Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Research Paper (National Research Council of Canada. Division of Building
Research); no. DBR-RP-628, 1975-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=f4cd148c-9fd1-4356-9fb6-3442fcc87dae
https://publications-cnrc.canada.ca/fra/voir/objet/?id=f4cd148c-9fd1-4356-9fb6-3442fcc87dae
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001757
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Equilibrium composition of fire atmospheres
Tsuchiya, Y.; Williams-Leir, G.
Ser
TH1
N 2 l r 2
NATIONAL RESEARCH COUNCIL OF CANADA
no.
628
CONSEIL NATIONAL DE RECHERCHES D U CANADA
c .
2
.-
-
BLDG
EQUILIBRIUM COMPOSITION O F FIRE ATMOSPHERES
by
Y.
Tsuchiya and G. Williams-Leir
4
Reprinted from
Journal of Fire and Flammability
Vol. 6, January 1975
12 p.
Research Paper No. 628 of the
Division of Building Research
OTTAWA
LA COMPOSITION DES ATMOSPHERES DE COMBUSTION
EN ETAT D'EQUILIBRE
La composition des produits de combustion d'un feu dans une enceinte en dktermine dans une large mesure la toxicite, la visibilitk et le degage- ment de chaleur. L'homogenkite et I'equilibre chimique a une tempera- ture uniforme supposes, on calcule la composition, celle-ci etant fonction du genre de combustible utilise, de I'approvisionnernent en oxygene et de la temperature. Les elkments de combustible consideres sont le carbone, I'hydrogkne et I'oxygene. On presente graphiquement les rksultats de solutions numkriques sirnultankes des equations de la conservation du matkriau, de l a conservation de la chaleur et des equili- bres chirniques. Les ternpkratures critiques de formation du noir de fumee sont indiqukes pour quatre combustibles. On indique, pour la cellulose prise comme combustible la quantitk des differents produits de combustion et le rapport de I'oxyde de carbone au bioxyde de carbone, avec comme variables independantes la temperature et le defi- cit en oxygene.
Fire Research Section
National Research Council o f Canada Division of Building Research Ottawa, Canada
r-
\
--~
A
L
ZED
Y
EQUl LIBR IUM COMPOSITION OF
FIRE ATMOSPHERES"
( R e c e i v e d A p r i l 29, 1974)
ABSTRACT: The composition of the combustion products of a fire in an enclosure determines t o a large extent toxicity, visibility and heat release. Composition, which depends on the nature of the fuel, the oxygen supply and the temperature, has been calculated by assuming homogeneity and chemical equilibrium at a uniform temperature. Elements considered in fuels are carbon, hydrogen and oxygen. The results of simultaneous numer- ical solutions of the equations of material conservation, heat conservation and chemical equilibria are presented graphically; and critical temperatures for soot formation are shown for four fuels. With cellulose as fuel, quan- tities of the individual combustion products and ratio of carbon monoxide t o carbon dioxide are shown, with temperature and oxygen d' eficit as independent variables.
W
H E N COMBUSTIBLE GASES or vapors burn i n air, oxygen is consumed, products of combustion are formed, and heat i s released. If this occurs in an enclosure, the composition of the resulting atmosphere determines i t s t o x i c i t y for anyone encountering it; and the concentration of solid or liquid particles, if formed, determines visibility. Released heat, which i s also strongly influenced by the composition, may damage the enclosing structure.For these reasons it i s useful t o study the composition of fire atmospheres under conditions representative of building fires. Such studies may be experimental or theoretical; i n fact, both are indispensable - the theoretical for understanding, the
experimental for testing the validity of theory. The processes occurring in a fire are quite complicated, and include thermal decomposition of fuel, diffusion o f oxygen and products, and a serie; of oxidation reactions. A kinetic approach would not be successful with present knowledge of elemental reactions and their kinetic data.
The study now reported is concerned w i t h a theoretical calculation by chemical thermodynamics of the composition of the atmosphere produced by a fire. It assumes that the mixing of all the components i s complete, that all the reactions * T h i s p a p e r i s a c o n t r i b u t i o n f r o m t h e D i v i s i o n o f B u i l d i n g Research, N a t i o n a l R e s e a r c h C o u n c i l o f Canada, a n d i s p u b l i s h e d w i t h t h e a p p r o v a l o f t h e D i r e c t o r o f t h e D i v i s i o n .
have enough time t o reach equilibrium both chemically and thermally, and thatthe gases emerge a t a uniform temperature and composition. I n i t s application t o com- partment fires, this i s certainly not true for the compartment as a whole, since air entering is not instantaneously mixed, but there is a smaller space between the fire and the exhaust where it is valid. In a real situation that does not meet these conditions, the thermodynamic equilibrium calculation is still useful in indicating the limiting compositions beyond which the reactions will not proceed. The afore- mentioned assumptions alone are sufficient t o determine the relation of: nature of fuel, adequacy of oxygen supply, temperature, quantity of each combustion prod- uct, and quantity of combustion heat. Of these five groups of variables, i f any three are given, the remaining two may be found by simultaneous solution of the equa- tions of material conservation, heat conservation, and chemical equilibrium. There is already available considerable literature dealing with similar problems i n specific fields, but to the authors' knowledge none in the field of building fires.
METHOD
The five groups of variables will be explained in more detail in relation t o this study.
Nature
of
Fuel'Only fuels having the elements carbon and hydrogen, with or without oxygen, were considered. Cellulose was adopted as representative of the fuels most com- monly found in building fires. Methane, ethane and benzene were chosen as typical carbon-lean and carbon-rich hydrocarbons and because they are often produced by the thermal decomposition of various fuels. The calculation is concerned only with the ratios of C/H/O atoms in, and the heat content of, each fuel.
Adequacy
of
Oxygen SupplyWhen oxygen supply i s more than stoichiometric, essentially all the carbon and hydrogen in a fuel are converted t o carbon dioxide and water. Calculation of the composition of products and quantity of combustion heat is quite straightforward in this case, unless the reaction
i s considered, and this reaction is practically negligible under building fire condi- tions.
I n the greater part of the course of most developed building fires combustion rate i s controlled by natural convection of air through openings. Under these cir- cumstances it is well known that the amount of oxygen available for the reaction is
found in experimental fires demonstrates this. A parameter called "oxygen deficit," a, i s used in this stucly:
i f
R
= quantity of oxygen required for complete combustion,and
P
= total quantity of oxygen plesent both in the supplied air and in tht. fuel molecule,then a = - R - P
R
Oxygen deficiency in actual fires depends on the rate of fuel supply t o tile reaction system in relation t o the rate of oxygen supply. I n a fire in which the fuel i s a volatile liquid, for example, oxygen deficit is expected t o be quite large because of the rapid supply o f fuel t o the gas phase b y vaporization. The maximum value of oxygen deficit at which combustion can continue i s determined by the uppet. flammable limit of the fuel vapor air mixture; thus i t depends on the kind o f fuel and the temperature of the system. Oxygen deficit has not been determined exper- imentally; in this study i t i s a given variable.
Temperature
The temperatures found i n ordinary building fires are not very high. I n the present study the main interest i s in the range
500
t o1300°~,
but some results are given t o1 600°
K.Quantity o f Combustion Products
I n fire conditions where temperature i s modest, pressure i s atmospheric and oxygen enters in the f o r m of air, there is a limited number of species of products that need t o be considered. For the work t o be described the following have been taken into account: carbon, carbon monoxide, carbon dioxide, hydrogen, water and methane. Thermodynamic data for these compounds were obtained f r o m the literature
[I,
21.
Quantity of Combustion Heat
The heat evolved i n a building fire is partly lost t o the surroundings at a rate depending on various factors such as the material of the enclosure, the size of openings, the temperatures of the surroundings and o f the fire. The present study introduces a parameter
0,
the ratio o f the enthalpy increase of the products t o thc heat released by the reaction; thus,0
=1
refers t o an adiabatic condition.The six products o f combustion have been listed. The quantities of each of these and that of free oxygen plus temperature are the eight unknowns in the problem. Available for its solution are equations representing several possible reactions among the molecular species considered; four are independent. There are also three independent equations for material conservation and one for heat conservation, making eight in all.
Y.
Tsuclriya a n d G. Williams-LeirThe procedure for solution of the equations was as follows: certain equations were used t o eliminate variables from the others, reducing the problem t o five equations in which the unknowns were carbon dioxide, hydrogen, water, temper- ature, and total moles of gas.
To produce the curves shown as Figures 1 to 6, one parameter i n each case was progressively varied while the others were held constant. A t each point the equa- tions were solved simultaneously by the method developed by Powell
[3].
This demands a rough initial estimate of the solution. Once solutions were available for two values of whatever parameter was being varied, linear extrapolation provided new initial estimates for a third value. By iteration, the curves were built up. The equations used are more fully described in Appendix A.ACCURACY
Accuracy of the result naturally depends on the quoted data. The precision of the calculation was tested by recalculating the equilibrium constants from the results, which were recorded to four places. All the deviations can be explained by errors not exceeding
0.0003
mole per carbon mole; and the maximum error is believed t o be not much, if at all, in excess of this.RESULTS AND DISCUSSION
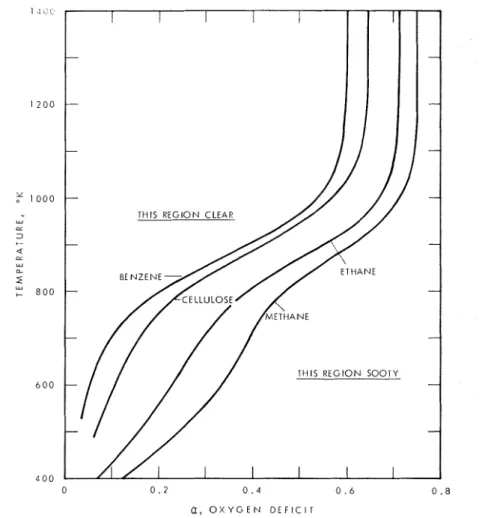
The relations between the variables might be shown as a set of three-dimensional models, one for each product, with a and temperature measured along perpendic- ular axes in a horizontal plane, and quantity of each product, or ratio of products, vertical. Each product or ratio would then be represented by a surface. The figures may be regarded as sections of such a model. Figure 1 shows where the carbon surface meets the origin plane for four different fuels.
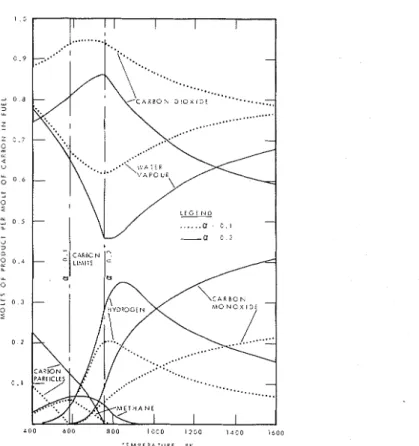
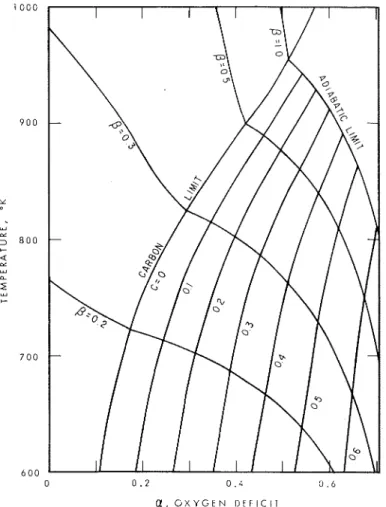
Figure 2 i s a combination of vertical sections perpendicular t o the a axis and showing six products. Figure 3 is a set of horizontal sections showing the carbon surface, i.e. a contour map. Figure 4 does the same for carbon monoxide, and Figure 5 for hydrogen. Figure 6, which is a set of vertical sections like Figure 2,
shows the ratio of carbon monoxide t o carbon dioxide.
The products of combustion comprise seven gases, including nitrogen from air and free oxygen, and one solid phase, carbon. The latter disappears at higher temperatures because the equilibria
go t o the right-hand side. There is a critical temperature at which carbon disappears for any given proportion of oxygen. This i s shown i n Figure 1 as a function of LY for four fuels. Above the critical temperature the flame is clean; below, i t i s sooty.
-
I
I
I
- - .- - - - THlS REGION CLEAP-
THlS REGION SOOTY --
Figure I . Critical temperatures for soot formation for compounds burning in ox ygen-deficien t a tmospheres.
Fuels of high C/H ratio have sooty flames at wider ranges of temperature and oxygen deficiency.
The proportion of each product is shown i n Figure 2 as a function of temper- ature at two levels of a for cellulose. I t may be seen that the patterns of distribu- tion of components are quite different on the two sides of the critical temperature.
I n the sooty-flame region, carbon decreases with increasing temperature while the other three carbon compounds increase. Close to the critical temperature CO in- creases rapidly. Above the critical temperature the equilibrium
Y.
Tsuchiya and G. Williams-LeirI 0
I I
I
II
I1
Figure 2. Products from combustion of cellulose in ox ygen-deficient atmospheres.
The quantity o f free oxygen i s not given i n Figure
2
because it i s always very small. I t increases with increasing temperature and decreasing oxygen deficit, but even at1600'~
and a =0.1
i t s mole quantity was less thanlU7.
The mole fractions of carbon, carbon monoxide and hydrogen, taking the carbon i n the fuel as unity, are shown i n Figures 3, 4 and
5,
respectively, as contour graphs for different values of a and temperature. The contours of0
are shown i n Figures 3 and6.
The graphs extend t o the adiabatic l i m i t and t o zero air supply. The temperature of the system cannot exceed the adiabatic limit unless heat i ssupplied from some external source and such sources are rare i n building fires. Oxygen deficit, a, does not go beyond
12/17,
or0.706,
because cellulose contains oxygen in i t s molecule. As shown in Figure 3, carbon particles increase rapidly with increasing oxygen deficit at fixed temperatures. I f/3
is kept constant, temperature drops with increasing oxygen deficit and this causes carbon particles t o increase even more rapidly than at constant temperature.LL
3 B O O
Figure 3. Carbon (particles) from combustion of cellulose.
Quantity of CO is shown in relation to temperature and oxygen deficit i n Figure
4. CO increased with increasing temperature for a fixed value of
a.
A t a fixed value of temperature with increasing a, CO increased in clean flames to a maximum a t the critical point for carbon formation and subsequently decreased in sooty flames. Quantity of hydrogen, as shown in Figure 5, has a trend similar to that of CO in sooty flames. In clean flames at a fixed value of a, there i s a maximum at a certain temperature.Figure 6 shows ratios of carbon monoxide and carbon dioxide. I n a clean flame the ratio increased with increasing temperature or increasing oxygen deficit while the other parameter was fixed. In a sooty flame this ratio increased with temper- ature increase, but was almost independent of oxygen deficiency.
Y.
T s u c h i j ~ a atzd G. Williams-Leit0 . 2 0 . 4 0 . 6
a ,
O X Y G E N D E F I C I TFigure 4. Carbon monoxide from combustion of cellulose.
CONCLUSION
Theoretical calculation of fire atmospheres under oxygen deficient conditions produced the following results. There was a critical temperature for carbon particle formation for any given proportion of oxygen. Fuels of high C/H ratio had sooty flames at wider ranges of temperature and oxygen deficiency. Above the critical temperature the flame was clean and the composition of the atmosphere was mainly determined by the equilibrium
Figure 5. Hydrogen from combustion of cellulose.
Among the components of the fire atmosphere, carbon monoxide increased with increasing temperature. A t a fixed value of temperature with increasing oxygen deficit, CO increased in clean flame t o a maximum at the critical point for carbon formation, and subsequently decreased in sooty flame. The ratio CO/COZ increased with increasing temperature or increasing oxygen deficit in a clean flame. In a sooty flame this ratio increased with temperature increase, but was independent of oxygen deficiency.
APPENDIX A Equations for Calculation The over-all reaction considered is:
4 0 0 6 0 0 8 0 0 1 0 0 0 1 2 0 0 1 4 0 0 1 6 0 0 T E M P E R A T U R E , ' K
Figure 6. Ratio of carbon monoxide to carbon dioxide from combustion of cellulose.
where p, q and a are given, and a, b, c, d, e, f, g, and h are unknown variables. Material conservation provides the following equations:
The equation for thermal energy conservation is:
(Heat of reaction) X
fl=
(Heat content of products at T'K) - (Heat content of products at 2 9 8 ' ~ ) where T is the temperature of equilibrium.There are several equilibria between the species of the combustion products. Among them, four are independent. I n principle, any four could serve, but stable and efficient computation demands an appropriate selection.
Examples are:
2CO =
co2
+
CThe equilibrium constants for these equilibria are functions of temperature only, since pressure is atmospheric and treated as constant. These nine equations are sufficient to determine the quantities of eight components and the temperature.
REFERENCES
1. J. H. Perry, C. H. Chilton, and S. D. Kirkpatrick, (Ed.) Chemical Engineers' Handbook.4th Ed. McGraw-Hill, 1963.
2. D. R. Stull and H. Prophet, (Ed.) JANAF Therrnochemical Tables, Office of Standard Reference Data, National Bureau of Standards, Washington, DC 1971.
3. M. J. D. Powell, Computer J. Vol. 7, p. 303, 1965.
Y. Tsuchiya
Yoshio Tsuchiya i s a Research Officer of the Division of Building Research, National Research Council of Canada. He received his Bachelor of Engineering and Doctor of Engineering degrees in Applied Chemistry from the University of Tokyo in 1953 and 1962 respectively. He joined the National Research Council in 1965. He has experience in the fields of industrial explosives, organic peroxides, and fire research.
G. Williams-Leir
G. Williams-Leir is a Research Officer of the Division of Building Research, National Research Council of Canada. He received his B.Sc. degree i n Physics from the University of Bristol, England. He joined the National Research Council in 1953, and has experience in applications of numerical methods, non-linear regres- sion, and fire protection of buildings.
This publication i s being distributed by the Division of Building Re- search of the National Research Council of Canada. I t should not be reproduced i n whole or in part without permission of the original pub- lisher. The Division would be glad t o be of assistance i n obtaining such permission.
Publications of the Division may be obtained by mailing the appropri- ate remittance ( a Bank, Express, or Post Office Money Order, 01. a cheque, made payable t o the Receiver General of Canada, credit NRC) to the National Research Council of Canada, Ottawa. KIAORG. Stamps are not acceptable.
A list of all publications of the Division i s available and may be ob- tained from the Publications Section, Division of Building Research, National Research Council of Canada, Ottawa. KIAORG.