Université de Sherbrooke
The hnRNP Al and Alb proteins
regulate alternative splicing in vivo
Par
Xiaoming Yang
Département de microbiologie
Mémoire présenté à la Faculté de Médecine en vue de l'obtention du grade de
Maître ès Sciences (M.Sc.) Avril 1994
l+I
National Libraryof Canada Bibliothèque nationale du Canada Acquisitions and Direction des acquisitions et Bibliographie SeNices Branch des seNices bibliographiques
395 Wellington Street Ottawa, Ontario K1AON4 395, rue Wellington Ottawa (Ontario) K1AON4
The author has granted an
irrevocable non-exclusive licence
allowing the National Library of
Canada to reproduce, loan,
distribute or sell copies of
his/her thesis by any means and
in any form or format, making
this thesis available to interested
persans.
The author retains ownership of
the copyright in his/her thesis.
Neither the thesis nor substantial
extracts from it may be printed or
otherwise reproduced without
his/her permission.
Your file VO/Te référence Our file Noire référence
L'auteur a accordé une licence
irrévocable et non exclusive
permettant
à
la Bibliothèque
nationale
du
Canada
de
reproduire, prêter, distribuer ou
vendre des copies de sa thèse
de quelque manière et sous
quelque forme que ce soit pour
mettre des exemplaires de cette
thèse
à
la disposition des
personnes intéressées.
L'auteur conserve la propriété du
droit d'auteur qui protège sa
thèse. Ni la thèse ni des extraits
substantiels de celle-ci ne
doivent être imprimés ou
autrement reproduits sans son
autorisation.
RESUMÉ
Titre: Les protéines hnRNP Al et Alb régissent l 'épis sage alternatif in vivo.
De récentes études in vitro suggèrent que la protéine hnRNP Al module l 'épissage alternatif du pré-mRNA du gène ElA de !'adénovirus. Plus précisément, Al favoriserait la sélection des si tes d 'épissage 5 ' distaux. Nous avons utilisé une lignée cellulaire érythroleucémique de souris (CB3C7), déficiente dans l'expression de hnRNP Al afin d'étudier l'action des protéines Al et Alb sur l'épissage alternatif in vivo.
Dans la lignée cellulaire CB3C7, le pré-mRNA de E lA est épissé principalement à partir des sites d'épissage 5' proximaux 13S et 12S. D'autre part, le site d'épissage 5' distal 9S, est préférentiellement utilisé dans les lignées DP27-17 et DP28-9 qui expriment Al. L'expression transitoire d'un cDNA de Al ou Alb dans les cellules CB3C7 rétablit un ni veau normal de la protéine et entraine l'utilisation préférentielle du site d'épissage 5' distal 9S. La synthèse de la protéine Al est essentielle puisqu'aucun
effet sur la sélection des sites d'épissage n'est observé lorsqu'un cDNA de Al contenant une mutation causant un changement de cadre de lecture dans l'exon 2 est utilisé. Cet effet stimulateur de hnRNP Al sur l'utilisation des sites d'épissage 5' distaux fut aussi observé lors de l'expression d'un pré-mRNA de la région précoce du virus SV40. Ces résultats suggèrent donc que des variations dans les niveaux de la protéine hnRNP Al peuvent influencer la sélection des sites d'épissage 5' in vivo.
ABSTRACT
Recent in vitro results suggest that the heterogeneous nuclear ribonucleoparticles (hnRNP) Al protein modulates alternative splicing by favoring distal 5' splice site selection on the adenovirus ElA pre-mRNA. We used a mouse erythroleukemic cell line (CB3C7) deficient in hnRNP Al expression to investigate whether variations in the hnRNP Al and Alb proteins could affect alternative splicing in vivo.
In the CB3C7 cell line, the ElA pre-mRNA was spliced principally by the use of the proximal 13S and 12S 5' splice sites. In contrast, the distal 9S 5' splice site was preferentially used in Al-expressing DP2 8-9 and DP2 7-17 cells. Transiently expressing the Al or Alb cDNA in CB3C7 cells restored normal levels of Al proteins and shifted 5' splice site selection toward the more distal 9S donor site. The hnRNP Al protein synthesis was required for this ef fect since expressing an Al cDNA harboring a frameshift mutation in exon 2 failed to displace 5' splice site selection. The capacity of the hnRNP Al protein to activate distal 5' splice sites was also demonstrated on the early SV40 pre-mRNA. These results suggest that variations in hnRNP Al protein level can influence 5' splice site selection in vivo.
TABLE OF CONTENTS RESUME ABSTRACT TABLE OF CONTENTS LIST OF FIGURES LIST OF TABLES ABBREVIATIONS INTRODUCTION
MATERIALS AND METHODS
I. Recombinant plasmids II. Methods of cloning III. Sequencing
IV. Western blot analysis V. Transfection assays VI. RNA extraction
VII. RT-PCR assays and oligonucleotides VIII. 'Touchdown' PCR program
IV I III IV VI VIII IX 1 17 17 20 22 22 23 23 24 25
RESULTS 27 I. CB3 cells are deficient in hnRNP Al expression 27 II. In vivo splicing of ElA pre-mRNA in MEL
cell lines
III. Transient expression of the Al and Alb cDNAs in CB3C7 cells
IV. ElA pre-mRNA splicing in CB3C7 cells restored for Al expression
V. The hnRNP Al affect SV40 pre-mRNA splicing DISCUSSION ACKNOWLEDGMENTS REFERENCES V 30 34 37 40 43 47 48
LIST OF FIGURES
Fig. 1. Pre-mRNA splicing pathway
Fig. 2. A model of regulated alternative splicing by the Sxl protein in Drosophila
Fig. 3. HnRNP particles separated by two-dimensional gel electrophoresis
Fig. 4. Pre-mRNA consensus splicing signals and splicing factors
Fig. 5. The model of hnRNP Al protein on
alternative splicing of ~-globin in vitro
Fig. 6. Patterns of ElA pre-mRNA splicing
Fig. 7. Patterns of SV40 early pre-mRNA splicing Fig. 8. Undetectable hnRNP Al protein in CB3 cells Fig. 9. The structure of recombinant pCMVSVElA
VI Pages 2 4 5 8 10 13 14 15 17
Fig. 10. pCMVSVAl, pCMVSVAlb and pCMVSVAlfs constructs
and their products 19
Fig. 11. Expression of hnRNP Al protein in MEL cells 28
Fig. 12. Al mRNA levels in CB3C7 cells 29
Fig. 13. In vivo ElA splicing in MEL cells 31 Fig. 14. Identification of ElA spliced products 33 Fig. 15. Abundance of Al and Alb mRNAs in CB3C7 cells
transiently restored for Al expression 36
Fig. 16. ElA splicing in Al- and Alb-transfected
CB3C7 cells 39
Fig. 17. Alternative splicing of the SV40 early
LIST OF TABLES
Table 1. Effects of SF2 and hnRNP Al on alternative
splicing in vitro. page 11
A ATP bp BSA
c
cDNAoc
dNTP DNA DNase ElA EDTA E. coli Fig. G g kb kd L M MELABBREVIATIONS
adenosine adenosine triphosphate base pair (of DNA) bovine serum albumin cytosine complementary DNA degree celsius deoxynucleotide triphosphate deoxyribonucleic acid deoxyribonucleaseadenovirus ElA pre-mRNA
ethylenediaminetetraacetic acid Escherichia coli figure guanosine gram kilobase (1000 bp) kilodalton litre molar (moles/litre) murine erythroleukemic IX
mRNA ml mM ng nt 32p PAGE PBS PCA PCR RT-PCR poly A pmole RNA RNase SDS SV40 T TE Tris µg µl µM 3'SS 5'SS messenger RNA milliliter (l0-3 L) millimolar ( 10-3 M) nanogram ( l0-9 gram) nucleotide
radioactive isotope of phosphorus. polyacrylamide gel electrophoresis phosphate buf fered saline
phenol:choroform:isoamyl alcohol (25:24:1) polymerase chain reaction
reverse transcription-polymerase chain reaction poly adenosine
picomole
ribonucleic acid ribonuclease
sodium dodecyl sulfate simian virus 40 thymidine 10 mM Tris, 1 mM EDTA, pH 7.9 tris(hydroxy methyl)aminoethane microgram (l0-6 gram) microlitre (l0-6 L) micromolar ( 10-6 M) 3' splice site 5' splice site X
INTRODUCTION
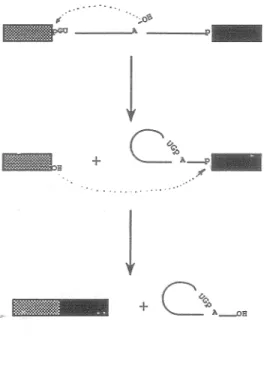
In eukaryotic nuclei, most primary transcripts produced by RNA polymerase II are termed heterogeneous nuclear RNAs (hnRNAs) and are equivalent to mRNA precursors (pre-mRNAs). The formation of f unctional protein-coding mRNAs of ten requires the removal of intervening RNA sequences (introns) from pre-mRNA and rejoining of coding sequences (exons) by a process called RNA splicing. This process refers to a two-step mechanism (Ruskin et al., 1984; Green, 1986; Padgett et al., 1984 and 1986; Fig. 1). The first step entails cleavage at the conserved 5'SS sequence to yield a 5' exon intermediate with a free 3'-0H terminus. Concomitantly, the guanosine (G) residue at the 5' end of the intron is joined, via a 2 '-5' phophodiester bond, to an adenosine residue within the intron known as the branch site located within 100 nt upstream of the 3'SS. This reaction forms a lariat intermediate containing a branched intron with the attached 3' exon. In the second step of splicing, cleavage at the 3'SS occurs; the two exons are joined by ligation via a 3'-5' phosphodiester bond. This reaction yields the mature mRNA and the excised lariat intron with a 3'-0H terminus.
~
__
_..···,a~----l
l
Fig. 1. Pre-mRNA splicing pathway. Exons are sho~-n as boxes and the intron as a
line.
Splicing is also a generator of diversity. In higher eukaryotes some pre-mRNAs undergo alternative splicing, which increases the number of protein products of a single gene and provides addi tional mechanisms for regulating gene expression. This process may be a central point of post-transcriptional control of gene expression and occurs in a temporal, tissue-specific, or developmental fashion (Leff et al.; 1986). Pre-mRNA alternative splicing involves the joining of one splice site
(either 5' or 3') to one of several complementary splice sites so that multiple structurally related but functionally distinct proteins can be generated from a single primary transcript. This results in a more economic use of the genetic information and is
of particular importance for viruses which have small genomes and need to compress the coding information to more eff iciently support replication. Another variation of alternative splicing unique to pre-mRNAs that contain multiple introns is the occasional "skipping" of specific exons.
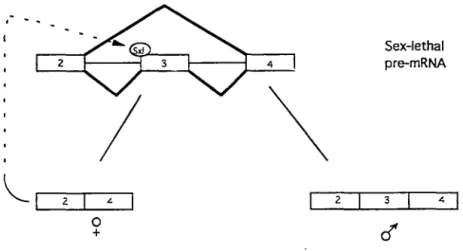
Alternative splicing can occur by a variety of modes: multiple S'SS for a single 3'SS as in the adenovirus ElA and SV40 early pre-mRNAs (Berk and Sharp, 1978a and 1978b); a common S'SS for multiple 3'SS as in the Drosophila transformer (Boggs et al., 1987) and the double-sex gene (Burtis and Baker, 1989); exon "jumping" as in the rat calcitonin/calcitonin gene related peptide gene (Amara et al., 1982); mutually exclusive exon "skipping" as in the rat a- or j3-tropomyosin genes (Helfman et al., 1986; Wieczorek et al., 1988); and intron "skipping" as in the Drosophila P element transposase (Laski et al., 1986) and the suppressor-of-white-apricot genes (Su(Wa)(Chou et al., 1987). Most importantly, alternative splicing can be regulated or modulated. Evidence for this has been reported for some adenovirus genes during the course of infection (Ziff, 1980). Several examples of regulated splicing suggest the involvement of trans-acting factors to control the various alternative splicing patterns. For example, sex determination in Drosophila species is controlled by sex-specif ic RNA splicing mediated by the products of the sex-lethal
( sxl) and transformer-2 ( tra-2) genes. Sxl and tra-2 proteins regulate sex-specif ic alternative splicing by, respectively,
blocking and activating distinct 3' splice sites (Bell et al., 1988; Nagoshi et al., 1988; Baker, 1989; Inoue et al., 1990; Fig. 2) •
\_ l.---2
~,
-.l.----, 0 + 2 Sex-lethal pre-mRNA 3 .l. 1Fig. 2. A mode! of regulated alternative splicing by the Sxl protein in Drosophila. The Sxl pre-mRNA is alternative spliced in females to exclude exon 3, but in males to include exon 3. The binding of alternative splicing factor Sxl in females to sequences 5' to exon 3 blacks exon inclusion and affects the sex determination pathway of Drosophila.
Regulated and constitutive spliced pre-mRNAs associate with a specif ic subset of proteins to form hnRNP complexes called heterogeneous nuclear ribonucleoparticles (hnRNPs). In growing vertebrate cells, the hnRNP proteins are nearly as abundant as histones. HnRNP complexes are likely to be important for the
hnRNP complexes have been isolated with monoclonal antibodies specific to some of the major hnRNP proteins. Purification of hnRNPs f rom nucleoplasm of growing He La cells has revealed approximately 20 major hnRNP proteins (Fig. 3). Their sizes range from 34 kd (Al) to 120 kd (U) (Pifiol-Roma et al., 1988). The Al, A2, Bl, B2, Cl and C2 proteins form the "core" hnRNP proteins
(Beyer et al., 1977). 116K-' 95K-68K- K,
-45K- C2-- •"}F
c1 ... •
30K-u / -L R '\ M T /--- }r
- -02-·1 -
-01 ; E.
---
/ A2 81 I • _...s"
N 82 I ~....
\ A1 G 1-Fig. 3. HnRNP particles separated by two-dimensional gel electrophoresis. The hnRNP particles were irnmunopurified wi th monoclonal antibody 4F4 specific to the c proteins from the nucleoplasm of [35s ]methionine-labelled He la cells, and then
separated by non-equilibrium pH gel electrophoresis in the first dimension and by SDS-PAGE in the second dimension and visualized by fluorography (from Dreyfuss et al., 1988).
The structural element of the hnRNP complex is a monomer particle isolated as 30S-40S structure from sedimentation assay in sucrose gradients and is of size of about 2 0 nm in diameter (Conway et al., 1988). HnRNPs are visualized as a linear array of globular monoparticles along each pre-mRNA molecule (Dreyfuss, 1986). Each monoparticle contains all the core hnRNP proteins and are arranged in a nonrandom manner on specif ic regions of pre-mRNAs in vivo (Beyer et al., 1977; Chung and Wooly, 1986; Dreyfuss, 1986). Reconstitution studies have demonstrated that a monoparticle packages approximately 700 nucleotides of RNA regardless of sequence (Conway et al., 1988). At least 75 percent of the hnRNP is associated with 30S particles, and most of this RNA sequence is accessible to nuclease. The hnRNPs can serve as an "operating table" for hnRNA processing. Therefore, the hnRNPs may be important in both the packaging of the hnRNA and in pre-mRNA splicing by functionning in substrate presentation and in positioning of the splicing intermediates (Yang et al., 1986).
Mammalian pre-mRNA splicing take place in the spliceosome which contains small nuclear ribonucleoprotein particles (snRNPs) Ul, U2, U4/U6, and U5 and a large number of non-snRNP proteins, including hnRNP proteins and other RNA-binding proteins. The snRNPs recognize conserved pre-mRNA sequences and are the best characterized factors required for splicing (Steitz et al., 1988). They play a key role in at least two regions of the pre-mRNA molecule and are important for the specif icity and ef f iciency of
RNA splicing. The Ul snRNP recognizes the 5 'SS (Mount et al., 1983; Kramer et al., 1984; Chabot and Steitz, 1987). The Ul and U2 snRNPs interact by base pairing with the 5 'SS and branch site sequences, respectively (Parker et al., 1987; Zhuang et al., 1989; Wu and Manley, 1989). The non-snRNP factor U2AF binds to the polypyrimidine tract and perhaps the 3'SS AG independent of the other spliceosome components to promote the interaction of U2 snRNP with the branch site during spliceosome assembly (Ruskin et al., 1988). A pre-existing (U4/U6/U5) particle is then assembled into the complex. The U6 and US snRNPs recognize exon sequences adjacent to 5 'SS and 3 'SS (Wyatt et al., 1992; Sontheimer and Steitz, 1993). Prior to the first step of splicing, a comforrnational change destabilizes the association of U4 snRNP with the complex. In addition, SR proteins, defined as conserved serine-and arginine-rich nuclear phosphoproteins, are splicing factors for constitutive splicing (Zahler et al., 1992). The hurnan SF2/ASF and SC35 proteins belong to SR proteins and contain an RS domain (serine and arginine repeat) and an RRM domain (RNA recognition motif). Those demains of SC35 and SF2/ASF proteins are highly homologous to the Drosophila splicing regulators tra, tra-2 and su(wa). SF2/ASF is a protein essential for the first cleavage-ligation step in constitutive pre-rnRNA splicing. SC35 is required for specific interactions between Ul snRNP and the U2 snRNP-containing complex assembled at the 3'SS.
3'SS
U2
_J
UNCURAC (mammalian)•
UACUAAC (Yeast)
Fig. 4. Pre-mRNA consensus splicing signais and splicing factors. The pre-mRNA is shown with exons as boxes and intron as a line. The consensus 5' and 3'SS, polypyrimidine tract (PPT), as well as consensus mammalian and yeast branchpoints are shown. Very highly conserved positions are underlined. R, purine; Y, pyrimidine; N, any base. The binding sites of hnRNP A 1, Cl /CZ, hnRNP 1 and UZAF, snRNP are shown.
Several findings are consistent with the involvement of hnRNP proteins in RNA splicing. Antibodies against hnRNP C proteins block in vitro splicing and depletion of hnRNP C proteins from HeLa extracts reduces splicing activity (Choi et al., 1986; Sierakowska et al., 1986). In vitro studies under splicing conditions showed that at least four proteins, core hnRNP proteins Al and Cl/C2, as well as of non-core hnRNP proteins D and I/PTB, preferentially associate with the polypyrimidine stretch at the 3'SS (Kumar et al., 1987; Swanson and Dreyfuss, 1988; Buvoli et
al., 1990; Gilet al., 1991; Patton et al., 1991; Bennett et al., 1992; and Ghetti et al., 1992). HnRNP I/PTB has also been found associated with various 5' splice site sequences and binding to a specific intron region upstream of the f3-tropomyosin exon 7 appears to negatively regulate exon inclusion (Mulligan et al., 1992). The transient association of hnRNP proteins with pre-mRNA suggests that these proteins modulate the interaction of splicing factors at these sites (Bennett et al., 1992; and Bennett et al., 1992).
The hnRNP Al protein specifically binds to the 3' end of introns between the branch si te and the 3 'SS . Its sequence-specif ic binding to the polypyrimidine-rich region is dependent on the AG dinucleotide at the 3 'SS. Mutations at 3 'SS AG abolish hnRNP Al protein binding (Swanson and Dreyfuss, 1988; Buvoli et al., 1990). In nuclear extracts, efficient ultraviolet-induced cross-linking of hnRNP Al proteins, as well as hnRNP C proteins, to pre-mRNA requires the presence of intact Ul and U2 snRNPs (Mayrand and Pederson, 1990). Another study revealed that Al stably binds to the U2, U4 snRNP and pre-mRNAs (Buvoli et al., 1992). The hnRNP Al protein displays RNA annealing activity (Munroe and Dong, 1992; Pontius and Berg, 1992; Casas-Finet et al., 1993). The multiple interaction and RNA annealing activity of Al proteins are consistent with direct or indirect roles of Al in pre-mRNA splicing. More significantly, alternative splicing of the hnRNP Al pre-mRNA can produce two mRNA encoding the Al (34 kd) and
Alb (38 kd) proteins (Buvoli et al., 1990). The Alb protein is generated by the mRNA including a cassette exon 7B and the abundance of Alb protein is 5% that of Al (Buvoli et al., 1990). However, the affinity of the Alb protein for ssDNA is significantly higher than Al (Buvoli et al., 1990). The extra 53 amino acids encoded by the cassette exon of Alb may affect its capacity to interact with RNA and with other proteins.
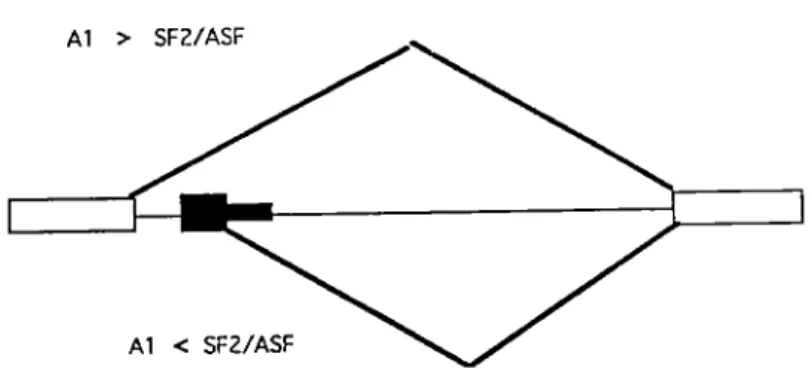
Al > SFZ/ASF
Al < SFZ/ASF
Fig. S. The mode! of hnRNP A 1 protein on alternative splicing of
~-globin in vitro. The boxes present as the ~-globin exons and lines as introns. Black areas represent the duplicated ~-globin exon and intron sequences. The two types of splicing patterns generated from the use of distal or proximal S' SS are shown. The ratio of A 1 /SF2 determines 5' splice site selection.
A regulatory role for hnRNP Al protein in alternative splicing has recently received support from the demonstration that Al affects splice site selection in vitro. When pre-mRNA
containing competing 5 'SS are spliced in vitro, the relative concentrations of the hnRNP Al and the essential splicing factor SF2 determine which 5 'SS is selected (Fig. 5 and Table 1). In general, an excess of hnRNP Al favors distal 5 'SS, whereas as excess of SF2 results in utilization of proximal 5'SS (Mayeda and Krainer, 1992).
SF2/ASF hnRNP Al
-+
+-(proximal 5' SS) (distal 5' SS)
• Cryptic 5' SS
(Human jl-globin) • Duplicated 5' SS (Human jl-globin) • Duplicated 5' SS
(Human jl-globin) • Chimeric jl-globin and SV40 (t) • SV40 early seq. (T/t)
• Adenovirus. • Adenovirus E1 A
Table 1. Effects of SFZ and hnRNP A 1 on alternative splicing in vitro. The activities of SFZ/ ASF and hnRNP A 1 protein in alternative splicing have been detected in the jl-globin pre-mRNAs containing duplicated 5' SS, a jl-thalassemic pre-mRNA using three adjacent cryptic S' SS, adenovirus early ElA sequences, chimeric S' SS downstream with SV40 small t and jl-globin consensus 5' SS. SFZ favors the selection of proximal S' SS. HnRNP A 1 protein favors the selection of distal
Furthermore, the phenomena of hnRNP Al protein antagonizing the activity of the splicing factor SF2/ASF is also found with splicing factor SC35 (Ge and Manley, 1990; Krainer et al., 1990; Fu et al., 1992; Mayeda and Krainer, 1992; Mayeda et al., 1993). The opposing activity of SR and hnRNP Al proteins in 5 'SS selection combined with the distinct tissue-specific distribution of SR protein family members (Zahler et al., 1992 and 1993), and the most recent evidence that hnRNP Al increases exon skipping in vitro in some but not all the pre-mRNAs tested (Mayeda et al., 1993) suggest an important role of Al protein in the regulation of alternative splicing.
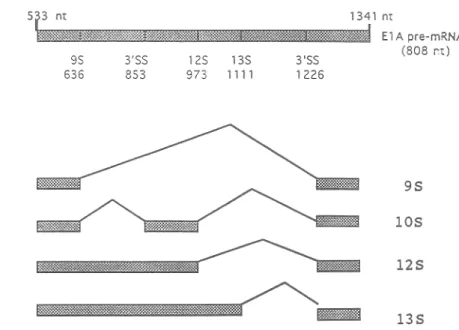
Yet, i t remains to be shown whether the concentration-dependent ef fects of hnRNA alternative splicing previously observed in vitro can also be seen in vivo. To investigate differences in S'SS selection between MEL extracts, we used the adenovirus ElA pre-mRNA which yields the 9S, 12S and 13S mRNAs through alternative S'SS selection (Fig. 6). In addition, a lOS mRNA is generated by the removal of a common intron specified
(Brockmann et al. 1990) by the 9S S'SS and a 3'SS located 216 nt downstream (Fig. 6, Fig. 12 and Fig. 16). The !OS mRNA accumulate late during infection and has been observed in vitro (Stephens and Harlow, 1987; Ulfendahl et al., 1987; Gattoni et al., 1988). The 9S mRNA becomes most abundant at late times of viral infection
(Spector et al., 1978; Chow et al., 1979; Svensson et al., 1983) and the swi tch toward 9S mRNA production can be reproduced in
vitro by reducing the ionic strength (Schmitt et al., 1987) or by addition of late viral transcripts which act by sequestering splicing factors (Gattoni et al., 1991).
s~
9$ 636 3'SS 853 973 12$ 1111 13$ 3'SS 1226 1341 ntS
El A pre-mRNA (808 r't) 95 105 125 135Fig. 6. Patterns of ElA pre-m.RNA splicing. The diagram shows the structure of spliced early region of adenovirus ElA from position 533 to 1341. Caret symbols represent the splicing reactions of joining two exons to generate 13S, 12S, lOS and 9S m.RNAs.
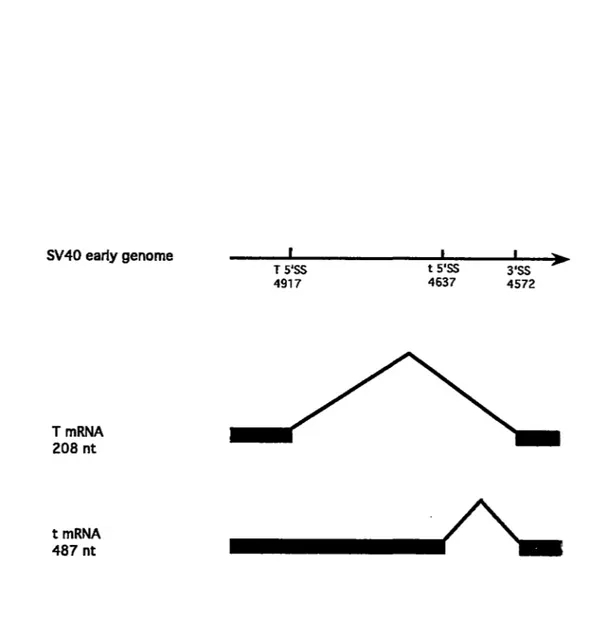
We also use the SV40 early pre-mRNA which is alternatively spliced to produce large T and small t mRNAs by joining either of two alternative 5' splice sites to a single shared 3'SS. In SV40-infected cells in vivo, use of the large T 5'SS is more efficient than use of the small t 5 'SS, al though bath are utilized relatively inefficiently in vitro (Van Santen and Spritz, 1986).
SV40 ear1y genorne T rnRNA 208 nt t rnRNA 487 nt T S'SS 4917 t S'SS 4637 3'SS 4572
----A~
Fig. 7. Patterns of early SV40 pre-mRNA splicing. The SV40 early pre-mRNA from 4401 to 4954 generates two spliced products, large T and small t mRNAs. The caret symbols indicate splicing of the two exons.
In order to detect the effect of the hnRNP Al proteins in vivo, we employed a MEL cell line lacking the Al protein (CB3C7). It has been reported that the hnRNP Al locus is a frequent target for retroviral integration by Friend leukemia virus (Ben-David et al., 1992). In the CB3 cell line (Fig. 8) and CB3C7 cell derivatives (Fig. 11) , hnRNP Al protein expression was undetectable by Western blot analysis as a result of a Friend Murine Leukemic Virus (F-MuLV) integration ln the 3'
transcriptional demain of the Al locus and deletion of the other allele. DP2 8-9 and DP2 7-17 MEL cell lines normally express the hnRNP Al proteins since the Al loci are unal tered. These cell lines serve as two different types of specif ic trans-acting environments with the hnRNP Al being present or absent.
-r.c ., ~ - --
-
-
50 -Al ~5-Fig. 8. Undetectable hnRNP Al protein in CB3 cells. Total protein extracted from CB3, DP27-17 and HeLa cells were separated on an SOS-PAGE (9%). After transfer to a nitrocellulose membrane, the filter was probed with polyclonal anti-hnRNP Al antibody and detected with 135!-protein A. This
antiserum also reveals at least two unknown higher molecular-weight bands (from Ben-David et al., 1992).
The resul ts presented in this "mémoire" have been accepted for publication in
Sciences of the U.
the Proceeding of the National Academy of S. A.. Our results constitute the first demonstration of a mammalian protein capable of affecting alternative splicing in vivo.
MATERIALS AND METRODS
I. Recombinant plasm.ids.
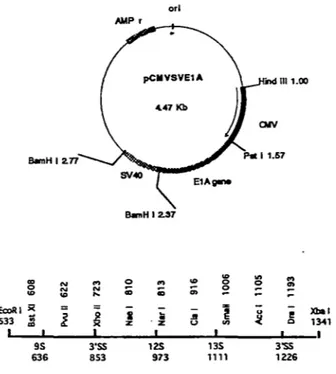
The pCMVE lA was generated by inserting the Klenow-filled EcoRI-XbaI fragment of pSP4-ElA (kindly supplied by J. Stevenin, Strasbourg, France) into the Bine II site of pCMVSV after treatment with the Klenow enzyme. This substrate contains the adenovirus segment the 135 5'55, 125 5'55, 95 5'55, and the 3'55 from positions 533 to 1342. The pCMV5V vector contains the promoter/enhancer region (-525 to +52) from the immediate early gene of human cytomegalovirus (CMV) for high level transcription and another 400 bp fragment containing the 5V40 polyadenylation regions. orl AMP r ... -.---. pCllVSVE1A 1111.00 4.47 Kb BlmH 12.37 0 "' "' "'
...
CD N...
...
0 0 en 0 N N éD éD en 2 "' "' ... EcoRIX = - - -i
-
- Xbal l. ~ 1 li u ! 533 Ji z ·z .li u :t 1341 "' 1 9S 3'SS 12S 13S 3'SS 636 853 973 1111 1226Fig· 9. The structure of recombinant pCMVSVElA. The promoter CMV, SV40 polyadenylation sequences, and different 5' and 3 'SS are represented. The
The pCMVT was a gift from Dr. A. Wildeman and was made by inserting the SV40 fragment (nucleotides 5027-4310) into a site of the polylinker of pCMVSV. This construction contains the large T intron (346 nt) and small t intron (65 nt). The large T (206 nt) and small t (487 nt) cDNAs can be generated by the PCR technique with the oligonucleotides SV4401 and SV4954.
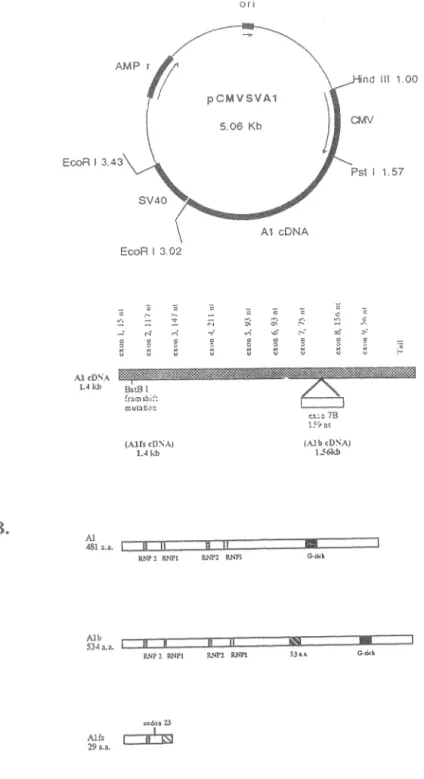
The pCMVAl and pCMVAlb were constructed by inserting into pCMVSV a 1.4 Kbp and 1.6 Kbp cDNA fragment of the murine Al and Alb cDNA, respectively. The Al and Alb cDNA were isolated by screening a Âgtll cDNA expression library made from MEL poly(A)+ RNA. The Âcloning and sequence determination was accomplished by the Ben-David's laboratory in Toronto, Canada.
The pCMVAlf s contains a f rameshift mutation in the Al cDNA and was produced by digestion of pCMVAl with BstBI, filling with Klenow and screening for plasmids which had lost the BstBI site. Sequencing the appropria te reg ion of one pCMVAlfs clone revealed the loss of one nucleotide in codon 23.
A. EcoR 1 AJ.z:DSA i.A kb orl
~'
.\
AMP r(
EcoR 1 3.02 -~ c -~=
cr ,..-;-(AHs cD'.'.-\J lAkb pCfiAVSVA1 5.06 i{b - - 0 - ~ -. C' "' "' ,.; ,.., g 0 ~ 0 0 u\
c - " ~ ,-: OÔ (Alb cD'.'A) l.56'Kbft:sa.
~i:-~~...----,,---;!;rl -·~ RNP2 RNP1 RNP2 RNP1 codvn 23 Alfsc:rn
29 a.a. Hind Ill 1.00 -~-Fig. 10. pCMVSVAl, pCMVSVAlB and pCM"vSVAlfs constructs and their products. The Al and AlB cDNAs were inserted into the polylinker Hinc II si te between the prornoter CMV and the SV40 polyadenylation sequences. Alb cDNA included an extra exon 7B of 159 nt. 'I'he .Alfs includes a fr&"ï\eshift mutation at nt 12 6. The of the exons and differences are ( B) Structure of the lü, Alb and Alfs proteins. The RNPl, RNP2 and r ich demain are
shm1n1. A frameshift mutation at codon 23 shown in Alfs should result in a mutant Al only 22 arnino acids in co:mmon wi th Al.
II. Methods of cloning:
1. Plasmid DNA preparations:
Small-scale plasmid DNA preparations were performed using the alkaline lysis technique as described in Sambrook et al. (1989). Large-scale preparations of plasmid DNA were also accomplished using the alkaline lysis technique but were further purif ied by cesium chloride density gradient centrifugation (Chabot, 1994).
2. Restriction enzyme analyses
Plasmid DNA and amplif ied DNA produced f rom PCR were digested with restriction enzymes purchased from Pharmacia, New England Biolabs, Promega, Amersham and were performed according to the manufacturer's instruction.
3. Purification of fragment and vector DNAs:
The DNA fragments and vector DNAs were eluted directly f rom agarose gels purified on DEAE columns, extracted with PCA (phenol:chloroform:isoamyl alcohol, 25:24:1), and precipitated with 2 volumes of ethanol. Samples were dissolved in TE (pH 7.6).
4. Klenow treatment:
The hnRNP Al DNA restriction fragments wi th recessed 3' termini were filled with the Klenow fragment of E. coli DNA polymerase I. In a 10 µl reaction, 0.2-0.5 µg DNA was added along wi th 1 µl of 1 mM dNTP and 1 unit of Klenow enzyme in lX OPA
buffer. The Klenow enzyme was inactivated by heating to 65oc for 10 minutes or extracted with PCA.
5. CIP treatment:
The vector DNA carrying compatible cohesive termini was treated with phosphatase as follow: after digestion with restriction enzymes, add 2.5 µlof 100 mM Tris-HCl (pH 8.3), 10 mM
ZnCl2, and 0 .25 unit of calf intestinal alkaline phosphatase (CIP). Incubation was for 30 minutes at 37oc. Samples were extracted with PCA and precipitated with ethanol.
6. Ligation:
Cloning DNA into expression vector was performed by using standard protocols of DNA ligation (Sambrook et al., 1989). The mixture contained fragment DNA (0.1-0.5 µg) and the molar ratio of insert DNA: plasmid vector were approximately 2:1 (4:1 for blunt-end DNA). Parallel controls included indepblunt-endent samples of insert
and vector DNAs. The final volume of the aliquots were 10 µl or less. On another assay, the final volume was increased to 50 µl to perform self-ligation of vector DNA.
III. Sequencing:
The T7sequencing™ kit from Pharmacia was used to confirm the mutation in pCMVAlfs. Sequencing was performed according to the manufacturer's instructions.
IV. Western blot analysis.
Approximately 5 x 106 cells were lysed in 400 µl of TNEN buffer (10 mM Tris-HCl pH 7.5, 100 mM NaCl, 1 mM EDTA, 1% NP40). The Al proteins were immunoprecipitated by pre-binding 0.5 µl of 9Hl0 monoclonal anti-hnRNP Al antibody (kindly provided by G. Dreyfuss, Philadelphia) to 50 µl of Protein A-Sepharose (5 mg) swelled in TNEN buffer. Beads were washed twice with TNEN buffer and added to the cell lysate. Following antibody binding for 1 hr at 4 °c, the beads were washed, the pellet was resuspended in 50 µl of Laemmli dye and heated 10 min at 37 oc. After spinning, the supernatant was boiled 5 min and the proteins separated on a 12.5% polyacrylamide/SDS gel. After transfer to nitrocellulose filters, the hnRNP Al proteins were detected using a 1: 1000 dilution of 9H10 antibody and the ECL detection kit (Amersham) according to the manufacturer' s instructions. Prior immunoprecipi tation of
hnRNP Al was essential to obtain clean and reproducible immunoblots with the transfected CB3C7 samples.
V. Transfection assays.
MEL cells were grown in a-Modif ied Eagle Medium ( a-MEM; GIBCO) supplemented with 5% fetal bovine serum. Approximately 7.5 x 106 cells were rinced sucessively with PBS and Optimem (GIBCO). In parallel, a mixture containing 25 µl of a DNA solution in water and 0.3 ml of Optimem was mixed with 0.31 ml of Optimem containing 10 µlof Lipofectin (GIBCO). The mixture was used to resuspend the cell pellet and, following an incubation at 37°C for 1 hr, 5 ml of a-MEM containing 10% fetal bovine serum was added. RNA was extracted 48 hr post-transfection using the following extraction procedure. Lipofection of pCMVElA was performed with 1 µg of DNA. In co-transfection experiments, either 10 µg of pCMVAl, or 10 µg of pCMV Alfs or 10 µg of pCMVAlB was also included.
VI. RNA extraction.
RNA was extracted from the dif ferent cells by the procedure of guanidinium-HCl (Chabot, 1994). To eliminate contaminating plasrnid DNA, RNA samples were treated with 20 units of DNase I
(Pharmacia) in the presence of 100 units of RNAguard (Pharmacia) and 4 mM DTT. Following extraction with PCA and ethanol
precipitation, RNA concentration was determined on agarose gels stained with ethidium bromide.
VII. RT-PCR assays and oligonucleotides.
2.5 µg of RNA was reverse transcribed at 37°C into cDNA with 4 units of AMV-reverse transcriptase (Promega) in a buffer (50 µl) containing 10 mM Tris-HCl pH 7.5, 1.5 mM MgCl2, 50 mM KCl, 250 µM of each dNTP, 30 µg/ml of BSA, 4 mM DTT, 50 units/ml of RNAguard and 2 µg/ml of the appropria te downstream oligonucleotide. A mixture containing 5 0 ng of each oligonucleotide, 3 µCi of radiolabeled [a-32p]dCTP (800 Ci/mmole; Amersham) and 2 units of Taq DNA polymerase (Pharmacia) was then added. Amplification was performed according to the 'touchdown' procedure for a total of 45 cycles as described below. Following electrophoresis of the PCR products on 5% polyacrylamide gels, the gels were dried and exposed on film. Quantitative estimation of the intensities of bands was accomplished by densitometric scanning of gels exposed without an intensifying screen.
The oligonucleotides used in the RT-PCR assays were as follows:
E1A569: 5'-ATTATCTGCCACGGAGGTGT-3'; E1A1315: 5'-GGATAGCAGGCGCCATTTTA-3';
A1701: 5'-CAATTTTGGTCGAGGAGGGA-3'; Al893: 5'-TCCCTTCATCGGCCCAAAAT-3'; A1B3: 5'-CCTCCGCCTCCGTTGTTATA-3'. SV4954: 5'-TCAACCTGACTTTGGAGGCT-3'; SV4401: 5'-AGGAAAGTCCTTGGGGTCTT-3'.
VIII. Touchdown PCR program:
(modified from Don et al., 1991)
1. 94oc, 1.5 min. ----;.. 71°C, 2 min. ----;.. 72°C, 3 min. 2. 94oc, 1.5 min. ----;.. 69°C, 2 min. ----;.. 72°c, 3 min. 3. 94oc, 1.5 min. ----;.. 67°C, 2 min. ----;.. 72°C, 3 min. 4. 94oc, 1.5 min. ----;.. 65°c, 2 min. ----;.. 72°C, 3 min. 5. 94oc, 1.5 min. ----;.. 63°c, 2 min. ----;.. 72°C, 3 min. 6. 94oc, 1.5 min. ----;.. 61°C, 2 min. ----;.. 72°C, 3 min. 7. 94oc, 1.5 min. ----;.. 59°c, 2 min. ----;.. 72°C, 3 min. 8. 94oc, 1.5 min. ----;.. 57°C, 2 min. ----;.. 72°C, 3 min. 9. 94oc, 1.5 min. ----;.. 55°C, 2 min. ----;.. 72°C, 3 min.
11. 94oc, 1.5 min. ~ 51°C, 2 min. ~ 12°c, 3 min.
12. 94oc, 1.5 min. ~ 50°c, 2 min. ~ 72°C, 3 min.
13. go to step 12 and repeat 15X (or more, e. g. 24X, 30X, 45X, etc.)
14. 72°C, 15 min.
RESULTS
I. CB3 cells are deficient in hnRNP Al expression.
The MEL cell lines used in this study were derived from spleen colonies of mice infected with F-MuLV (CB3 cell line) and the FV-P strain (DP28-9 and DP27-17 cell lines) of Friend virus (Shibuya and Mak, 1983; Ben-David et al., 1990). Here, we have used the CB3C7 cell line which was subcloned from the original CB3 cell line by limi ting dilution. In CB3 and CB3C7 cells, Al proteins were not detected by Western blot analysis using either anti-hnRNP Al polyclonal (Ben-David et al., 1992; Fig. 8) or monoclonal antibodies (Fig. 11, lane 3). In MEL cell lines DP27-17 and DP28-9, the 34 kDa Al and the 38 kDa Alb proteins were readily detected (Fig. 11, lane 1). Alb typically represents less than 10% of all Al proteins and is produced by alternative splicing of a cassette exon of the hnRNP Al pre-mRNA (Buvoli et al., 1990). Al though northern blot analysis failed to reveal hnRNP Al transcripts in CB3C7 cells (Ben-David et al., 1992), a RT-PCR assay allowed the detection of minute amounts of hnRNP Al transcripts in these cells (Fig. 12). The intensity of the Al band in the CB3C7 sample was compared with Al signals obtained from a serially diluted DP27-17 RNA sample. The level of Al mRNA in CB3C7 cells was estimated to be at least 200-fold lower than in DP27-17 cells (Fig. 12) . Thus, even though hnRNP Al expression is not
completely silenced by the viral integration event, the hnRNP Al defect in CB3C7 cells is severe.
+-
B
1
2
3
4
5
6
Fig. 11. Expression of hnRNP Al proteins in MEL cells. Total proteins from DP27-17, CB3C7 and Al-transfected CB3C7 cells (CB3C7-Al) were immunoprecipitated with the anti-hnRNP Al monoclonal antibody ~HlO, separated on gel, transfered on nitrocellulose and probed with the monoclonal anti-Al antibody (lanes 1-3). As a control, total proteins were used in an immunoblot performed with the monoclonal Yl2 antibody which recognizes the 28 kd snRNP-associated B protein ( lanes 4-6). Only the relevant portions of the innnunoblots are shown.
A1701 ~
·-f
6=:J-
c·o ..
r··7B
..J~~-c=a i-~ ~ AllB3 A1893B
]x bp l 2 3 4 5 6Fig. 12. Al roIDJA levels in CB3C1 cells. (A) Diagram indicating the position of the primers used in RT~PCR assays" (B) RT-PCR assays using straight CB3C7 or diluted DP27-17 RNA and the A1701/Al893 pair of primerso All diluted DP27-17 RNA samples were mixed with a constant amount of CB3C7 RNA, equiv·alent to the mr~unt used in lane 1. The amplified Al product co-migrated with the product
alllplified from a cloned Al cDNA (lane 7). A product corresponding to the Alb
mRNA is not detected in this assay. The identi ty of the band labelled x is unlmown. An ethidium bro:m..ide staining of straight CB3C7 and DP27~17 RNA samples separated on an ag1.;u::-ose is shoirm on the left; major bands are the 28S and 18S rRNAsc
II. In vivo splicing of ElA pre-mRNA in MEL cell lines.
To test whether splicing differences could be observed in vivo, we transiently expressed the adenovirus ElA gene in MEL cells. Because the efficiency of transfection was very low in MEL cells, ElA splicing was detected by RT-PCR analysis. The primers we chose for the RT-PCR assay map downstream of the major 3'SS and upstream of the ElA 9S 5'SS and are shown in Fig. 13A. In the two MEL cell lines that expressed normal level of Al protein (DP27-17 and DP28-9), the majority of the ElA amplified products were derived from 9S species and accounted for more than 80% of the all amplified ElA products (Fig 13B, lanes 1-4). In CB3C7 cells, ElA pre-mRNA splicing led to considerably higher relative levels of 13S, 12S and lOS products, the 9S species representing less than 30% of the total amplified isoforms (Fig. 13B, lanes 6 and 7). Although the use of the 9S 5'SS is required to generate lOS from 12S, we scored the lOS species as the product of proximal 5'SS selection because it is produced through a subsequent splicing event that does not involve competition between 5'SS. The 10S:l2S ratio did not significantly change between DP27-17 and CB3C7 cells. Thus, the use of the proximal 5'SS was favored in cells that lacked the hnRNP Al proteins.
A B !'1A569
.r:_.~il!ldi-*-mfi
CMV 156 276 493 63: ~9 ~? ::p :0? 9S 636 3 3'SS 853 4 125 13S 973 1112 5 6 3'SS 1226 SV40 ~ pCMVSVE1A E1A1315 SIS lOS l2S 13S ~l ~12s ~lOS ~gs 7Fig. 13. In vivo splicing assays using MEL cells. (A) Diagram representing the structure of pCMVSVElA, the ElA spliced isoforms and the size of the corresponding RT-PCR products obtained using oligonucleotide E1A1315 and E1A569 are indicated. (B) Every independent duplicate samples of the three cell lines were extracted after forty-eight heurs lipofection with pCMVSVElA. Total RNA was treated with DNase I and analyzed for ElA splicing by RT-PCR. As a control, a reaction was performed with RNA from the ElA-expressing cell line 293 (lane 7). The position of the amplified products corresponding to each ElA mRNA is indicated by the arrows. The numbers in the box indicate for each lane the percentage of amplified 9S product relative to all ElA isoforms.
The identi ty of the amplif ied products obtained by RT-PCR analysis was confirmed by their cc-migration with amplified products obtained from ElA-expressing 293 cells (Fig. 13, lane 7) and restriction enzyme analysis (Fig. 14).
156 276 493 631
""":&Â
~ Oal916 .., c 631 95 637..
Q) < ....i
~
Cl) 3·ss 8SZ 5 fil ·-Vl M !JS --493 12S -;ï,, 1 o:; .._ 156 95 · -12S 135 974 1112 3'ss 1226 "' .... u Vl Vl Vl Ill Ill "' .... 0 .... "' .... N-
...
-~ El A 1315 9S liJS :2s 135 Vl Ill 0 "'...
-=
(156 bp) (130; 146 bp) (34 7; 146 bp) (347; 284 bp)Fig. 14. Identification of ElA spliced products. The 13S, 12S, lOS and 9S RT-PCR products were purified separately. Then, these radioactive samples were digested with the restriction enzyme Cla I and separated in a 5% acrylamide gel in TBE. The top diagram shows all products generated from the Cla I
III. Transient expression of the Al and Alb cDNAs in CB3C7 cells:
The striking difference in ElA pre-mRNA splicing between CB3C7 and Al-containing MEL cells is consistent with the ability of the hnRNP Al protein to affect ElA splicing in vivo. However, the comparison involves cell lines differing in more than their Al expression profiles (Ben-David et al., 1991; and Ben-David et al., 1992). To rule out the contribution of other factors, we demonstrated that restoring Al expression in CB3C7 cells also influences ElA pre-mRNA splicing. The strong cytomegalovirus (CMV) promoter was used to express the murine Al or Alb cDNA in CB3C7 cells. Transfection ef f iciencies on all MEL cell lines were estimated to be approximately 0. 5% based on transfections of a CMV-lacZ reporter plasmid (data not shown). RT-PCR analysis was used to estimate the abundance of Al and Alb mRNAs in DNAse-treated RNA samples from transfected CB3C7 cells (Fig. 15). Levels of Al and Alb mRNAs in RNA samples derived from transfected CB3C7 cells were approximately 10- and 5-fold lower, respectively, than DP27-17 Al and Alb RNA levels (Fig. 15A and 15B). Given the efficiency of transfection, the Al and Alb mRNAs were therefore more abundant in successfully transfected CB3C7 cells than in DP27-17 cells (approximately 20- and 40-fold more, respectively). Des pi te the apparent abundance of Al and Alb mRNAs, hnRNP Al proteins remained difficult to detect in transfected CB3C7 cells. Western blots analysis performed with the 9Hl0 monoclonal antibody indicated that the level of Al protein in transf ected CB3C7 cells
Assuming a 0.5% efficiency of transfection, the abundance of Al protein in a transfected CB3C7 cell was therefore very close to the level found in a DP27-17 cell. We failed to detect the Alb protein in Alb-transfected CB3C7 cells (data not shown), suggesting that the level of Alb protein was also considerably less than predicted f rom the estimated amount of Alb transcripts and thus possibly closer to the level found in DP27-17 cells. The discrepancy between Al RNA and Al protein levels may be due to a less efficient recovery of Al proteins in samples with little Al. However, the absolute levels of Al and Alb proteins in stable CB3C7 transfectants were identical to the levels found in DP27-17 cells (B. Chabot, unpublished results), suggesting that post-transcriptional events regulate Al and Alb proteins levels (see Discussion).
Fig. 15. Abundance of Al and Alb mRNAs in CB3C7 cells transiently restored for Al expression. (A) Total RNA was prepared from DP27-l 7, CB3C7 and CB3C7 transfected with the ElA and either the Al or the Alfs expression vectors. RT-PCR assays using primers Al 701 and Al893 were performed wi th straight or diluted RNA samples. (B) Straight or diluted RNA from DP27-17, CB3C7 and Alb transfected CB3C7 cells were assayed for Alb expression by RT-PCR using primers Al701 and AlB3 (Fig. 13A). RNA samples in panels A and B were treated with DNase I and RNA concentrations were estimated on agarose gels by ethidium bromide staining. The position of the Al and Alb PCR products is indicated and was conf irmed by cc-migration of products amplif ied from the corresponding cDNAs (data not shown). The identities of the bands labelled x and x' are unknown.
IV. E lA pre-mRNA splicing in CB3C7 cells restored for Al expression.
The relative abundance of the ElA mRNAs produced from the above co-transfections in CB3C7 cells was estimated by RT-PCR. In the f irst set of duplicate transfections, Al expression was associated with a 2-fold increase in the relative abundance of the distal 9S RT-PCR product (Fig. 16, lanes 1-4). In a second set of transfections, even though the basal level of the 9S isoform was higher, Al expression also provoked a 2-fold increase in the relative level of the 9S product (Fig. 16, lanes 7-9). The percentage of 9S products in these samples did not change when the amount of total RNA was reduced or when amplification runs were shortened (data not shown). A 2-fold increase in the percentage of 9S product upon Al expression was noted in three other sets of duplicate transfections while another set revealed a 4-fold increase (data not shown). Variations in the baseline production of 9S mRNA in CB3C7 cells could be due to a variety of factors that may include dif ferences in cell growth of transfected cells and in the expression levels of the ElA pre-mRNA.
Transient expression of the Alb cDNA in CB3C7 cells produced an intermediate shift toward the production of 9S mRNA (Fig. 16, lanes 10 and 11). Co-expression of Al and Alb isoforms in CB3C7 cells did not stimulate 9S 5'SS selection more than expression of the Al isoform alone (data not shown).
To demonstrate that high levels of Al mRNA in transfected cells were not responsible for the shift in ElA 5'SS selection by sequestering regulatory splicing factors (Gattoni et al., 1991), we transfected CB3C7 cells with an Al cDNA containing a frameshift mutation at codon 23 in the hnRNP Al coding sequences. The level of Al transcripts generated from this mutated Al cDNA (Alfs) was equivalent to the level produced with the wild-type Al cDNA (Fig. 16, compare lanes 5-8 with lanes 9 and 10) but Al proteins could not be detected by western analysis. Expression of Alfs in CB3C7 cells did not stimulate distal 5' SS selection on the ElA pre-mRNA
(Fig. 16, lanes 5 and 6). This result demonstrates that the shift in the selection of ElA 5'SS was not due to high levels of Al mRNA and that synthesis of Al proteins was required to favor distal 5'SS selection.
1 2 3 4 5 6 7 8 9 10 11 :=n 18 54 53 21 34 53 53 98 75 86 +-IUtSP1.1Ce:d +-13S 1-- 12S ~lOS +- 9S % 9S
Fig. Hi. E lA spli.cing in Al- and Alb-transfected CB3C7 cells. Duplicate transfections were performed with pCMVElA alone (CB3C7), 10:1 mixtures of pCMVAl:pCMVElA (CB3C7 +Al), 10:1 mixtures of pCMVAlb:pCM\.'ElA (CB3C7 + AlB) or 10:1 mixtures of pCMVAlfs:pCK"VElA (CB3C7 + Alfs). Samples in lanes 1-6 and lanes 7-11 represent two indepedently performed transfection assays. DNase I-treated RNA samples prepared from transfected cells were assayed by RT-PCR using the ElA-specific primers. In the RT-PCR assays shown here, doublets were often obtained for the 12S and lOS products. Repeated RT-PCR assays with the same and other samples processed from similarly transfected cells ei ther yielded only the lower band for the 12S and lOS products or varied in the ratio of upper and lower 12S bands.We attributed the appearance of the upper
12S and l OS bands to RT~PCR artifacts and the se bands were not further characterized.
V. The hnRNP Al protein affects SV40 early pre-mRNA splicing. To assess the role of hnRNP Al in the selection of SV40 S'SS in vivo, we compared the ratio of SV40 rnRNAs produced following SV40 early expression in Al- and Al+ MEL cells. Expression of the SV40 early transcription unit was accomplished by transfection of plasmid pCMVT which contains the SV40 early region under the control of the CMV promoter. The relative abundance of small t and large T rnRNAs was assessed by RT-PCR analysis following transient expression. Transfection in DP2 7-17, DP2 8-9 and CB3C7 cells revealed no significant change in the small/large T rnRNA ratio between these cells (Fig. 17, lanes 1-7). Likewise, the stable expression of Al and Alb in CB3C7-Al and CB3C7-Alb cells, respectively, did not modify the relative abundance of the SV40 amplification products. In contrast, co-transfection experiments with the Al expression plasmid promoted a clear reduction in the relative abundance of small t amplified product. The decrease in the selection of the proximal small t S'SS was approximately 3-fold in the first set of transfection (Fig. 17. lanes 8 and 9), and averaged 10-fold in the other set (lanes 10-13). These results suggest that transient expression of Al reduced the selection of proximal small t S'SS. Transient expression Alb and Alfs led to no detectable changes in the SV40 splicing profile (data net shown).
large T 5'ss 2;j4567 small t 5'ss T:r l Tr 2 S 'IO 12 9 i rn +--unspliced 553 +--small t 487 T 20!3
Fig. 17. Alternative splicing of the SV40 early pre-mRNA in MEL cells. The position of the SV40 primers and a schematic structure of the early SV40 splicing unit is depicted above. MEL cells were transfected with the SV40 expression vector alone (lanes 1-7, 8, 10, and 11) or co-transfected with a 10:1 mixture of Al and SV40 expression plasmid (lanes 9, 12 and 13). Forty-eighth hours later, RNA was extracted, treated with DNase I and 2.5 µg was assayed for SV40 early pre-mRNA splicing by RT-PCR using oligonucleotides SV4401 and SV4954. The position of the PCR products corresponding to unspliced RNA, large T and small t mRNAs are indicated. Their identity was confirmed by restriction enzyme analysis and sequencing. In the right panel, lanes 8-9 and lanes 10-13 represent two independent transfection experiments. The identity of the band labelled "x" is unknown.
Discussion
Since Mayeda and Krainer (1992) originally reported that the hnRNP Al protein antagonizes the activity of the splicing factor SF2/ASF in vitro, the role of hnRNP Al protein regulating RNA splicing has become a hot focus in the RNA processing field. It is increasingly important to know whether hnRNP Al can act as an alternative splicing factor in mammalian cells. The objective of my study was to examine the role of hnRNP Al in the modulation of RNA splicing in vivo.
We showed in this mémoire that the alternative splicing profile of the adenovirus ElA pre-mRNA is altered in a MEL cell line (CB3C7) deficient in hnRNP Al proteins. In agreement with previous in vitro results (Mayeda and Krainer, 1992), the deficiency in Al proteins is also associated with an increase in the selection of proximal 5'SS on the ElA pre-mRNA in vitro (B. Chabot, unpublished data). the ability to detect such differences in nuclear extracts suggests that hnRNP Al proteins affect splicing rather than the transport or the stability of the ElA mRNAs.
Transiently restoring Al expression in CB3C7 cells promoted a shift toward distal 5 'SS selection. Specifically, the relative
abundance of the distal 9S amplified product in ElA-transfected CB3C7 cells reproducibly doubled upon transient Al co-expression.
Despite large differences in Al protein levels between Al- and Al+ MEL cells, the magnitude of the effects on ElA pre-rnRNA splicing appeared relatively small. More important differences in ElA splicing may be prevented by a combination of factors. First, hnRNP proteins form a large family of RNA-binding proteins in which individual members display overlapping binding specificity (Dreyfuss et al., 1993). Thus, other hnRNP proteins expressed in CB3C7 cells may partially compensate for the absence of Al resulting in a nuclear environrnent that offers Al-like activity. Second, the activity of Al may vary depending on the pre-rnRNA tested. For example, we did not detect variations in the splicing profiles of the alternate cassette exons in the endogenous c-myb and c-kit pre-rnRNAs between DP27-17 and CB3C7 cells. Recent work indicates that hnRNP Al does not promote exon skipping with the sarne efficiency on all substrates tested in vitro (Mayeda et al., 1993). The substrate specificity associated with the activity of Al suggests that a subset of pre-rnRNAs rnay be more sensitive than ElA to variations in Al protein levels.
The Alb protein also influenced 5'SS selection. When the Alb isoforrn was transiently expressed in CB3C7 cells, we observed a signif icant but less important shift toward the production of 9S
mRNA. The reduced effect may be due to the lower abundance of Alb protein in transfected CB3C7 cells. Further in vivo and in vitro work will be required to determine whether the Al and Alb proteins display subtle differences in function.
While our experiments clearly demonstrate that increased levels of transiently expressed hnRNP Al proteins influence S'SS selection, it remains to be shown whether modulation of Al activity is a genuine strategy used by cells to control alternative splicing. Our results suggest that the level of Al proteins is tightly regulated in a cell. For instance, the abundance of Al proteins in transient and stable CB3C7 transfectants remained equivalent to DP27-17 levels even though the level of Al and Alb transcripts in CB3C7 transfectants exceeded the level found in DP27-17 cells. Moreover, we were unable to stably or transiently overexpress Al in cells already expressing Al (i.e., DP27-17, 293, HeLa and NIH3T3 cells; B. Chabot, unpublished results). These observations suggest that post-transcriptional events may prevent Al proteins to reach exceedingly high levels. Although the mechanism responsible for this regulation is not known, excess hnRNP Al protein may be degraded or may have a negative feedback effect on the transport or translation of the hnRNP Al mRNAs. The recent demonstration that hnRNP Al proteins shuttle between the nucleus and the cytoplasm (Pifiol-Roma and Dreyfuss, 1992) is consistent with the notion that Al may regulate post-transcriptional processes.
In contrast to transient assays, restoring stable Al expression in CB3C7 cells did not affect ElA pre-mRNA splicing. It is possible that the survival of the original CB3 cell line and of stable transf ectants may in each case have required and selected for compensatory changes in other proteins. As we set out to investigate the events responsible for this apparent loss of Al activity in stable transfectants, the CB3C7 cell line will constitute a valuable tool to ascertain the precise role of hnRNP Al proteins in alternative splicing and other processes.
ACKNOWLEDGEMENTS
I would like to thank my superviser Dr. Benoit Chabot for his invaluable help in my graduate study as well as his constant sound advice in my research. I also wish to show my great appreciation for accepting me to study in his laboratary such that result in the beginning of my new field on the science.
I thank Hélene Chabot and Johanne Toutant for their help with a number of techniques especially the laborious transfection, sequencing analysis of hnRNP Al DNA and western blot assay.
I thank Jocelyn Côté, Lavanya Narasiah, Alain Lavigueur and Isabelle Lapierre for their friendship and stimulation. Especially, Jocelyn and Lavanya offered me a lot of help.
Finally, I thank professors Benoit Chabot, Joseph Weber and Jean-Pierre Perreault for having accepted to review this "mémoire". Their comments and criticisms were greatly appreciated.
REFERENCES
Amara, G. G., V. Jonas, M. G. Rosenfeld, E. S. Ong, and R. M. Evans ( 1982). Alternative RNA processing in calcitonin gene expression generates rnRNAs encoding dif f erent polypeptide products. Nature 298: 240-244.
Baker, B. S. (1989). Sex in flies: the splice of life. Nature 340: 521-524.
Bell, L. R., E. M. Mainne, P. Schedl, and T. W. Cline (1988). Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding protein. Cel! 55: 1037-1046.
Ben-David, Y., M. Bernstein (1992).
R. Bani . B . Chabot, A. De Koven, and A. Retroviral insertions downstream of the heterogeneous nuclear ribonucleoprotein Al gene in erythroleukemia cells: Evidence that Al is not essential for cell growth. Mol. Cel!. Bio!. 12: 4449-4455.
Ben-David, Y., E. B. Giddens, K. Letwin, and A. Bernstein (1991). Erythroleukemia induction by Friend murine leukemia virus: insertional activa tien of a new member of the ets gene f amily, Fli-1, closely linked to c-ets-1. Genes Dev. 5: 908-918.
Bennett, M., S. Michaud, J. Kingston, and R. Reed (1992). Protein components specifically associated with prespliceosome and spliceosome complexes. Genes Dev. 6: 1986-2000.
Bennett, M., S. Pifiol-Roma, D. Stakinis, G. Dreyfuss, and R. Reed (1992). Differential binding of heterogeneous nuclear ribonucleoproteins to mRNA precursors prior to spliceosome assembly in vitro. Mol. Cell. Biol. 12: 3165-3175.
Berk, A. J., and P. A. Sharp (1978a). Spliced early mRNAs of
simian virus 40. Proc. Natl. Acad. Sei. USA 75: 1274-1278.
Berk, A. J., and P. A. Sharp (1978b). Structure of the adenovirus 2 early mRNAs. Cell 14: 695-711.
Beyer, A. L., M. E. Christensen, B. W. Walker, and W. M. LeStourgeon (1977). Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell 11: 127-138.
Boggs, R. T., P. Gregor, S. Idriss, J. M. Belote, and M. McKeown (1987). Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell 50: 739-747.
Brockmann, D., B. Tries, and H. Esche (1990). Isolation and characterization of novel adenovirus type 12 ElA mRNAs by cDNA PCR technique. Virology 179: 585-590.
Burtis, K. C., and B. S. Baker (1989). Drosophila doublesex gene control somatic sexual differentiation by producing alternative spliced mRNAs encoding related sex-sepecific polypeptides. Cell 56: 997-1010.
Buvoli, M., F. Cobianchi, and S. Riva (1992). Interaction of hnRNP Al with snRNPs and pre-mRNAs: evidence for a possible role of Al RNA annealing activity in the first steps of spliceosome assembly. Nucl. Acids Res. 20: 5017-5025.
Buvoli, M., F. Cobianchi, G. Biamonti, and S. Riva ( 1990). Recombinant hnRNP prote in Al and i ts N-terminal domain show preferential aff ini ty for oligodeoxynucleotides homologous to intron/exon accepter sites. Nucl. Acids Res. 18: 6595-6600.
Buvoli, M., F. Cobianchi, M. G. Bestagno, A. Mangiarotti, M. T. Bassi, G. Biamonti, and S. Riva (1990). Alternative splicing in the human gene for the core protein Al generates another hnRNP protein. EMBO J. 9: 1229-1235.
Casas-Finet, J. R., J. D. Smith, Jr, A. Kumar, J. G. Kim, S. H. Wilson, and R. L. Karpel (1993). Mammalian heterogeneous ribonucleoprotein Al and its constituent domains. Nucleic acid interaction, structural stability and self-association. J. Mol. Biol. 229: 873-889.
Chabot, B. (1994). Synthsis and purification of RNA substrates. RNA processing - A Practical Approach, edited by Higgins, S. J. and B. D. Hames (IRL press, Oxford), p. 1-30.
Chabot, B. and J. Steitz (1987). Multiple interactions between the splicing substrate and small nuclear ribonucleoproteins in spliceosomes. Mol. Cell. Biol. 7: 281-293.
Choi, Y. D., P. J. Grabowski, P. A. Sharp, and G. Dreyfuss (1986). Heterogeneous nuclear ribonucleoproteins: role in RNA splicing. Science 231: 1534-1539.
Chou, T. B., Z. Zachar, and P. M. Bingham (1987). Developmental expression of regulatory gene is programmed at the level of splicing. EMBO J. 6: 4095-4104.
Chow, L. T., T. R. Broker, and J. B. Lewis (1979). Complex splicing patterns of RNAs from the early regions of adenovirus 2. J. Mol. Biol. 134: 1534-1539.
Chung, S. Y., and J. Wooly ( 198 6) • Set of novel, conserved proteins fold pre-messenger RNA into ribonucleosomes. Proteins 1: 195-210.
Conway, G., J. Wooley, T. Bibring, and W, M, LeStourgeon (1988). Ribonucleoproteins package 700 nucleotides of pre-mRNA into a repeating array of regular particles. Mol. Cell. Biol. 8: 2884-2895.
![Fig. 3. HnRNP particles separated by two-dimensional gel electrophoresis. The hnRNP particles were irnmunopurified wi th monoclonal antibody 4F4 specific to the c proteins from the nucleoplasm of [ 35 s ]methionine-labelled He](https://thumb-eu.123doks.com/thumbv2/123doknet/5427305.127039/17.920.108.799.91.1035/separated-dimensional-electrophoresis-particles-irnmunopurified-monoclonal-nucleoplasm-methionine.webp)