Development of Stimulus-responsive Polydiacetylene Systems for
Molecular Colorimetric Detection
by
Yunfei Zhang
B. S. in Chemical PhysicsDepartment of Chemical Physics, University of Science and Technology of China Hefei, Anhui Province, P. R. China
M. S. in Analytical Chemistry
Department of Chemistry, University of Florida Gainesville, FL, U. S.
Submitted to the Department of Chemistry in Partial Fulfillment of the Requirements for the Degree of
DOCTOR OF PHILOSOPHY
at theMASSACHUSETTS INSTITUTE OF TECHNOLOGY June 2018
C2018 MASSACHUSETTS INSTITUTE OF TECHNOLOGY All Rights Reserved
Signature of Author
Certified by
T. Alan Hatton Professor of Chemical Engineering Thesis Supervisor
Accepted by MASACHSETS ISTIUTESignature
redacted
MASSACHUSETTS INSTITUTE
OF TECHNOLOGY
JUN 20 2018
Robert W. Field Chairman, Departmental Committee on Graduate Students UJ
Signature redacted
1
3epartment of Chemistry/ 2 4
Signature redacted
This doctoral thesis has been examined by a committee of the Department of Chemistry and Department of Chemical Engineering as follows:
Signature redacted
Professor T. Alan Hatton
Professor Sylvia Ceyer
Professor Bradley D. Olsen
Professor Jianshu Cao
Thesis Supervisor;Department of Chemical Engineering
Signature redacted
Thebis Chair; Depadment of Chemistry
____S ignature redacted
Depadtmient of Chemical Engineering
Signature redacted
C,
Department of ChemistrySignature redacted
Department of Cheical Engineering Dr. Lev Bromberg
Development of Stimulus-responsive Polydiacetylene Systems for
Molecular Colorimetric Detection
by Yunfei Zhang
Submitted to the Department of Chemistry on June, 2018 in partial fulfillment Of the requirements for the Degree of Doctor of Philosophy in Chemistry
ABSTRACT
Direct and convenient detection of hazardous molecules in the environment, agriculture, and industry plays a critical role in humanity's health and safety. In order to achieve this goal, I have rationally designed polydiacetylene systems that possess unique optical properties such as color changing in the presence of external stimulus. These advanced polydiacetylene systems are conjugated with functional groups having specific targeting ability toward targeted hazardous molecules to build up a stimulus-responsive structure for molecular colorimetric detections.
In the first part of the work, pyridine-2-aldoxime, capable of binding pesticide malathion, was conjugated with diacetylene monomer, followed by the conjugate polymerization under UV irradiation to form a stimulus-responsive system. The rapid, specific and sensitive colorimetric detection of malathion was demonstrated both in aqueous suspension and on solid support. Within seconds, the successful detection of malathion through color changes observed by the naked eye was accomplished, with a detection limit of mmol/L for liquid suspensions and nmol/cm2 for solid membranes. Furthermore, density functional theory (DFT) calculations on this system before and after binding to malathion unveiled the mechanism of spectral change as the detection progressed. The second part of the work describes the conjugation of dipicolylamine to diacetylene and the polymerization of as-conjugated diacetylene. This polydiacetylene-dipicolylamine system has visible colorimetric response to lanthanide ions exposure within one minute, and this response is highly selective toward lanthanide ions evidenced by the absence of colorimetric response to the control metal ion Hg2+. Remarkably, through the color change observation by naked eye, the lanthanide ions (Gd3+ Ce3' Er3') detection limit was shown to be as low as pmol/L in aqueous
suspensions and nmol/cm2 on solid supports.
In summary, two polydiacetylene systems were developed and demonstrated to be rapid, sensitive and selective sensors for the colorimetric detection of their target molecules. The mechanism of the colorimetric response elucidated by DFT calculations, which is beneficial for future molecular sensor design. These research results not only develop the efficient sensor systems for the detection of pesticide and lanthanide ions but also be valuable for the design of more advanced polydiacetylene sensors of varying target molecules in the future.
Thesis supervisor: Dr. T. Alan Hatton
Title: Ralph Landau Professor of Chemical Engineering and Director of David H. Koch School of Chemical Engineering Practice
Acknowledgments
First of all, I would like to express my sincerest gratitude to my exceptional research advisor and thesis supervisor -Professor T. Alan Hatton -for his enlightening guidance and highly useful advices in my whole Ph. D. study at Massachusetts Institute of Technology. His precious advice, inspiration and encouragement strongly encourage me to be a more confident person, and his knowledge and instructions are valuable and strongly influence me to be a better scientist. I am grateful for his precious supporting to me fighting against all the difficulties I faced in these years, which will definitely have a great impact throughout my life and career.
Moreover, I would like to sincerely thank my Ph.D. thesis committee chair -Professor Sylvia Ceyer -for her valuable advices and precious suggestion to my graduate study and research projects. She is always ready to help me whenever I faced difficulties during my study in MIT. I am so grateful for all her precious time and kindest support.
I would like to thank my Ph. D. thesis committee member -Professor Bradley Olsen - for his good suggestions to my course study and research projects. Professor Olsen taught me Polymer Synthesis at the second year of my Ph. D. study, which brought me to the fantastic world of polymer and helped me so much in building up my knowledge of polymers.
I would like to especially thank my Ph. D. thesis committee member - Dr. Lev Bromberg, for the countless help he has offered me during my Ph. D. study. It is so lucky for me to work with such a great, smart and helpful scientist, and what I have learned from him will benefit my whole scientific career.
I would like to thank my Ph. D. thesis committee member -Professor Jianshu Cao -for all his precious help and kindest support to me during my Ph. D. years. I cannot appreciate more. This thesis is a result of successful collaboration with many great scientists in different areas. I especially would like to thank Prof. Troy Van Voorhis for his kindest support of collaborating with the theoretical calculation. I want to greatly thank Dr. Zhou Lin for her help in molecular modeling and DFT calculation. I want to thank Dr. Paul Brown for the guidance, tremendous help and friendship during my first two years of research. I want to thank Dr. Jie Wu for his patient guidance to teach me and help me in organic synthesis. I am grateful to Dr. Tonghan Gu for the many helpful discussions on different projects as a knowledgeable friend. I am thankful to Fan He for being there whenever I need any help or advice.
It has been a great time and experience in the Hatton research group, and it is my great pleasure to meet so many kind and nice friends here. I would like to thank Dr. Emily Chang, Dr. Xianwen Mao, Dr. Wenda Tian and others for their friendship and help. Each of them has made the time very enjoyable and pleasant.
I would like to thank Dr. Yong Zhang for his help of transmission electron microscopy and Dr. Patrick Boisvert for his help of scanning electron microscopy.
Also, I am deeply grateful to my husband, Dr. He Wei, for his supporting to all my important choice in my life. I appreciate all the things he has dedicated for our family, and for our future. Finally, I owe a huge debt of gratitude to my parents, for their sincerest love, encouragement, and support to me. Their endless love and constant guidance make me who I am. I really
appreciate their dedication to me all this time. I also want to thank my lovely daughter Tina; she is the most beautiful sunshine in my life, forever and ever.
Table of Contents
A ckn ow ledgm en ts ... 7
L ist of F ig u res ... 13
L ist of T ab les ... 16
Chapter 1. Introduction to conjugated polymers and polydiacetylene for the development of sen so r s...17
1.1 Development of chemical sensors... 17
1.2.Introduction to conjugated polymers ... 19
1.3 Conjugated polymer-based chemical sensors ... 22
1.4 Structure of polymerized diacetylene ... 23
1.5 Optical properties of polydiacetylene ... 25
1.6 Polydiacetylene-based chemical sensors ... 27
Chapter 2 Polydiacetylene functionalized with 2-pyridine aldoxime for colorimetric detection of malathion...30
2 .1 Introdu ction ... 3 1 2.2 Molecular design, synthesis, copolymerization and material characterization...34
2.2.1 Molecular design of diacetylene-pralidoxime (DA-PAM) ... 34
2 .2 .2 M aterials ... 34
2.2.3 Synthesis of diacetylene-bromine(DA-Br)...35
2.2.4 'H-NMR of DA-Br ... 36
2.2.5 13C-NMR of DA-Br... 37 2.2.6 Mass Spectrometry of DA-Br...38
2.2.7 Synthesis of diacetylene-pralidoxime (DA-PAM)... 39
2.2.8 'H-NMR of DA-PAM ... 40
2.2.10 Mass Spectrometry of DA-PAM...42 2.2.11 Photo polymerization of DA/DA-PAM suspension...43 2.2.12 Electron microscopy of the polydiacetylene-pralidoxime (PDA-PAM) vesicles...44 2.2.13 Conductivity and dynamic light scattering (DLS) diameters of DA/DA-PAM
suspension at varying concentrations ... 46 2.3 Colorimetric detection of organophosphate by PDA-PAM...47
2.3.1 Photo polymerization of DA/DA-PAM suspension and its color change after m alathion binding...48
2.3.2 Colorimetric detection of organophosphate by PDA-PAM. ... 48 2.3.3 Colorimetric detection of organophosphate by PDA-PAM at different temperatures.
... 5 0 2.3.4 Colorimetric detection of organophosphate by PDA-PAM towards different
ch em icals ... 5 1 2.3.5 Colorimetric detection of organophosphate by PDA-PAM in the presence of other chem ical com pounds. ... 52 2.3.6 Colorimetric response of PDA-PAM absorption change ... 53 2.3.7 Colorimetric detection of organophosphate by PDA-PAM embedded Teflon
m em brane ... 56 2.3.8 Dynamic light scattering (DLS) diameters of DA-PAM, PDA-PAM and PDA-PAM w ith M alathion . ... 57
2.3.9 Circular dichroism (CD) spectrum measurement of DA/DA-PAM, PDA-PAM and M alathion bound PD A -PA M ... 59
2.3.10 Zeta potential measurement of DA/DA-PAM, PDA-PAM and Malathion bounded P D A -P A M ... 6 1 2.4 Density Functional Theory (DFT) calculation of polydiacetylene-pralidoxime with
m alathion binding ... 63 2.4.1 Structure optimization of PDA-PAM before and after malathion binding ... 63
2.4.2 Excitation energies of PDA-PAM before and after malathion binding ... 65
2 .5 S um m ary ... 6 8 Chapter 3 Polydiacetylene functionalized with dipicolylamine for colorimetric detection of lan th an id e ion s. ... 70
3.1 Introdu ction ... 70
3.2 Molecular design, synthesis, copolymerization and material characterization...74
3.2.1 Molecular design of diacetylene-dipicolylamine (DA-DPA)...74
3 .2 .2 M aterials...74
3.2.3 Synthesis of D A -D PA ... 75
3.2.4 M ass Spectrom etry of DA-DPA ... 76
3.2.5 Photo co-polymerization of DA/DA-DPA suspension ... 76
3.2.6 Electron microscopy of the polydiacetylene-dipicolylamine (PDA-DPA) vesicles ... 77
3.3 Colorimetric detection of lanthanides by PDA-DPA...79
3.3.1 Colorimetric detection of Gd 3 ion by PDA-DPA in aqueous suspension...79
3.3.2 Colorimetric detection of Er3 ion by PDA-DPA in aqueous suspension...80
3.3.3 Colorimetric detection of Ce31 ion by PDA-DPA in aqueous suspension...81
3.3.4 Colorimetric detection of control metal ion Hg2+ by PDA-DPA in aqueous su sp en sion . ... 82
3.3.5 Spectrum change of PDA-DPA with different concentrations of Gd3 + .. . . . 83
3.3.6 Spectrum change of PDA-DPA with different concentration of Er3... . . . 85
3.3.7 Spectrum change of PDA-DPA with different concentration of Ce3 .. . . . 87
3.3.8 Spectrum change of PDA-DPA with control metal ion Hg2+. . . . 89
3.2.9 Colorimetric Response of PDA-DPA with different lanthanide ions (Mercury as co n tro l) ... 9 0 3.3.10 Dynamic light scattering (DLS) diameters of DA-DPA, PDA-DPA and PDA-DPA w ith lanthanide m etal ions... 92
3.3.11 Colorimetric detection of Gd3
+ by PDA-DPA on solid state ... 94
3.3.12 Colorimetric detection of Er3+ by PDA-DPA on solid state... 95
3.3.13 Colorimetric detection of Ce3+ by PDA-DPA on solid state ... 96
3.3.14 Colorimetric detection of Hg2+ by PDA-DPA on solid state ... 98
3 .4 Sum m ary ... 100
Chapter 4 Conclusion ... 101
Chapter 5 Future Perspective...103
5.1 Optimization of the solid state colorimetric detection systems ... 103
5.2 Degradation of hazardous molecules on solid support ... 105
5.3 Antibody functionalized polydiacetylene for detection of biohazards ... 107
List of Figures
Figure 1. 1 Schematic representation of the conjugated polymer-amplified molecular detection. ... 2 0
Figure 1. 2 Examples of a-conjugated polym ers ... 21
Figure 1. 3 Colorimetric response of polythiophene in the presence of adenosine triphosphate(ATP) and polyphenylene vinylene in different organic solvents . ... 22
Figure 1.4 Structure of commercially available diacetylene-carboxylic acid monomers...24
Figure 1.5 Polymerization of 10,12-pentacosadiynoic acid with functional group...25
Figure 1.6 Colorimetric response of polydiacetylene in the presence of target molecules...26
Figure 2.1 Molecular design of diacetylene-pralidoxime (DA-PAM) ... 34
Figure 2.2 Synthesis of diacetylene-bromine (DA-Br)... 35
Figure 2.3 'H NM R of DA-Br (DM SO-d6)... 36
Figure 2.4 13C NM R of DA-Br (DM SO-d6). ... 37
Figure 2.5 ESI-M S spectra of DA -Br ... 38
Figure 2.6 Synthesis of diacetylene-pralidoxime (DA-PAM)...39
Figure 2.7 'H NMR of DA-PAM(DMSO-d 6)...40
Figure 2.8 13C-NMR of DA-PAM(DMSO-d 6). ... 41
Figure 2.9 ESI-M S Spectra of DA-PAM ... 42
Figure 2.10 Copolymerization of DA and DA-PAM suspension and its color change...43
Figure 2.11 Scanning Electron Microscopy (SEM) image of PDA-PAM. ... 44
Figure 2.12 Transmission Electron Microscopy (TEM) image of PDA-PAM. ... 45
Figure 2.13 Conductivity and dynamic light scattering (DLS) diameters of DA/DA-PAM suspension at varying concentrations. ... 47
Figure 2.14 Copolymerization of DA and DA-PAM suspension and its color change upon addition of m alathion...48
Figure 2.15 Colorimetric response of PDA-PAM to malathion in aqueous suspensions...49
Figure 2.16 Colorimetric response of PDA-PAM to malathion in aqueous suspensions at 4 'C an d 4 0 C . ... 50
Figure 2.18 Colorimetric response of PDA-PAM to malathion in aqueous suspensions in the
presence of a herbicide interference. ... 53
Figure 2.19 Spectrum change of the PDA-PAM vesicle suspension upon malathion addition. ... 55
Figure 2.20 Colorimetric response of the PDA-PAM vesicle suspension upon malathion addition. ... 5 5 Figure 2. 21 Colorimetric detection of organophosphate using PDA-PAM impregnated Teflon m em b ran e. ... 57
Figure 2.22 Volume-average DLS diameter distribution of DA-PAM, PAM, and PDA-PA M com plexed w ith m alathion...58
Figure 2.23 Circular dichroism (CD) spectrum of PDA-PAM before polymerization, after polym erization and after m alathion binding... 60
Figure 2.24 Zeta Potential of PDA-PAM before polymerization, after polymerization and after m alathion binding. ... 62
Figure 2.25 DFT-optimized ground state molecular structures for the model trimer systems...64
Figure 2. 26 The simulated UV-Vis single-photon spectra for the model trimer systems ... 67
Figure 3.1 M olecular design of DA-DPA ... 74
Figure 3.2 Synthesis of diacetylene dipicolylamine amine (DA-DPA) ... 75
Figure 3.3 Mass Spectrometry of DA-DPA ... 76
Figure 3.4 Photo co-polymerization of DA/DA-DPA suspension ... 77
Figure 3.5 Transmission Electron Microscopy (TEM) images of PDA-DPA...78
Figure 3.6 Colorimetric detection of Gd 3 by PDA-DPA suspension in aqueous suspension. ... 79
Figure 3.7 Colorimetric detection of Er3' by PDA-DPA suspension in aqueous suspension...80
Figure 3.8 Colorimetric detection of Ce3+by PDA-DPA in aqueous suspension. ... 81
Figure 3.9 Colorimetric detection of control metal ion Hg2+ by PDA-DPA in aqueous suspension. ... 8 2 Figure 3. 10 Colorimetric detection of Gd3+ by PDA-DPA...84
Figure 3. 11 Colorimetric detection of Er3+ by PDA-DPA ... 86
Figure 3. 12 Colorimetric detection of Ce3+ by PDA-DPA ... 88
Figure 3.14 Colorimetric response of DA-DPA, PDA-DPA and PDA-DPA with lanthanide ions
(m ercury ion as control)...91
Figure 3. 15 DLS measurements of DA-DPA, PDA-DPA and PDA-DPA with lanthanide ions (m ercury ion as control)...93
Figure 3. 16 Colorimetric detection of Gd3+ by PDA-DPA on solid membrane...94
Figure 3. 17 Colorimetric detection of Er3' by PDA-DPA on solid membrane...95
Figure 3. 18 Colorimetric detection of Ce3' by PDA-DPA on solid membrane ... 97
Figure 3. 19 Colorimetric detection of Hg2+ by PDA-DPA on solid membrane...98
Figure 5.1 Solid support immobilization of polydiacetylene. ... 104
Figure 5.2 Schematic represent of organophosphate degradation on solid support. ... 106
Figure 5.3 Schematic represent antibody functionalized polydiacetylene for detection of biological hazards. ... 108
List of Tables
Table 1.1 Polydiacetylene based molecular sensors and their applications ... 28
Table 2.1 The excitation energies , the dominant transitions , and the dimensionless oscillator strengths for the five most intense single-photon absorptions for the model trimer systems...66
Chapter 1. Introduction to conjugated polymers and polydiacetylene
for the development of sensors.
1.1 Development of chemical sensors
Environmentally hazardous substances are always one of the world's leading problems that needs solving. Millions of synthetic organic and inorganic compounds are synthesized every year. [1] Consumed in industry, agriculture, and defense sectors, these compounds include plastics, pesticides, solvents, surfactants, and even chemical warfare agents. Due to the worldwide over-usage of synthetic compounds with improper handling practices, many of these compounds leak to the environment and then cause pollution and damage human health. Some contaminants with persistent, long half-lives can become a long-term serious problem. For example, pesticides are one of the most commonly overused chemicals that applied directly to plants and soils [2]. The chemically stable pesticides can migrate through large regions of soil and reach water resources, where they present a long-term threat to human health. In addition, industrial waste containing heavy metal compounds pollutes surface and underground water sources, which also results in considerable soil pollution, and the animals that feed by contaminated plants and drink from polluted waters will accumulate such metals in their tissues, and milk. Humans are taking heavy metals by consuming contaminated plants and animals, which cause various harmful effects on human health[3, 4].
There is an increasing demand for developing direct, rapid, sensitive and convenient detection methods for monitoring the potential environmental toxic substance. Traditional methods for detection and identification of environmental risk factors such as mass-spectrometry
limitations, but lack the convenience to be of field usage because they require costly and heavy devices, long analysis time, and complex pretreatment of the analyzed sample.
Chemosensors, which are generally understood to be devices that transform chemical information into analytically signal readouts [9], have been developed for sensing various analytes [10]. A typical stimulus-responsive colorimetric chemosensor usually contains two parts: a target recognition site as the receptor, and a linked fluorophore that translates the recognition event into the fluorescence signal [11]. Synthesized chemosensors have the advantages of high sensitivity, high selectivity, and rapid response.
Binding affinity is one of the most important key factors in chemosensor design. By increasing the binding affinity between the "receptor" part of chemosensor and the targets, the sensitivity of the sensor can be improved. However, if the analyte is present at high concentration that saturate the chemosensor, the detection cannot be quantified by the saturated signal output because the fluctuations in concentration cannot produce sufficiently high signal changes. The chemosensor with the target stability constant (Ks) equal to approximately the inverse of the median guest concentration for the concentration range of interest will be ideal for the molecular design [12].
Another important issue in the chemosensor design is the choice of chromophore or fluorophore used to output the analyte binding signals. Visible colorimetric response, fluorescence or luminescence are always preferred as the measurements can be easily accomplished with simple devices, or even without any optical devices, i.e., visualized by the naked eye.
Considering all these factors in detection system design, conjugated polymers with their unique physical and chemical properties, have shown their great potential in chemosensor
1.2. Introduction to conjugated polymers
Conjugated polymers are a group of polymers with backbone chains consisting of alternating double- and single-bonds [13]. Due to the substantial i-electron delocalization and resonance along the conjugated polymer backbone, a "molecular wire"-like polymer structure forms at the molecular level [14].
Because the alternating saturated and unsaturated bonds along backbone contain spi or sp2
-hybridized atoms [15], the p-orbital overlap results in fascinating chemical and physical properties for the a-conjugated polymers, including but not limited to electroconductivities such as semiconductive properties and optical properties such as electroluminescence [16].
With these unique properties, 7r-conjugated polymers are always an attractive research object in both academia and industry. Furthermore, a-conjugated polymers are relatively sensitive to the external stimuli, which induce the alteration of their spectroscopic and electrochemical properties. For the optical absorption in 2-conjugated polymer, the excitation energy E(,) of the lowest excited state depends on the number of repeat units(n) in the following equation[ 17]:
E(n) = E + (El - E.)e[-a(n1)]
E, are the measured excitation energies for the monomer and E are the measured excitation energies for the polymer. The effective conjugation length of the polymer is defined
as neff repeat units that mark the onset of the saturation ( E(n > neff) E, ). Alternation in
intramolecular conformation and intermolecular packing in 2-conjugated polymers can change effective conjugation length of the polymer, resulting in changes in absorption, fluorescence, and conductive properties making them well-suited as signal transducers. The electron delocalization
and resonance also offer the advantage of signal amplification to a-conjugated polymers compared to small molecule-based sensors [18]. While a binding event occurring with a small molecule only triggers a change in fluorescence wavelength of ingle molecule , the same signal can be significantly amplified by a-conjugated polymers via information transportation along the resonance backbone, which is very beneficial for sensing applications because the efficient detection of target molecules at low concentrations is required [18].
Molecular Probe Target Molecule K d [Sensor - Target] [Sensor] [Target] Target Molecule
0
Conjugated Polymer Probe
One-to-one Molecular Detection
(Kd Limited Detection)
Enhanced Molecular Detection
(Molecular Wire Amplification)
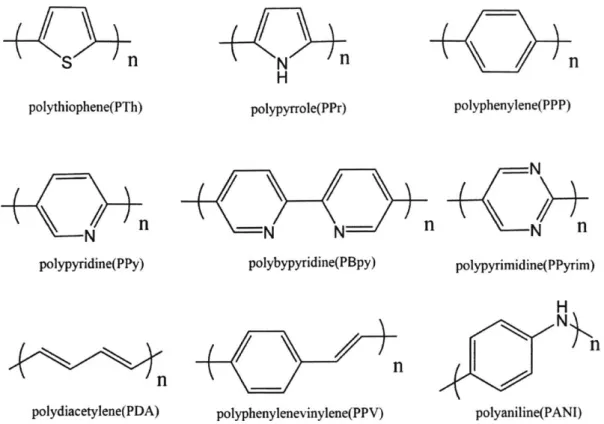
Figure 1. 1 Schematic representation of the conjugated polymer-amplified molecular detection. A number of conjugated polymers have been investigated for the development of sensors, such as polythiophenes [19], polypyrroles [20], polyacetylenes [21], and polydiacetylenes [22].
nS
n
polythiophene(PTh) N polypyridine(PPy). N
n
H polypyrrole(PPr) polyphenylene(PPP) polybypyridine(PBpy) polypyrimidine(PPyrim)nn
polydiacetylene(PDA) polyphenylenevinylene(PPV)Figure 1. 2 Examples of a-conjugated polymers.
H N_
polyaniline(PANI)
Conjugated polymers have shown their great potential as transduction materials by readily transforming a chemical stimulus into an electrical or optical signal output. The output signal can be amplified by the electron excitation transportation along the conjugated chain [23]. Conjugated polymers can also transport charge signal when oxidized or reduced, which makes them an excellent platform for the signal transportation. [24].
I
1.3 Conjugated polymer-based chemical sensors
Due to the unique optical properties of 2-conjugated polymers, many colorimetric detection systems have been built by the modification of these polymers. In 2005, Li et al. reported a modified polythiophene system for the colorimetric detection of adenosine triphosphate (ATP) with the detection limit at the scale of nmol/L [25]. In 2013, Yagai et al. reported using n-conjugated compound oligo(p-phenylene vinylene) that exhibited tunable luminescence in the solid state in the presence of different organic solvents. [26] It was demonstrated that the
21-conjugated systems with proper amphiphilicity can form different metastable self-assembled phases with discrete packing states with unique emission properties [26]. The 2-conjugated polymers have shown their great potential in molecular sensor development and in photonic device build-up, attracting increasing interest from both academic and industrial research groups.
Polythiophene
S SI /\ S /
S S
Poly(p-phenylene vinylene)
Figure 1. 3 Colorimetric response of polythiophene in the presence of adenosine triphosphate(ATP) [25] and polyphenylene vinylene in different organic solvents [26].
1.4 Structure of polymerized diacetylene
Since the discovery of polyacetylenes in 1969 by Wegner et.al [27]with their unique features including UV-induced polymerization, and stimuli-responsive colorimetric/fluorometric chromism [28], polydiacetylene offer great potential as stimulus-responsive materials. Because of the easy preparation, convenient modification, and its large output signal in the visible spectrum range, diacetylenes (R-C-C-C=C-R') are particularly suitable for sensor design[29]. Diacetylene molecules can be photopolymerized by 254 nm UV irradiation via a 1,4-addition reaction, resulting in polydiacetylene with an alternating ene-yne polymer chain, and this polymerization process needs neither initiators nor a catalyst [30].
Polydiacetylene based sensors can provide dual signals through absorbance spectrum change and fluorescence spectrum change. After UV polymerization, the produced polydiacetylene has a pure blue color, which is non-fluorescent. With the stimulus-induced backbone structure change, the absorbance of polydiacetylene has a blue shift, which enables blue-violet-red color change. The polydiacetylenes in the red form are strongly fluorescent. The color change of polydiacetylene is a unique property that has given rise to their application in sensors. This color change has been reported in response to heat [31], mechanical stress [32], and in response to the binding with various "target" molecules [33].
Several diacetylenic-carboxylic acids are commercially available, including 5,7-hexadecadiynoic, 10,12-tricosadiynoic, and 10,1 2-pentacosadiynoic acids.(Fig. 1.4)
OH 5,7-Hexadecadiynoic acid H 10, 12-Tricosadiynoic acid 0 OH 10,12-pentacosadiynoic acid
Figure 1.4 Structure of commercially available diacetylene-carboxylic acid monomers
10,12-Pentacosadiynoic acid with a carbon chain and a carboxylic acid group (Fig. 1.5)has been studied in my research. The amphiphilic molecular structure enables the monomer to self-assemble into vesicle, bi- and multilayer vesicles, etc. In addition, the carboxylic acid termini can be readily modified with other functional groups for the specific binding towards different target molecules.
0
O0 -Functional Group
Hydroprobic Lipid Tail Diacetylene Conjugation Specific Binding Site
0
hv
/,, ;Ii// ,' 1,4 addition polymerization -:' -
'-Figure 1.5 Polymerization of 10,12-pentacosadiynoic acid with functional group.
Diacetylene monomers with functional head groups and hydrophobic alkyl chain can self-assemble into micelles, vesicles, or layer-by-layer structures. After exposure to UV irradiation, diacetylene monomers are polymerized to form the polydiacetylene vesicles.
1.5 Optical properties of polydiacetylene
The polymer chains of the blue phase are planar with the so-called "ene-yne" bond alternation, corresponding to a relatively long effective conjugation length (Fig. 1.6).
Phase * Red *--* Phase . OL-O 04Z' .. e' Functional group H O, HO , 0 --- OH O Target molecule ,HO -O HO 'HO H H/HO 8 0 0 H0 H I- HO HO V0 Binding Group
Delocalized 7-electron resonance
Target induced backbone twist
Figure 1.6 Colorimetric response of polydiacetylene in the presence of target molecules.
In the presence of the target molecule, the binding between the functional group and the target affect the packing of side chains, in turn, relieves strain in the "ene-yne" conjugated backbone. This structural change is often claimed to be related to a decrease of order (nonplanarity), which would cause a shortening of the effective conjugation length of polydiacetylene, thus causing a blue shift in polydiacetylene spectrum and a blue-to-red color change on polydiacetylene visualized color [34]. Usually, the blue and red phases have maximum absorption near 640 and
1.6 Polydiacetylene-based chemical sensors
Target molecular binding can cause the polydiacetylene backbone chain conformation changes and therefore, trigger remarkable color transitions. The unique structural and optical properties of polydiacetylene make them excellent materials for sensing chemical or biological targets. A lot of conjugated polymer systems have been investigated for analytical applications. Among them, polydiacetylene based chemosensors seem to be more promising to compete with the currently available techniques.
The first polydiacetylene based sensing system was reported in 1993 by Charych et al.[35]. In their work, polydiacetylene was modified by a sialic acid for a specific response to the influenza virus. Since then, polydiacetylene based sensors have been developed for various target analytes such as cations [36], anions [37], surfactants [38], and even gas molecules [39].
Polydiacetylene Molecular Structure Analyst Detection Ref Limitation Pb2+
200 ppb
[36]
OH 0 OH ATP 5.88 ppm [37] Cationic 10 PM [38] 0 ~ surfactants OH 0 OH 0 OH 0 HCl gas 2 ppm [39]In order to accomplish the effective detection of target molecules, there are three requirements for the development of sensors: fast response, high specificity and low detection limit. Most of the detection specificity depends on the functional group conjugated to the termini of the polydiacetylene, which can be designed by its interactions with analytes. In my thesis, two polydiacetylene systems were developed with different functional groups and were demonstrated to be rapid, sensitive and selective sensors for the colorimetric detection of their specific target molecules.
Chapter 2 Polydiacetylene functionalized with 2-pyridine aldoxime
for colorimetric detection of malathion
(The work reported in this chapter has been published as "Polydiacetylenefunctionalized with charged termini for device-free colorimetric detection of malathion" by Yunfei Zhang, Lev Bromberg, Zhou Lin, Paul Brown, Troy Van Voorhis, TAlan Hatton. Journal of Colloid and Interface Science, 528 (2018) 27-35.)
In this work, we introduce 10,12-Pentacosadiynoic acid conjugated with pyridine-2-aldoxime (pralidoxime, PAM) as a positively charged amphiphilic molecule capable of forming a polymer with polydiacetylene (PDA-PAM) via 1,4-addition photopolymerization of self-assembled PAM-modified monomers. PDA-PAM stabilized colloidally by unmodified pentacosadiynoic acid in a basic aqueous medium via electrostatic interactions exhibits a
colorimetric transition in the presence of malathion. The malathion detection limit, through color change observed by the naked eye, is in the mM range (liquid suspension) or at nmol/cm2 levels (solid membrane); the response to exposure to malathion was rapid, within seconds. Density functional theory (DFT) calculations on the PDA-PAM system before and after binding with the target molecule (malathion) demonstrate that the large blue absorption shift of 0.42 eV observed in the malathion-bound configuration of the polymer is due to the dissociation of the positive charge center on the pralidoxime functional group from the negative charge center on the carboxylic terminus.
2.1 Introduction
Organophosphate pesticides are among the most common chemical hazards in the environment. Over 3 million cases of pesticide poisoning occur every year, which result in more than 250,000 deaths annually[40]. Several methods have been reported for the detection of organophosphate compounds, including, but not limited to, potentiometry[41, 42], chromatography[43-46], mass spectroscopy [47-50], and fluorescence spectrometry [51-55]. However, these detection techniques require complex and bulky devices that cannot be readily employed outside of a laboratory and are time-consuming (-hours). One of the simplest and most convenient signal readouts of chemical detection is an optical signal such as a change in color. In this work, a 7n-conjugated amphiphilic polymer, polydiacetylene[56-58], was selected and modified to function as a colorimetric organophosphate sensor. The structure of the diacetylene monomer consists of two parts: a hydrophobic hydrocarbon tail that includes diacetylene triple bonds and a terminal hydrophilic group [59]. As amphiphilic molecules, diacetylene fatty acid monomers self-assemble into vesicles, micelles or tubular structures in aqueous systems [58, 60, 61]. Polydiacetylene is formed via 1,4-addition photopolymerization of self-assembled diacetylene monomers, resulting in a highly ordered conjugated backbone. The photopolymerization occurs only when the diacetylene monomers are arranged densely in the appropriate crystalline structure at an intermolecular distance of 4.7-5.2
A
[62]. Prior to polymerization, self-assembly is driven by partitioning of the hydrophobic tails away from the aqueous phase, thus exposing the functional groups to the outside aqueous environment. The optical properties of polydiacetylene result from the electron resonance on the in-conjugated backbone [63]. Suspensions of polydiacetylene colloids exhibit electronic absorption with maxima at 630 nm (blue) and 550 nm (red).Polydiacetylene (PDA) exhibits a remarkable ability to change color in solution or suspension, including color transition and red fluorescence emission after contact with a target substrate, which enables its application in sensoric devices[22, 57, 64, 65]. PDA is typically blue in color when it is photopolymerized. However, substituted PDA derivatives undergo a dramatic color change when they are exposed to a variety of environmental perturbations, including changes in temperature and solvent polarity, and mechanical stress[57]. This color change is due to the induced backbone twisting [66], which leads to a planar to non-planar conformation transition in the backbone. This phenomenon is termed mechanochromism, a very attractive self-signaling property for sensor applications. When a target molecule species binds to the PDA assembly surface, the effective conjugation length of the polymer backbone is reduced, leading to a chromic transition [67]. The polydiacetylene serves as the colorimetric "tranducer" of the target recognition on the vesicle surface by the color change from blue to red [68].
In 2012, Lee et al. [47] incorporated the 4-methylbenzaldehyde oxime moiety into the
-COOH terminus of pentacosadiynoic acid for the colorimetric detection of diisopropylfluorophosphate (DFP) and diethylchlorophosphate (DCP) in a pH neutral environment. The molecule they developed was non-ionic (electronically neutral) and, together with 4-methylbenzaldehyde- and 2-hydroxy-4-4-methylbenzaldehyde-modified diacetylene molecules comprising major constituents, self-assembled into micelles or vesicles in aqueous media maintained at neutral pH. The aqueous suspension of the obtained oxime-modified micelles or vesicles changed color, and hence, sensed 160 ppb of DFP in vapor, and 0.1 M of DCP and DFP in solution[47, 48].
As the degradation of organophosphate can be enhanced at basic pH by nucleophilic hydroxyl anions, we focused on the design of a polydiacetylene-based detection system that
functions in a basic environment. In this case, the PDA will be negatively charged at pH above its pKa of 9.5-9.9 [69]. To enhance the colloidal stability of the oxime-modified polydiacetylene in water, and to further understand the mechanism of the PDA color change, we improved upon the design by incorporating a positively charged pyridine-2-aldoxime (pralidoxime, or PAM) group in the terminus of 10,12-pentacosadiynoic acid, which itinteracts electrostatically, and is stabilized by the unmodified pentacosadiynoic acid via ionic interactions in a basic aqueous medium. The mixture of two different diacetylene monomers co-assemble colloidally with both the pralidoxime and acetic acid groups on the surface of the vesicle.
Pralidoxime is a member of the oxime family of compounds that was specifically designed as an antidote to organophosphate poisoning because of its ability to act as acetylcholinesterase (AChE) reactivators [70, 71]. Malathion, a common organophosphate pesticide that is often used as a less-toxic simulant of VX (a combat warfare agent), poisons and inactivates the AChE by binding onto its active serine-histidine-glutamate site. Because of PAM's high nucleophilicity, it can displace the phosphoryl group from the catalytic serine, thus hydrolyzing the 0-phosphorylated serine to an active free serine.
The novel PDA molecule with a positively charged pralidoxime terminus reported in the present work is soluble in basic media, polymerizes under UV irradiation and responds selectively to the target molecules with a color change. The detection limit by the naked eye is as low as in the mM range in solution, or at the nmol/cm2 level on a solid support, with a response time of 2 min or less. We carried out density functional theory (DFT) calculations for the PAM-modified PDA before and after binding to the target molecule to shed light on the detection mechanism. The synthesis of the PAM-modified diacetylene, its self-assembly and its colorimetric response in a basic aqueous environment, and DFT binding model, are described below.
2.2 Molecular design, synthesis, copolymerization and material characterization 2.2.1 Molecular design of diacetylene-pralidoxime (DA-PAM)
0 Br
N C
'OH
Lipid Tail
Diacetylene
Linker
Ligand
ON
hv
1,4 additlon polymerizatlon
Figure 2.1 Molecular design of diacetylene-pralidoxime (DA-PAM) 2.2.2 Materials
10,12-pentacosadiynoic acid
(
> 97%), 3-bromo-1-propanol(
3 97%), 4-dimethylaminopyridine (:99%), N,N'-dicyclohexylcarbodiimide (399%) , chlorosulfuron (399%), anhydrous methylene chloride( 3 99.8%), anhydrous magnesium sulfate ( > 99.5%),
pyridine-2-aldoxime methochloride (.97%) were obtained from Sigma-Aldrich Chemical Co. and were of the highest purity available. Pentacosadiynoic acid was purified from its polymerized admixtures by dissolution in acetonitrile followed by filtration and drying under vacuum, and stored sealed in aluminum foil. Other reagents were used as received.
2.2.3 Synthesis of diacetylene-bromine(DA-Br)
0 0
OH-^Br'Br H30
DCM, reflux2 Figure 2.2 Synthesis of diacetylene-bromine (DA-Br).
A mixture of 10,12-Pentacosadiynoic acid (1.873g, 5 mmol), 3-bromopropanol (1.808 mL, 20 mmol), and N,N-dimethylaminopyridine (30.5mg, 0.05mmol) was purged by nitrogen bubbling to remove moisture. Then 50 mL of anhydrous methylene chloride was added via syringe. A red-colored solution (solution A) was obtained.
A solution of dicyclohexylcarbodiimide (DCC), an activating agent (1.134 g, 5.5 mmol) in 25 mL anhydrous CH2Cl2 (solution B, chilled on ice) was mixed with ice chilled solution A.
Solution B was added into solution A via a speed-controlled funnel within 30 min. The resulting mixture was stirred on ice for 30 min and then stirred overnight at room temperature. Bromine-terminated diacetylene (DA-Br) was obtained from the supernatant formed in the above mixture after a precipitate was filtered out; the supernatant was repeatedly washed by 1 M HCl, saturated aqueous NaHCO3 solution and then by 1 M HCl and water. The supernatant was dried by
MgSO4 and the solvent was evaporated under reduced pressure, followed by dissolution of the product in ethyl acetate. The DA-Br was further purified on a silica column (hexane/ethyl acetate =10:1) with TLC (Rf= 0.3; 10% ethyl acetate/ hexane). The DA-Br formed yellow crystals after solvent evaporation. Yield, 49 %.
2.2.4 'H-NMR of DA-Br
1H NMR of the DA-Br was conducted in DMSO-d6 on Varian Inova-500 NMR
I r I y '-1I "r f
f/-i f/-i
-/i DA-Br Hi ii ~- V / Ss e e 4.6 4.4 4.2 4.0 3.8 3.6 3.4 3.2 3.0 2.8 '2.6 2.4 2%2 2.0 '1.8 1.6 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 -5000 3s000 -30000I
-20000 15000 -10000Figure 2.3 'H NMR of DA-Br (DMSO-d6).
'H-NMR (DMSO, 500 MHz), 8: 0.84 (3H, t), 1.10-1.30 (26 H, m), 1.40 (4H, m), 1.50 (2H, m),
2.2.5 "C-NMR of DA-Br
13C NMR of the DA-Br was conducted in DMSO-d6 on Varian Inova-500 NMR
M2 ill
Iii
iI~
III I
10 ISO 140 130 IN 110 100 s
f1 WK 70 60
" 40 x 2 10 -10
Figure 2.4 13C NMR of DA-Br (DMSO-d
6). 13C-NMR (101 MHz, DMSO-d6) 0: 173.29, 78.39, 78.36, 73.53, 65.82, 62.13, 40.63, 40.55, 40.42, 40.21, 40.01, 39.80, 39.69, 39.59, 39.49, 39.38, 33.86, 31.78, 31.71, 31.39, 29.49, 29.43, 29.34, 29.19, 28.99, 28.91, 28.87, 28.84, 28.75, 28.63, 28.61, 28.17, 24.86, 22.58, 18.75, 14.42. 210 m I ISO 170
i1i H i
I
2.2.6 Mass Spectrometry of DA-Br
ESI-MS of the DA-Br was conducted in acetonitrile on Agilent 5973N Gas Chromatograph/Mass Spectrometer. The spectrum results confirmed the formation of DA-Br.
6000000 A 5000000 4000000 3000000 2000000 1000000 0 [M147. L8L 518.2657 S12-3015 1.31 5l0.-I2 SZO.2651 513 515 517 519 521 523 525 ml
Figure 2.5 ESI-MS spectra of DA-Br.
2.2.7 Synthesis of diacetylene-pralidoxime (DA-PAM)
A mixture of DA-Br (crystallized, 496 mg, 1 mmol) and 2-pyridine aldoxime methyl chloride, (122 mg, 1 mmol) in aqueous acetonitrile (20 mL) was stirred overnight at room temperature. Acetonitrile was vacuum-evaporated and formation of a white crystalline product was observed. 0 0 OH-^Br Br H 2H DCM, reflux DCM, r.t. 0 Br N 3 OH
2.2.8 'H-NMR of DA-PAM
'H NMR of the DA-PAM was conducted in DMSO-d6 on Varian Inova-500 NMR
4il **v
Pl-l
IiI
I
VT V 4 AT?' T1 ~ 4 is 0 1 a it to 9 S 7 ,14 S 4 3 2 1 0 -1 -2 -3 Figure 2.7 1H NMR of DA-PAM(DMSO-d 6). 'H-NMR (DMSO, 500 MHz), 8: 0.86 (3H, m), 1.25-1.46 (32 H, m), 2.11 (2H, t), 2.26-2.32(6H, m), 3.56 (2H, t), 4.11 (2H, t), 7.56 (2H, m), 8.25 (1H, t), 8.60 (2H, m), 11.80 (1H, d). 0 100 go2.2.9 "C-NMR of DA-PAM
13C NMR of the DA-PAM was conducted in DMSO-d
6 on Varian Inova-500 NMR
1111
Ut
.:. t. x
I
Iii
0I
Olmomom
30 too 170 too ISO 14 1i 0 io lie 100 90 60 M 0 U 40 X U 10
Figure 2.8 "C-NMR of DA-PAM(DMSO-d6). "C-NMR (101 MHz, DMSO) d 173.31, 150.35, 147.06, 141.05, 121.13, 99.98, 78.42, 65.82, 62.14, , 33.86, 31.77, 31.70, 31.42, 29.47, 29.41, 29.33, 29.17, 28.97, 28.84, 28.74, 28.62, 28.59, 28.15, 24.86, 22.57, 18.74, 14.43. 21 200 0 -W
Ili 11
Ii
2.2.10 Mass Spectrometry of DA-PAM
ESI-MS of the DA-Br was conducted in acetonitrile on Agilent 5973N Gas Chromatograph/Mass Spectrometer. The spectrum results confirmed the formation of DA-PAM.
3 -5*07
a~n
4UI . Ii
300 400 s0 t00 $00 1000 10 /a
Figure 2.9 ESI-MS Spectra of DA-PAM
[M]' m/z calculated., 537.41; found, 537.40. W be~q 2,5..07 1. 0.07 5.0e404 0. t00 700 000 I I I
2.2.11 Photo polymerization of DA/DA-PAM suspension
A suspension of DA and DA-PAM (mol ratio of 9:1) in aqueous 50 mM HEPES buffer (pH 9.0) with final concentrations of 0.9mM DA and 0.1mM DA-PAM was prepared. The suspension was sonicated at 80 'C for 30 min, filtered through a 0.8 im syringe filter and stored at 4'C. The monomer mixture was copolymerized by UV irradiation at 254 nm for 30 s. After the UV irradiation, a colorless-to-blue color change was observed. A blue polydiacetylene-pralidoxime suspension was prepared(PDA-PAM)
H
H
H UV 254nm 30s
H
2.2.12 Electron microscopy of the polydiacetylene-pralidoxime (PDA-PAM) vesicles The shape and structure of the PDA-PAM aggregates were elucidated by electron microscopy. SEM (FEI/Philips XL30 FEG Environmental Scanning Electron Microscope) and TEM (JEOL 2010 FEG Analytical Transmission Electron Microscope) were utilized to
characterize PPAM suspension that was prepared by polymerizing a mixture of DA and DA-PAM (mol ratio of 9:1) in aqueous Milli-Q water. The UV source was a hand-held UV lamp and the UV light was applied directly to the sample for 30 s.
Figure 2.11 Scanning Electron Microscopy (SEM) image of polydiacetylene-pralidoxime (PDA-PAM).
Figure 2.12 Transmission Electron Microscopy (TEM) image of PDA-PAM.
SEM images revealed a nearly spherical structure of the PDA-PAM vesicles sized in the 120-180 nm range, while TEM indicated a vesicle-like structure of the PDA-PAM aggregates with a bilayer membrane. The bilayer thickness of PDA-PAM vesicle is around 20 nm under TEM, which is the same scale compared to the thickness of polydiacetylene (~5nm)[72].
2.2.13 Conductivity and dynamic light scattering (DLS) diameters of DA/DA-PAM suspension at varying concentrations.
In order to determine hydrodynamic properties of the diacetylene-pralidoxime suspension in aqueous solution, dynamic light scattering (DLS) was also used for the measurement of both critical micelle concentration (CMC) and hydrodynamic diameter.
The solution of DA and DA-PAM (1 mM, mol ratio 9:1) was prepared in HEPES buffer
(50 mM, pH=9.0) and diluted from 1mM to 50 pM. Both conductivity and the DLS diameter of
the aggregate suspensions were monitored (Fig.2.13). The results shows that the assembling pattern of the DA/DA-PAM has certain change at 330 pM and 100 pM. The critical micelle concentration of DA/DA-PAM is around 100 pM.
200 3304;0 600
Concentration(pM)
800 1000
Figure 2.13 Conductivity (black dot) and dynamic light scattering (DLS) diameters (grey bar) of DA/DA-PAM solution at varying concentrations.
2.3 Colorimetric detection of organophosphate by PDA-PAM.
In this study, we conjugated the positively charged pralidoxime with the diacetylene terminus. The resulting modified polymer responds to the presence of organophosphate by changing its conformation. The DA/DA-PAM suspension exhibited an obvious colorless-to-blue color change after UV irradiation, which indicated the formation of the 7-conjugated backbone of the polymer structure. The blue color formation was visible within seconds and was stable for weeks under ambient conditions. The addition of the target molecule malathion caused the PDA-PAM suspension color to change immediately from blue to purple and then to red. This process occurred within seconds and could be observed directly by the naked eye.
42 -40 -387 36- 34- 32-30 0 Q
IO
n
IF
400 350 300 250 200 150 100 50 0 co EI
100 28 I 0 dd MP W MWM 0
g-M-Ji- 0
- -M-- Emma 282.3.1 Photo polymerization of DA/DA-PAM suspension and itscolor change after malathion binding.
A suspension of DA and DA-PAM (mol ratio of 9:1) in aqueous 50 mM HEPES buffer (pH 9.0) with final concentrations of 0.9mM DA and 0.1mM DA-PAM was prepared. The suspension was sonicated at 80 C for 30 min, filtered through a 0.8 pm syringe filter and stored at 40C. The monomer mixture was copolymerized by UV irradiation at 254 nm for 30 s. After the UV irradiation, a colorless-to-blue color change was observed. A blue polydiacetylene-pralidoxime suspension was prepared (PDA-PAM)
N o 000 UV 254nm 30s Malathion ON 0"0 ON ~oN w
Figure 2.14 Copolymerization of DA and DA-PAM suspension and its color change upon addition of malathion.
2.3.2 Colorimetric detection of organophosphate by PDA-PAM.
The colorimetric changes in the PDA-PAM aqueous suspension with varying
blue PDA-PAM suspension was prepared in aqueous 10 mM HEPES buffer (pH 9.0) in eight PCR tubes each containing 50 pL. Malathion/acetonitrile solution of varying concentrations (2 gL total) was added into each tube.
Figure 2.15 Colorimetric response of PDA-PAM to malathion in aqueous suspensions
Upon addition of certain concentrations of malathion (from 1mM to 64mM) to a polydiacetylene-pralidoxime aqueous suspension, a dramatic blue-to-red color change was observed in seconds. As shown in Figure 2.15, at 8mM the color change occurred and, starting from 16 mM of malathion, a significant blue-to-red color change could be observed by the naked eye. As the reported acetylcholinesterase inhibition concentration (IC50) of malathion is
approximately 60 mM,[73] the colorimetric response of our PDA-PAM suspension is in a useful range of detection with a good sensitivity and fast response.
2.3.3 Colorimetric detection of organophosphate by PDA-PAM at different temperatures.
Figure 2.16 Colorimetric response of PDA-PAM to malathion in aqueous suspensions at 4 'C and 40 'C.
The colorimetric malathion detection capability of this PDA-PAM system was found to be relatively constant over a wide range of temperatures. The PDA-PAM suspension and malathion/acetonitrile solution were preheated in an oven to 40 'C or precooled in a refrigerator to 4'C, at which temperatures the colorimetric tests were performed. As shown in Figure 2.16, the same significant blue-to-red color change was observed at these temperatures as at 20 *C, over the concentration range of 1 to 64 mM, indicating the potential for PDA-PAM to be used in a sensoric system for hazard detection over a wide range of field temperatures.
2.3.4 Colorimetric detection of organophosphate by PDA-PAM towards different chemicals The possible confounding of malathion detection with positive colorimetric responses of PDA-PAM to other chemical species was also explored. Some compounds selected for these tests (HCl, Acetic Acid, H2SO4, H3PO4,) mimic the different acidic environments found in
polluted aqueous sources in which the byproducts of air and water pollution are present, while hydrogen peroxide (H202) was included to evaluate the oxidative stress due to environmental pollutants. Acetonitrile was also selected as it is used in malathion solution preparation to examine the possibility of it inducing a false reading during malathion detection.
A blue PDA-PAM suspension was prepared in aqueous 10 mM HEPES buffer (pH 9.0) in eight PCR tubes each containing 50 pL. Different chemicals/water solution (malathion, HC,
H2SO4, acetatic acid, HNO3, H202, acetonitrile) was added into each tube. The final
concentration of malathion was 20 mM while the other control chemicals had the final concentration of 100 mM.
20mM
100mM
0ank
Wttakhi6h H
CI Acetic Hsbt
4H
2 2aP
nitrile
I
As can be seen in Figure 2.17, the PDA-PAM suspension has a blue-to-red color change in the presence of only malathion, while almost no color change was observed with the control group compounds.
2.3.5 Colorimetric detection of organophosphate by PDA-PAM in the presence of other chemical
compounds.
Colorimetric detection of organophosphate by PDA-PAM in the presence of cholrsulfuron, an herbicide that is commonly used together with malathion but has much lower toxicity, was also conducted. A blue PDA-PAM suspension was prepared in aqueous 10 mM HEPES buffer (pH 9.0) in eight PCR tubes each containing 50 tL. Chlorsulfuron as was added to each tube to a final concentration of 100 mM. Malathion/acetonitrile solutions of varying concentrations (2 ptL total) were added to tubes. The final concentration of malathion varied from 1 to 64 mM. No color change was observed after the addition of chlorsulfuron. After the addition of malathion the color changed visually within seconds. The colorimetric response of PDA-PAM towards malathion was not affected when cholrsulfuron was added to the solution, as shown in Figure 2.18.
With
100mM Chlorsulfuron
Malathion:
Figure 2.18 Colorimetric response of PDA-PAM to malathion in aqueous suspensions in the presence of a herbicide interference.
2.3.6 Colorimetric response of PDA-PAM absorption change
The color change in the PDA-PAM suspension in aqueous medium (pH 9.0) could be quantified spectrophotometrically. The PDA-PAM suspension was titrated with the malathion/acetonitrile solution. The PDA-PAM suspension (400 p.L, 1 mM) was prepared as described previously. Because of the poor aqueous solubility of malathion, a malathion/acetonitrile solution was prepared (100 mg/ml). For each titration, 2.67 d of the malathion was added to the 400 tL PDA solution, which resulted in 2mM addition in the final concentration of malathion.
With the addition of malathion, the absorbance of the PDA-PAM suspension above 600 nm decreased, while the absorbance between 400 nm and 600 nm increased. An isosbestic point at 630 nm was also detected, indicating a one-to-one conversion of PDA-PAM during the titration. The degree of color change is readily quantified by the electronic absorption at specific wavelengths. As shown in Figure 2.19, the blue-colored solution had a strong absorption maximum
at 645 nm. After interaction with malathion, the absorption at 550 nm increased with a concurrent decrease in the maximum at 645 nm, resulting in the blue-to-red color shift. The visible spectrum of the solution before exposure to malathion was analyzed as [35]
BO 645
,550 +1645 (Eq.1)
where Bo is defined as the intensity of absorption at 645 nm divided by the sum of the absorption intensities at 550 nm and 645 nm.. After exposure to malathion a new measure of absorbance arises:
B= '645
I550 +I645 (Eq.2)
By represents the new ratio of absorption intensities.Then the colorimetric response (CR) can be expressed as
CR=(By-Bo)/Bo (Eq.3)
0.8
300
400
500
600
700
800
Wavelength
X
(nm)
Figure 2.19 Spectrum change of the PDA-PAM vesicle suspension upon malathion addition.
0
5
10
Malathion Concentration (mM)
15
Figure 2.20 Colorimetric response of the PDA-PAM vesicle suspension upon malathion addition.
12 miM
10 mM
14 mM
6rnM
16 mM
6mM
4
mM
2mM
PDA-PAM
Only
C) (U 0 C,0.6
0.4
0.2
0
C 0 CL (a 0 UE
0 0 00.5
0.4
0.3
0.2
0.1
With the addition of malathion, the absorbance of the PDA-PAM suspension at 640 nm decreased, while the absorbance between 400 nm and 600 nm increased. An isosbestic point at 630 nm was also detected, indicating a one-to-one conversion of PDA-PAM during the titration.
2.3.7 Colorimetric detection of organophosphate by PDA-PAM embedded Teflon membrane Immobilization of polydiacetylene onto solid supports would be a convenient construct to maximize its signal transduction[74] and enhance its storage stability.[75] The solid state
detection of malathion was demonstrated with a membrane strip coated with a PDA-PAM suspension.
A Teflon membrane was impregnated with a solution of DA and DA-PAM (mol ratio of 9:1) in acetonitrile, and then dried in a hood. Upon UV irradiation at 365 nm for 30s, a white-to-blue color change on the Teflon membrane indicated polymerization of the monomers. 10 piL drops of malathion/acetonitrile solution were applied onto the membrane, with the final
concentration of malathion varying from 1.25nmol/cm2 to 1.28 pimol/cm2.
As is evident in Figure 2.21, a colorimetric response was observed by the naked eye even at malathion concentrations in the nmol/cm2 range on the PDA-PAM/Teflon membrane.
Malathion concentrations as low as nmol/cm2 on the PDA-PAM/Teflon membrane lead to a colorimetric response that could be observed directly by eye.
PDA-PAM Only 1280 nmol kn
4!2o42mm
1 t2.5 nmol IC etonitrile/H
Figure 2. 21 Colorimetric detection of organophosphate using PDA-PAM impregnated Teflon membrane.
2.3.8 Dynamic light scattering (DLS) diameters of DA-PAM, PDA-PAM and PDA-PAM with Malathion.
The structural change in the DA-PAM, PDA-PAM and PDA-PAM with Malathion suspension was followed by dynamic light scattering measurements. The suspension was sonicated at 80 0C for 30 min, filtered through a 0.8 pim syringe filter and stored at 40C. The monomer mixture was copolymerized by UV irradiation at 254 nm for 30 s. After the UV irradiation, a colorless-to-blue color change was observed. A blue polydiacetylene-pralidoxime suspension was prepared (PDA-PAM). The DLS diameter of DA/DA-PAM (mol ratio of 9:1 in 10mM HEPES buffer, pH 9.0), PDA-PAM(lOmM HEPES buffer, pH 9.0) and malathion bound PDA-PAM (10mM HEPES buffer, pH 9.0) were measured on NanoBrook ZetaPALS Potential Analyzer.
As shown in Figure 2.22, the hydrodynamic diameter of the diacetylene (DA/DA-PAM) monomer aggregates was ~ 270 nm in aqueous medium, while after polymerization, the vesicles appeared to shrink to an average diameter of 190 nm. After binding to malathion, the apparent average diameter of the mixed PDA-PAM and PDA colloid expanded to 240 nm.
120 100 80 60 40 0 PDA-PAM
PDA-PAM Malathion DA-PAM
200 300
Diameter(nm)
100 400 500
Figure 2.22 Volume-average DLS diameter distribution of DA-PAM (black), PDA-PAM (blue), and PDA-PAM complexed with malathion (red) in 50 mM HEPES buffer (pH 9.0).
a)

![Figure 1. 3 Colorimetric response of polythiophene in the presence of adenosine triphosphate(ATP) [25] and polyphenylene vinylene in different organic solvents [26].](https://thumb-eu.123doks.com/thumbv2/123doknet/14744197.577584/22.917.114.761.620.974/colorimetric-response-polythiophene-presence-adenosine-triphosphate-polyphenylene-different.webp)






