Publisher’s version / Version de l'éditeur:
Highway Research Record, 62, pp. 106-118, 1965-05-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
A study of length changes of compacts of Portland cement on
exposure to H
₂O
Feldman, R. F.; Sereda, P. J.; Ramachandran, V. S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=234c2cc6-e96d-4c5e-a4c8-7dc730c1b25c https://publications-cnrc.canada.ca/fra/voir/objet/?id=234c2cc6-e96d-4c5e-a4c8-7dc730c1b25c
S e r TKI. N 2 l r 2
no.
248 c . 2BLDG
NATIONAL RESEARCH COUNCIL CANADA
DIVISION O F BUILDING RESEARCH
A Study of Length Changes of Compacts of
Portland Cement
on
Exposure to
W 2 0
"Y
R. F . FELDMAN, P. J. SEREDA and V. S. RAMACHANDRAN
Reprinted f r o m
Highway R e s e a r c h R e c o r d Number 62 (1964) Highway R e s e a r c h Board, Washington, D. C . , U. S. A.
p . 106 - 118 R e s e a r c h P a p e r No. 248 of the Division of Building R e s e a r c h P r i c e 25 c e n t s NRC 8514 OTTAWA May 1965
E T U D E D E L'HYDRATATION DU CIMENT PORTLAND PAR LA METHODE DES VARIATIONS D E LONGUEUR DES
ENSEMBLES COMPACTS SO MMAIRE L e s d i f f g r e n t e s mCthodes d e s t i n C e s 'a llCtude d e l a v i t e s s e d ' h y d r a t a t i o n d u c i m e n t P o r t l a n d s o n t p a s s C e s e n r e v u e , c o m p t e t e n u p a r t i c u l i k r e m e n t d e s m e s u r e s v o l u m C t r i q u e s . L a n o u v e l l e mCthode dCcrite p e r m e t d ' b t u - d i e r l l a c t i o n r t c i p r o q u e d e l ' e a u , d u c i m e n t P o r t l a n d e t d e s e s c o n s t i t u - a n t s e n s u i v a n t l e s v a r i a t i o n s d e l o n g u e u r e n fonction d u t e m p s d l u n e n - s e m b l e c o m p a c t c o m p o s 6 dlCICments non h y d r a t C s . L a f o r m a t i o n e t l e s p r o p r i C t 6 s d e s e n s e m b l e s c o m p a c t s s o n t Cgalement indiquCes d a n s l e s g r a n d e s l i g n e s . On d i s c u t e d e s m g r i t e s de c e t t e mCthode, s o n p r i n c i p a l a v a n t a g e Ctant que l e s p a r t i c u l e s d e c i m e n t non hydratC s o n t en c o n t a c t d i r e c t l e s u n e s p a r r a p p o r t aux a u t r e s . C e c i p e r m e t d e dCtecter immC- d i a t e m e n t , p a r l a v a r i a t i o n d e l o n g u e u r , l a f o r m a t i o n d e c o r p s hydratCs a u t o u r d e l a p a r t i c u l e e t d l C t u d i e r l a r C a c t i o n d ' h y d r a t a t i o n e t l e s a u t r e s e f f e t s d k s que c o m m e n c e l ' h u m i d i f i c a t i o n . Un Cchantillon dont l e s d i m e n - s i o n s s o n t de l ' o r d r e d e 1 x 0 . 2 5 x 0 . 0 6 p c e p e r m e t un c o n t r 6 l e p r C c i s d e l a t e m p C r a t u r e e t c o m p o r t e c o m m e avantage s u p p l C m e n t a i r e l a p o s s i - bilit6 d ' Ctudier l l h y d r a t a t i o n e n p h a s e v a p e u r . On donne l e s r C s u l t a t s d e s v a r i a t i o n s de l o n g u e u r d u e s 'a l ' e x p o s i t i o n 'a l l e a u e n p h a s e v a p e u r s o u s une h u m i d i t 6 r e l a t i v e d e 20, d e 50 e t de 85%. D e s r C s u l t a t s s o n t a u s s i prCsentCs p o u r l l h y d r a t a t i o n e n p h a s e l i q u i d e , y c o m p r i s d e s o b s e r v a t i o n s f a i t e s s e u l e m e n t une m i n u t e apr'es l e dgbut d e l l h y d r a t a t i o n a i n s i q u e l l e f f e t s u r l l h y d r a t a t i o n subsCquente e n p h a s e l i q u i d e , d e l ' e x p o s i t i o n p r C a l a b l e a u x t r o i s d e g r ' e s dlhumiditC p o u r une durCe a l l a n t d e 5 m i n u t e s
'a 5 j o u r s . L e s e x p h r i e n c e s ont dbmontrC que l ' e x p a n s i o n c o m m e n c e a u m o m e n t m E m e d e l ' e x p o s i t i o n 'a l l e a u ( e n p h a s e l i q u i d e ou v a p e u r ) e t s e continue a v e c l e t e m p s , e t que l ' e x p o s i t i o n d e s s p g c i m e n s m E m e 'a d e s d e g r C s dlhurniditC peu 6levi.s a f f e c t e l a v i t e s s e d e l ' h y d r a t a t i o n s u b s b - quente. L a s i g n i f i c a t i o n d e s t e r r n e s " p r i s e " e t " d u r c i s s e m e n t " e s t d i s
-
cutCe e n c e qui c o n c e r n e l e s e n s e m b l e s c o m p a c t s .A St~ldy of Length Changes of Compacts of
Portland
Cement on
Exposure
to
H z 0
R. F. FELDMAN, P. J. SEREDA and V. S. RAMACHANDRAN
Respectively, Research Officer and Head, Inorganic Materials Section, Division of Building Research, National R e s e a r c h Council of Canada; and P o s t Doctorate Fellow, C e n t r a l Building R e s e a r c h Institute, Roorkee, India
The various methods f o r studying the hydration r a t e of portland cement a r e reviewed, with particular reference to volumetric m e a s u r e m e n t s . A new method is described for studying the in- teractionwithH,Oof portland cement and i t s constituents by fol- lowing the length change with time of a compact of unhydrated m a t e r i a l ; the formation and properties of the compacts a r e a l s o outlined. The advantages of this method a r e discussed, the chief being that the particles of unhydrated cement a r e i n d i r e c t contact with oneanother; this enables the length change to reflect i m m e - diately the formation of hydrated products a t the particle bound- a r i e s and the study of hydration reaction and other effects within the f i r s t seconds of wetting. Sample s i z e of the o r d e r of 1 by 0 . 2 5 by 0.06 in. allows close t e m p e r a t u r e control and the possibility of a study of vapor phase hydration i s cited a s a f u r t h e r advantage. Results of length changes a r e presented f o r vapor phase exposure a t 20, 50 and 85 percent RH. Results a r e presented also for liquid phase hydration, including observations made only one minute after the s t a r t of hydration and including the effect of pre-exposure f o r periods f r o m 5 m i n t o 5 days a t the t h r e e humidities on s u b s e - quent liquid phase hydration. The experiments showed that expan- sion begins immediately on exposure to water (Liquid o r vapor) and continues with time and that pre-exposure of the specimens even a t low humidities has an effect on subsequent hydration r a t e s . The meaning of "setting" and "hardening" with r e g a r d to compacts i s discussed.
.THE HYDRATION r a t e of portland cement has been studied by many methods, one of these being the strength development of concrete a s a function of time. Other methods include the microscopic examination of pastes and X-ray diffraction analysis of the un- hydrated cement in hardened pastes. Whereas the l a t t e r method may eventually be b e s t f o r measuring the r a t e of hydration of the individual phases, other techniques will always add to the knowledge concerning the mechanism of hydration. These techniques, listed a s indirect methods ( I ) , a r e concerned with the measurement of a change in the physical and chemical p r o p e r t i e s with time. Among these a r e the evolution of the heat of hydra- tion, the change in the non-evaporable w a t e r , the development of specific s u r f a c e , and the formation of calcium hydroxide. The volume change method, in addition to being a valuable adjunct to other methods, has s o m e advantages because i t r e f l e c t s not only the chemical, but a l s o the physical, changes.
Paper sponsored by Committee on Basic Research P e r t a i n i n g t o P o r t l a n d Cement and Concrete.
VOLUME CHANGE METHODS
When portland cement is in the presence of w a t e r , the chemical and physical changes that occur a r e accompanied by a reduction i n the total absolute volume of the s y s t e m . This was shown f i r s t by Le Chatelier (2) and l a t e r by Kuhl (3) and has a l s o been shown f o r other hydrating s y s t e m s (4). Le ~ h a t e ~ i e r a l s o observed the apparent volume in- c r e a s e of the solid during hydration a s made evident by the bursting of the inclosing v e s s e l s . A. H. White (5) reviewed the considerable experimental work (6, 7, - 8, - 9)done
before 1915 on measuring the volume changes of neat cement and c o n c r e t e b a r s . The r e s u l t s of this work showed that cement expanded continuously when kept in water and contracted markedly when maintained in a i r . This e a r l y work led to volume change studies which may be divided according to t h r e e methods:
1. Measurement of absolute volume change of the s y s t e m by the dilatometer, 2. Measurement of apparent volume change of s o l i d s , and
3. Measurement of the sedimentation r a t e of paste and m o r t a r s .
Method 1 was refined by a succession of w o r k e r s (2, 4, 10, 11) and was applied to p l a s t e r of p a r i s , various types of portland cement, the effect of additives on portland cement, and gelatin. Although a good relationship between heat of hydration and volume d e c r e a s e f o r portland cement was not obtained, a m e a s u r e of the r a t e of hydration was found, including the effect of gypsum a s the r e t a r d e r .
Much of the work relating to Method 2 has been standardized (12) and applied to study of apparent volume change f r o m the aspect o i volume stability (13). In his investigations of varying compositions of f r e e lime, magnesia, reactive s i l i c a i n portland cement and various clinker compositions, Bogue (14) did, i n effect, study the magnitude and r a t e of hydration of s o m e of these componentsin cement. T h i s volume change work extended into autoclave reactions, alkali reactivity of cement aggregates, and sulfate r e s i s t a n c e of cements (15). More s u c c e s s in correlating volume change with reaction r a t e was ob- tained by L. C h a s s e v e n t (11) f o r the p l a s t e r of p a r i s s y s t e m and recently in this labora- tory f o r p l a s t e r of p a r i s
(16)
and lime (17). Where the study dealt with a single chemical compound, the s u c c e s s wasunderstandable a s compared with multicomponent s y s t e m s .Method 3, used mainly by P o w e r s (18) and Steinour (19), indicated that the s i z e , shape, and number of cement particles-are not a l t e r e d appreciably by the reactions oc- c u r r i n g within the f i r s t hour o r s o a f t e r contact with water. This method produced i m - portant physical evidence i n opposition to the theory of Baikov (20) and Rehbinder (21) that a t e a r l y hydration t i m e s , the cement p a r t i c l e s a r e d i s p e r s e d o r frcolloidized. " A S pointed out by Powers (22), the evidence on which the theory was based, the rapid d e - velopment of a r e a a t e a f i y hydration t i m e s , may be explained by the thin layer of g e l on the unhydrated cement particles.
In the development of the new volume change method, a n attempt was made to over- come s o m e of the chief limitations of the previous methods f o r measuring the apparent volume change, e . g.
,
control of the t e m p e r a t u r e throughout the m a s s of the sample dur- ing hydration, and the ability to m e a s u r e the length change f r o m the moment the water comes in contact with the solid. This method affords the added f e a t u r e of observing volume change when cement is hydrated with water i n the vapor phase.A NEW VOLUME CHANGE METHOD
It has been shown (23 -9 A 24) that d r y , powdered, inorganic m a t e r i a l s , such a s calcium
carbonate, s i l i c a , plaster of p a r i s and calcium oxide (17), can be compacted to produce rigid porous bodies; f u r t h e r , these bodies can be u s e d & - the study of their length change isotherms. Compacts of unhydrated portland cement have now been produced and t h e i r length changes w e r e m e a s u r e d during the period of hydration.
Materials
The cement used was a Type I with the chemical and physical properties shown in Table 1. It was obtained directly f r o m the plant and s t o r e d in s m a l l sealed containers
indesiccators over magnesium perchlorate. The extent of carbonation is indicated in Table 1.
TABLE 1
ANALYSES O F UNHYDRATED PORTLAND CEMENT Analysis Percent Chemical: Cao SiQ A L 4 Fe2 4 MgO SQ Naz 0 K2 0 Free l i m e CO, Potential Mineralogical: c3s Physical:
Blaine surface area = 3,240 sq cnv'gm Autoclave expansion = 0 . 0 1 percent Initial set Vicat = 3 h r 45 m i n
Initial s e t Gillmore = 3 h r 3 0 nlin Final set Gillmore
-
5 h r 15 min Compressive strength:at 3 days 1,445 psi
at 7 days 2 , 6 1 7 psi
Experimental
-
-Fabrication of Compacts. -The com- pacts were fabricated in a mold 1 . 2 5 in. in diameter, c a r e being taken to insure that the m a t e r i a l was evenly distributed s o that the load could be evenly applied. Compacts were produced under p r e s s u r e s varying from 8,150 to 118,000 psi. The entire procedure was performed in a d r y box using magnesium perchlorate (anhy- r o u s ) a s a drying agent; i t was found that the relative humidity in the box was main- tained well below 1 percent. Sufficient m a t e r i a l was compacted to give a final thickness of 0.06 in. and a specimen of rectangular shape, approximately 1 by 0.25 in.
,
was cut from it. The sample normally weighed l e s s than one g r a m .Extensometer and Hydration Cell. -
Because of the s m a l l sample s i z e , an a c - curate means of length change rneasure- ment had to be used. This was achieved by a modified Tuckerman gage extensom- e t e r (Fig. 1). This optical instrument measured the length change with an accu- racy of 2 x in./in. The sample (1) was held against the knife edges (2 and 3 )
by a light spring (4), the sample resting on a holder (5). The optical system con- sisted of a fixed m i r r o r (6) and a tilting m i r r o r (7) which was one face of the rock- ing lozenge. The whole assembly was supported by a stand. The final assembly remained in the dry box during and after mounting of the sample on the extensom- e t e r and then was carefully placed in the
hydration cell. This c e l l (Fig. 2) i s made Figure 1. Extensometer.
s o that the sample can be subjected to high vacuum; ground g l a s s joints and valves
a r e used in i t s construction to exclude COz. The cell is provided with an optical window through which the extensometer can be read. A pipette arrangement i s used f o r inject- ing water a t a controlled r a t e when hydration in the liquid phase i s to begin. E x c e s s water may a l s o be withdrawn f r o m the sample when required.
When the sample and assembly a r e placed in the cell, the optical window assembly i s placed on the ground joint (4) and the valve (2) i s opened to vacuum (Fig. 2). After evacuation the c e l l i s removed from the d r y box and placed in a temperature controlled bath.
Measurement of Length Change a s Function of Hydration Time. -The measurement of length change during the vapor phase exposure of the compact can be achieved a t any desired humidity by joining the cell through the ball and socket joint to a vacuum s y s t e m ; f r o m this s y s t e m water vapor a t any d e s i r e d humidity may be obtained. Because this exposure proceeds with the exclusion of a l l g a s e s except water vapor, the diffusion r a t e of the vapor is rapid and the desired humidity is attained quickly.
F i g u r e 2 . Hydra-tion c e l l (1, o p t i c a l riin- do1.r; 2 , v a l v e t o v a c u w ; 3, c o n t r o l l i n g v a l v e ; 4, l a r g e g r o u ~ d g l a s s j o i n t ; 5 ,
ba1.l and s o c k e t j o i n t ; 6, p i p e t t e ) .
Liquid phase hydration i s a l s o achieved without exposure of the s a m p l e t o a i r . The pipette containing liquid water i s placed on the joints (Fig. 2); the assembly i s then placed in the constant t e m p e r a t u r e bath until the pipette has reached bath t e m p e r a - ture. Water i s then injected by the con- trolling valve (3) until the s a m p l e is en- t i r e l y i m m e r s e d . This procedure i s c o m - pleted in 1 5 s e c , a f t e r which readings of length change begin. After a given t i m e , the level of the water i s lowered below the level of the s a m p l e by admitting s o m e a i r into the cell.
Results
Mercury P o r o s i m e t r y . -Plots of void fraction against the logarithm of the com- paction p r e s s u r e have yielded c h a r a c t e r - i s t i c linear relationships f o r s e v e r a l mate- r i a l s (23, 25) and this i s a l s o o b s e r v e d f o r
-
- the unhydrated cement used in these ex-F i g u r e 3. R e l a t i o r i s h i p o f t o t a l p o r e v o l - Lune v s compaction p r e s s u r e f o r u n h y d r a t e d
cement.
8,125 LB/IN'
TOTAL PORE VOL= 0 151 CC/G
48,750 L8/lN2
TOTAL PORE VOL: 0 106 CC/G
81,250 LB/IN' TOTAL PORE VOL: 0 0 9 0 CC/G
PORE SIZE DIAMETER, MICRONS
Fi.gure 4. E f f e c t of compaction p r e s s u r e on p o r e s i z e d i s t r i b u t i o n and p o r e volume of
periments. Figure 3 i s a plot of this relationship f o r compaction p r e s s u r e s varying f r o m 8,150 to 118,000 psi. Mercury penetration r e s u l t s shown in Figure 4 illustrate how the pore s i z e distribution i s changed and the pore volume is lowered with i n c r e a s - ing p r e s s u r e of compaction.
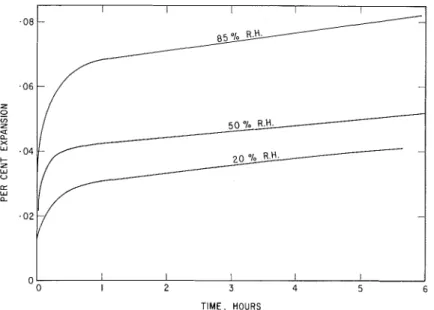
Vapor Phase Exposure. -After initial degassing, during which only slight contraction was observed, vapor phase measurements w e r e taken a t t h r e e relative humidities: 85, 50, and 20 percent on compacts formed a t 49,000 psi. Although readings were taken after 1 min, i t was observed that an expansion began on exposure of the sample to water vapor.
F o r each humidity, the high initial r a t e of expansion was followed by a continually decreasing r a t e reaching a negligible value a f t e r 5 d a y s , when the experiments w e r e terminated. The higher the humidity, the g r e a t e r was the initial r a t e of expansion and the final value f o r the expansion. The c u r v e s in Figure 5 a r e the mean values obtained f r o m 1 0 to 30 experiments showing the g e n e r a l c h a r a c t e r i s t i c s . Results varied some- what depending on the bottle of unhydrated cement used even though f r o m the s a m e batch; deviation f r o m the mean a t each humidity was a s much a s 20 percent. It was observed, however, that expansions attained mean values of approximately 0. 14, 0. 08, and 0.05 percent a f t e r exposure to 85, 50, and 20 percent RH, respectively. The reproducibility i s thus good enough to make the differences a t the various humidities significant.
Liquid P h a s e Hydration. -Results w e r e obtained f o r the expansion of the compacted
--
specimen a s a function of time from the f i r s t minute a f t e r they w e r e submerged i n w a t e r a t 70 F . These r e s u l t s and those f o r s a m p l e s subjected to pre-exposure to water vapor a r e included in F i g u r e s 6, 7 and 8. Although the expansion r a t e d e c r e a s e d with time, a measurable r a t e was observed f o r a s long a s m e a s u r e m e n t s were taken (12 days). Expansion of up to 0.30 percent was obtained within a 24-hr period.
Effect of Vapor Exposure on Expansion Due to Liquid P h a s e Hydration. -Results showing the effect of pre-exposure a t 85, 50, and 20 percent RH f o r t i m e s of approxi- mately 5 min, 6 h r , 24 h r and 5 days a r e shown in F i g u r e s 6, 7, and 8. Rates a r e very much reduced f o r the 85 percent humidity curves and f o r the 5-day exposure the r a t e of expansion i s almost negligible. Even f o r the 5-min exposure the r a t e w a s considerably reduced, indicating considerable interaction a t s o s h o r t a time. At 50 percent RH, r e - s u l t s s t i l l indicate a considerable interaction, although to a much l e s s extent than a t 85 percent; exposure f o r 5 min a t 85 percent will reduce the initial r a t e (length change f o r
I I I I I
I
0 I 2 3 4 5 6
TIME, HOURS
0,04
1
0 ' h0 I 2 3 4 5 6
TIME, HOURS
Figure 6. Expansion during submersion of cement pre-exposed to 85 percent NI for various time intervals.
TIME, HOURS
Figure 7. Expansion during submersion of cement pre-exposed to 50 percent RH for various time intervals.
0
1
1
1
0 I 2 3 4 5 6
T I M E , HOURS
F i c u r e 8. Expansion during sul~mersion of ce!ncnt pre-exposed t o PO p e r c e n t RFI f o r various time i n t e r v a l s .
TIME OF PRE - E X P O S U R E , DAYS
F i g u r e 9. Expansion a f t c r f i r s t ~ l l i n u t e of submersi.on vs prc-expos~u-e time t o va.rious r e l a t i v e h u m i d i t i e s .
the f i r s t minute) a s much as a t 50 p e r c e n t f o r 5 h r (Fig. 9). Continued exposure a t 50 p e r c e n t f o r g r e a t e r than 5 h r does not g r e a t l y a l t e r the i n i t i a l r a t e of expansion. This i s a l s o indicated by the r e s u l t s of the vapor phase hydration (Fig. 5). R e s u l t s of e x - posure a t 20 p e r c e n t RH show a s i m i l a r t r e n d . It is significant, a s shown by the vapor phase r e s u l t s , that t h e r e is considerable interaction a t s u c h low humidities between the w a t e r vapor and the unhydrated cement.
T h e r e s u l t s obtained f r o m s u b m e r g i n g s a m p l e s i n w a t e r a r e reproducible f o r t h e s a m p l e s that w e r e pre-exposed f o r 5 h r , 24 h r , and 5 d a y s a t 85 p e r c e n t RH. F o r the s a m p l e s pre-exposed f o r 5 h r a t 50 p e r c e n t RH, the value of the expansion a t 1 min may v a r y by 15 percent, whereas those pre-exposed a t the s a m e t i m e a t 20 percent humidity may v a r y by about 3 0 percent. Lf, however, s a m p l e s w e r e taken f o r which the r e s u l t s f o r vapor hydration did not v a r y m o r e than 10 p e r c e n t , the reproducibility of r e s u l t s of liquid phase hydration w a s within 1 0 p e r c e n t . Although the cement may b e affected t o different d e g r e e s by the m o i s t u r e and COz contamination, t h e possibility of a variation of the c l i n k e r composition f r o m s a m p l e to s a m p l e is v e r y r e a l . This is a disadvantage in using s m a l l s a m p l e s . Despite t h e s e difficulties, the t r e n d s shown a r e c o n s i d e r e d to b e significant.
Discussion
P o r e S t r u c t u r e of Compact. -It h a s been shown
(26)
that t h e compaction behavior of powders depends markedly on t h e i r h a r d n e s s and that t h e i r r e s u l t s a r e d e s c r i b e d by two n e a r l y independent probabilistic p r o c e s s e s . The f i r s t is the filling of holes of the s a m e o r d e r of s i z e a s the o r i g i n a l p a r t i c l e s . T h i s o c c u r s p r i m a r i l y by p a r t i c l e s sliding p a s t one another, which may r e q u i r e slight f r a c t u r i n g . The second p r o c e s s is associ- a t e d with the filling of voids substantially s m a l l e r than the original p a r t i c l e s by p l a s t i c flow o r by fragmentation. T h i s p r o c e s s , however, does not add significantly t o the s u r - f a c e a r e a of the m a t e r i a l(2).
The p o r e s t r u c t u r e of t h e compact is quite reproducible and, a s shown i n F i g u r e s 3 and 4, m a y be defined by the compaction p r e s s u r e . The s t r e n g t h and rigidity of this body is probably d e r i v e d m o r e f r o m physical than c h e m i c a l f o r c e s , and a s shown by C z e r n i n (27), compacted bodies c a n develop f a i r s t r e n g t h s .T h e compact, with i t s p a r t i c l e s i n d i r e c t contact and i t s n a t u r a l rigidity, p o s s e s s e s the p r i m a r y s t r u c t u r e f r o m which the f i n a l s t r u c t u r e is developed on hydration (28, - - 29). The important c h a r a c t e r i s t i c is that i t p o s s e s s e s this s t r u c t u r e before any contact with w a t e r , and the effective w a t e d c e m e n t r a t i o will be definedby p o r e v o l u m e of thecompact.
Control of Conditions. -Because of t h e i r s i z e . t h e s e bodies can be m a d e i n controlled ~ - - - -
humidity conditions and e x p e r i m e n t s c a n b e p e r f o r m e d i n vacuum. T e m p e r a t u r e control can be e a s i l y maintained externally but i t is within a s a m p l e that c o n t r o l is difficult. The hydration of c e m e n t is a n e x o t h e r m i c r e a c t i o n and u n l e s s the heat c a n b e removed rapidly f r o m the s i t e of the reaction, the t e m p e r a t u r e will r i s e . The likelihood of this happening depends on the m a s s of the s a m p l e f o r the quantity of heat liberated and on the thickness of the s a m p l e f o r the r a t e of heat t r a n s f e r f r o m within. Optimum condi- tions a r e achieved i n t h i s method by having a v e r y thin s a m p l e ; this is not usually pos- sible i n any of the o t h e r volume change methods.
The u s e of s m a l l s a m p l e s a l s o h a s the advantage of avoiding g r a d i e n t s of s t r e s s . When using l a r g e b a r s i n the t e s t , although of g r e a t e r p r a c t i c a l importance, the volume changes would not c o r r e c t l y r e f l e c t the p r o g r e s s of a reaction. Volume change gradients through the l a r g e r body c a n c a u s e d e c r e a s e i n s t r e n g t h , and finally, d e s t r u c t i o n of the body.
M e a s u r e m e n t s a t E a r l y T i m e s . -The p r i m a r y advantage of this method is the ability to o b s e r v e within the f i r s t minute the volume change when w a t e r c o m e s i n contact with the unhydrated c e m e n t p a r t i c l e s . The s a m p l e is under vacuum when w a t e r i s injected and t h i s f o s t e r s the rapid distribution of w a t e r i n t h e p o r e s and consequent r a p i d wetting of a l l the s u r f a c e . B e c a u s e the p a r t i c l e s a r e i n contact with e a c h o t h e r , any physical changes in the p a r t i c l e s due to r e a c t i o n will b e reflected a s a n apparent volume change of t h e s y s t e m . F o r t h e s e r e a s o n s the m e a s u r e m e n t of length change of a compact should r e f l e c t the r a t e of r e a c t i o n without any lag in t i m e .
C e m e n t p a s t e , a s is u s e d in the o t h e r volume change methods, i s f o r m e d during s e v e r a l m i n u t e s of mixing and handling; i t is a p l a s t i c cohesive m a s s composed of-an
aqueous solution and p a r t i c l e s of clinker. Owing to the nature of the paste, molding techniques w e r e used and sometimes, in the m o r t a r b a r methods, readings w e r e n i t taken t i l l 24 h r a f t e r the initial wetting. In other methods, however, readings w e r e taken after 1 5 min o r l e s s ; in the sedimentation technique, the t i m e may be l e s s .
The p r i m a r y s t r u c t u r e of cement paste i s the network of d i s c r e t e p a r t i c l e s formed by the p r o c e s s of mixing the p a r t i c l e s with water. These p a r t i c l e s a r e s e p a r a t e d f r o m each other by water and until they come into contact with each other, a n expansion due to initial formation of hydrate product on the s u r f a c e of the p a r t i c l e cannot b e observed. In fact, i t has been reported
(29)
that the e a r l i e s t t i m e at which an expansion h a s been observed in portland cement paste is 1% h r a f t e r mixing. Measurements in a i r of vol- ume changes of normally mixed p a s t e s have shown marked and extended contractions f r o m e a r l y ages. Thus, no volume change representing the formation and movement of the hydrated product f r o m the f i r s t instant of wetting c a n b e made by o t h e r methods f o r liquid phase hydration.Vapor Phase Exposure. -Measurement of volume change during vapor exposure can only be made by this method. The cement particles m u s t never come into contact with water before the study, and the p r i m a r y s t r u c t u r e m u s t not only be established but m u s t be r e p r e s e n t e d by particle contact s o that the limited hydration which may take place can be detected and measured. This d i r e c t contact of the cement p a r t i c l e s avoids a l l contraction during vapor phase exposure even a t low humidities.
The discussion of p r i m a r y s t r u c t u r e and particle-to-particle contact leads to the sub- ject of the "setting" o r "hardening" of cement. A s was pointed out by Steinour (30), these t e r m s a r o s e f r o m p r a c t i c a l considerations and a r e not theoretical.
everth he less,
t h e r e has been s o m e debate a s to the difference in the nature of the hydration p r o c e s s before and a f t e r setting. When the t e r m s a r e applied to the hydration of compacted portland cement, one is forced to conclude that setting has taken place before hydration has begun o r a t l e a s t within a few minutes a f t e r exposure to water.
s t u d ; of Effect of E x t e r n a l Agents o r
~dditives.- he
effect of r e t a r d e r s o r a c c e l e r - a t o r s on e a r l y hydration can b e followed by volume changes i n a s i m i l a r manner a s by the other methods: either by mixing the additive with t h e unhydrated cement powder orby dissolving i t i n the water to be introduced to the s a m p l e .
G r e a t e r versatility is obtained in this method, however, i n the study of e x t e r n a l agents. Effect of carbonation f r o m the g a s phase can b e studied during vapor phase exposure with confidence of attaining uniform carbonation throughout the sample.
Interpretation of Results. -The initial expansion of the unhydrated cement compact may be attributed to the phenomena of s t r e s s relaxation, dispersion on wetting, physical adsorption, and hydration of the cement p a r t i c l e s . In can b e expected that d r y powders when compacted should be found in a s t a t e of s t r e s s and that this s t r e s s may be relaxed through i r r e v e r s i b l e expansion when the body i s wetted by liquids. Cyclic wetting by organic liquids, such a s methanol, f o r s i m i l a r s y s t e m s did not r e s u l t in i r r e v e r s i b l e expansion, thus indicating that the r e s i d u a l s t r e s s i s negligible f o r the p r e s s u r e r a n g e s used in these experiments.
Compacts of s o m e m a t e r i a l s d i s p e r s e when i m m e r s e d in water. Such compacts, however, will behave a s rigid bodies up to high humidities a s was shown (23, 24) f o r compacts of calcium carbonate. F o r p l a s t e r of p a r i s i t has been found ( 1 6 m X a s m a l l amount of hydration on the p a r t i c l e s by exposure to vapor will prevent a n y d i s i n t e g r a t i o n on submersion in water. Compacts of unhydrated cement, even without exposure to vapor, show no tendency to d i s p e r s e when i m m e r s e d i n water.
T h e r e i s no doubt that s o m e expansion will occur due to physical adsorption; a t 20 percent RH, expansion may be due entirely to this, because s o m e previous work (31) has suggested f r o m m e a s u r e m e n t s of "evaporable and non-evaporable water" t h a t n o interaction other than physical adsorption takes place a t this humidity. The concept of "evaporable w a t e r , " however, is somewhat a r b i t r a r y a s f a r as an absolute definition of physically o r chemically sorbed w a t e r is concerned. The amount of reaction product formed a t 20 percent RH in all likelihood would be v e r y s m a l l and may not be of the s a m e nature a s in liquid phase hydration; s o m e f o r m of chemisorption may be occurring. The formation of s m a l l quantities of hydration product formed on the s u r f a c e of each particle may be sufficient to be detected by this technique.
I I I I I 0 5 M I N - - \ \ - \ \ 0 \ \ \ \ \ \ \ \
i
- 0 , I I I I I 0 I 2 3 4 5 6 T I M E , D A Y S F i g u r e 10. Expansion on e x p o s u r e to 20 p e r c e n t HI 2nd contrac1;ion on e v u c u a t i o n .The evidence f o r considering that chemical interaction takes place a t 20 percent RH is: 1. Expansion o c c u r r e d continuously, although at a decreasing r a t e f o r a s long a s ob- servations w e r e taken (5 days); this is illustrated in Figures 5 and 10 and presented in the r e s u l t s ; f o r physical adsorption a t low humidities, equilibrium is attained in a few hours.
2. Vapor phase exposure affects subsequent liquid phase exposure (Fig. 9); this m u s t be attributed to chemical reaction because physical adsorption could not have this influence and water vapor pre-exposure was c a r r i e d out in a high vacuum apparatus where CO, effects would b e eliminated.
3 . Expansion due to physical adsorption was observed
(23)
on compacts of various m a t e r i a l s including precipitated calcium carbonate of p a r t i c l e s i z e 2 to 5 p; expansion a t equilibrium when exposed to 20 percent RH was 0.018 percent. The particle-size distribution of the unhydrated cement, although varying between 1 to 50 p, is such that the majority of the p a r t i c l e s is about 10 p. Physical adsorption could not be expected to produce a l a r g e expansion under these conditions but if a s m a l l quantity of hydrate product was already f o r m e d on the p a r t i c l e s , a significant expansion would be expected. If this is the case, however, the sample must possess adsorbed water in equilibrium with the humidity where hydration can no longer occur (30 percent RH) and this s a m p l e on evacuation should produce contractions g r e a t e r than those observed on subsequent exposure to 20 percent RH. On evacuation before exposure, contraction was in the o r d e r of 0.002 percent.4. Results obtained by exposing four different s a m p l e s to 20 percent RH f o r 5 min, 6 h r , 1 day, and 3% days, respectively, a r e shown in F i g u r e 10. These s a m p l e s w e r e evacuated a t high vacuum until no f u r t h e r contraction o c c u r r e d . The time required f o r this was usually 24 h r . It is observed that a f t e r evacuation the sample remained sub- stantially longer than the original length. The i r r e v e r s i b l e portion i n c r e a s e d with t i m e of exposure. This is consistent with the assumption that some chemical interaction is taking place during exposure to 20 percent RH, producing a high s u r f a c e a r e a product, the formation of which c a u s e s an i r r e v e r s i b l e expansion. As m o r e high s u r f a c e a r e a product is formed, however, m o r e contraction should occur on drying due to i t s higher s u r f a c e a r e a . If this effect was due to physical adsorption, the expansion should be r e v e r s i b l e f o r a l l exposure t i m e s .
The concept of hydration occuring a t low humidities is not new. Calcium oxide has been shown to hydrate completely a t humidities as low as 20 percent (17, - - 32) and the
p r e s e n c e of a "liquid phase" has been considered unnecessary due to the possibilities of "direct1' o r "solid-state" mechanisms. Several authors (33, 34, 35) have discussed the possibility of this mechanism and even a t 50 percent RHEismay-e operative be- cause capillary water would not exist a t this humidity: the s a m p l e s w e r e evacuated be- f o r e exposure and, thus, a r e on the "adsorption" p a r t of the i s o t h e r m .
A portion of the expansion taking place on exposure of the unhydrated cement com- pacts to vapor is thus considered to be due to hydration. The vapor interacts with the unhydrated cement particles on the e x t e r n a l s u r f a c e , causing a p r e s s u r e on the next particle due t o growth of the hydrate product outward. ~ ~ d r a t i o n may a l s o take place within s m a l l c r a c k s in the individual p a r t i c l e s , causing each particle to expand individ- ually; this may m o r e easily explain the immediate expansion of the p a r t i c l e s on expo- s u r e to vapor.
The initial expansion occurring on submersion of the compact in water may be due t o both hydration and p a r t i a l relaxation of the compacts. The hydration that is presumed to take place on vapor phase exposure even a t 20 percent humidity, however, and the effect of reduction of the expansion r a t e on subsequent submersion indicates that the hydration product s u r r o u n d s the p a r t i c l e s with rapid setting of the cement taking place even in the vapor phase. The fairly high r a t e of expansion f o r the f i r s t minute even on vapor phase exposure leads to a n expectation of a high expansion r a t e f o r the s u b m e r - sion experiments. It is concluded that the high expansion in the f i r s t minute of submer- sion even f o r s a m p l e s without pre-exposure to vapor is due predominantly to hydration f r o m the s u r f a c e of the particles. This rapid initial expansion may be correlated to the rapid heat evolution that h a s been observed during the f i r s t few minutes of the hydration of cement (3 6).
The f a c t t h a t hydration a p p e a r s to proceed even a t low relative humidity but a t a d e - creasing r a t e with time indicates that products f o r m a n effective l a y e r around the un- hydrated m a t e r i a l . At a higher humidity, the hydration may proceed f u r t h e r . This behavior differs f r o m that of calcium oxide which hydrates completely in the vapor phase, though a t a lower r a t e than in the liquid phase. This occurs even a t relative humidities below 20 percent (17).
In the liquid phase, the rapid initial expansion, with decreasing rate, does not explain the dormant period and reacceleration observed by c a l o r i m e t r i c m e a s u r e m e n t s during e a r l y hydration (36). The f a c t that the effective water/cement r a t i o of the compact is approximately 0.10 may provide an explanation. Cement is a complex mixture of dif- f e r e n t compounds, however, and not until the behavior of each of them c a n be studied by this method can any interpretations involving the mechanism of hydration and the method of t r a n s p o r t of the hydration product be made.
C ONC LUSIONS
1. This method pro,vides a means f o r following both by vapor and liquid phase the immediate formation of hydrated product a t the s u r f a c e s of cement p a r t i c l e s . Owing to the s m a l l s i z e of the s a m p l e s , control of conditions may be exercised.
2. Cement compacts appear to hydrate and expand immediately when exposed to relative humidities a s low as 20 percent. No contractions a r e observed on vapor phase hydration of cement compacts.
3. Pre-exposure to water vapor h a s a considerable retarding effect on subsequent liquid phase hydration and this effect is a function of both relative humidity and exposure time.
4. Expansion due to liquid phase hydration of compacts begins immediately on wetting and continues a t a decreasing r a t e f o r a t l e a s t 12 days.
ACKNOWLEDGMENT
The authors wish to thank H. F. Slade and S. E. Dods f o r collecting the data and setting up the apparatus required f o r these studies.
REFERENCES
Copeland, L. E . , Kantro, D. L . , and Verbeck, G . , "Chemistry of Hydration of Portland Cement, 11
-
Kinetics of the Hydration of P o r t l a n d Cement."
P r o c . 4th Internat. Symp. on the Chem. of Cement, Washington, p. 429-465 (1960). L e C h a t e l i e r , H.,
"Sur l e s Changements d e Volume qui Accompagnent l e D u r c i s -s e m e n t de C i m e n t s .
"
Bull. Soc. de l 7 E n c o u r a g e m e n t pour 1'Ind. Nat.,
5th S e r .,
5:54 (1900).Kuhl, H . , "Die U r s a c h e d e s T r e i b e n s d e r Zemente." Torund. Zeit., 36:1331 (1912). Neville, H. A.
,
and J o n e s H. C.,
"The Study of Hydration Changes by a VolumeChange Method.
"
Colloid Symp. Monograph (VI), p. 309-318 (1928).White, A. H.
,
"Volume Changes in C o n c r e t e . " Struct. Conservation, p. 2 -16 (1915).Bauschinger, J . , "Untersuchung M e h r e r e S o r t e P o r t l a n d C e m e n t . " Mitt. Mech. Tech. Lab. d . T . H. Miinchen, Heft 8 (1879).
Considere, A . , "Variations d e Volume d e s M o r t i e r s de Ciment d e P o r t l a n d Re- sultant d e l a P r i s e e t de 1'Etat Hygrometrique." ComptesRendus, 129:467 (1899). Campbell, E . B . , and White, A. H . , "Some Conditions Influencing Constancy of
Volume in Portland C e m e n t s . " J o u r . A m e r . Chem. S o c . , 28:1273 (1906). White, A. H . , "Destruction of C e m e n t M o r t a r s and C o n c r e t e Through Expansion
and Contraction.
"
P r o c . ASTM, 11:531 (1911).P o w e r s , T. C . , "Absorption of W a t e r by P o r t l a n d Cement P a s t e During the Hardening P r o c e s s . " Lnd. Eng. C h e m . , 27:790-794 (1935).
Chassevent, L . , "Study of the Variations i n Volume of P l a s t e r s During and After Hardening." Rev. M a t e r . C o n s t r . , Ed. C , 405:188-194, 406:219-224, 407:267- 272, 408:304-308 (1949).
ASTM DesignationC 490-62T, "Tentative Specifications f o r Apparatus f o r Use in M e a s u r e m e n t of Volume Change of Cement P a s t e , M o r t a r , and Concrete." p. 28-32 (1962).
Vironnaud, L.
,
"The E a r l y Stages of C o n c r e t e ."
Ann. Inst. Tech. B2ti. T r a v . P u b . , 154:1003-1016 (1960).Bogue, R. H.
,
"Studies on the Volume Stability of P o r t l a n d Cement P a s t e s . I 'PCA Fellowship at Nat. B u r . S t d . , p. 1-90 (1949).
Thorvaldson, T . , "Chemical A s p e c t s of the Durability of Cement Products." P r o c . 3 r d Internal. Symp. on t h e Chem. of Cement, London, p. 436-466 (1952). S e r e d a , P. J . , Feldman, R. F . , and Ramachandran, V. S . , submitted f o r
publication.
Ramachandran, V. S . , S e r e d a , P. J . , and Feldman, R. F . , "Mechanism of Hydration of Calcium Oxide.
"
To b e publ.P o w e r s , T . C.
,
"The Bleeding of P o r t l a n d C e m e n t P a s t e , M o r t a r and C o n c r e t e ."
PCA R e s . Dept. Bull. 2 (1939).Steinour, H. H.
,
" F u r t h e r Studies of the Bleeding of P o r t l a n d Cement P a s t e s ."
PCA R e s . Dept. Bull. 4 (1945).Baikov, A. A.
,
"On the Theory of Hardening Hydraulic C e m e n t s ."
Compte Rendus, 182:128-129 (1926).Rehbinder, P. A . , "Physico-Chemical Concepts of the Mechanism of Setting and Hardening of M i n e r a l B i n d e r s .
"
(In R u s s i a n ) State Publ. Lit. on Struct. M a t e r .,
Moscow, pp. 125-137 (1956).P o w e r s , T. C . , Discussion of p a p e r " E a r l y Hydration Reactions of Portland Cement" by K. T. G r e e n e . P r o c . 4th Internat. Symp. of t h e Chem. of Cement, Washington, p. 376 (1960).
S e r e d a , P. J . , and Feldman, R. F . , ''Compacts of Powdered M a t e r i a l a s P o r o u s Bodies f o r Use in Sorption Studies.
"
Jour. Appl. Chem.,
13:150-158 (1963). Feldman, R. F . , and S e r e d a , P. J . , "Use of Compacts t o Study the SorptionC h a r a c t e r i s t i c s of Powdered P l a s t e r of P a r i s . " J o u r . Appl. C h e m . , 13:158- 167 (1963).
Amberg, C. H . , and Echigoya, E . "Pelletization and Diffusibility Studies on Silver C a t a l y s t s . " J o u r . Chem. E n g . , 39:215-218 (1961).
Cooper, A. R.
,
and Eaton, L. E . , "Compaction Behavior of S e v e r a l C e r a m i c Powders." J o u r . A m e r . C e r a m . S o c . , 45(3):97-101 (1962).Czernin, W.
,
Discussion of principal p a p e r s (In German). Zement Beton 16:18 (1959).Giertz-Hedstrom, S. "The P h y s i c a l S t r u c t u r e of Hydrated Cements. " P r o c . Symp.
on the Chem. of Cements, Stockholm, p. 505-534 (1938).
P o w e r s , T . C . , "Some Physical Aspects of the Hydration of Portland Cement." Jour. PCA R e s . and Develop. Lab.
,
3(1):47-56 (1961).Steinour, H. H . , "The Setting of Portland Cement. A Reveiw of Theory P e r f o r - mance and Control." PCA R e s . Bull. 98, p. 1-124 (1958).
P o w e r s , T . C . , "A Discussion of Cement Hydration i n Relation t o the Curing of Concrete.
"
P r o c . HRB, 27:178 (1947).Glasson, D. R.
,
"Reactivity of Lime and Related Oxides. 11-Sorption of Water Vapor on Calcium Oxide." J o u r . C h e m . , 8:798-803 (1958).Jeffery, J. W . , " P r a c t i c a l Implications of Fundamental R e s e a r c h i n Cement Hydration." Chem. I n d . , p. 1756-1763 (1955).
Hansen, W. C . , "Solid-Liquid Reactions in Portland Cement P a s t e s . " Mater. R e s . Std.
,
2(6):490-493 (1962).Kelly, R . , "Solid-Liquid Reactions: P a r t 1. The Determination of Solid-Liquid Reaction Mechansims." Can. J o u r . of C h e m . , 38:1209-1216 (1960).
Lerch, W . , "The Influence of Gypsum on the Hydration and P r o p e r t i e s of Portland Cement P a s t e s . " P r o c . ASTM, 46:1252-1292 (1946).