Publisher’s version / Version de l'éditeur:
Cement and Concrete Research, 5, November 6, pp. 617-630, 1975-11-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1016/0008-8846(75)90062-9
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Strength development in magnesium oxychloride and other cements
Beaudoin, J. J.; Ramachandran, V. S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=cdbca9f9-b5e6-4767-b174-eadda7005d7e https://publications-cnrc.canada.ca/fra/voir/objet/?id=cdbca9f9-b5e6-4767-b174-eadda7005d7e
MENT AND CONCRETE RESEARCH. Vol
.
5 , p p . 617-630, 1975. Pergamon Press, ~ n c , i n t e d i n t h e United S t a t e s .STRENGTH DEVELOPMENT IN MAGNESIUM OXYCHLORIDE AND OTHER CEMENTS
J.J. Beaudoin and V.S. Ramachandran, Division of Building Research, National Research Council of Canada, Ottawa, Canada.
(Communicated by D. M. Roy)
(Received Aug. 18, 1975; i n f i n a l form Sept. 1 2 , 1975)
Measurements of compressive strength, modulus of elasticity, and
microhardness were made.on magnesium oxychloride cement systems having a wide range of porosity. Analysis of the plots of log mechanical property versus porosity gave expressions interrelating mechanical properties which were independent of porosity. Comparison of these results with those of the portland cement system show that magnesium oxychloride cement paste has a higher value of modulus of elasticity and microhardness over the porosity range studied. Microhardness of magnesium oxychloride cement paste is also greater than that for all gypsum plaster preparations in the porosity range studied. Mechanical behaviour of magnesium oxychloride cement compacts is improved when heated in water at 85 OC. It appears that this heat treatment results in formation of a porous body consisting mainly of Mg(OH)2. Among the systems magnesium oxychloride, portland cement, gypsum and magnesium hydroxide, it appears that magnesium hydroxide forms the strongest
On mesure la rEsistance B la compression, le module d'Elasticit6 et la
microduretEdeciments d'oxychlorure de magngsium de porositE trSs vari6e. Une analyse des proprigt6s mkaniques logarithmiques et des diagrammes de porositg produit des expressions qui relient entre elles des proprigt6s mBcaniques indgpendantes de la porosit6. Une compa- raison de ces rgsultats et de ceux du ciment portland montre que la pste de ciment d'oxychlorure de magn6sium a un module d1Glasticit6 et une microduretg plus 6lev6s pour les variations de poyosit6 Gtudiges. La microduretg de la p$te de ciment d'oxychlorure de magngsium est 6galement plus 6levge que celle de toutes les pr6parations de plstre de gypse pour les variations de porositg 6tudiBes. Lorsque des comprim6s de ciment d'oxychlorure de magnEsium sont chauffgs dans de l'eau 2 8 5 ' ~ ,leur comportement m6canique est am6lior6. I1 semble que ce traitement thermique produise un corps poreux comportant surtout du Mg(OH)2. I1 semble que l'hydroxyde de magngsium produise des corps plus rgsistants que l'oxychlorure de magngsium, le ciment portland ou
divided magnksium oxide with an aqueous solution of magnesium chloride.- Magnesium oxychloride cement possesses many properties superior to those of portland cement. It has high fire resistance, low thermal conductivity, high resistance to abrasion, and high compressive and flexural strengths. Many organic and inorganic aggregates, which may not be suitable for making portland cement concrete, can be used in combination with the oxychloride cement.
Depending on the conditions of preparation, four types of oxychloride complexes are known to form in the MgO - MgC12
-
H20 system. They are 5Mg(OH)2.MgC12.8H20 (5-form)*, 3Mg(OH)2aMgC12.8H20 (3-form)*, 2Mg(OH) 2.MgC12-5H20 (2-form)* and 9Mg(OH)2,MgC12-6H20 (9-form)* (2).Continuous exposure of the oxychloride to air results in the formation of magnesium chlorocarbonate of formula Mg(OH)2.MgC12-2MgC03.6H20.
The use of oxychloride cement is not so widespread as it might be because it loses strength on prolonged exposure to water. The stabilization of oxychloride against attack by water is therefore a matter of importance and an understanding of the mechanism of strength development for the oxychloride system is necessary. The use of compacted systems has led to some success in understanding the bonding mechanism in portland cement and gypsum pastes (3, 4, 5, 6). This technique was applied to oxychloride cements to investigate the probable bonding mechanism that occurs and to compare it with the bonding mechanisms of other cementitious systems as portland cement and gypsum pastes.
Experiment a1 Materials
The following materials were used:
1. Magnesium Oxide. The MgO powder was supplied by Basic Chemicals, Cleveland, Ohio. It had the following characteristics: N2 surface area, 20m2/g; active CaO, 1.5%; ignition loss 4%; fraction passing through 200 mesh, 98%. The material satisfied the requirements of A.S.T.M. C-275-61.
2. Magnesium Chloride Solution. Aqueous solution of specific gravity 1.18 was prepared by mixing MgCl2-6H20 in a dry form with distilled water. Measurements
Helium pycnometer was used to measure the solid volume of the cement specimens of known geometry. Porosity was determined from the
apparent volume and the solid volume. This avoids the problem of dissolution that arises when water is used as the displacement medium. Samples were conditioned at 11% relative humidity for each measurement. Application of this technique to other inorganic cement systems is described elsewere (7). Porosity was also determined using methanol displacement employing
Archimedes principle.
...
.,...
'ead as it might be The s t a b i l i z a t i o n :ter of importance jment f o r t h e Isterns has l e d t o )rt land cement and :o oxychloride it occurs and t o .ous systems a s
ic Chemicals,
N2 surface a r e a , sing through 200 mesh,
.
C-275-61. g r a v i t y 1 . 1 8 illed water. d volume of t h e ined from t h e " roblem of d i s s o l u t i o n m. Samples were t . Application o f ribed elsewere ( 7 ) . en~ployingSTRENGTH, MAGNESIUM OXYCHLORIDE, CEMENTS
Modulus of e l a s t i c i t y was measured on d i s c s with a d i a m e t e r o f cm and a t h i c k n e s s o f 1 . 3 mm. This procedure i n v o l v e s measuring t h e e c t i o n o f a specimen when i t i s loaded a t i t s c e n t r e and supported a t e e p o i n t s l o c a t e d on t h e circumference o f a c i r c l e 2 . 5 cm i n d i a m e t e r ( 8 ) . h v a l u e r e p r e s e n t s an average o b t a i n e d f o r t h r e e specimens. The same c s used f o r measuring t h e modulus of e l a s t i c i t y were c o n d i t i o n e d a t 50% e l a t i v e humidity f o r microhardness d e t e r m i n a t i o n . Microhardness was
e a s u r e d w i t h a L e i t z microhardness t e s t i n g machine with a Vickers i n d e n t e r . ach microhardness v a l u e recorded h e r e i n i s an average of t h r e e d i s c s ; f i v e magnesium oxychlori.de p a s t e samples was measured f o r 5 . 1 cm cubes.
D i f f e r e n t i a l thermograms were o b t a i n e d u s i n g t h e 990 Dupont thermal a l y s i s system. Twenty mg of t h e m a t e r i a l were used f o r each r u n . The r a t e
h e a t i n g was 20 "c/min (T = 150 s e c / i n ) and t h e s e n s i t i v i t y was
= 5 m c a l / s e c / i n .
The m i c r o s t r u c t u r e of some of t h e samples was examined u s i n g a Cambridge S t e r e o s c a n Mark 2 A . The examination was done on f r a c t u r e d s u r f a c e s .
X-Ray examinations were c a r r i e d out with a Debye S c h e r r e r Camera. c r o d e n s i t o m e t e r t r a c e s were made from t h e X-ray f i l m s . S u r f a c e a r e a was t a i n e d by means of a numinco-0rr s u r f a c e a r e a - p o r e volume a n a l y s e r with N2
s t h e a d s o r b a t e . Powdered samples were d r i e d f o r 3 hours a t 110 "C p r i o r
o measurements.
C h l o r i d e e s t i m a t i o n was done u s i n g a c h l o r i d e i o n s e l e c t i v e l e c t r o d e a c c o r d i n g t o a method d e s c r i b e d by Berman ( 9 ) .
System I
Cement p a s t e samples were prepared a t MgC12-6H20/Mg0 s o l u t i o n - o l i d r a t i o s of 0.59, 0.64, 0.71, 0.77 and 0.86 by weight. Mixes were c a s t
cube molds and cured a t 50% r e l a t i v e humidity f o r p e r i o d s of up t o t e n n t h s ; t h i s was t o e n s u r e t h a t t h e p a s t e s prepared a t d i f f e r e n t s o l u t i o n
-l i d r a t i o s were a-lmost comp-lete-ly h y d r a t e d .
Discs 3 . 2 cm i n d i a m e t e r , 1 . 3 mrn t h i c k were c u t f o r measurements of microhardness and modulus o f e l a s t i c i t y from 3 . 2 cm c o r e s from cube samples.
The cement p a s t e with a s o l u t i o n - s o l i d r a t i o of 0.59 (hydrated t h s ) was f i n e l y ground and used f o r f a b r i c a t i o n of compacts. Compacts
i n d i a m e t e r , 1 . 3 mrn t h i c k were made a t compaction p r e s s u r e s of Pa t o 1665 MPa, g i v i n g a wide r a n g e of p o r o s i t i e s .
System 11 compacts were immersed i n w a t e r a t 22 "C and were s l o w l y o r 30 min t o 85 O C and maintained t h e r e f o r 5 hours. T h i s t r e a t m e n t
J .
J.
Beaudoin,V. S. Ramachandran
Other Cement Systems
The p r e p a r a t i o n of p o r t l a n d cement p a s t e s and hydrated p l a s t e r systems have been described i n e a r l i e r works ( 3 , 4 , 5 , 6 ) .
Results P o r o s i t y - Mechanical Property Relationships
The logarithms of modulus of e l a s t i c i t y , microhardness and
compressive s t r e n g t h were p l o t t e d a g a i n s t p o r o s i t y ; t h e p l o t s f o r a l l systems i n d i c a t e l i n e a r r e l a t i o n s h i p s (Figs. 1, 2 , 3 ) .
Modulus of e l a s t i c i t y , microhardness and compressive s t r e n g t h f o r a l l magnesium oxychloride p r e p a r a t i o n s s t u d i e d obeyed t h e general r e l a t i o n - s h i p , E , H, S = (Eo, H0. So) eXP [ ( - b E , H , S )p] where E , H , S r e f e r t o modulus of e l a s t i c i t y , microhardness and compressive s t r e n g t h r e s p e c t i v e l y and p i s p o r o s i t y . An a n a l y s i s of l i n e a r r e g r e s s i o n f o r magnesium oxychloride and o t h e r systems is recorded i n Table I . The s l o p e of t h e l i n e r e p r e s e n t i n g
TABLE 1
Regression Analysis of -~odulits of Elasticity, Microhardness and Compressive Strength Data
E H S E H S
Magnesium Oxychloride
Paste (System I) 45.39 0.035 317.0 0.089 238.0 0.047 1.085 1.068 1.020 85.06 98.2'5 98.22 1.48 1.95 Z.150 Magnesium Oxychloride
Compacts (SystemII) 46.56 0.096 130.8 0.132 - - 1.092 1.159 - 97.12 97.08 - 1.94 2.06
-Portland Cement Paste 29.30 0.030 90.0 0.046 170.0 0.048 - - - .
Portland Cement 29.30 0.050 90.0 0.046 - - - - -Compacts of Bottle - - -Hydrated Plaster 63.3 0.114 108.9 0.106 - -52.4 0.030 121.3 0.069 - - - - -- 800 - - - - 1.067 - - 98.0 - - 2.26
-* Units for E, H, S are MPa x MPa x 10-I and MPa Standard error of estimate
Correlation coefficient Tf 90% confidence limits
log modulus of e l a s t i c i t y versus p o r o s i t y f o r t h e compacted System I 1 i s
about t h r e e times g r e a t e r than t h e slope of t h e corresponding l i n e f o r t h e i n s i t u hydrated p a s t e of System I (Fig. 1 ) . The d a t a f o r System I11 compacts (heat t r e a t e d i n water a t 85 O C ) were included i n t h e d a t a
population f o r System I . A summary of t h e mechanical p r o p e r t i e s f o r System 111 compacts i s recorded i n Table 11. Heat treatment of System I 1 compacts i n water r e s u l t e d i n a p o r o s i t y i n c r e a s e of 6 3 t o 189%, microhardness i n c r e a s e of 18 t o 64%, and modulus of e l a s t i c i t y i n c r e a s e of 64%. Length
STRENGTH, MAGNESIUM OXYCHLORIDE
,
CEMENTSTABLE IT
Mechanical P r o p e r t i c s o f System 111 Compacts Treated a t 85 'C i n H20
n i t i a l F i n a l
ardness and
l o t s for a l l systems
essive strength for e general
relation-,
s
refer t o modulus spectively and nesium oxychloride t h e line representingI
ressive Strength Data,acted System 11 i s ;pending line for the
for System 111
?d in the data
properties for System
of System I 1 compacts
4, microhardness ease of 64%. Length
change due t o h e a t treatment was between 0.66 and 0.90%. Surface a r e a increased from 4.95 t o 8.99 m2/gm. The heat t r e a t e d compacts o f system 111 have a narrow p o r o s i t y range (21.1 t o 29.1%)
.
Extrapolated d a t a f o r systems I and 111 may n o t be c o l l i n e a r with each o t h e r (Fig. 1) over t h e e n t i r e p o r o s i t y range. The l i n e r e p r e s e n t i n g t h e e x t r a p o l a t e d modulus o f e l a s t i c i t y f o r system I1 compacts i n t e r s e c t s t h a t of system I a t approximately zero p o r o s i t y . The modulus o f e l a s t i c i t y f o r i n s i t u hydrated magnesium oxychloride p a s t e (system I ) i s approxi-mately 30 t o 50% g r e a t e r than t h a t f o r hydrated portland cement p a s t e over t h e p o r o s i t y range studied. I t should be noted t h a t values o f modulus of a s t i c i t y f o r both mpacted portland 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 0 4 8 1 2 16 2 0 24 28 POROSITY, % FIG. 1 Modulus o f e l a s t i c i t y v s p o r o s i t y f o r magnesium oxychloride and o t h e r inorganic cement systemso SYSTEM I
SYSTEM II
A SYSTEM Ill
-
PORTLAND CEMENT PASTE.
... COMPACTS OF BOTTLE HYDRATED PLASTER (REF 3)Vol. 5, Na. 6
J. J.
Beaudoin,V.
S. Ramachandrancement paste and in situ hydrated portland cement paste lie on the same line (8). The line for portland cement paste intersects the line for compacted magnesium oxychloride (system 11) at about 7% porosity. The line for modulus of elasticity of compacts of bottle hydrated plaster is nearly collinear with the line for compacted magnesium oxychloride (system 11) and intersects the portland cement paste line at approximately 9%porosity.
The line for in situ hydrated plaster compacts (compacted first, then hydrated) lies slightly above the line for in situ hydrated magnesium oxychloride paste (system I).
Microhardness data for the magnesium oxychloride systems studied show the same trend as modulus of elasticity data (Fig. 2). Microhardness values for compacted magnesium oxychloride (system 11) gradually approach those for in situ hydrated paste (system I) as porosity decreases. The slope of the line for log microhardness versus porosity for system I1 is nearly 50% greater than that for system I. The extrapolated lines for systems I and I1 do not intersect. The line for portland cement paste intersects the line for compacted magnesium oxychloride (system 11) at approximately 5% porosity.
The line representing log microhardness versus
-porosity for compacts of bottle hydrated plaster intersects the extrapo-lated line for system I1 compacts at about 7%
porosity. Modulus of elasticity at a given porosity is greater for hydrated plaster compacts than hydrated magnesium oxychloride paste
(system I); the converse is true for microhardness.
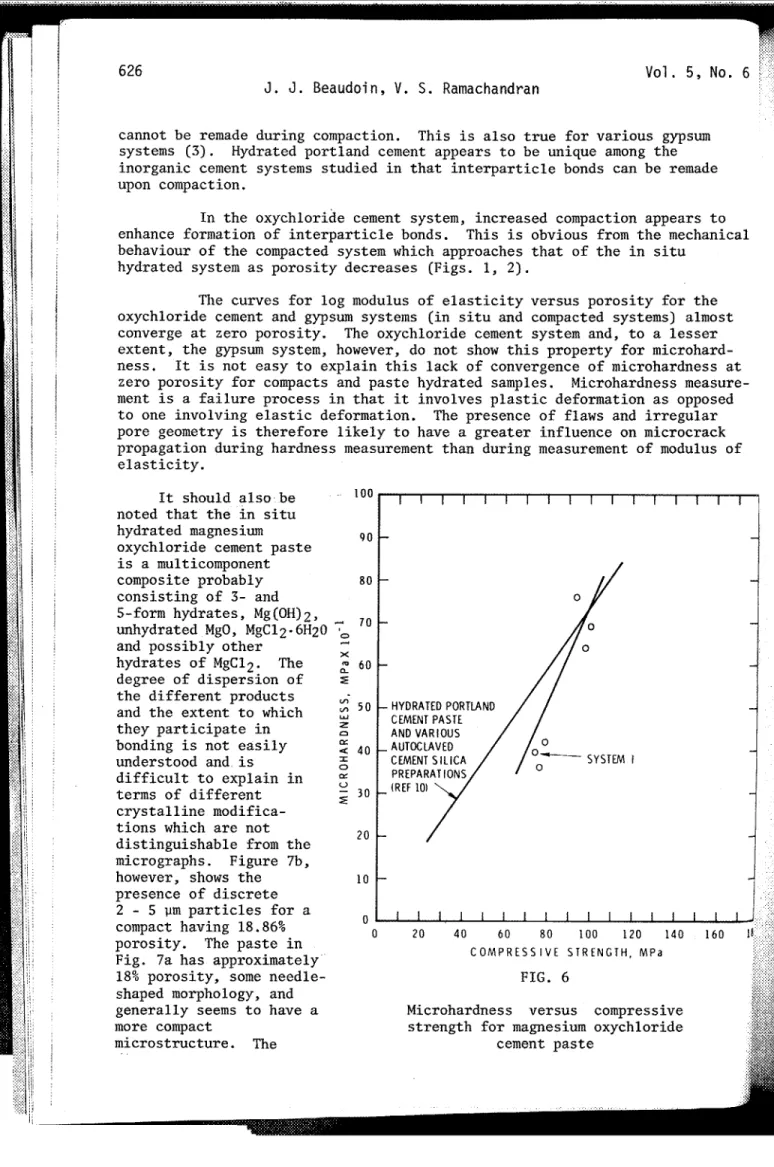
The plot of log compressive strength versus porosity for in
situ hydrated magnesium '
- oxychloride cement paste
A SYSTEMIII (system I) results in a
M g (OH)2 COMPACTS
straight line (Fig. 3).
-
PORTLAND CEMENT PASTE- ... COMPACTS OF BOTTLE HYDRATED PLASTER (REF 3) - Values of compressive
--
IN SITU HYDRATED PLASTER COMPACTS (REF 31 strength for oxychloridepaste are approximately
1 I I I I I I f I I I f I I I I I I I I I I 30% greater than for
;
4 8 1 2 1 6 2 0 l 4 2 8 I 2 ) * hydrated portland cementP O R O S I T Y , % paste of equal porosity.
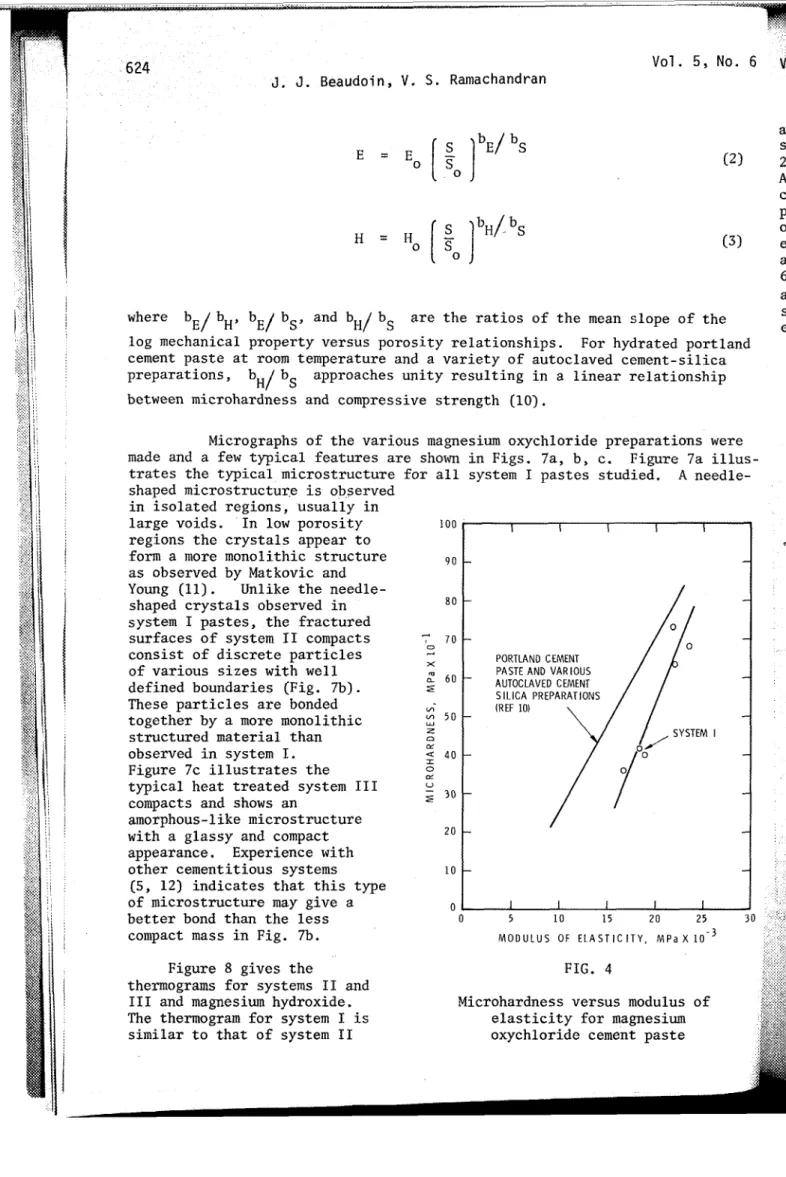
FIG. 2 Figure 4 is the plot
Microhardness versus porosity for of microhardness versus magnesium oxychloride and other modulus of elasticity for
Vol. 5, No.
6
J . J. Beaudoin,
V.
S. Ramachandranwhere bE/ bH, bE/ bS, and bH/ bS are the ratios of the mean slope of the log mechanical property versus porosity relationships. For hydrated portland cement paste at room temperature and a variety of autoclaved cement-silica preparations, bH/bS approaches unity resulting in a linear relationship between microhardness and compressive strength (10).
Micrographs of the various magnesium oxychloride preparations were made and a few typical features are shown in Figs. 7a, b, c. Figure 7a illus- trates the typical microstructure for all system I pastes studied. A needle-shaped microstructur.e is observed
in isolated regions, usually in large voids. In low porosity regions the crystals appear to
form a more monolithic structure 9 0
-as observed by Matkovic and
Young (11). Unlike the needle- -shaped crystals observed in 80
system I pastes, the fractured
surfaces of system I1 compacts ': 7 0
-0
consist of discrete particles of various sizes with well defined boundaries (Fig. 7b). These particles are bonded together by a more monolithic structured material than observed in system I. Figure 7c illustrates the typical heat treated system 111 compacts and shows an
amorphous-like microstructure with a glassy and compact appearance. Experience with
other cementitious systems 10
-(5, 12) indicates that this type of microstructure may give a better bond than the less
compact mass in Fig. 7b. M O O U L U S O F E L A S T I C I T Y , MPa x
Figure 8 gives the
thermograms for systems I1 and
I11 and magnesium hydroxide. Microhardness versus modulus of
The thermogram for system I is elasticity for magnesium similar to that of system I1 oxychloride cement paste
625
(2)
(3)
3 mean s l o p e o f t h e
For hydrated portland Laved cement-silica Linear r e l a t i o n s h i p ide p r e p a r a t i o n s were b, c . Figure 7a i l l u s - ?S studied. A needle-E L A S T I C I T Y . M P a X [ G . 4 rersus modulus of f o r magnesium : cement p a s t e
STRENGTH, MAGNESIUM OXYCHLORIDE, CEMENTS
and i s n6t presented. There i s an i n d i c a t i o n o f an endothermic e f f e z t i n system I1 a t 120 O C and medium s i z e d endothermic peaks a t about 165 C and
225 O C . Large endothermal e f f e c t s a r e p r e s e n t a t about 365 O C and 420 O C .
An a d d i t i o n a l endothermal e f f e c t i s i n d i c a t e d a t about 460 O C . This i s c l e a r e r i n t h e DTA t r a c e . The peaks may be i n t e r p r e t e d a s r e p r e s e n t i n g t h e presence of magnesium hydroxide, t h e 3-form o r 5-form o r both forms o f
oxychloride. According t o Cole and Demediuk (13) t h e 5-form gives a moderate endotherlilal e f f e c t i n t h e range o f 450 t o 500 OC. The endothermic e f f e c t a t about 460 O C is u s u a l l y a t t r i b u t e d t o t h e presence o f t h e 5-form o r MgC12,
6H20 o r both. The p a s t e s were a l l prepared a t a high Mg(OH)2/MgC12 r a t i o and hence t h e 5-form i s expected t o be p r e s e n t . The system I11 compacts show only a broad v a l l e y i n t h e range of about 75 'C t o 250 O C and a l a r g e endothermic peak a t about 425 O C .
Heat t r e a t e d system
I11 compacts had X-ray d i f f r a c t i o n p a t t e r n s i n d i c a t i n g an increased amount of Mg(OH)2 a s
20 - - compared t o u n t r e a t e d
system I1 compacts (Fig.
2).
A s t r o n g peak a t d = 7.5 A rn p r e s e n t i n system I1 compacts i s absent i n X m oxychloride comple 5 -C O M P R E S S I V E S T R E N G T H , M P aModulus of e l a s t i c i t y versus compressive s t r e n g t h f o r magnesium oxychloride
J. J. Beaudoin, V. S. Ramachandran
cannot be remade during compaction. This is also true for various gypsum systems (3). Hydrated portland cement appears to be unique among the inorganic cement systems studied in that interparticle bonds can be remade upon compaction.
In the oxychloride cement system, increased compaction appears to enhance formation of interparticle bonds. This is obvious from the mechanical behaviour of the compacted system which approaches that of the in situ
hydrated system as porosity decreases (Figs. 1, 2).
The curves for log modulus of elasticity versus porosity for the oxychloride cement and gypsum systems (in situ and compacted systems) almost converge at zero porosity. The oxychloride cement system and, to a lesser extent, the gypsum system, however, do not show this property for microhard- ness. It is not easy to explain this lack of convergence of microhardness at
zero porosity for compacts and paste hydrated samples. Microhardness measure- ment is a failure process in that it involves plastic deformation as opposed to one involving elastic deformation. The presence of flaws and irregular pore geometry is therefore likely to have a greater influence on microcrack propagation during hardness measurement than during measurement of modulus of elasticity.
It should also be noted that the in situ hydrated magnesium oxychloride cement paste is a multicomponent composite probably consisting of 3- and 5-form hydrates, Mg(OH)2, unhydrated MgO, MgC12.6H20 and possibly other
hydrates of MgC12. The degree of dispersion of the different products and the extent to which they participate in bonding is not easily
understood and is CEMENT SILICA
difficult to explain in terms of different crystalline modifica- tions which are not distinguishable from the micrographs. Figure 7b, however, shows the presence of discrete 2
-
5 pm particles for a compact having 18.86% porosity. The paste inC O M P R E S S I V E S T R E N G T H , M P a
Fig. 7a has approximately
18% porosity, some needle- FIG. 6
shaped morphology, and
generally seems to have a Microhardness versus compressive
more compact strength for magnesium oxychloride
nds can be remade
paction appears to s from the mechanical f the in situ
porosity for the ted systems) almost
and, to a lesser erty for microhard-
of microhardness at icrohardness measure-ormation as opposed aws and irregular ence on microcrack rement of modulus of
STRENGTH, MAGNESIUM OXYCHLORIDE, CEMENTS
FIG. 7a FIG. 7b
Magnesium oxychloride Magnesium oxychloride cement paste - system I compact
-
system I1FIG. 7c
Magnesium oxychloride compact
-
system 111latter may account for the increased strength of system I at a given porosity. Microhardness in system 111 is higher than that in system 11; the micro- structure of system I11 presents a more compact matrix.
It was thought that treatment in water would enhance the strength development in system I11 due to a recrystallization effect as in gypsum (14).
425 "C was due only to Mg(OH)2 (Fig. 8). A comparison of this curve with pure Mg(OH)2 under similar conditions confirmed this. X-ray diffraction results of system I11 show that lines due to oxychloride complex were absent and that peaks due to the presence of Mg OH)2 at d = 1.80, 2.37 and 4.75
k
all increased in intensity. Chlorideestimation of system I1 and system 111 were also compared and it was seen that system 111
contained only about 10% of the .-.
1
,,.,,,
,,,,,
total chloride that was containedin system 11. The true density
-of system I11 was found to be2.31 g/cc which is closer to \ I
the density of pure Mg(OH)2. As shown previously, this
material contained mainly T E M P E R A T U R E . "C
Mg(OH)2; it would appear that the strength of system I11 was
main1 y due to the Mg (OH) 2 FIG. 8
component which was left after
the oxychloride complex became Di-fferential scanning calorimetry traces unstable in hot water. To
verify that pure Mg(OH)2 had similar strengths, some compacts
of different porosities were made from it. Their microhardness was found to be slightly above the line representing system I11 (Fig. 2). Although
systems I and 111 are of different composition, they are collinear with respect to the plot of log mechanical property versus porosity. This coincidence in the behaviour of the two systems appears to be fortuitous.
Conclusions
For a particular porosity, in situ hydrated oxychloride cement has a mechanical behaviour superior to that of hydrated portland cement paste. Interparticle bonds are not remade during compaction of magnesium oxychloride cement which seems to have a bonding mechanism similar to that of the gypsum system. The mechanical behaviour of magnesium oxychloride cement compacts improves when treated in water at 85 OC and the surface area increases from 4.95 to 8.99 m2/g. Among the gypsum, magnesium oxychloride, portland cement, and magnesium hydroxide systems, it appears that magnesium hydroxide forms the strongest bonds in the porosity range studied.
A porosity as low-as 21% is obtained in a sample of the magnesium hydroxide system by preparing a compact of powdered magnesium oxychloride paste at 550 MPa and heating in water at 85 "C. A compact with a porosity of 32% resulted when a pressure of 550 MPa was applied to a powder of Mg(OH)2. This would indicate that indirect methods may be successfully used if a low porqsity system cannot be obtained by direct compaction. It appears that the system containing essentially Mg(OH)2 (system III), formed by leaching of
Vol.
5,No.
6J . J .
Beaudoin,
V.
S.
Ramachandran
The constants have physical significance in that EO, So, and Ho are modulus of elasticity, compressive strength and microhardness at zero porosity
respectively; b ~ /b ~ ,b ~ /b ~ ' b ~ /b~ are ratios of the slopes of the log mechanical property versus porosity function. Mechanical properties expressed
in terms of porosity provide a good basis for comparison of cementitious
Acknowledgment
The authors wish to thank P. J. Sereda for helpful discussion and
J.J. Wood who performed most of the experiments. The assistance of Messrs. G.M. Polomark, G.A. O'Doherty, P.J. Lefebvre, E.G. Quinn and R.E. Myers is also appreciated. This paper is a contribution from the Division of Building Research, National Research Council of Canada, and is published with the approval of the Director of the Division.
References 1. S. S0rel- Compt. Rend.
-
65, 102 (1867).2. W.F. Cole 6 T. Demediuk. Aust. J. Chem.
8,
234 (1955). 3. I. Soroka E P.J. Sereda. J. Am. Ceram. Soc. 51, 337 (1968).4. I. Soroka E P.J. Sere&. proc. Fifth Intl.
sip.
Chem. Cem. Tokyo, Part 111, Val, 111, 67 (1968).5. A. Traetteberg E V.S. Ramachandran. J. Appl. Chem. Biotechnol.
24,
157 Paper I1-
3, (1974).
7. R.F. Feldman. J. Cem. Tech. 3, 5 (1972).
8. P.J. Sereda, R.F. Feldman 6 E.G. Swenson. Highway Res. Board, Washington. Special Report 90, 58 (1966)