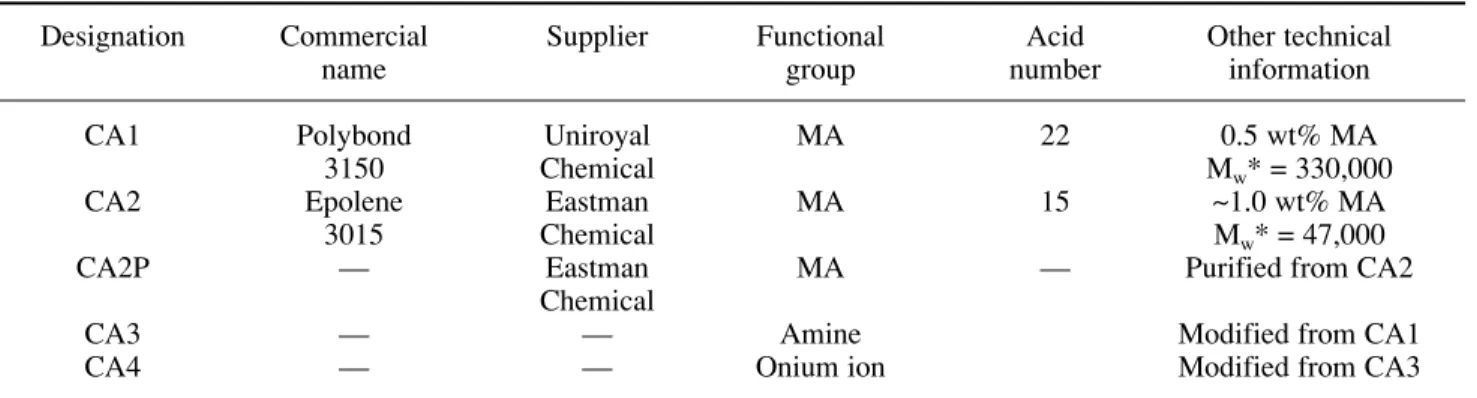
Table I – Characteristics of the coupling agents used
Designation Commercial Supplier Functional Acid Other technical
name group number information
CA1 Polybond Uniroyal MA 22 0.5 wt% MA
3150 Chemical Mw* = 330,000
CA2 Epolene Eastman MA 15 ~1.0 wt% MA
3015 Chemical Mw* = 47,000
CA2P — Eastman MA — Purified from CA2
Chemical
CA3 — — Amine Modified from CA1
CA4 — — Onium ion Modified from CA3
repeated for some samples. A JEOL JSM-840A SEM and a Hitachi H9000 TEM were employed to evaluate the disper-sion of the materials.
A Du Pont 983 DMA instrument was used to character-ize the mechanical behaviour of the material at different temperatures. Specimens with dimensions L/T > 10 (where L is the length and T is the thickness) were prepared by compression moulding. The dynamic properties were stud-ied in a fixed frequency mode at a frequency of 1 Hz and the strain amplitude was 0.2 mm. The samples were heated in the temperature range from –40 to +160 °C at a heating rate of 5 °C/minute.
Flexural properties were evaluated according to ASTM test method D790 using an Instron 5500R. The crosshead speed was set at 1.2 mm/minute. All tests were done at room temperature (23 °C).
RESULTS AND DISCUSSION Rheological Behaviour
In general, it is difficult to compare the torque values for different blends because the torque is strongly determined by the total volume of material in the mixing chamber. Since it is difficult to control the volume of the system, the amount of sample introduced into the chamber was con-trolled by weight. Since each ingredient has a different den-sity, the total volume differed for the different blends, even though the total weight was constant. It is therefore more meaningful to compare the trend of the torque curve during mixing. In the first five minutes of mixing, the system is not stable because it takes some time to introduce the ingre-dients one by one into the chamber and also to heat and melt them. Thus, it is more reasonable to look at the change of the torque in the later stage after all the ingredients have been added, melted and well mixed.
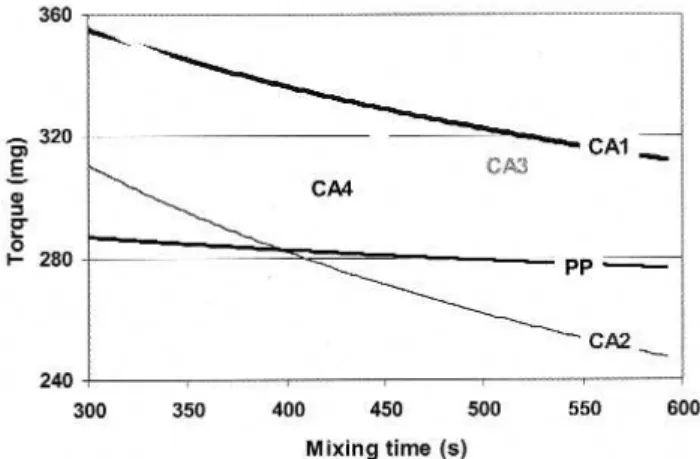
Figure 1 shows the torque curves of the PP and its blends with different coupling agents. Some obvious dif-ferences can be seen. Since the temperature of the chamber was very stable in all the experiments, these changes are likely due to changes in the material itself. The torque curve of PP became relatively stable after 5 minutes of mix-ing and showed only a slight downward trend with time
indicating little change in the viscosity of the sample. A similar trend was observed for the blend containing cou-pling agent CA4. However, the other coucou-pling agents all resulted in a more pronounced downward trend. The gener-al order of the effect of the coupling agent is CA2 ª CA3 > CA1 > CA4 ª none. The lowering of viscosity with time is most likely related to a reduction in molecular weight caused by oxidation and/or degradation of the matrix. The grafting process of MA into PP is based on a free radical reaction with an excess amount of reactants; it is impossible to remove all the residuals and radicals from the material. It is well known that MA-g-PP is in general not highly ther-mally stable. Of the four coupling agents, CA2 and CA3 had the greatest detrimental effect. In the case of CA2, solvent extraction showed it to contain approximately 0.2 wt% of soluble impurities, whereas for CA1, the amount was found to be negligible; this could explain the larger effect of the CA2. The higher content of MA groups in CA2 could also be a factor. The CA3 was prepared by the reaction between CA1 and Jeffamine, so the low stability observed for CA3 could be related to residual amine groups. CA4 was obtained by soaking CA3 in an acid environment at a high temperature which might be expected to remove amine residues and other impurities. This may explain the improved stability for CA4 compared to the CA1 from which it originated. Figure 2 shows the torque curves for the composites prepared with Cloisite 15A in addition to the coupling agents. The order of the effect of the coupling agent on the degradation is now CA2 > CA1 > CA3 > CA4
Fig. 1. Torque time curves of the blends with different coupling agents. Table II – Characteristics of the nanoclay
Commercial Organic Modifier concentration Gallery distance Supplier
name modifier (meq/100 g) (Å)
Cloisite 2M2HT* 125 31.5 Southern Clay
15A Products Inc.
S.G. LEI, M.-T. TON-THAT, S.V. HOA, K.C. COLE and I. PESNEAU
> none. This is generally similar to the trend without nan-oclay except that CA3 has improved somewhat relative to the others.
Dispersion Behaviour
In XRD curves of nanoclays, the position (angle 2q) of the 001 peak is related to the clay gallery spacing and thus the degree of intercalation, whereas the peak intensity is an indicator of the amount of intercalated clusters or by dif-ference, the amount of non-intercalated (exfoliated) mater-ial. Figure 3 shows the XRD curves of the Cloisite 15A alone and the composites containing the coupling agent. Cloisite 15A has three distinct peaks at 2.9°, 4.8° and 7.3° which can be related respectively to the different extents of clay intercalation of high, poor and none. Generally speak-ing, it appears that in the composite samples, all three peaks shift to lower angles compared to those of the starting clay and the intensity of the peaks also decreases to different extents. A shift of the peaks to lower angles proves that intercalation has taken place during mixing. A reduction in the peak intensity indicates that the amount of intercalated clay has decreased or, in other words, the dispersion has been improved by the breakdown of clusters or even
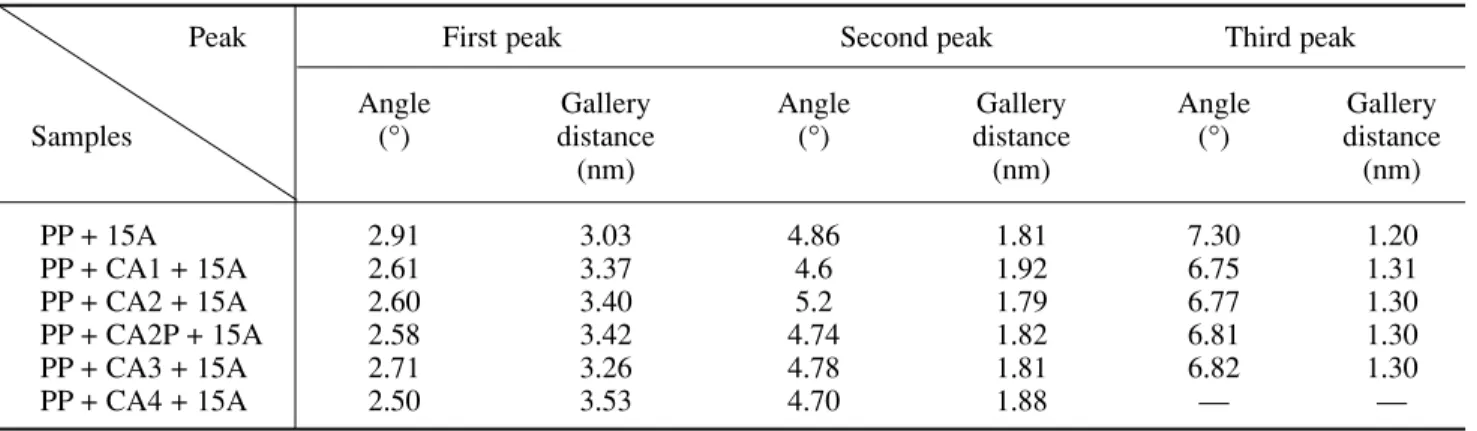
exfo-liation. From these two aspects, it may be concluded that some intercalation and exfoliation have taken place simul-taneously in the composites obtained, although it is far from complete. The gallery distances of the composites are summarized in Table III which provides clearer evidence of the quality of the intercalation of the clays by the coupling agent. The gallery distance of 3.0 nm for the onium-treated Cloisite 15A was slightly extended to 3.3-3.5 nm in the nanocomposites depending on the type of coupling agent. Undoubtedly, the coupling agent plays an important role in forming an acceptable interface between the matrix and the clay surface. It is not unreasonable to conclude that the hydrophilic groups of the coupling agents have created some strong van der Waals bonds and even hydrogen bonds with the hydroxyl groups on the clay surface. This kind of interface is like a bridge to help hydrophobic PP molecules penetrate the hydrophilic galleries of the clay to form inter-calated nanocomposites. The efficiency of each coupling agent is strongly dependent on its chemistry.
Among the composites (Figure 3), the CA2 system had a first peak with a rather high intensity, while the second peak shifted to a higher angle. This indicates that the clay was poorly dispersed in the matrix of this sample. 36
Fig. 2. Torque time curves of the composites.
Fig. 3. Effect of a coupling agent on the X-ray diffraction results.
Table III – Gallery distances of the clays in the composites
Peak First peak Second peak Third peak
Angle Gallery Angle Gallery Angle Gallery
Samples (°) distance (°) distance (°) distance
(nm) (nm) (nm) PP + 15A 2.91 3.03 4.86 1.81 7.30 1.20 PP + CA1 + 15A 2.61 3.37 4.6 1.92 6.75 1.31 PP + CA2 + 15A 2.60 3.40 5.2 1.79 6.77 1.30 PP + CA2P + 15A 2.58 3.42 4.74 1.82 6.81 1.30 PP + CA3 + 15A 2.71 3.26 4.78 1.81 6.82 1.30 PP + CA4 + 15A 2.50 3.53 4.70 1.88 — —
Surprisingly, when the impurities in CA2 are removed, the dispersion is greatly improved since the intensity of all three peaks in the sample CA2P is noticeably weaker. It is also interesting to observe in Figure 3 that in the CA4 composite, the first peak shifted farthest to the left with a corresponding gallery distance of 3.53 nm showing that CA4 results in a slightly better intercalation of the clay. In addition, the inten-sities of the second (poorly intercalated) and third (non-intercalated) peaks in this sample are almost negligible sug-gesting that the amine cation in the CA4 may actively open up the non-intercalated clay galleries in the molten state. It can be understood that this could occur through the reaction of the amine cations of the CA4 with anionic sites on the sur-face of the non-intercalated clay via an ion exchange reac-tion. CA1 and CA3 provided more or less the same level of dispersion, although CA1 led to a slightly better intercalation as the peak shifted slightly to the left, while CA3 led to bet-ter exfoliation as the peak intensity decreased. However, one may wonder if the oxidation/degradation of some of the for-mulation discussed earlier may contribute to these differ-ences. Further experiments have been conducted in the same formulations under a much better control of oxidation and degradation at a low temperature of 180 °C and under nitro-gen atmosphere. However, a similar observation has been obtained indicating that the oxidation/degradation of the matrix did not have a significant impact on the dispersion in this case.
SEM observation showed that in all the samples, the clays were dispersed into the PP matrix in the form of large and small aggregates. It is very difficult to estimate the size of the aggregates in order to quantify the efficiency of the coupling agents because the aggregates are non-isometric and randomly dispersed in the matrix. The size of the observed aggregates is therefore strongly dependent on the orientation of the particles to the observing plane. Figure 4 is an example of a SEM micrograph of the nanocomposite con-taining CA4. TEM studies of the nanocomposite confirm the presence of aggregates with different sizes. Figure 5 is a TEM picture of the nanocomposite containing CA1 which represents all the samples. As clearly seen in this figure, the clay platelets are bent and the outer layers are peeling off from the aggregates. From the TEM study, the gallery dis-tance of the clay aggregates was found to be between 2.8 and 3.5 nm which is in good agreement with the X-ray results.
Dynamic Mechanical Properties
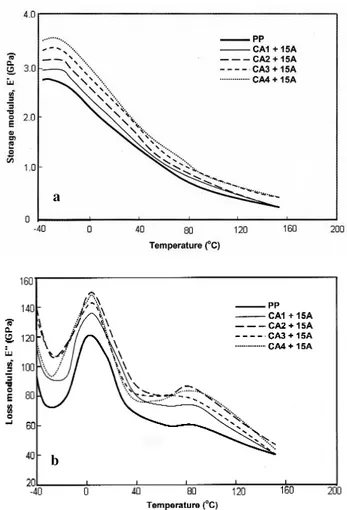
Figures 6a and 6b show the temperature dependence of the storage modulus E¢ and the loss modulus E¢¢ of systems with different coupling agents. All the nanocomposite samples had a higher storage modulus E¢ than pure PP over the entire range of temperature. Among them, the CA4 sample had the highest E¢ of almost 40% higher than pure PP at room tem-perature. The CA3 sample also showed good performance. The CA1 and CA2 samples (both maleic anhydride grafted PP) gave results intermediate between PP and CA4. The
sig-nificant improvement of the modulus for CA3 and CA4 could be due to a better interface and dispersion in these samples as discussed earlier.
Two obvious changes occur in these systems, a sharp peak in E¢¢ around 5 °C and a broader weaker peak above 80 °C (Figure 6b). The first peak is related to the glass transition of the amorphous phase in the PP matrix, while the second is attributed to a softening of the matrix. From Figure 6b, the glass transition (Tg) and the softening point (Ts) of the nanocomposites were determined as the peak temperatures of the E¢¢ curves and are listed in Table IV. There was no sig-nificant difference in the Tgof the different samples, but the Ts showed some variation as being somewhat higher for CA3 and CA4. Again, this is probably related to their better quality of dispersion and interface. On the other hand, the oxidation/degradation as mentioned earlier may have some
Fig. 4. SEM observation of the CA4 nanocomposite.
S.G. LEI, M.-T. TON-THAT, S.V. HOA, K.C. COLE and I. PESNEAU
impact on these properties. Experiments have been per-formed for the blend of PP and the coupling agent in the absence of nanoclays. A similar observation has been obtained indicating that the latter case seems not to play a significant role.
Flexural Properties
Figure 7 shows the flexural strength and modulus of nanocomposites based on 15A with five types of coupling
agents. As shown in this figure, flexural modulus increased significantly with the presence of a coupling agent, but the level of improvement depends on the type of coupling agent. No significant improvement of strength, except with PP/CA4/15A, was observed. The differences of the com-patibility efficiencies of the coupling agents have been dis-cussed earlier which are likely related to their chemistry, molecular weight and grafting level. The PP/CA4/15A nanocomposite exhibits the best flexural properties which can be due to the better chemical interaction with the clay as described in Figure 8. As a result, the quality of disper-sion (Figure 3) and interface must be improved which is related to the increase of flexural modulus and flexural strength, respectively. The improvement of flexural modu-lus is in good agreement with DMA results (Figure 6).
CONCLUSIONS
From this preliminary study, several conclusions can be drawn. In contrast with conventional composites, the role of coupling agents in PP nanocomposites is not only to improve the interface but also to improve the clay disper-38
Fig. 6. DMA curves for the nanocomposites: a) storage modulus and b) loss modulus.
a
b
Fig. 7. Effect of coupling agents on flexural properties.
Fig. 8. The chemical interaction of coupling agent CA4 with the clay. Table IV – Effect of coupling agent on Tgand Ts
Sample Tg(°C) Ts(°C) PP 6.2 86.6 PP + 15A 5.6 85.3 PP + CA1 + 15A 5.2 84.4 PP + CA2 + 15A 5.0 84.5 PP + CA3 + 15A 5.1 87.4 PP + CA4 + 15A 5.5 89.0
sion in the PP matrix. However, coupling agent character-istics like the molecular weight and the type and amount of functional groups can significantly affect the compatibility with the matrix and the clay and thus the performance of the nanocomposites. Among the five types of coupling agents used in this study, amine and amine cation terminat-ed coupling agents lterminat-ed to a better dispersion and better per-formance than maleic anhydride terminated coupling agents. The mechanism of the interaction between these coupling agents and the clays is not clear at this point; fur-ther experiments have been designed in order to obtain a better understanding of these effects.
ACKNOWLEDGEMENTS
The authors would like to thank Manon Plourde and Chantal Coulombe (NRC-IMI) and Monique Riendeau (McGill University) for their help in the characterization work.The authors also would like to thank NSERC for financial support (Grant N00784).
REFERENCES
1. M. Alexandre and P. Dubois, “Polymer – Layered Silicate Nanocomposites: Preparation, Properties and Uses of a New Class of Materials”, Materials Science and Engineering,
2000, vol. 28, p. 1.
2. H.R. Dennis, D.L. Hunter, J.W. Cho, D.R. Paul, D. Cheng, S. Kim and J.L. White, “Guidelines for the Production of Polypropylene Nanocomposites”, Proc. SPE ANTEC, May
2000, Orlando, Florida, U.S.A.
3. M. Kawasumi, N. Hasegawa, M. Kato, A. Usuki and A. Okada, “Preparation and Mechanical Properties of Polypropylene-Clay Hybrids”, Macromolecules, 1997, vol.
30, p. 6333.
4. N. Hasegawa, M. Kawasumi, M. Kato, A. Usuki and A. Okada, “Preparation and Mechanical Properties of Polypropylene-Clay Hybrids Using a Maleic Anhydride-Modified Polypropylene Oligomer”, Journal of Applied Polymer Science, 1998, vol. 67, p. 87.
5. N. Hasegawa, H. Okamoto, M. Kato and A. Usuki, “Preparation and Mechanical Properties of Polypropylene-Clay Hybrids Based on Modified Polypropylene and Organophilic Clay”, Journal of Applied Polymer Science,
2000, vol. 78, p. 1918.
6. J.G. Doh and I. Cho, “Synthesis and Properties of Polystyrene-Organoammonium Montmorillonite Hybrid”,
Polymer Bulletin, 1998, vol. 41, p. 511.
7. J.S. Ma, Z.N. Qi and Y.L. Hu, “Synthesis and Characterization of Polypropylene/ Clay Nanocomposites”,
Journal of Applied Polymer Science, 2001, vol. 82, p. 3611.
8. E. Manias, A. Touny, L. Wu, K. Strawkecher, B. Lu and T. C. Chung, “Polypropylene/ Montmorillonite Nanocomposites. Review of the Synthetic Routes and Materials Properties”,
Chemistry of Materials, 2001, vol. 13, p. 3516.
9. M. Kato, A. Usuki and A. Okada, “Synthesis of Polypropylene Oligomer – Clay Intercalation Compounds”,
Journal of Applied Polymer Science, 1997, vol. 66, p. 1781.
10. A. Oya, Y. Kurokawa and H. Yasuda, “Factors Controlling Mechanical Properties of Clay Mineral / Polypropylene Nanocomposites”, Journal of Materials Science, 2000, vol.
35, p. 1045.
11. R. Peter, N. Hansjorg, K. Stefan, B. Rainer, T. Ralf and M. Rolf, “Poly(propylene)/ Organoclay Nanocomposite Formation: Influence of Compatibilizer Functionality and Organoclay Modification”, Macromolecular Materials and Engineering, 2000, vol. 275, p. 8.
12. M.-T. Ton-That, F. Perrin, P. Lacand, K.C. Cole, J. Denault and G. Enright, “Preparation and Performance of Nanocomposites Based on Polypropylene and Layered Nanoclays”, Polymer Nanocomposites 2001, November
14-16, 2001, Montreal, Canada.
13. A. Usuki, M. Kato, A. Okada and T. Kurauchi,“Synthesis of Polypropylene-Clay Hybrid”, Journal of Applied Polymer Science, 1997, vol. 63, p. 137.
14. Nanocor Inc. website: www.nanocor.com
15. Southern Clay Products Inc. website: www.nanoclay.com 16. I. Pesneau, “Reactive Extrusion: Materials Customization”,
Polymer Technology Symposia, April 9, 2003, Boucherville,
Quebec, NRC Industrial Materials Institute and Quebec Materials Network.