Publisher’s version / Version de l'éditeur:
Journal of the American Concrete Institute, 80, 3, pp. 235-241, 1983-05
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Adsorption and hydration behavior of tricalcium aluminate- water and
tricalcium aluminate-gypsum-water systems in the presence of
superplasticizers
Ramachandran, V. S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=d659e50d-2505-420e-9842-c8b2157f7d01 https://publications-cnrc.canada.ca/fra/voir/objet/?id=d659e50d-2505-420e-9842-c8b2157f7d01
Ser
THlN21d
National Research
Conseil national
l$r
Council Canada
c.
2
de recherches Canada
BLDG
~
ADSORPTION AND HYDRATION BEHAVIOR OF TRICALCIUM ALUMINATE-WATER
AND TRICALCIUM ALUMINATE-GYPSUM-WATER SYSTEMS I N THE PRESENCE
OF SUPERPLASTICIZERS by V.S. Ramachandran
ANALYZED
Reprinted from ACI Journal May-June 1983 p. 235-
241 DBR Paper No. 1115Division of Building Research
.-a.
"BLDG.
RE<
'
L I B R A R Y
03-
0,-
,3
8
B~BLIOTWEQUE
Rech.
Bairn.
C N R C-
t C I U 1 III
r
lukd
L ' a d d i t i o n de 1, 2 e t
4%
de mglamine sulfon'ee ou d e formald'ehyde d e n a p h t a l h e r e t a r d e l ' h y d r a t a t i o n d e C A e t l a c o n v e r s i o n d e l ' a l u m i n a t e h y d r a t ' e h e x a g o n a q e n a l u m i n a t e hydrat'e cubique. Cet e f f e t est p l u s prononc'e l o r s q u e l a c o n c e n t r a t i o n du s u p e r p l a s t i f i a n t e s t p l u s 6lev'ee. La v i t e s s e d ' a d s o r p t i o n du s u p e r p l a s t i f i a n t e s t p l u s grande dans l e systBme C3A-H20 que dans l a phase cubique ou hexagonale. L ' a d s o r p t i o n e s t i r r h e r s i b l e . En m i l i e u non aqueux, s e u l e l a phase hexagonale a d s o r b e d e p e t i t e s q u a n t i t g s de s u p e r p l a s t i f i a n t .La r d a c t i o n e n t r e l r a l u m i n a t e t r i c a l c i q u e e t l e gypse, a v e c f o r m a t i o n d ' e t t r i n g i t e , e s t acc'el'erde e n prl'esence d'un s u p e r p l a s t i f i a n t . La r'eaction exothermique dans c e syst'eme, due
3
1'excBs d ' a l u m i n a t e t r i c a l c i q u e , e s t retard'ee e n prdsence d'un s u p e r p l a s t i f i a n t . Des e s s a i s d ' a d s o r p t i o n s u r un mglange a l u m i n a t e t r i c a l c i q u e g y p s e pr'ehydrat'e pendant d i f f ' e r e n t e s p g r i o d e s montrent que l a v i t e s s e d ' a d s o r p t i o n d6croPt g'engralement dans l ' o r d r e d d c r o i s s a n t : 0, 1 h , 2 j, 15 min,39
min e t5
min. En m i l i e u non a q u e u x , s e u l e s d e p e t i t e s q u a n t i t ' e s d e s u p e r p l a s t i f i a n t s o n t *adsorbGes. Dans t o u s l e s c a s,
1 ' a d s o r p t i o n 6 t a i t i r r ' e v e r s i b l e . In!w$~fllp~
III
r
~~IIII
- --3
80 01026
4Title
no.
80-25Adsorption and Hydration Behavior of Tricalcium Aluminate-
Water and Tricalcium Aluminate-Gypsum-Water Systems in the
Presence of Superplasticizers
by
V.S.
Ramachandran
Additions of I , 2, and 4 percent suuonated melamine or naphthalene formaldehyde retard the hydration of C,A and the rate of conversion of hexagonal to cubic aluminate hydrate. This effect is more pro- nounced at high dosages of the superplasticizer. Rate of adsorption of the superplasticizer is higher in the C d - H 2 0 system than in the hex- agonal or cubic phase; adsorption is irreversible. In a non-aqueous medium only the hexagonal phase adsorbs small amounts of super- plasticizer.
Reaction between tricalcium aluminate and gypsum to form etlrin- gite is accelerated in the presence of superplasticizer. The exothermal reaction occurring in this system as a result of the reaction of excess tricalcium aluminate is delayed in the presence of the superplasticizer. Adsorption experiments on the tricalcium aluminate-gypsum mixture prehydrated for different periods show that the rate of adsorption is generally in the decreasing order 0, 1 hr, 2 days, I5 min, 30 min, and 5 min. In the non-aqueous medium only smull utnounis of superplasti- cizer are adsorbed. Adsorption was irreversible in all cases.
Keywords: adsorption; calcium aluminate hydrates; calorimeters; conductivity;
CIA; ettringite; gypsum; hydration; melamine resins; napthalene compounds; plasticizers; sulfonation.
Added in small amounts to concrete, superplasticizers or high-range water reducers are capable of reducing water requirements by as much as 25 to 30 percent and promoting workability significant!^ at normal water-ce- ment ratios. Most work on superplasticizers is based on either sulfonated melamine formaldehyde (SMF) or sul- fonated naphthalene formaldehyde (SNF).
Water reduction and workability attained with super- plasticizers depend on the physical and chemical nature of portland cement. Slight variations in its composition may sometimes significantly alter their effects. In spite
I
of continuing investigation, the exact role of superplas- ticizers in adsorption and hydration of cement is notI
completely understood.'-s From a practical standpoint, knowledge of the effect of superplasticizers on portland cement is important. Admixtures, however, act in a complex way, and it has proved to be more valuable to study the action of an ad- mixture on the adsorption and hydration characteristics of individual cement components and then extend theknowledge gained to cement itself. A study of the action of SMF and SNF on the adsorption and hydration char- acteristics of the C,A and C,A
+
gypsum systems is pre- sented. These components are known to influence the behavior of concrete in the early periods of setting and hardening.EXPERIMENTAL Materials
Tricalcium aluminate was made by firing stoichio- metric amounts of CaCO, and A120,. It had a Blaine surface area of 4350 cm2/g. The sample contained 0.1 percent Na20. The mixture of C3A and gypsum was made by combining C3A and CaS04.2H20 in the ratio
1:0.25 by weight. Gypsum was of reagent quality. The hexagonal phase (C2AH,
-
C,AH,) was prepared by hydration of C3A at 2 C (36 F) for 5 days. The cu- bic calcium aluminate hydrate (C,AHd was obtained by steam curing C3A a t a pressure of 100 Ib/in.2 (0.69 MPa) for 24 hr.The sulfonated melamine formaldehyde (SMF) and sulfonated naphthalene formaldehyde (SNF) were com- mercial qudi:jr.
~ e t h o d
Adsorption of SMF on some of the samples was de- termined at different intervals at a water to C3A ratio of 10. A typical mixture consisted of 2 g C,A, 0.5 g CaS04.2H,0, and 20 cc water. At specific intervals the suspensions were centrifuged, a known amount of su- pernatant solution was diluted, and the concentration of SMF was estimated by the spectrophotometric method at a wavelength of 219 nm. The difference in the amount of SMF originally added and that left in the solution gave the percentage (on solid basis) of SMF adsorbed by
Received Aug. 23, 1982, and reviewed under Institute publication policies.
Presented at the 1983, ACI Annual Convention, Los Angeles, Mar. 24, 1983.
Copyright O 1983, American Concrete Institute. All rights reserved, ~ncluding
the making of copies unless permission is obtained from the copyright proprie-
tors. Pertinent discussion will be published in the March-April 1984 ACI JOUR-
NAL if received by Dec. 1, 1983.
ACI JOURNAL I May-June 1983
V.S. Rarnachandron is head of the Building Materials Section, Division of Building Research, Nation01 R,esearch Council of Canada. For 30 yeors he has been engaged in reseorch concerning catalysis, clay mineralogy, lime, gypsum, cement chemistry, and concrete technology. Dr. Ramachondron has published four books, over 100 research papers, and is a fellow of the Royal Society of
Chemistry and Ceramic Society, U.K., and also of the American Ceramic Soci- ety.
Fig. I-Conduction calorimetric curves of C,A in the presence of SMF
the solid, viz., C3A, hexagonal phase, cubic phase, or C3A
+
gypsum. Similar experiments were carried out using SNF, and the amount of SNF in the solution was determined at a wavelength of 292 nm. Desorption ex- periments were conducted from each point on the ad- sorption curve by successively diluting the solvent and determining the amount of superplasticizer bound by the solid. Some experiments'were also carried out using di- methyl sulfoxide (DMSO) as the non-aqueous solvent.Hydration of C3A and C3A
+
gypsum was carried out at ambient temperature at a water to C3A ratio of 2 in the presence of 0, 1, 2, and 4 percent SMF or SNF. At specified intervals each sample was placed in excess cold acetone, washed with cold acetone, subsequently evacu- ated for 24 hr using liquid air in the trap, and subjected to thermal analysis. A differential scanning calorimetric (DSC) cell supplied as a module to a DuPont 990 ther- m a l analysis system was used to obtain thermograms. The rate of heating was maintained at 20 C h i n (36 F/ min).The rate of heat development during hydration was determined by a conduction calorimeter having a sensi- tivity of 20 mV/W. The water to C3A ratio used in these experiments was 2.
Surface area of some samples was determined with
N,
as the adsorbate by a Numinco Orr Surface Area-Pore Volume Analyzer.
RESULTS AND DISCUSSION
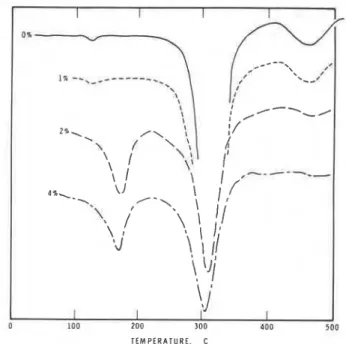
Hydration and adsorption of superplasticizers in The rate of hydration of C3A in the presence of 0, 1 , 2, and 4 percent superplasticizers was followed by con- duction calorimetric and differential scanning calori- metric techniques. Fig. 1 compares the conduction ca- lorimetric curves of C3A hydrated in the presence of 2 percent SMF with that of C3A hydrated without the ad-
T E M P E R A T U R E . C
Fig. 2-DSC curves of C,A hydrated in the presence of \
SMF for 1 day
mixture. Results suggest that within a few seconds of contact with water there is a rapid rate of heat devel- opment, with a peak at about 8 to 9 min for the sample - without admixture. Although the initial rate of heat development is marginally higher in the sample con- taining 2 percent SMF, the total amount of heat gener- ated in the first 30 min and the amplitude of the peak are lower in comparison with those in the sample with- out SMF. It is thus evident that SMF acts as a retarder for the hydration of C3A. Sulfonated naphthalene formaldehyde is also known t o retard hydration of C3A.6,7
The DSC curves also indicate that SMF retards the hydration of C3A to t h e hexagonal phase as well as the conversion of the hexagonal to the cubic phase. DSC curves of C3A hydrated for a day in the presence of 0, 1 , 2, and 4 percent SMF illustrate the relative retarding ef- fects of different amounts of SMF (Fig. 2). Curves of C3A hydrated in the presence of 0 or 1 percent SMF in- dicate that at 1 day C3A is converted to the cubic phase, as evidenced by a large endothermal effect at about 320 to 340 C (600 t o 640 F). The corresponding peak for samples containing 2 or 4 percent SMF is of lower mag- nitude. Existence in these samples of an endothermal peak at about 170 to 185 C (340 to 365 F) is due to the presence of the hexagonal phase. It is thus evident that at higher concentrations of SMF, retardation effects in the C,A-H,O system are enhanced. Published results indicate that a SNF admixture has a similar effect on the hydration of C3A. Present results show that SNF does not retard the hydration of C3A as effectively as SMF.
Adsorption experiments indicate that in an aqueous medium C3A adsorbs rapidly almost all the SMF and SNF present in the solution. Typically, 12.4 percent of both superplasticizers (based on CIA) are adsorbed in 6 to 18 min. Results are similar to those observed for the ACI JOURNAL I May-June 1983
0 10 2 0 30 4 0 50 6 0
T l h l E , rnin
Fig. 3-Adsorption of SMF and SNF on hexagonal cal- cium aluminate hydrate
adsorption of calcium lignosulfonate on hydrating C3A.' Retardation of C3A hydration to form the hexagonal phase is possibly due to a complex of the superplasti- cizer that forms on the hydrating C3A. At lower concen- trations of the admixture retardation is not intense enough to stop the conversion altogether because, after initial adsorption, the particles are dispersed to expose fresh surfaces for further hydration. Some of the ad- mixture is adsorbed by the hydrated products, and the remaining small amounts retard the C,A hydration. At higher concentrations hydration may be retarded very efficiently or even inhibited in a manner similar to that occurring in the C3A-calcium lignosulfonate-H,O sys- tem. In the presence of a non-aqueous medium such as DMSO containing SNF, the C3A phase does not absorb the admixture, indicating that the retarding action is re- lated to adsorption on the hydrating C3A surface. The action is similar to that of calcium lignosulfonate on C3A.9
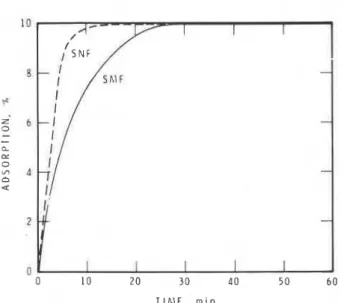
Adsorption of SMF and SNF on the synthetically pre- pared hexagonal phase is compared as a function of time in Fig. 3. Adsorption is rapid, and within 25 min almost all the superplasticizer in the aqueous medium has been adsorbed. SNF is more rapidly adsorbed than SMF, possibly due to the more efficient dispersive action of the SNF on the hexagonal aluminate. Surface area of the hexagonal aluminate was 5.5 m2g. Surface areas were 6 and 7.2 m2/g, respectively, after adsorption of SMF and SNF. Adsorption of both superplasticizers was irrever- sible owing to the formation of a chemical complex be- tween the hexagonal phase and the superplasticizers.
In a non-aqueous medium the hexagonal phase ad- sorbs small amounts of SNF irreversibly (Fig. 4). Stabi- lization or retardation of the hexagonal phase, with re- spect to the cubic phase, may be related to the adsorp- tion of SMF or SNF on the hexagonal phase. Many or- ganic compounds form complexes with the hexagonal phase.'0s" The retardation or interconversion effect probably occurs by the adsorption of superplasticizers
ACI JOURNAL I May-June 1983
i
Z
H E X A G O N A L P H A S E C3AH6. C3A. CaS04. 2 H 2 0
0 1 2 3 4 5
T I M E , d a y s
Fig. 4-Adsorption of SNF on C,A, CaSO,
.
2 H 2 0 , C d H , and hexagonal phase in a non-aqueous medium (Adsorption is zero on C,A, CaSO,.
2H20, and C d H J1 2 3 4 5 6
TIME, d a y s
Fig. 5-Adsorption of SMF and SNF on C 4 H 6
that inhibit the free movement of the interlayer ions re- sponsible for conversion to the cubic form.
Adsorption results of SMF and SNF on the cubic phase are shown in Fig. 5. Adsorption occurs at a slower rate.than in either the C,A or hexagonal phase. SNF is adsorbed more rapidly than SMF. It is possible that in addition to its dispersion effect, SNF is adsorbed to a greater extent than SMF because it has a lower molecu- lar weight. Adsorption effects of calcium lignosulfonate on C3AH6 have shown that adsorption per unit surface is 53 percent less than that on the hexagonal phase. Ad- sorption of superplasticizers on the cubic phase was ir- reversible.
Hydration and adsorption of superplasticizers in the C&-gypsum-H,O system
Fig. 6 gives conduction calorimetric curves of the C,A
+
gypsum mixture hydrated for up to 30 min in the presence of 0, 1,2, and 4 percent SMF. Peaks signifying the maximum rate of heat development occur within 4Fig. 6-Conduction calorimetric curves of C,A
+
gyp- sum+
H 2 0 containing SMFFig. 7-Conduction calorimetric curves for C3A
+
gyp- sum+
Hz0 containing SMFmin for all samples, caused mainly by the formation of ettringite. The peak corresponds to a heat evolution rate of about 1 cal/g/min for C3A
+
gypsum, compared to a value of 3.6 to 3.7 cal/g/min for C3A hydrated with- out gypsum (Fig. 1). It follows that gypsum retards the hydration of C3A. In the presence of SMF the amplitude of the peak is higher and the total,heat developed in the first 30 min is larger than that in samples hydrated with- out the admixture. It appears, therefore, that SMF ac- celerates the reaction between C3A and gypsum. Slan- icka,I2 on the other hand, has reported that at high con- centrations of SMF, the reaction between C3A and gyp- sum is retarded. The .amount of gypsum used in these experiments was nearly 50 percent and this may partly' explain the discrepancy.
.Samples containing SMF exhibit a second pkak at about 20 hr for 1 percent SMF, at about 23 hr for 2 per- cent SMF, and at about 15 hr for 4 percent SMF (Fig. 7). A continuous hump of lower amplitude is registered for th'e sample containing no admixture. A sharper peak may result depending on the water: solid and C3A to gypsum ratio. This second peak is known to occur after all gypsum has been exhausted and is attributed to the formation of low sulfoalurninate (from C3A
+
ettrin- gite reaction), hexagonal aluminate, or their solid solu- tion. The calorimetric curves show that in the presence of SMF this reaction is delayed.Differential thermal investigations showed that con- version of excess C3A to low sulfoaluminate and alumi- nate hydrate is delayed by the addition of SMF. At 3 days of hydration all samples showed the presence of low sulfoaluminate and hexagonal aluminate hydrate. The x-ray, diffraction results of the C3A
+
gypsum+
0
0 1 2 3 4 5 6
TIME, h
Fig. 8-Adsorption of SMF on
C,A
+
gypsum prehy- drated for different periodsH 2 0 system hydrated at different times in the presence
of SMF confirm that formation of the low sulfoalumi- nate phase is retarded compared to that without SMF.
The conduction calorimetric curves of C3A
+
gypsum hydrated in the presence of SNF showed results gener- ally similar to those for samples containing SMF. At 1 percent SNF there was a slight retardation in the forma- tion of ettringite; at other concentrations acceleration was indicated. Collepardi et al.13 reported that with 0.6 percent SNF the hydration rate of C3A+
gypsum+
Ca(OH), remained the same as that of the sample with- out SNF. Conversion of ettringite to monbs~lfate and/ or the aluminate hydrates was, however, delayed as it was for the system with SMF. Thermal anlaysis indi- cated that formation of low sulfoaluminate is debyed with 1 percent SNF, and less hexagonal phase is formed in the presence of SNF.The increased rate of reaction of gypsum and C3A to produce ettringite is initially due to the dispersion of hy- drating C3A, which promotes the exposure of higher surface area. The addition of superplasticizer may inter- fere with the type of ettringite layer that forms on hy- drating C3A. In addition, adsorption of SMF takes place on this surface, delaying the conversion of excess ettrin- gite and C3A to the monosulfate phase. The relative . times of appearance of the second peak in the conduc- tion calorimetric curve may depend on a number of fac- tors: the extent of initial dispersion of hydrates, thick- ness and crystallinity of ettringite, amount of adsorp- tion of the superplasticizer on C3A and the ettringite layer, and orientation of the adsorbed layer. The depen- dence of these factors on the reaction of excess C3A has yet to be resolved.
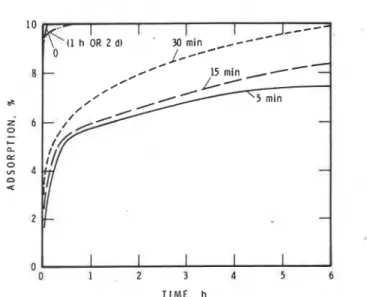
Fig. 8 represents adsorption of SMF on the products obtained by prehydrating C,A
+
gypsum for periods of 0,5,10, and 30 min and 2 days. The gypsum component adsorbs practically no SMF (Fig. 4). The mixture that was not prehydrated adsorbs almost all SMF present in the solution within a few minutes. The sample prehy- drated for 5 min adsorbs about 5 percent SMF in 30 minand the rate is slower thereafter; the amount of adsorp- tion (on the basis of C3A
+
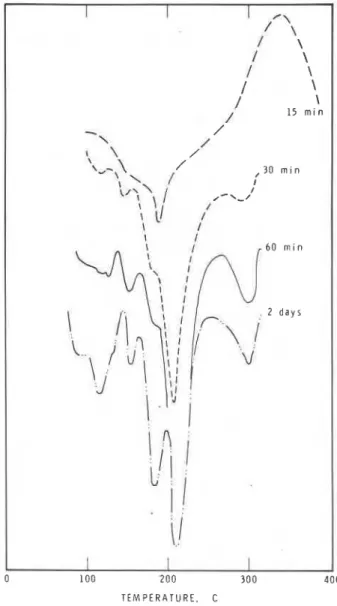
gypsum) at 6 hr is 7.6 per- cent SMF. As the prehydration time is increased, the rate and amount of adsorption increase and at a prehydra- tion period of 1 hr or 2 days, the rate and amount of ad- sorption are high.Fig. 9 presents the DSC curves of the C3A
+
gypsum mixture prehydrated to different times. The unhydrated sample exhibits a typical double endothermic effect within the temperature range 125 to 150 C (257 to 300 F) caused by the stepwise dehydration of gypsum. At 5 min of hydration the intensity of the gypsum is de- creased, signifying formation of sulfoaluminate, and a new endothermic peak occurs in the vicinity of 200 C (390 F). The intensity of this peak decreases at 15 min. It and the peak at about 260 to 275 C (500 to 530 F) can be attributed to the presence of a mixture of low sulfoaluminate and hexagonal aluminate. The down- ward slope of the curve occurring below 100 C (212 F) in prehydrated samples is indicative of ettringite. Its presence is indicated by the endothermal peak at about 125 C (260 F) for the sample prehydrated for 60 min. At low water to C3A ratios the formation of ettringite occurs as a prelude to formation of low sulfoalumi- nate. The samples in Fig. 9 were all hydrated at a water to C3A ratio of 10. Dispersion effects, competition for the adsorption of SMF on hydrating C3A (promoted especially at higher water to C,A ratios), are responsi- ble for large adsorption effects for samples that were not prehydrated.The rate and amounts of adsorption are lower for the C3A + gypsum mixture prehydrated for 5 to 30 min. In these samples products are formed on the surface of C3A so that diffusion of SMF is lowered. The hexagonal hy- drates adsorb at a lower rate than does the hydrating C3A (Fig. 3). It is possible that eventually all the added SMF will be adsorbed. At 6 hr to 2 days the main phases present are the low sulfoaluminate and C,A. There is evidence that low sulfoaluminate adsorbs large amounts of SNF and SMF.I4 Desorption experiments showed that in all cases most SMF is irreversibly adsorbed. This in- -dicates that there is a surface-chemical or chemical in- teraction between hydrating C3A and the C3A-gypsum
I mixture with SMF.
The superplasticizing effect on flowing concrete is generally known to be enhanced when the superplasti- cizer is added a few minutes after the ingredients have been mixed with water. Otherwise, when superplasti- cizer comes directly into contact with C3A
+
gypsum, most of the admixture will be rigidly attached, with a , very small amount available for dispersion of the silicate phases. The present work shows that partly hydrated C3A+
gypsum adsorbs at a sldwer rate and in smaller amounts (Fig. 8). In concretes, with the late addition there will be enough SMF left in the solution to promote dispersion of the silicate phases and lower the viscosity of the system.Fig.10 refers to the DSC curves of C3A
+
gypsum samples that were exposed to six hr of adsorption, as described in Fig. 8. A comparison of the curves for sam- ples unexposed to SMF (Fig. 9) and the correspondingACI JOURNAL I May-June 1983
I I I I T I U N H Y D R A T E D C J A 4 25%. G Y P S U M H Y D R A T E D 5 m l n C j A + 25'b G Y P S U M H Y D R A T E D 6 0 lnln L I I L I I L 1 1 I 5 0 150 2 5 0 3 5 0 4 5 0 T E M P E R A T U R E . C
Fig. 9-DSC curves of C,A
+
gypsum hydrated for dif- ferent periodsones that were exposed to SMF for six hr (Fig. 10) indi- cates that during the adsorption experiments C,A re- acted with gypsum. Only small amounts of gypsum are left in the products. The amounts of low sulfoalumi- nate, hexagonal aluminate hydrate, and ettringite, how- ever, are present in different amounts.
Fig. 1 1 represents the adsorption of SNF is on the C3A-gypsum system prehydrated for 0, 5, 15, and 30 min and 2 days. The trend in the results is similar to that obtained for SMF. As in other systems presented in Fig. 3 and 5, it appears that the rate of adsorption of SNF is greater than that of SMF.
In a non-aqueous medium SNF adsorbed by the pre- hydrated products, its amount increasing as prehydra- tion time is increased. Unhydrated C3A-gypsum does not adsorb SNF, indicating that superplasticizers are ad- sorbed on the hydrating C3A. The lower adsorption amounts of SNF in a non-aqueous medium compared with adsorption in an aqueous medium (Fig. 12 and 11) are due to competition between DMSO and SNF for ad- sorption on the surface of the hydrated surfaces.
/ \ / \ / \
/
\ / \ 1 15 m i n -\ / 1 \ / / / ,30 m i n 60 r n i n . 2 d a y s 0 100 '200 300 400 TEMPERATURE. CFig. 10-DSC curves of C3A
+
25 percent gypsum hy- drated for different periods and exposed toSMF
for 6 hrc
0 1 2 3 4 5 6
TIME, h
Fig. 11-Adsoprtion of SNF in the C3A-gypsum-H,O system 240 TIME, d a y s 8 z' 0
-
C n. w=:
0 a 1 0 0Fig. 12-Adsorption of
SNF
on C,A+
gypsum+
H,O system in a non-aqueous medium-
-
3 --
2 --
-,--
---
30 AND 60 min-
L'-------
'
,
-
-
-
-
-
2 5 m i n --
- -
-
'
I k 5 m i n I 4 I CONCLUSIONSThe rate of hydration of C3A to the hexagonal phase and conversion of the hexagonal to the cubic phase are retarded by the addition of both sulfonated melamine formaldehyde and sulfonated naphthalene formalde- hyde. Large amounts and high rates of adsorption (irre- . versible) of superplasticizers on the hydrating C3A and hexagonal phase suggest that intereonversions and the '
rate of hydration are retarded due to chemical interac- tions between the superplasticizer and the hydrating phases.
The rate of hydration of C,A
+
gypsum to form et- tringite is accelerated by the superplasticizer, but con- version to low monosulfate is retarded. Dispersion ef- fects and interference by the type of ettringite formed on the surface of the C3A-gypsum mixture may explain the acceleration effect. Retardation of the ettringite-C3A re- action to produce low sulfoaluminate may be caused by adsorption of the superplasticizer on the hydrating C3A surface.The C,A-gypsum mixture, on contact with a solution of superplasticizer, adsorbs large amounts of the admix- ture, whereas the mixture prehydrated for a few minutes adsorbs substantially lesser amounts. In -concrete tech- nology the superplasticizer is known to act more effec- tively if it is added a few minutes after the mixing water has been added to concrete. By this late addition lesser amounts of superplasticizer are adsorbed by C3A
+
. gypsum, and the superplasticizer remaining in solution is capable of dispersing the silicate phases.0 1 2 3 U 5 6
ACKNOWLEDGMENTS
The author wishes to thank G.M. Polomark for experimental assis- tance. This paper is a contribution from the Division of Building Re- search, National Research Council Canada, and is published with the approval of the Director of the Division.
REFERENCES
1. Ramachandran, V. S.; Feldman, R. F.; and Beaudoin, J . J . , Concrete Science. Heyden and Son Limited, London, 1981,427 pp.
2. Rixom, M. R., Chemical Admixtures in Concrete, E . & F. N .
Spon Limited, London, 1978,234 pp.
3. Superplasticizers in Concrete, SP-62, American Concrete lnsti-
tute, Detroit, 1979, 427 pp.
4. Developments in the. Use of Superplasticizers, SP-68, American Concrete Institute, Detroit, 1981, 561 pp.
5. Ramachandran, V. S., "lnfluence of Superplasticizers on the Hydration of Cement," Proceedings, 3rd International Congress on Polymers in Concrete, Koriyama, 1981, V. 2, pp. 1071-1081.
6. Sakai, E.; Raina, K.; Asaga, K.; Goto, S.; and Kondo, R., "In- fluence of Sodium Aromatic Sulfonates on the Hydration of Trical- cium Aluminate With or Without Gypsum," Cement and Concrete
Research. V . 10, No. 3, May 1980, pp. 31 1-319.
7. Massazza, F.; Costa, U.; and Corbella, E., "Influence of 0-
Naphthalene Formaldehyde Condensate Superplasticizing Admixture
on CIA Hydration," Seminar on Reaction of Aluminates During the .
Setting of Cement, Eindhoven, Apr. 1977.
8. Ramachandran, V. S., Feldman, R. F., "Effect of Lignosulfo- nate on Tricalcium Aluminate and Its Hydration Products," Mate-
rials and Structures, Research and Testing (RILEM, Paris), V . 5, No.
26, Mar.-Apr. 1972, pp. 67-76.
9. Ramachandran, V. S., and Feldman, R. F., "Adsorption of Ca- Lignosulfonate on CIA and Its Hydrates in a Non-Aqueous Me- dium," Cement Technology (London), V . 2, No. 4, July-Aug. 1971, pp. 121-129.
10. Dosch, W., "Intercrystalline Adsorption of Water and Organic Substances by Tetracalcium Aluminate Hydrates (Adsorption Inter- cristalline d'Eau et de Substances Organiques par les Aluminates Tet- racalciques Hydrates)," Neue Jahrbuch fur Mineralogie Abhandhun-
gen. V . 106, 1967. pp. 200-239.
11. Young, J. F., "Effect of Organic Compounds on the Intercon- versions of Calcium Aluminate Hydrates. Hydration of Tricalcium Aluminate," American Ceramic Society Journal. V . 53, No. 2, Feb. 1970, pp. 65-69.
12. Slaniaka, S., "Influence of Water-Soluble Melamine Formal-
dehyde Resin on Hydration of C3S, CIA
+
Ca.30,-2H,O Mixes and Ce-ment Pastes," Proceedings. 7th International Congress on the Chem-
istry of Cement (Paris, 1980). Editions Septima, Paris, 1980, V. 2, pp.
161-166.
13. Collepardi, M.; Corradi, M.; and Baldini, G., "Hydration of C3A in the Presence of Lignosulfonate-Carbonate System or Sulfo- nated Naphthalene Polymer," Proceedings, 7th International Con- gress on the Chemistry of Cement (Paris, 1980), Editions Septima, Paris, 1980, V. 4, pp. 524-528.
14. Massazza, Franco; Costa, Umberto; and Barrila, Alessandro," Adsorption of Superplasticizers on Calcium Aluminate Monosulfate Hydrate," Developments in the Use of Superplasticizers, SP-68, American C-oncrete Institute, Detroit, 1981, pp. 499-514.
Authorized Reprint From
American Concrete I n s t i t u t e
This publication i s being d i s t r i b u t e d by the Division of Building R e s e a r c h of the National R e s e a r c h Council of Canada. I t should not b e r e p r o d u c e d i n whole o r in p a r t without p e r m i s s i o n of the original publisher. The Di- vision would b e glad to b e of a s s i s t a n c e i n obtaining s u c h p e r m i s s i o n .
Publications of the Division m a y b e obtained by m a i l - ing the a p p r o p r i a t e r e m i t t a n c e ( a Bank, E x p r e s s , o r P o s t Office Money O r d e r , o r a cheque, m a d e payable t o the R e c e i v e r G e n e r a l of Canada, c r e d i t NRC) to t h e National R e s e a r c h Council of Canada, Ottawa. K1A OR6.
Stamps a r e not acceptable.
A l i s t of allpublications of the Division i s available and m a y be obtained f r o m the Publications Section, Division of Building R e a e a r c h , National R e s e a r c h Council of Canada, Ottawa. KIA OR6.