HAL Id: hal-02438076
https://hal.archives-ouvertes.fr/hal-02438076
Submitted on 3 Dec 2020HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Discovery of hydrazone containing thiadiazoles as
Mycobacterium tuberculosis growth and enoyl acyl
carrier protein reductase (InhA) inhibitors
Hilal Doğan, Şengül Dilem Doğan, Miyase Gözde Gündüz, Vagolu Siva
Krishna, Christian Lherbet, Dharmarajan Sriram, Onur Şahin, Emin Sarıpınar
To cite this version:
Hilal Doğan, Şengül Dilem Doğan, Miyase Gözde Gündüz, Vagolu Siva Krishna, Christian Lherbet, et al.. Discovery of hydrazone containing thiadiazoles as Mycobacterium tuberculosis growth and enoyl acyl carrier protein reductase (InhA) inhibitors. European Journal of Medicinal Chemistry, Elsevier, 2020, pp.112035. �10.1016/j.ejmech.2020.112035�. �hal-02438076�
1
doganDiscovery of hydrazone containing thiadiazoles as Mycobacterium tuberculosis growth and enoyl acyl carrier protein reductase (InhA) inhibitors
Hilal Doğana,b, Şengül Dilem Doğanb*
, Miyase Gözde Gündüzc, Vagolu Siva Krishnad, Christian Lherbete, Dharmarajan Sriramd, Onur Şahinf, Emin Sarıpınara
a
Department of Chemistry, Faculty of Science, Erciyes University, 38039, Kayseri, Turkey
b
Department of Basic Sciences, Faculty of Pharmacy, Erciyes University, 38039, Kayseri, Turkey
c
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Hacettepe University, Sıhhiye, 06100, Ankara, Turkey
d
Department of Pharmacy, Birla Institute of Technology and Science-Pilani, 500078, Hyderabad, India
e
LSPCMIB, UMR-CNRS 5068, Université Paul Sabatier-Toulouse III, 236 cours Eugène Cosserat, 31062 Toulouse Cedex, France
f
Scientific and Technological Research Application and Research Center, Sinop University, 57000 Sinop, Turkey
*Corresponding author
Dr. Şengül Dilem Doğan
Address: Erciyes University Faculty of Pharmacy
Department of Basic Sciences 38039
2
E-mail address: dogandilem@gmail.com Phone number: +90 352 2076666-28032
Abstract
Tuberculosis, caused by Mycobacterium tuberculosis, is a serious infectious disease and remains a global health problem. There is an increasing need for the discovery of novel therapeutic agents for its treatment due to the emerging multi-drug resistance. Herein, we present the rational design and the synthesis of eighteen new thiadiazolylhidrazones (TDHs) which were synthesized by intramolecular oxidative N-S bond formation reaction of 2-benzylidene-N-(phenylcarbamothioyl)hydrazine-1-carboximidamide derivatives by phenyliodine(III) bis(trifluoroacetate) (PIFA) under mild conditions. The compounds were characterized by various spectral techniques including FTIR, 1H NMR, 13C NMR and HRMS. Furthermore, the proposed structure of TDH12 was resolved by single-crystal X-ray analysis. The compounds were evaluated for their in vitro antitubercular activity against M. tuberculosis H37Rv. Among them, some compounds exhibited remarkable antimycobacterial activity, MIC=0.78-6.25 µg/mL, with low cytotoxicity. Additionally, the most active compounds were screened for their biological activities against M. tuberculosis in the nutrient starvation model. Enzyme inhibition assays and molecular docking studies revealed enoyl acyl carrier protein reductase (InhA) as the possible target enzyme for the compounds to possess their antitubercular activities.
3
1. Introduction
Tuberculosis (TB) is a fatal infectious disease caused by various strains of mycobacteria. Among them, Mycobacterium tuberculosis remains the leading cause of TB, which is responsible for affecting one-third of the world’s population [1]. World Health Organization (WHO) has declared TB as the leading cause of death due to a single infectious agent and also estimated that approximately 10 million people developed TB in 2018 [2]. Despite the availability of many commercial anti-TB drugs; the disease still causes an enormous amount of deaths worldwide. The limited efficacy of these traditional drugs against extensively drug-resistant and multidrug-resistant TB makes the treatment of the disease complicated [3]. Thus, there is an emergent need to discover new therapeutic agents with safer toxicity profiles to combat the spread of TB.
Compounds possessing hydrazide-hydrazone functionality have been reported to display significant anti-TB activity [4,5], in addition to activities against further infectious diseases such as bacterial infections and malaria [6,7]. Therefore, these structural motifs demonstrate an absolute ability to interfere with vital biochemical processes of infectious agents. Consequently, introducing the hydrazone moiety to the structure of new drug candidates as well as marketed antimycobacterial agents such as isoniazid [8], ciprofloxacin [9] and pyrazinamide [10] is a rational and frequently used approach to obtain novel antitubercular molecules with reduced toxicity (Figure 1).
4
Figure 1. Antimycobacterial hydrazone derivatives of well-known antitubercular drugs.
Thiadiazole scaffold stands as a distinguished pharmacophore integrated into the structures of lead drug molecules for the treatment of various pathologies ranging from tuberculosis to diabetes [11,12]. Particularly, 1,2,4-thiadiazole isomer represents an excellent core structure for different therapeutic purposes including antitubercular as well as antibacterial agents exemplified by cefozopran (Figure 2), which is a commercial member of β-lactam antibiotics [13,14].
Figure 2. Chemical structure of cefozopran.
The enoyl acyl carrier protein reductase, InhA, is one of the enzymes that are employed in the mycobacterial fatty acid biosynthesis pathway. This enzyme is essential for the bacterial growth of Mycobacterium tuberculosis and has been also identified as the target of two anti-tubercular drugs; isoniazid and ethionamide. Therefore, InhA attracts great interest as a target for the development of new anti-tubercular agents [15].
The binding site of InhA contains three key regions that are occupied by its inhibitors. Site I, the catalytic site, contains the ribose group of the NAD cofactor and a tyrosine amino acid. In this site, the inhibitors interact with the enzyme via forming hydrogen bonds with the hydroxyl group of Tyr158 and the ribose ring of NAD. Site II forms a hydrophobic pocket close to the side chain of Tyr158. This region hosts the hydrophobic rings of the inhibitors for hydrophobic interactions. The Site III is a relatively unexplored one but the hydrophobic rings of the inhibitors lie close to the phosphate groups of NAD as well as Gly96 and Phe97 at van der Waals distance (Figure 3) [16]. These hydrophobic moieties of InhA inhibitors contribute to the antimycobacterial activity not only interacting with the enzyme but also increasing the lipophilicity to enable the compounds to easily cross the biological membranes [17].
5
Figure 3. Molecular surface representation of InhA with the co-crystallized ligand and NAD
(PDB code:4TZK) represented as pink and blue sticks, respectively (A). Enlarged view of InhA active site: the ligand is displayed as pink stick, residues contributing ligand binding are represented as lines, hydrogen bonds are shown as green dotted lines (B).
In light of these considerations, we aimed to synthesize new thiadiazolylhidrazones (TDH) and increase the lipophilicity of these compounds by introducing two (substituted) phenyl rings to obtain better antimycobacterial activity (Figure 4). In order to establish the mechanism of antitubercular activity, we tested the title compounds against InhA as they possess the required structural moieties (two hydrophobic rings and hydrogen bond acceptor atoms to form hydrogen bonds to NAD and Tyr158) to interact with this enzyme.
6
2. Results and discussion
2.1. Synthesis
The syntheses of the target compounds were performed as outlined in Scheme 1. The condensation of benzaldehydes (I) in the presence of sodium hydroxide with aminoguanidine nitrate (II), produced 2-benzylidenehydrazinecarboximidamides (III). N-[(phenylamino) thioxomethyl]-2-(phenylmethylene)hydrazinecarboximidamide derivatives (IV) were synthesized by the reaction of substituted phenyl isothiocyanates with compounds III in acetonitrile under reflux conditions. The target compounds, 3-(2-benzylidenehydrazinyl)-N-phenyl-1,2,4-thiadiazol-5-amine derivatives TDH1-18, were synthesized by the oxidative cyclization of imidoyl thiourea group in IV using hypervalent iodine (III) reagent [13,18,19], phenyliodine(III) bis(trifluoroacetate) (PIFA) as oxidant. Treatment of IV with PIFA in CH2Cl2 in the presence of trifluoroacetic acid (TFA) as initially described by Mariappan et al. [20] afforded the desired cyclized compound TDH1-18 (Table 1) via intramolecular N-S bond formation reaction but in modest yield (52-91%). In all cases, the final products were purified by silica gel chromatography. The plausible reaction mechanism for the formation of title compounds is shown in Scheme 2.
Scheme 1. Reagents and conditions: (i) H2O, NaOH, rt, 1 h. (ii) various phenyl isothiocyanates (PhNCS), ACN, reflux. (iii) PIFA, TFA, DCE, rt, 3-5 mins.
7
Scheme 2. The plausible reaction mechanism for the formation of TDH1-18
The structures of the target compounds were confirmed by 1H-NMR, 13C-NMR, HRMS and FTIR and that of TDH12 was confirmed by single-crystal X-ray analysis. 1H-NMR and 13 C-NMR spectra of TDH1-18 are provided as supplementary materials.
Table 1. Structural features and physical properties of the synthesized compounds
Compound R1 R2 R3 Yield (%) m.p (ºC) Molecular
Weight TDH1 H H H 73 249-251 295 TDH2 H H CH3 71 220-222 309 TDH3 H H OCH3 88 240-242 325 TDH4 H H NO2 45 237-239 340 TDH5 H H Br 83 224-226 374 TDH6 H H F 82 237-239 313 TDH7 H H I 79 69-71 421 TDH8 H Cl Cl 90 213-215 364 TDH9 H H CF3 55 131-133 363 TDH10 CH3 H H 87 223-225 309 TDH11 CH3 H CH3 83 204-206 323 TDH12 CH3 H OCH3 91 205-207 339 TDH13 CH3 H NO2 52 268-270 354 TDH14 CH3 H Br 88 233-235 388 TDH15 CH3 H F 63 215-217 327 TDH16 CH3 H I 76 235-237 435 TDH17 CH3 Cl Cl 63 190-192 378 TDH18 CH3 H CF3 57 117-119 377
8
2.2. X-Ray Structure Determination
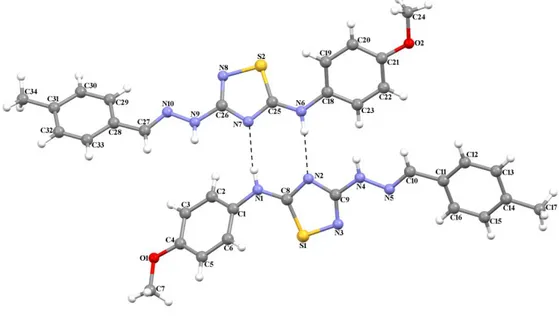
The three-dimensional structure of TDH12 with the atom labeling is shown in Figure 5.
Figure 5. The molecular structure of TDH12 showing the atom numbering scheme.
The asymmetric unit contains two symmetry-independent molecules with no significant difference in their structures. The N=C bond distances lie in the range 1.256 (16)-1.258 (16) Å, respectively. The 1,2,4-thiadiazole rings make dihedral angles of 16.61 (4) and 40.16 (2)° with the two phenyl rings in one molecule, and 21.27(5) and 41.71(3)° in the other. The dihedral angles of the phenyl rings are 56.18(3) and 62.75(3)°, respectively. The 1,2,4-thiadiazole rings are approximately planar, with maximum deviation from the least-squares plane being 0.0114(49) Å for atom N3 and 0.0086(74) Å for atom N7. The molecules of TDH12 are connected by N-H∙∙∙N hydrogen bonds (Table 2).
Table 2. Hydrogen-bond parameters (Å, º)
D-H· · ·A D-H H···A D···A D-H···A N1—H1···N7 0.86 2.09 2.944 (14) 172 N6—H6A···N2 0.86 2.15 2.982 (14) 163
9
2.3. Mycobacterium tuberculosis growth inhibition and cytotoxicity evaluation
The obtained compounds were evaluated for their in vitro antitubercular activity against M.
tuberculosis H37Rv utilizing the Microplate Alamar Blue Assay (MABA) method. Isoniazid,
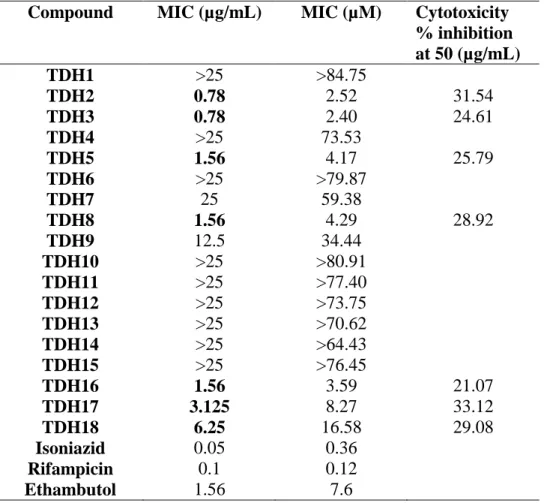
rifampicin, and ethambutol were used as positive control. As the discovery of new antimycobacterial agents with low cytotoxicity is of utmost importance, the most active compounds were also screened for their cytotoxic effects against RAW 264.7 cells at 50 μg/mL concentration using MTT assay. The MIC values of the compounds, as well as the cytotoxicity data for the most active compounds, are provided in Table 3.
Table 3. Antitubercular activity and cytotoxicity data of TDH1-18
Compound MIC (µg/mL) MIC (µM) Cytotoxicity
% inhibition at 50 (µg/mL) TDH1 >25 >84.75 TDH2 0.78 2.52 31.54 TDH3 0.78 2.40 24.61 TDH4 >25 73.53 TDH5 1.56 4.17 25.79 TDH6 >25 >79.87 TDH7 25 59.38 TDH8 1.56 4.29 28.92 TDH9 12.5 34.44 TDH10 >25 >80.91 TDH11 >25 >77.40 TDH12 >25 >73.75 TDH13 >25 >70.62 TDH14 >25 >64.43 TDH15 >25 >76.45 TDH16 1.56 3.59 21.07 TDH17 3.125 8.27 33.12 TDH18 6.25 16.58 29.08 Isoniazid 0.05 0.36 Rifampicin 0.1 0.12 Ethambutol 1.56 7.6
10
According to the obtained MABA results, seven compounds exhibited MIC values at ≤ 6.25 µg/mL. TDH2 and TDH3 stand as excellent antitubercular agents in this series with MIC value of 0.78 µg/mL. Additionally, TDH5, TDH8 and TDH16 also exhibited MIC values at 1.56 in the same manner as ethambutol. One of the primary differences between the synthesized compounds is the presence or absence of the methyl substituent on the benzylidene moiety. It is obvious that the introduction of this methyl group into the structure did not mediate an increase in the mentioned activity because the compounds carrying non-substituted benzylidene group
(TDH1-TDH9) generally have lower MIC values. When the obtained results are examined in terms of the
substituents on the N-phenyl ring, it is noteworthy that the di-substitution of this ring made a positive contribution to antimycobacterial activity. Besides that, the most active two compounds carry methyl (TDH2) and methoxy (TDH3) groups suggesting that electron-donating properties of these substituents can play a role in exhibiting their antitubercular activities.
The seven compounds, which have MIC values ≤ 6.25µg/mL, were also screened for their in
vitro cytotoxicities by utilizing MTT assay. According to the obtained inhibition percentages, all
tested compounds were found to be non-toxic with <50% inhibition.
2.4. Nutrient starvation model of Mycobacterium tuberculosis H37Rv
The first-line drugs used for the treatment of TB are able to clear Mycobacterium tuberculosis in individuals but they cannot prevent the spread of the bacteria. This situation can be partly explained by the fact that these drugs are not effective in killing inactive or persistent bacteria, which can also survive in oxygen and supplement deprived conditions. Thus, it is crucial to carry out biological assays that intimately mimic the conditions where the bacterium resides. For this purpose, M. tuberculosis culture was supplement starved in phosphate buffer saline (PBS) for six weeks. Afterward, the culture was treated with TDH2 and TDH3 (at 10 µM concentrations) which have the lowest MIC values obtained from MABA. The obtained results are provided in Figure 6.
11
Figure 6. Biological activities of TDH2 and TDH3 against M. tuberculosis in the nutrient
starvation model. Bacterial count estimation (Mean ± S.D., n = 3) for control and treated groups conducted by using the MPN (most probable number) assay. Both the compounds gave significant inhibition of growth of M. tuberculosis in this model as compared to the control (p < 0.0001, two way ANOVA using GraphPad Prism Software.
The tested compounds, TDH2 and TDH3, showed 2.4 and 2.2 log reductions in growth, respectively. As new drugs should also be effective against latent or dormant M. tuberculosis, it is important to note that these compounds significantly inhibited the growth of M. tuberculosis in the nutrient starvation model.
2.5. InhA inhibition assay
In order to gain insights into the antitubercular activity mechanism of the synthesized compounds, they were evaluated for their in vitro inhibition of InhA from M. tuberculosis at 50 μM using triclosan as positive control. This enzyme was chosen as the possible target of
TDH1-18 as the title compounds fulfill all required structural moieties to interact with the enzyme
binding pocket in the same manner as one of well-known inhibitors of InhA (Figure 7). Additionally, thiadiazole-based compounds were reported to be InhA inhibitors in recent studies [21,22]. These data further constituted a valid basis for the selection of this enzyme.
12
Figure 7. Pharmacophore similarities between the co-crystallized ligand of InhA, PDB code:
4TZK, (top) and the title compounds (bottom)
The compounds possessing more than 60% inhibition were also tested at 5 µM concentration. The obtained results are presented in Table 4.
Table 4. Enzyme inhibition values for TDH1-18 derivatives. Results are expressed as a
percentage of InhA inhibition.
Compound % Inhibition at 50 µM of compounds (in bracket at 5 µM) TDH1 16 TDH2 75 (48) TDH3 46 TDH4 35 TDH5 61 (39) TDH6 54 TDH7 Not soluble TDH8 38 TDH9 50 TDH10 34 TDH11 21 TDH12 50 TDH13 8 TDH14 46 TDH15 67 (26) TDH16 34 TDH17 62 (48) TDH18 64 (35) TCL >99
13
According to the obtained values; most of the compounds exhibited from moderate to good InhA inhibitory effects. One of the most active compounds (TDH2) with the lowest MIC value from MABA assay was found to be the best InhA inhibitor, as well. Generally, introducing a halogen to N-phenyl ring led to an increase in the mentioned activity. However, the most active compound carries methyl group at the same locus suggesting that the interaction types with the binding site of the enzyme play a more important role than the electronic features of the substituents. As the enzyme inhibition values are consistent with the results obtained from MABA assay, it can be concluded that InhA is an important target for those compounds that possess antitubercular activities.
2.6. Molecular docking
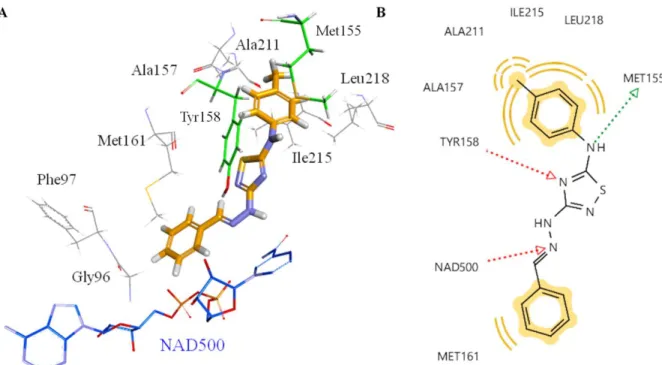
The most effective InhA inhibitor compound, TDH2, was selected for the docking studies. In order to investigate the interacting atoms/groups and the type of interactions, TDH2 was docked into the binding pocket of InhA. The orientation of THD2 in the active site of the enzyme and 2D representations of the pharmacophores are shown in Figure 8.
Figure 8. Proposed binding mode of THD2 (orange stick) in the active site of InhA enzyme (A).
2D depiction of enzyme-ligand interactions: Hydrogen bond acceptor and donor interactions are represented as red and green arrows, respectively. Hydrophobic interactions are shown by yellow spheres (B).
14
When the obtained results are analyzed, it can be seen that TDH2 forms two key hydrogen bonds with NAD500 and Tyr158 via the nitrogen atoms in the hydrazine functionality and the thiadiazole ring, respectively in the same manner as the co-crystallized ligand. It is important to note that THD2 interacts with the enzyme by forming one more hydrogen bond with the sulfur atom of Met155 through the amine group. The hydrophobic pocket formed by Met155, Ala157, Tyr 158, Ala211, Ile215 and Leu218 hosted one of the lipophilic phenyl rings. The other phenyl ring, which is the part of hydrazine functionality, was also responsible to form hydrophobic interactions. Additionally, the ring is oriented towards to the phosphate groups of NAD as well as Gly96 and Phe97 at van der Waals distance. Consequently, THD2 fulfills all structural requirements to possess InhA inhibitory activity.
3. Conclusions
As tuberculosis threats considerable amount of lives worldwide, the discovery of new therapeutics, preferably with novel structures and mechanisms of action, is of utmost importance. For this purpose, we presented the rational design and synthesis of hydrazone derivatives of thiadiazoles as Mycobacterium tuberculosis growth inhibitors in this study. We obtained very effective compounds, which are also able to act on latent or dormant forms of the bacteria. Additionally, the compounds were demonstrated to show their mode of action by inhibiting InhA, which catalyzes an essential step in fatty acid biosynthesis of Mycobacterium tuberculosis. Altogether, our data can serve as a template for designing future agents against Mycobacterium
tuberculosis for the successful clinical control of the disease.
4. Experimental
4.1. Chemistry
4.1.1. Materials and methods
Unless otherwise specified, all materials were obtained from commercial sources and used without purification. Reaction time and purity of the products were determined by thin-layer chromatography (TLC) with fluorescent indicator visualizable at 254 nm and 365 nm. Melting points were determined using open glass capillaries and were uncorrected. Infrared (IR) spectra were obtained via ATR diamond in the range 4000–600 cm−1. 1H NMR (400 MHz) spectra and 13
15
CDCl3 or DMSO-d6 as the solvent. Coupling constants, J, are reported in hertz (Hz). MS spectra were carried out on an LC/MS High-Resolution Time of Flight (TOF) Agilent 1200/6530 instrument at the Atatürk University-East Anatolian High Technology Research and Application Center (DAYTAM).
4.1.2. General procedure for the synthesis of 2-benzylidenehydrazine-1-carboximidamide derivatives (III)
These compounds were synthesized according to the modified literature procedure [23,24]. Benzaldehydes (I) (0.1 mmol) and aminoguanidine nitrate (II) (0.11 mmol) in 15 mL water were mixed at room temperature for 1 h. The suspension was neutralized with excess of 2 N NaOH. The precipitated material was filtered off, washed with water and dried to afford crystals. Obtained compounds were used for the next step without any purification process.
4.1.3. General procedure for the synthesis of 2-benzylidene-N-(phenylcarbamothioyl)hydrazine-1-carboximidamide derivatives (IV)
2-Benzylidenehydrazine-1-carboximidamide derivatives (III) (0.1 mmol) and various isothiocyanates (0.11 mmol) in acetonitrile (5 mL) were stirred at 80 ºC for 2 h as described in the literature [25,26]. Upon cooling, formed crystals were separated and recrystallized from ethanol. The obtained compounds (IV) were used the next step without any purification process.
4.1.4. General procedure for the synthesis of 1,2,4-thiadiazol-5-amine derivatives (TDH1-18).
A solution of PIFA (1.3 equiv.) in 15 mL of CH2Cl2 was added at 0 °C to a solution of 2-benzylidene-N-(phenylcarbamothioyl)hydrazine-1-carboximidamides (IV) (1 equiv.) and TFA (3 equiv.) in 20 mL of CH2Cl2, and the resulting solution was stirred for 2 h. (Reaction was followed by TLC). The reaction was quenched with saturated aqueous NaHCO3 and the mixture was diluted with EtOAc and extracted with EtOAc. The organic layers were washed with water and brine and dried over MgSO4. Then, the solvent was evaporated at reduced pressure, and the resulting residue was purified by column chromatography (EtOAc / hexanes, 2:3) followed by crystallization from suitable solvents to afford the title compounds.
16
4.1.4.1. 3-(2-Benzylidenehydrazinyl)-N-phenyl-1,2,4-thiadiazol-5-amine (TDH1)
White solid, yield: 73%. Mp 249-251 ºC; Rf(EtOAc:Hexane=4:7): 0.44. IR (ATR) 3391, 2988, 1650, 737. 1H NMR (400 MHz, DMSO-d6) δ 11.28 (s, 1H), 10.89 (s, 1H), 8.10 (s, 1H), 7.63 (d, J = 7.5 Hz, 2H), 7.55 (d, J = 8.0 Hz, 2H), 7.49 – 7.28 (m, 4H), 7.07 (d, J = 7.0 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 178.12, 163.42, 141.26, 140.34, 135.66, 129.74, 129.21, 129.16, 126.61, 123.19, 118.15. HRMS (EI): [M+H]+, found 296.0957. C15H13N5S requires 296.0970.
4.1.4.2. 3-(2-Benzylidenehydrazinyl)-N-(p-tolyl)-1,2,4-thiadiazol-5-amine (TDH2)
White solid, yield: 71%. Mp 220-222 ºC; Rf (EtOAc:Hexane=4:7): 0.43. IR (ATR) 3209.1, 2901.4, 1601, 737.71. 1H NMR (400 MHz, DMSO-d6) δ 11.26 (s, 1H), 10.74 (s, 1H), 8.09 (s, 1H), 7.62 (d, J = 7.5 Hz, 2H), 7.53 – 7.29 (m, 5H), 7.19 (d, J = 8.0 Hz, 2H), 2.28 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 178.27, 163.47, 141.17, 137.94, 135.67, 132.36, 130.14, 129.19, 129.16, 126.60, 118.30, 20.84. HRMS (EI): [M+H]+, found 310.1095. C16H15N5S requires 310.1048.
4.1.4.3. 3-(2-Benzylidenehydrazinyl)-N-(4-methoxyphenyl)-1,2,4-thiadiazol-5-amine (TDH3)
Cream-yellow solid, yield: 88%. Mp 240-242 ºC; Rf (EtOAc:Hexane=4:7): 0.32. IR (ATR) 3308.4, 2970.2, 1608.2, 761.83. 1H NMR (400 MHz, DMSO-d6) δ 11.23 (s, 1H), 10.62 (s, 1H), 8.09 (s, 1H), 7.62 (d, J = 7.6 Hz, 2H), 7.47 – 7.28 (m, 5H), 6.97 (d, J = 9.1 Hz, 2H), 3.75 (s, 3H). 13 C NMR (100 MHz, DMSO-d6) δ 178.76, 163.54, 155.76, 141.11, 135.72, 133.72, 129.18, 129.18, 126.61, 120.35, 114.98, 55.78.HRMS (EI): [M+H]+, found 326.1052. C16H15N5OS requires 326.0997. 4.1.4.4. 3-(2-Benzylidenehydrazinyl)-N-(4-nitrophenyl)-1,2,4-thiadiazol-5-amine (TDH4)
Yellow solid, yield: 45%. Mp 237-239 ºC; Rf (EtOAc:Hexane=4:7): 0.29. IR (ATR) 3371.2, 2987.3, 1620.9, 763.15. 1H NMR (400 MHz, DMSO-d6) δ 11.46 (s, 2H), 8.29 (d, J = 9.5 Hz, 2H), 8.12 (s, 1H), 7.81 (d, J = 9.5 Hz, 2H), 7.72 – 7.61 (m, 2H), 7.48 – 7.39 (m, 2H), 7.36 (d, J = 7.3 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 177.57, 163.47, 145.93, 141.83, 141.79, 135.51, 129.37, 129.20, 126.71, 126.01, 117.62. HRMS (EI): [M+H]+, found 341.0788. C15H12N6O2S requires 341.0742.
17
4.1.4.5. 3-(2-Benzylidenehydrazinyl)-N-(4-bromophenyl)-1,2,4-thiadiazol-5-amine (TDH5)
White solid, yield: 83%. Mp 224-226 ºC; Rf (EtOAc:Hexane=4:7): 0.44. IR (ATR) 3320.9, 2971.2, 1610, 751.6. 1H NMR (400 MHz, DMSO-d6) δ 11.36 (s, 1H), 10.96 (s, 1H), 8.09 (s, 1H), 7.63 (d, J = 7.6 Hz, 2H), 7.59-7.53 (m, 4H), 7.41 (t, J = 7.4 Hz, 2H), 7.35 (d, J = 7.2 Hz, 1H). 13C NMR (100MHz, DMSO-d6) δ 177.79, 163.41, 141.44, 139.62, 135.60, 132.43, 129.26, 129.17, 126.65, 120.01, 114.57. HRMS (EI): [M+H]+, found 374.0051. C15H12BrN5S requires 374.0075.
4.1.4.6. 3-(2-Benzylidenehydrazinyl)-N-(4-fluorophenyl)-1,2,4-thiadiazol-5-amine (TDH6)
White solid, yield: 82%. Mp 237-239 ºC; Rf(EtOAc:Hexane=4:7): 0.44. IR (ATR) 3324.3, 2988, 1619, 755.63. 1H NMR (400 MHz, DMSO-d6) δ 11.32 (s, 1H), 10.84 (s, 1H), 8.09 (s, 1H), 7.73 – 7.55 (m, 4H), 7.41 (t, J = 7.5 Hz, 2H), 7.35 (d, J = 7.1 Hz, 1H), 7.24 (t, J = 8.7 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 178.21, 163.39, 157.05, 141.33, 136.83, 135.62, 129.25, 129.18, 126.63, 120.04 (d, J = 7.9 Hz), 116.34 (d, J = 22.6 Hz). HRMS (EI): [M+H]+, found 314.0842. C15H12FN5S requires 314.0876. 4.1.4.7. 3-(2-Benzylidenehydrazinyl)-N-(4-iodophenyl)-1,2,4-thiadiazol-5-amine (TDH7)
White solid, yield: 79%. Mp 69-71 ºC; Rf(EtOAc:Hexane=4:7): 0.43. IR (ATR) 3361.5, 2952.9, 1612. 1H NMR (400 MHz, DMSO-d6) δ 11.33 (s, 1H), 10.92 (s, 1H), 8.10 (s, 1H), 7.71 (d, J = 8.8 Hz, 2H), 7.65 – 7.60 (m, 2H), 7.45 – 7.38 (m, 4H), 7.37 – 7.32 (m, 1H). 13C NMR (100 MHz, DMSO-d6) δ 177.75, 163.43, 141.43, 140.08, 138.23, 135.62, 129.26, 129.17, 126.65, 120.29, 86.23. HRMS (EI): [M+H]+, found 421.9900. C15H12IN5S requires 421.9936.
4.1.4.8. 3-(2-Benzylidenehydrazinyl)-N-(2,4-dichlorophenyl)-1,2,4-thiadiazol-5-amine (TDH8)
White solid, yield: 90%. Mp 213-215 ºC; Rf (EtOAc:Hexane=4:7): 0.63. IR (ATR) 3386.8, 2986.9, 1584.5, 752.79. 1H NMR (400 MHz, DMSO-d6) δ 11.32 (s, 1H), 10.42 (s, 1H), 8.41 (d, J = 9.2 Hz, 1H), 8.08 (s, 1H), 7.71 – 7.67 (m, 1H), 7.62 (d, J = 7.5 Hz, 2H), 7.52 – 7.46 (m, 1H), 7.44 – 7.29 (m, 3H). 13C NMR (100 MHz, DMSO-d6) δ 178.87, 162.85, 141.35, 136.05, 135.62, 129.61, 129.23, 129.16, 128.29, 128.04, 126.63, 124.46, 123.42. HRMS (EI): [M+H]+, found 364.0162. C15H11Cl2N5S requires 364.0190.
18
4.1.4.9. 3-(2-Benzylidenehydrazinyl)-N-(2-(trifluoromethyl)phenyl)-1,2,4-thiadiazol-5-amine
(TDH9)
White solid, yield: 55%. Mp 131-133 ºC; Rf (EtOAc:Hexane=4:7): 0.44. IR (ATR) 3217.4, 2987.2, 1596.2, 753.22. 1H NMR (400 MHz, DMSO-d6) δ 11.21 (s, 1H), 10.19 (s, 1H), 8.03 (s, 1H), 7.96 (d, J = 8.3 Hz, 1H), 7.85 – 7.70 (m, 2H), 7.59 (d, J = 8.2 Hz, 2H), 7.53 – 7.27 (m, 4H). 13 C NMR (100 MHz, DMSO-d6) δ 181.07, 163.03, 141.19, 137.69, 135.63, 134.20, 133.48, 129.21, 129.16, 128.04, 127.07, 127.02, 126.67, 126.59. HRMS (EI): [M+H]+, found 364.0816. C16H12F3N5S requires 364.0844. 4.1.4.10. 3-(2-(4-methylbenzylidene)hydrazinyl)-N-phenyl-1,2,4-thiadiazol-5-amine (TDH10)
White solid, yield: 87%. Mp 223-225 ºC; Rf (EtOAc:Hexane=3:7): 0.44. IR (ATR) 3220.3, 2986.8, 1589.5, 742.95. 1H NMR (400 MHz, DMSO-d6) δ 11.23 (s, 1H), 10.85 (s, 1H), 8.06 (s, 1H), 7.70 – 7.46 (m, 4H), 7.45 – 7.33 (m, 2H), 7.21 (d, J = 8.0 Hz, 2H), 7.07 (d, J = 7.8 Hz, 1H), 2.50 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 178.07, 163.51, 141.41, 140.34, 138.82, 132.93, 129.77, 129.76, 126.61, 123.19, 118.12, 21.41.HRMS (EI): [M+H]+, found 310.1101. C16H15N5S requires 310.1126. 4.1.4.11. 3-(2-(4-Methylbenzylidene)hydrazinyl)-N-(p-tolyl)-1,2,4-thiadiazol-5-amine (TDH11)
White-cream solid, yield: 83%. Mp 204-206 ºC; Rf(EtOAc:Hexane=3:7): 0.47. IR (ATR) 3180, 2980, 1610, 717.76. 1H NMR (400 MHz, DMSO-d6) δ 11.17 (s, 1H), 10.74 (s, 1H), 8.05 (s, 1H), 7.51 (d, J = 7.9 Hz, 2H), 7.41 (d, J = 8.1 Hz, 2H), 7.30 – 7.12 (m, 4H), 2.31 (s, 3H), 2.27 (s, 3H). 13 C NMR (100 MHz, DMSO-d6) δ 178.21, 163.51, 141.40, 138.84, 137.92, 132.91, 132.40, 130.14, 129.78, 126.60, 118.31, 21.40, 20.83. HRMS (EI): [M+H]+, found 324.1270. C17H17N5S requires 324.1283. 4.1.4.12. N-(4-methoxyphenyl)-3-(2-(4-methylbenzylidene)hydrazinyl)-1,2,4-thiadiazol-5-amine (TDH12)
Pink solid, yield: 91%. Mp 205-207 ºC; Rf(EtOAc:Hexane=3:7): 0.36. IR (ATR) 3185.6, 2926.8, 1602.9, 733.27. 1H NMR (400 MHz, DMSO-d6) δ 11.15 (s, 1H), 10.62 (s, 1H), 8.04 (s, 1H), 7.51 (d, J = 7.5 Hz, 2H), 7.43 (d, J = 8.4 Hz, 2H), 7.21 (d, J = 7.7 Hz, 2H), 6.96 (d, J = 8.3 Hz, 2H), 3.74 (s, 3H), 2.32 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 178.65, 163.56, 155.70, 141.21,
19
138.75, 133.70, 132.98, 129.77, 126.57, 120.28, 114.95, 55.75, 21.41.HRMS (EI): [M+H]+, found 340.1209. C17H17N5O5 requires 340.1232.
4.1.4.13. 3-(2-(4-methylbenzylidene)hydrazinyl)-N-(4-nitrophenyl)-1,2,4-thiadiazol-5-amine
(TDH13)
Yellow solid, yield: 52%. Mp 268-270 ºC; Rf(EtOAc:Hexane=3:7): 0.29. IR (ATR) 3259, 2900, 1611.5, 716.62. 1H NMR (400 MHz, DMSO-d6) δ 11.39 (s, 1H), 8.29 (d, J = 9.2 Hz, 2H), 8.08 (s, 1H), 7.81 (d, J = 9.2 Hz, 2H), 7.54 (d, J = 7.8 Hz, 2H), 7.23 (d, J = 7.8 Hz, 2H), 2.33 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 177.51, 163.54, 145.95, 141.93, 141.81, 138.98, 132.80, 129.81, 126.70, 126.00, 117.61, 21.42. HRMS (EI): [M+H]+, found 355.0948. C16H14N6O2S requires 355.0977.
4.1.4.2. N-(4-bromophenyl)-3-(2-(4-methylbenzylidene)hydrazinyl)-1,2,4-thiadiazol-5-amine (TDH14)
White solid, yield: 88%. Mp 233-235 ºC; Rf (EtOAc:Hexane=3:7): 0.42. IR (ATR) 3245.1, 2987.8, 1602, 714.50. 1H NMR (400 MHz, DMSO-d6) δ 11.27 (s, 1H), 10.95 (s, 1H), 8.05 (s, 1H), 7.66 – 7.41 (m, 6H), 7.23 (bs, 2H), 2.32 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 177.71, 163.45, 141.57, 139.62, 138.87, 132.88, 132.43, 129.79, 126.64, 119.98, 114.54, 21.41.HRMS (EI): [M+H]+, found 388.0198. C16H14BrN5S requires 388.0232.
4.1.4.15. N-(4-fluorophenyl)-3-(2-(4-methylbenzylidene)hydrazinyl)-1,2,4-thiadiazol-5-amine
(TDH15)
White solid, yield: 63%. Mp 215-217 ºC; Rf (EtOAc:Hexane=3:7): 0.44. IR (ATR) 3220.5, 2979.8, 1616, 714.68. 1H NMR (400 MHz, DMSO-d6) δ 11.24 (s, 1H), 10.83 (s, 1H), 8.06 (s, 1H), 7.67 – 7.46 (m, 4H), 7.38 – 7.03 (m, 4H), 2.32 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 178.13, 163.45, 141.43, 138.82, 136.85, 132.92, 129.77, 126.61, 119.95, 116.43, 116.21, 21.40.HRMS (EI): [M+H]+, found 328.1004. C16H14FN5S requires 328.1032.
20
4.1.4.16. N-(4-iodophenyl)-3-(2-(4-methylbenzylidene)hydrazinyl)-1,2,4-thiadiazol-5-amine
(TDH16)
White-cream solid, yield: 76%. Mp 235-237 ºC; Rf(EtOAc:Hexane=3:7): 0.44. IR (ATR) 3250, 2964.4, 1624, 700. 1H NMR (400 MHz, DMSO-d6) δ 11.26 (s, 1H), 10.92 (s, 1H), 8.05 (s, 1H), 7.70 (d, J = 8.3 Hz, 2H), 7.52 (d, J = 7.8 Hz, 2H), 7.40 (d, J = 8.5 Hz, 2H), 7.22 (d, J = 8.0 Hz, 2H), 2.31 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 177.65, 163.46, 141.55, 140.07, 138.86, 138.22, 132.88, 129.79, 126.63, 120.25, 21.42. (one carbon signal was overlapped). HRMS (EI): [M+H]+, found 436.0062. C16H14IN5S requires 436.0092.
4.1.4.17. N-(2,4-dichlorophenyl)-3-(2-(4-methylbenzylidene)hydrazinyl)-1,2,4-thiadiazol-5-amine (TDH17)
White solid, yield: 89%. Mp 190-192 ºC; Rf (EtOAc:Hexane=3:7): 0.67. IR (ATR) 3184.1, 2987.2, 1585, 725.69. 1H NMR (400 MHz, DMSO-d6) δ 11.25 (s, 1H), 10.41 (s, 1H), 8.40 (d, J = 8.9 Hz, 1H), 8.04 (s, 1H), 7.68 (d, J = 2.5 Hz, 1H), 7.57 – 7.40 (m, 3H), 7.20 (d, J = 7.9 Hz, 2H), 2.31 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 178.77, 162.90, 141.49, 138.83, 136.06, 132.90, 129.78, 129.61, 128.29, 127.99, 126.62, 124.40, 123.36, 21.41. HRMS (EI): [M+H]+, found 378.0316. C16H13CI2N5S requires 378.0347. 4.1.4.18. 3-(2-(4-methylbenzylidene)hydrazinyl)-N-(2-(trifluoromethyl)phenyl)-1,2,4-thiadiazol-5-amine (TDH18)
White solid, yield: 57%. Mp 117-119 ºC; Rf (EtOAc:Hexane=3:7): 0.44. IR (ATR) 3207.1, 2972.5, 1523.6, 725.14. 1H NMR (400 MHz, DMSO-d6) δ 11.15 (s, 1H), 10.19 (s, 1H), 8.02 – 7.90 (m, 2H), 7.84 – 7.66 (m, 2H), 7.57 – 7.38 (m, 3H), 7.21 (bs, 2H), 2.31 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 181.05, 163.11, 141.31, 138.80, 137.73, 137.70, 134.20, 132.93, 129.77, 128.05, 127.06, 127.01, 126.74, 126.65, 21.40.HRMS (EI): [M+H]+, found 378.0971. C17H14F3N5S requires 378.10003.
4.2. X-Ray diffraction analysis
Suitable crystal of TDH12 was selected for data collection which was performed on a D8-QUEST diffractometer equipped with a graphite-monochromatic Mo-Kα radiation at 296 K. The
21
structure was solved by direct methods using SHELXS-2013 [27] and refined by full-matrix least-squares methods on F2 using SHELXL-2013 [28]. The H atoms were located from different maps and then treated as riding atoms with C-H distances of 0.93-0.96 Å and N-H distances of 0.86 Å. The following procedures were implemented in our analysis: data collection: Bruker APEX2 [29]; program used for molecular graphics was as follows: MERCURY programs [30]; software used to prepare material for publication: WinGX [31]. Details of data collection and crystal structure determinations are given in Table 5.
Table 5. Crystal data and structure refinement parameters.
Empirical formula C17H17N5OS Formula weight 339.41 Crystal system Triclinic Space group P-1 a (Å) 7.3016 (11) b (Å) 11.236 (2) c (Å) 20.241 (4) α (º) 82.602 (9) β (º) 88.646 (10) γ (º) 89.32 (1) V (Å3) 1646.3 (5) Z 4 Dc (g cm-3) 1.369 μ (mm-1 ) 0.21 θ range (º) 3.1-22.8 Measured refls. 21604 Independent refls. 6416 Rint 0.091 S 1.25
22
4.3. Microplate Alamar Blue Assay for Mycobacterium tuberculosis
Briefly, the inoculum was prepared from fresh LJ medium re-suspended in 7H9-S medium (7H9 broth, 0.1% casitone, 0.5% glycerol, supplemented oleic acid, albumin, dextrose, and catalase [OADC]), adjusted to a OD590 1.0, and diluted 1:20; 100 µl was used as inoculum. Each drug stock solution was thawed and diluted in 7H9-S at four-fold the final highest concentration tested. Serial two-fold dilutions of each drug were prepared directly in a sterile 96-well microtiter plate using 100 µl 7H9-S. A growth control containing no antibiotic and a sterile control were also prepared on each plate. Sterile water was added to all perimetre wells to avoid evaporation during the incubation. The plate was covered, sealed in plastic bags and incubated at 37oC in normal atmosphere. After 7 days incubation, 30 µl of alamar blue solution was added to each well, and the plate was re-incubated overnight. A change in colour from blue (oxidised state) to pink (reduced) indicated the growth of bacteria, and the MIC was defined as the lowest concentration of drug that prevented this change in colour [32,33].
4.4. In vitro cytotoxicity screening
The in vitro cytotoxicity of the privileged antitubercular active analogues with lower MIC value were assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay against growth inhibition of RAW 264.7 cells at 50 μg/mL concentration.[1,2] Cell lines were maintained at 37 0C in a humidified 5% CO2 incubator (Thermo scientific). Detached the adhered cells and followed by centrifugation to get cell pellet. Fresh media was added to the pellet to make a cell count using haemocytometer and plate 100μl of media with cells ranging from 5,000 - 6,000 per well in a 96-well plate. The plate was incubated overnight in CO2 incubator for the cells to adhere and regain its shape. After 24hr cells were treated with the test compounds at 50 μg/mL diluted using the media to deduce the percentage inhibition on normal cells. The cells were incubated for 48 h to assay the effect of the test compounds on different cell lines. Zero hour reading was noted down with untreated cells and also control with 1% DMSO to subtract further from the 48hr reading. After 48 hr incubation, cells were treated by MTT (4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) dissolved in PBS (5mg/ml) and incubated for 3-4 hr at 37 0C. The formazan crystals thus formed were dissolved in 100μl of DMSO and the viability was measured at 540nm on a multimode reader (Spectra max). The
23
values were further calculated for percentage inhibition which in turn helps us to know the cytotoxicity of the test compounds [34,35].
4.5. Nutrient starvation model
A culture of M. tuberculosis H37Rv (O.D. of 0.8 - 1.0) grown in Middlebrook 7H9 medium supplemented with OADC was pelleted and washed twice with PBS. The pellet was resuspended in PBS in sealed bottles and incubated at 37 °C for 6 weeks. Aliquots of these cultures were then treated with standard drugs like INH, Rif and the lead compounds for 7 days at a concentration of 10 µg/mL. The frequency of persistors was enumerated by MPN (Most Probable Number) assay [33,36].
4.6. InhA activity inhibition
Triclosan and NADH were obtained from Sigma-Aldrich. Stock solutions of all compounds were prepared in DMSO such that the final concentration of this co-solvent was constant at 5% v/v in a final volume of 1 mL for all kinetic reactions. Kinetic assays were performed using trans-2-dodecenoyl-coenzyme A (DDCoA) and wild type InhA as previously described [37]. Briefly, reactions were performed at 25 °C in an aqueous buffer (30 mM PIPES and 150 mM NaCl pH 6.8) containing additionally 250 µM cofactor (NADH), 50 µM substrate (DDCoA) and the tested compound (at 50 µM or 5 µM). Reactions were initiated by addition of InhA (100 nM final) and NADH oxidation was followed at 340 nm. The inhibitory activity of each derivative was expressed as the percentage inhibition of InhA activity (initial velocity of the reaction) with respect to the control reaction without inhibitor. Triclosan was used as a positive control. All activity assays were performed in triplicate.
4.7. Molecular docking
The crystal structure of Mycobacterium tuberculosis enoyl-ACP reductase (InhA) with its inhibitor 1-cyclohexyl-N-(3,5-dichlorophenyl)-5-oxopyrrolidine-3-carboxamide (resolution: 1.62 Å) was obtained from Protein Data Bank under the PDB code 4TZK [38]. The chemical formula of THD2, the most effective inhibitor was drawn using ChemDraw Ultra 12.0 and saved as Simplified Molecule Input Entry System (SMILES) file. This file was transferred to LigandScout 4.2 [39], the structure was geometrically optimized and energy minimized to the 3D
24
structure using the MMFF94x force field. This compound was docked into the active site of InhA using AutoDock4.2, integrated into LigandScout 4.2, with default parameters. 3D pharmacophores from the co-crystallized ligand of 4TZK were generated using LigandScout. This pharmacophore model was employed to rank the obtained docking poses of THD2. The selection of the most plausible one was based on its ability to provide the highest amount of chemical interactions defined in this 3D pharmacophore model. Docking results were analyzed using LigandScout 4.2. The molecular docking figures in this study were generated using Maestro [40], DiscoveryStudio [41] and LigandScout 4.2.
Author Statements
HD and ŞDD synthesized the compounds and elucidated their structures. ES and ŞDD supervised the chemistry part of the study. OŞ carried out the X-ray analysis. VSK and DS were responsible for the MABA assay, nutrition starvation model and cytotoxicity experiments. CL performed InhA inhibition assay. MGG accomplished computational studies. MGG and ŞDD wrote the manuscript.
Conflict of Interest
The authors declared no potential conflicts of interest.
Acknowledgements
The author is indebted to the Research Foundation of Erciyes University (Grant No: FYL-2019-9164) and the Faculty of Pharmacy at Erciyes University for their financial support of this work. The authors acknowledge to Scientific and Technological Research Application and Research Center, Sinop University, Turkey, for the use of the Bruker D8 QUEST diffractometer. M.G.G. would like to thank Prof. Dr. Gerhard Wolber, Freie Universitat Berlin, for providing the license for LigandScout 4.2.
25
Supplementary Material
Crystallographic data for the structural analysis has been deposited with the Cambridge Crystallographic Data Centre, CCDC No. 1965137. Copies of this information may be obtained free of charge from the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or www: http://www.ccdc.cam.ac.uk).
References
[1] S. Tiberi, M. Muñoz-Torrico, R. Duarte, M. Dalcolmo, L. D’Ambrosio, G.-B. Migliori, New drugs and perspectives for new anti-tuberculosis regimens, Pulmonology. 24 (2018) 86–98. doi:10.1016/J.RPPNEN.2017.10.009.
[2] WHO | Global tuberculosis report 2019, WHO. (2019). https://www.who.int/tb/publications/global_report/en/.
[3] A. Campaniço, R. Moreira, F. Lopes, Drug discovery in tuberculosis. New drug targets and antimycobacterial agents, Eur. J. Med. Chem. 150 (2018) 525–545. doi:10.1016/J.EJMECH.2018.03.020.
[4] R.M. Beteck, R. Seldon, A. Jordaan, D.F. Warner, H.C. Hoppe, D. Laming, L.J. Legoabe, S.D. Khanye, Quinolone-isoniazid hybrids: synthesis and preliminary in vitro cytotoxicity and anti-tuberculosis evaluation, Medchemcomm. 10 (2019) 326–331. doi:10.1039/C8MD00480C.
[5] V. Velezheva, P. Brennan, P. Ivanov, A. Kornienko, S. Lyubimov, K. Kazarian, B. Nikonenko, K. Majorov, A. Apt, Synthesis and antituberculosis activity of indole–pyridine derived hydrazides, hydrazide–hydrazones, and thiosemicarbazones, Bioorg. Med. Chem. Lett. 26 (2016) 978–985. doi:10.1016/J.BMCL.2015.12.049.
[6] Ł. Popiołek, Hydrazide–hydrazones as potential antimicrobial agents: overview of the literature since 2010, Med. Chem. Res. 26 (2017) 287–301. doi:10.1007/s00044-016-1756-y.
[7] P. Kumar, K. Kadyan, M. Duhan, J. Sindhu, V. Singh, B.S. Saharan, Design, synthesis, conformational and molecular docking study of some novel acyl hydrazone based molecular hybrids as antimalarial and antimicrobial agents, Chem. Cent. J. 11 (2017) 115.
26
doi:10.1186/s13065-017-0344-7.
[8] Y.-Q. Hu, S. Zhang, F. Zhao, C. Gao, L.-S. Feng, Z.-S. Lv, Z. Xu, X. Wu, Isoniazid derivatives and their anti-tubercular activity, Eur. J. Med. Chem. 133 (2017) 255–267. doi:10.1016/J.EJMECH.2017.04.002.
[9] E. Vavříková, S. Polanc, M. Kočevar, K. Horváti, S. Bősze, J. Stolaříková, K. Vávrová, J. Vinšová, New fluorine-containing hydrazones active against MDR-tuberculosis, Eur. J. Med. Chem. 46 (2011) 4937–4945. doi:10.1016/J.EJMECH.2011.07.052.
[10] F.M.F. Vergara, C.H. da S. Lima, M. das G.M. de O. Henriques, A.L.P. Candéa, M.C.S. Lourenço, M. de L. Ferreira, C.R. Kaiser, M.V.N. de Souza, Synthesis and antimycobacterial activity of N′-[(E)-(monosubstituted-benzylidene)]-2-pyrazinecarbohydrazide derivatives, Eur. J. Med. Chem. 44 (2009) 4954–4959. doi:10.1016/J.EJMECH.2009.08.009.
[11] K.M. Dawood, T.A. Farghaly, Thiadiazole inhibitors: a patent review, Expert Opin. Ther. Pat. 27 (2017) 477–505. doi:10.1080/13543776.2017.1272575.
[12] P. A. Datar, T. A. Deokule, Development of Thiadiazole as an Antidiabetic Agent- A Review, Mini Rev. Med. Chem. 14 (2014) 136–153.
[13] L.M.T. Frija, A.J.L. Pombeiro, M.N. Kopylovich, Building 1,2,4-Thiadiazole: Ten Years of Progress, European J. Org. Chem. 2017 (2017) 2670–2682. doi:10.1002/ejoc.201601642.
[14] A. Castro, T. Castaño, A. Encinas, W. Porcal, C. Gil, Advances in the synthesis and recent therapeutic applications of 1,2,4-thiadiazole heterocycles, Bioorg. Med. Chem. 14 (2006) 1644–1652. doi:10.1016/J.BMC.2005.10.012.
[15] K. Rožman, I. Sosič, R. Fernandez, R.J. Young, A. Mendoza, S. Gobec, L. Encinas, A new ‘golden age’ for the antitubercular target InhA, Drug Discov. Today. 22 (2017) 492–502. doi:10.1016/J.DRUDIS.2016.09.009.
[16] A. Chollet, L. Maveyraud, C. Lherbet, V. Bernardes-Génisson, An overview on crystal structures of InhA protein: Apo-form, in complex with its natural ligands and inhibitors, Eur. J. Med. Chem. 146 (2018) 318–343. doi:10.1016/J.EJMECH.2018.01.047.
[17] G. Piccaro, G. Poce, M. Biava, F. Giannoni, L. Fattorini, Activity of lipophilic and hydrophilic drugs against dormant and replicating Mycobacterium tuberculosis, J. Antibiot. (Tokyo). 68 (2015) 711–714. doi:10.1038/ja.2015.52.
27
[18] A. Correa, I. Tellitu, E. Domínguez, R. SanMartin, Novel Alternative for the N−N Bond Formation through a PIFA-Mediated Oxidative Cyclization and Its Application to the Synthesis of Indazol-3-ones, /2006). doi:10.1021/JO060070+.
[19] J. Huang, Y. Lu, B. Qiu, Y. Liang, N. Li, D. Dong, One-Pot Synthesis of Substituted Isothiazol-3(2 H )-ones: Intramolecular Annulation of α-Carbamoyl Ketene- S , S -acetals via PIFA-Mediated N-S Bond Formation, Synthesis (Stuttg). 2007 (2007) 2791–2796. doi:10.1055/s-2007-983875.
[20] A. Mariappan, K. Rajaguru, N. Merukan Chola, S. Muthusubramanian, N. Bhuvanesh, Hypervalent Iodine(III) Mediated Synthesis of 3-Substituted 5-Amino-1,2,4-thiadiazoles through Intramolecular Oxidative S–N Bond Formation, J. Org. Chem. 81 (2016) 6573– 6579. doi:10.1021/acs.joc.6b01199.
[21] R. Šink, I. Sosič, M. Živec, R. Fernandez-Menendez, S. Turk, S. Pajk, D. Alvarez-Gomez, E.M. Lopez-Roman, C. Gonzales-Cortez, J. Rullas-Triconado, I. Angulo-Barturen, D. Barros, L. Ballell-Pages, R.J. Young, L. Encinas, S. Gobec, Design, Synthesis, and Evaluation of New Thiadiazole-Based Direct Inhibitors of Enoyl Acyl Carrier Protein Reductase (InhA) for the Treatment of Tuberculosis, J. Med. Chem. 58 (2015) 613–624. doi:10.1021/jm501029r.
[22] S.D. Joshi, U.A. More, D. Koli, M.S. Kulkarni, M.N. Nadagouda, T.M. Aminabhavi, Synthesis, evaluation and in silico molecular modeling of pyrroyl-1,3,4-thiadiazole inhibitors of InhA, Bioorg. Chem. 59 (2015) 151–167. doi:10.1016/J.BIOORG.2015.03.001.
[23] S. Titus, K.G. Sreejalekshmi, One-pot four-component synthesis of 4-hydrazinothiazoles: novel scaffolds for drug discovery, Tetrahedron Lett. 55 (2014) 5465–5467. doi:10.1016/J.TETLET.2014.08.033.
[24] E. da C. Petronilho, M. do N. Rennó, N.G. Castro, F.M.R. da Silva, A. da C. Pinto, J.D. Figueroa-Villar, Design, synthesis, and evaluation of guanylhydrazones as potential inhibitors or reactivators of acetylcholinesterase, J. Enzyme Inhib. Med. Chem. 31 (2016) 1069–1078. doi:10.3109/14756366.2015.1094468.
[25] Ş.D. Doğan, Copper-catalyzed NH/SH functionalization: A strategy for the synthesis of benzothiadiazine derivatives, Tetrahedron. 73 (2017) 2217–2224. doi:10.1016/J.TET.2017.02.063.
28
[26] S. Titus, K.G. Sreejalekshmi, Enriching biologically relevant chemical space around 2-aminothiazole template for anticancer drug development, Med. Chem. Res. 27 (2018) 23– 36. doi:10.1007/s00044-017-2039-y.
[27] G.M. Sheldrick, A short history of SHELX, Acta Crystallogr. Sect. A Found. Crystallogr. 64 (2008) 112–122. doi:10.1107/S0108767307043930.
[28] G.M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr. Sect. C Struct. Chem. 71 (2015) 3–8. doi:10.1107/S2053229614024218.
[29] APEX2, Bruker AXS Inc. Madison Wisconsin USA (2013).
[30] C.F. Macrae, I.J. Bruno, J.A. Chisholm, P.R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek, P.A. Wood, IUCr, Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures, J. Appl. Crystallogr. 41 (2008) 466–470. doi:10.1107/S0021889807067908.
[31] L.J. Farrugia, IUCr, WinGX and ORTEP for Windows: an update, J. Appl. Crystallogr. 45 (2012) 849–854. doi:10.1107/S0021889812029111.
[32] L. Collins, S.G. Franzblau, Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium., Antimicrob. Agents Chemother. 41 (1997) 1004–1009. doi:10.1128/AAC.41.5.1004.
[33] V.S. Krishna, S. Zheng, E.M. Rekha, L.W. Guddat, D. Sriram, Discovery and evaluation of novel Mycobacterium tuberculosis ketol-acid reductoisomerase inhibitors as therapeutic drug leads, J. Comput. Aided. Mol. Des. 33 (2019) 357–366. doi:10.1007/s10822-019-00184-1.
[34] J. van Meerloo, G.J.L. Kaspers, J. Cloos, Cell Sensitivity Assays: The MTT Assay, in: Humana Press, 2011: pp. 237–245. doi:10.1007/978-1-61779-080-5_20.
[35] G. Kumar, V.S. Krishna, D. Sriram, S.M. Jachak, Synthesis of carbohydrazides and carboxamides as anti-tubercular agents, Eur. J. Med. Chem. 156 (2018) 871–884. doi:10.1016/J.EJMECH.2018.07.047.
[36] J.C. Betts, P.T. Lukey, L.C. Robb, R.A. McAdam, K. Duncan, Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling, Mol. Microbiol. 43 (2002) 717–731. doi:10.1046/j.1365-2958.2002.02779.x.
29
[37] T. Matviiuk, J. Madacki, G. Mori, B.S. Orena, C. Menendez, A. Kysil, C. André-Barrès, F. Rodriguez, J. Korduláková, S. Mallet-Ladeira, Z. Voitenko, M.R. Pasca, C. Lherbet, M. Baltas, Pyrrolidinone and pyrrolidine derivatives: Evaluation as inhibitors of InhA and Mycobacterium tuberculosis, Eur. J. Med. Chem. 123 (2016) 462–475. doi:10.1016/J.EJMECH.2016.07.028.
[38] X. He, A. Alian, R. Stroud, P.R. Ortiz de Montellano, Pyrrolidine Carboxamides as a Novel Class of Inhibitors of Enoyl Acyl Carrier Protein Reductase from Mycobacterium
tuberculosis, J. Med. Chem. 49 (2006) 6308–6323. doi:10.1021/jm060715y.
[39] G. Wolber, T. Langer, LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters., J. Chem. Inf. Model. 45 (2005) 160–9. doi:10.1021/ci049885e.
[40] Schrödinger Release 2019-4: Maestro, Schrödinger, LLC, New York, NY, (2019). [41] Dassault Systèmes BIOVIA, Discovery Studio Visuliazer, Version 4.1.34. (2016).