Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Paper (National Research Council of Canada. Division of Building Research); no.
DBR-P-743, 1977-08
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=3d27e252-6987-4019-b57b-f4c44d006b2d
https://publications-cnrc.canada.ca/fra/voir/objet/?id=3d27e252-6987-4019-b57b-f4c44d006b2d
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001766
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Significance of HCN generation in fire gas toxicity
Ser
. -
no.
74
3
National Research
Conseil national
c .
2
Council Canada
de recherches Canada
SIGNIFICANCE OF HCN GENERATION IN
FIRE
GAS TOXICITY
02804
ANALYZED
by Yoshio Tsuchiya
/=r
Reprinted from
Journal of Combustion Toxicology VoL 4, August 1977
p. 271
-
282DBR Paper No. 743
Division of Building Research
Price
25cents
5
BUILDING
RESEARCH
-
LIBRARY
-
MAR
14
1978
NATIONAL RESARCH
comcn
SO MMAIRE
P l u s i e u r s produits de combustion de polym'eres sont rCputCs (ou soupfonnCs d ' z t r e ) plus nocifs que l e GO dans d i v e r s e s conditions de combustion ou de dCcornposition thermique. Dans l e p r e s e n t mCmoire, on Ctudie l e c a s du HCN, qui e s t l'un d e s composCs formCs p a r l a cornbustionde d i v e r s polyrn'eres contenant de l'azote. On p r g s e n t e un compte rendu d e s donnCes d'btudes effectuges a v e c d e s anirnaux, dans l e s q u e l l e s l e HCN peut avoir jouC un rBle tr'es i m p o r t a n t , a i n s i que d e s donnCes quantitatives r e l a t i v e s 'a l a production de HCN. On t r a i t e Cgalement d e s conditions de l a cornbustioncomme l a t e m p h r a t u r e e t l a quan- tit6 dloxyg'ene qui influent s u r l a production de HCN. On prCsente Cgalement d e s calculs thCo- r i q u e s portant s u r l a concentration
'a
1'Cquilibre du HCN et on l e s 6tudie e n r e l a t i o n avec l e s donn6es expCrimentales. On dCmontre l ' i m p o r - tance de l a contribution du HCN'a
l a toxicit6 d e s gaz d'incendie'a
l ' a i d e d'un f a c t e u r de toxicite. Le c l a s s e ment d e s matCriaux contenant de l'azote quant>
l e u r s propriCtCs de production de gaz toxiques peut Ctre variable p a r c e que l a quantit6 de HCN produite p r 6 s e n t e d'importantes v a r i a - tions s e l o n l e s conditions de l a combustion.YOSHIO T S U C H I Y A * Fire Research Section Division o f Building Research National Research Council o f Canada Ottawa, Canada K 1A
OR6
SIGNIFICANCE O F HCN GENERATION I N FIRE GAS T O X I C I T Y * "
Original manuscript received January 28, 1977 Revised manuscript received April 20, 1977
ABSTRACT: Several components i n c o m b u s t ~ o n products of polymers are k n o w n t o be or are suspected of being harmful than CO for various con- ditions of combustion or thermal decomposition. HCN. one such com- pound produced f r o m a variety o f nitrogen containing polymers, is dis- cussed i n this paper. Published data for animal exposure studies i n w h i c h HCN may have played a major role and q u a n t ~ t a t i v e data for HCN gener- ation are reviewed. The conditions of combustion, such as temperature and amount of oxygen, that affect generation of HCN, are discussed. Theoreti- I cal calculation of the equilibrium concentration of H C N is also presented I and discussed i n relation t o experimental data. Significance of H C N gener-
i
ation i n fire gas t o x i c i t y is shown b y using the t o x ~ c i t y factor. Ranking of ! nitrogen-containing materials based o n the propensity of t o x i c gas gerrera-t i o n can be variable because the q u a n t i t y of HCN generated varies widely depending o n the conditions of combustion.
I
N H A L A T I O N OF T O X I C gases produced during the combustion and pyrolysis ofmaterials is the major cause of fatalities in building fires. Although carbon mon- oxide has been and will continue to be the major toxicant, some other components may also be significantly harmful. Such components include HC!? produced from polyvinyl chloride, nitrogen oxides from nitro-cellulose, HCN from nitrogen- containing polymers, organic acids and aldehydes from cellulosic materials, and organic bicyclic phosphate from certain types of flame-retardant-treated polyure- thane. kllost of the large number of other components that have been identified and quantified, are not significant in either quantity or toxicity. The purpose of this paper is, firstly, to show the significance of HCN generation especially in compari- son with CO generation during the combustion of various nitrogen-containing poly- mers and, secondly, to show that the variation of HCN generation could alter the toxicity ranking of materials. For this purpose available data on HCN generation are reviewed in relatior: to the conditions of combustion. A theoretical calculation of HCN concentration in combustion gases is presented, taking the temper-
'Fire Research Section, D i v ~ s i o n of Building Research, National Research Council o f Can ada, Ottawa, K 1 A O R 6 .
"Based on a paper presented at the Second International Conference o n Fire Safety, U n i versity of San Francisco, San Francisco, California. January 24 t o 28. 1977.
Reprinted
from Journal of Con~bustiorz Toxicologl~,
Volume4 ,
(August1977)
a / /
qcd
C
Yoshio Tsuchiya
ature and oxygen supply as variables. Available data on animal experiments in which HCN may have played a major role are also reviewed. Using the toxicity factors, the significance of HCN generation i s shown.
SURVEY OF AVAILABLE DATA
The generation of HCN from nitrogen-containing polymers and i t s possible hazard at fires were recognized in the 1930s. After the great loss of life in the Cleveland Clinic fire due t o fumes from burning X-ray films, Olsen and others studied thermal decomposition products of films. They found that HCN from nitro-cellulose and from gelatin in the coating material was significant, though not the primary toxicant [ I ] . They also found that HCN was produced from the thermal decomposition of silk and wool [ 2 ] . I n the 1940s. Easton pyrolyzed and burned wool and silk, and determined the amount of HCN generated [ 3 ] . Later, a group of researchers from U.S. Bureau of Mines studied toxic gas production from various thermosetting polymers [ 4 ] and cellular acrylonitrile [5] for the purpose of evaluating materials used for ships. HCN was quantified along with other toxic components. In the 1950s Nagao undertook a systematic study on HCN generation from polyacrylonitrile, silk and wool [ 6 ] . In a review paper, Dufour [7] cited two unpublished reports in the early 1960s on HCN generation from polyurethane, silk and wool [8, 91.
Investigation of aircraft crashes in the United States in the 1960s drew attention t o the toxic effect of CO and other combustion gases. In 1970, blood samples from victims of an aircraft crash and fire were analysed for cyanide for the first time. Cyanide was found in most of the specimens but the contribution of HCN t o lethality was not conclusive [ l o ] .
In Japan, in 1968, a couple died in a small fire which involved a polyurethane foam mattress. Medical doctors found unusually high cyanide levels (23 and 7.2 ~ g l m l ) in the blood of the victims and reported that death was primarily due to HCN evolved from the mattress [ I I ] . This report initiated a series of discussions and stimulated research on the generation of HCN from polyurethane and other materials.
I n the combustion of nitrogen-containing polymers CO i s always produced in addition t o HCN, and the relative contribution of each t o fatality is often difficult t o judge. In controlled experiments of animal exposure t o combustion products, there are several cases in which HCN could have been a major toxicant. Hofmann et al [12, 131 exposed rats t o the thermal decomposition products of polymers and determined the fatality ratio of animals and carboxy-hemoglobin (CO-Hb) content in the blood of animals. For PVC, wool, felt and nylons, high fatality was observed despite the fact that the CO-Hb content was not high enough t o kill the animals. HCI from PVC and HCN from other polymers could have been responsible for the fatalities.
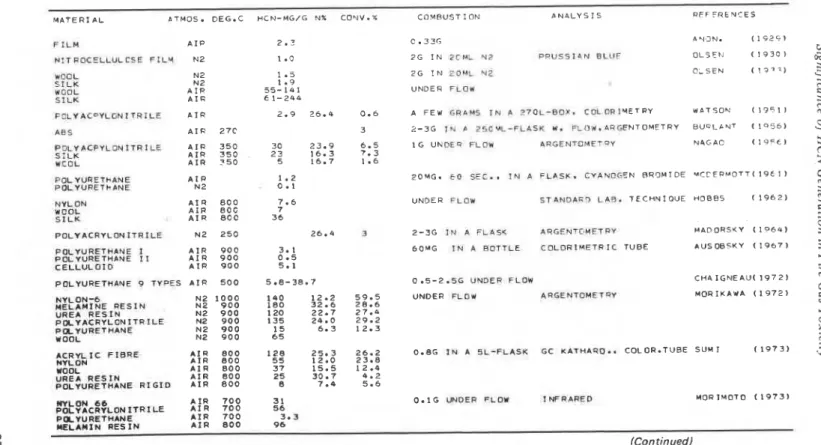
Table 1. HCN Generation from Nitrogen Containing Polymers. M A T E R I A L b T M O S . 0 E G . C H C N - M G / G N X C 0 Y V . X C O M R U S T i OW b N 4 L Y S I S R F F F R E N C E S F ILM A I R N I T R O C E L L U C C S E P I L Y N 2 WOOL C I L K WLUL N 2 N 2 A I P A I R C.33G 2 G I N Z t M L Y ? P n U S S 1 4 N aLLLF 2 G I N ?OWL Y 2 U N D E R F L O W b v 9 ~ . ( 1 4 2 4 ) O L S E N ( 1 9 3 0 ) @,SEN (11") F 3 C Y A C * Y C G N l T R i L E A I R AElS A I R 2 7 C P 3 L Y A C P Y L D N f T R I L E A I R 3 5 0 S:LK A I R 3 5 0 WCOL A I R 3 5 0 A I R N 2 H Y L O N A I R 8 0 0 WCOC A I R BOG S I L K A I R 8 0 0 P O L V U A E T H b N E 1 A I R 9 0 0 P O L Y U R E T U C N E I I A I R 9 0 0 C E L L U L O I D A I R 9 0 0 P O L Y U R E T W A N E 9 TYPES A I R 5 0 0 0 . 6 A F E W G R A Y S I N P ? 7 O C - f l 0 X . C O C G R I Y E T P Y W b T S O N ( 1 7 5 1 1 3 2 - 3 G 1 % 8 ? 5 C W - F L A S K Y. F-UWm4QGEPITOMETRY B U Q L & N T ( 1 9 5 6 ) 6.5 1 G UNDEW F L O W A R G E N T O H E T QY N A G A C ( : Y s t ) 7 . 3 1 . 6 ZOMG. C O SEC.. I N A F L A S K . C Y A N D G E N R R 9 M I D E u C C E R V O T T ( 1 9 C I ) U N D E R F L O W S T b N D A E 3 L A q . T E C H N I Q U E H O B B S ( 1 9 6 2 ) 3 2 - 3 G I N A F L A S < A R G E N + D H F T P Y M A D O R S K Y ( 1 0 6 4 ) 6 0 M G I N A R O T T t E C O L O R I M E T R I C T U B E A U S O B S K Y ( 1 9 6 7 ) A C R Y L I C F I B R E A I R 8 0 0 1 2 8 2 5 . 3 2 6 . 2 0.8G 1 N A 5 L - F L A S K GC K A T U & 9 O m . C O L 0 R . T U B E S U M 1 ( 1 9 7 3 ) N Y L O N A I R 8 0 0 55 1 2 . 0 2 3 . 8 WOOL AIR 8 0 0 3 7 1 5 . 5 1 2 . 4 U R E A RESIN A I R 8 0 0 2 5 3 0 . 7 4 . 2 P O L I U R E T H A N E R I G I D A I R 8 0 0 8 7 . 4 5 . 6 A I R 7 0 0 3 1 ~ ~ ~ : C X ~ L D N I T R I L E A I R 7 0 0 5 6 P Q Y U R E T H A H E A I R 7 0 0 3.3 ~ FA Y I U I m F S I M A I R 8 0 0 96 (Con tinuedl
Significance of HCN Generation in Fire Gas Toxicity
Similar experiments were conducted by Kishitani, who found that gases pro- duced from polyurethane killed mice even though the CO-Hb in blood was low
[ 1 4 ] . Sakai and co-workers [15] determined LD5 values for five different fabrics by exposing mice to combustion products. The products from polyacrylonitrile, nylon and wool, which are known t o produce HCN, were found to be more toxic than cottonand polyester. In experiments by Hilado et al, wool was found to be relatively more toxic than cotton under pyrolysis conditions in which the speci- mens were heated up to 6 0 0 ~ ~ . LDS, values for mice, based on pyrolyzed weight, were, for example, wool/cotton = 5 9/14 g
[ I
61.
Yamamoto's work gave more conclusive information on the relative contribution of HCN and CO [17]. He exposed rats and rabbits t o the combustion products of several polymers and determined both the CO-Hb and cyanide in their blood. The combustion products of polyacrylonitrile, Modacryl, wool, silk and polyvinylidene chloride were fatal t o the animals even though their CO-Hb levels were low. The cyanide levels in the blood from combustion experiments on silk, polyacrylonitrile and Modacryl were high enough t o kill animals.
Table 1 summarizes published analytical data for HCN generation from various nitrogen-containing natural and synthetic polymers. The table is arranged chron- ologically. The reported data vary widely: in general, early investigators found lesser amounts of HCN in their experiments than did later researchers from the same kinds of materials. This is probably because the significance of HCN gener- ation in fire gas toxicity was not well recognized at the beginning and researchers did not vary the condition under which the gas was produced.
Many factors could have affected the observations, such as the conditions re- quired for HCN generation and the methods of sampling gases and their analysis. Among the conditions that affect the generation of HCN, the temperature of de- composition of the material and the oxidizing capability of the atmosphere appear t o be the two main factors. I t is generally agreed that in a non-oxidizing atmosphere higher temperatures are more favourable for HCN generation, but this is not always true in an oxidizing atmosphere. A theoretical study carried out by Jellinek and Takada suggested a kinetic involvement of oxygen molecules in the initial stage of HCN evolution [18]. The effect of oxygen, however, appears to increase temper- atures by generating the heat of oxidation. A higher temperature may increase HCN but flaming may cause i t to decrease by consuming some of it.
Whether the decomposition performed in a static condition or in a dynamic condition i s another factor. I t appears that in a dynamic experiment, where the specimens are decomposed under a flow of air or inert gas and the decomposition products are swept away from the hot zone, more HCN i s found. In a static experiment, the products stay in a hot zone for a longer time and tend to decom- pose further. Chaigneau and LeMoan, for example, pyrolyzed various polyurethane samples in the presence of oxygen and found no HCN in static experiments, while varied amounts were found in dynamic experiments [19]. Bulygin et al compared
Yoshio Tsuchiya
free burning and burning with forced air blowing for polyurethane foams. They stated that HCN generated in free burning is readily sorbed by resinous non-volatile products and remains in the hot zone to be subjected to further decomposition
[20]. Thus, free burning produces less HCN than burning with forced air blowing. Polymers of higher nitrogen content are expected to produce more HCN than those with lower content. The relation between material composition and HCN generation was studied by Bulygin [21]. He found a reciprocal dependence be- tween the logarithm of the quantity of HCN generated and the oxygenlnitrogen ratio in polymer molecules. With higher oxygenlnitrogen ratio, e.g. polyurethane, further oxidation of HCN takes place, and the quantity of HCN i s reduced. Ashida et al studied the generation of HCN from variously formulated polyurethane foams. They found that the amount of HCN generated strongly depended on the types of isocyanates and that, among the foams of the same type, i t depended on the nitrogen content [22].
Morikawa, in his comprehensive studies of various nitrogen-containing polymers and low molecular weight compounds [23], found that the amount of HCN gen- erated was proportional to the nitrogen content of the compounds. In a nitrogen atmosphere, HCN production was increased by increasing the temperature of pyrol- ysis. In air, a maximum in HCN production was observed if the temperature was increased a t a fixed temperature. He commented that many reports of small quantities of HCN were because either the temperature of decomposition was too low or the air supply was excessive.
HCN generation i s not restricted to nitrogen-containing polymers; thereare re- ports of HCN generation from other nitrogen-containing materials, such as coal [24] and dried fish [22]. According to Morikawa, any nitrogen-containing compound, except nitro compounds, generates HCN at temperatures above 6 0 0 ~ ~ . Some types of compounds, nitriles for example, generate HCN at much lower temperatures, e.g., 300 to 4 0 0 ~ ~ [231.
EQUILIBRIUM CALCULATION OF HCN
A theoretical calculation is often useful in finding underlying rules when ex- perimental results are complicated.
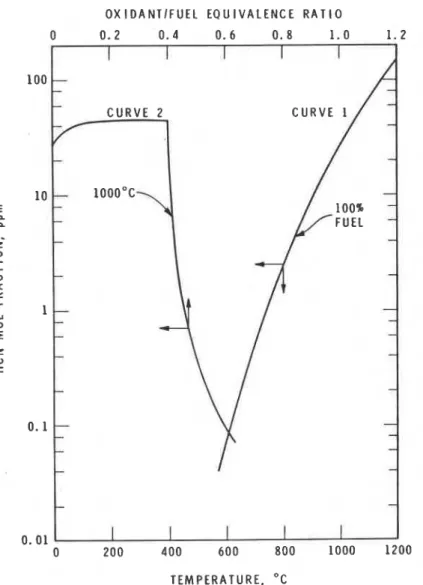
Figure 1 shows a theoretical equilibrium calculation by chemical thermo- dynamics of HCN production from pyrolysis and combustion of polyacrylonitrile. An equilibrium calculation assumes that the mixing of all the components is com- plete, and that all the reactions have enough time to reach equilibrium both chem- ically and thermally, and emerge at a uniform temperature and composition. Al- though these conditions are not satisified in a real fire situation, this calculation i s useful in indicating limiting composition beyond which the reactions will not pro- ceed. A computer program developed at NASA [25], that employs the principle of minimization of free-energy, was used in this study. Polyacrylonitrile was selected as an example of a fuel because it i s generally recognized as the highest HCN
Significance
o fHCN Generation in Fire Gas Toxicity
OX I O A N T l F U E L EQUIVALENCE R A T I O 0 0.2 0.4 0.6 0.8 1.0 1.21
-
-
-
C U R V E 2-
-
1000°C-
-
-
-
-
-
-
3 --
-
-
--
+-
L-
-
-
-
-
-
-
I
1
1
0 200 400 600 800 1000 1200 TEMPERATURE. O CFigure I . Equilibrium concentration of HCN in the combustion of polyacrylonitrile.
producing polymer. In this set of calculations the initial chemical compositions,
pressure (assumed p = 1 atmosphere) and temperature were given and the mole
fractions of various products were obtained. Air and polyacrylonitrile were the sole oxidant and fuel, respectively.
In Figure 1, curve 1 indicates that increasing the temperature increases HCN
production. This i s generally observed in experimental determinations of HCN pro-
duction. Curve
2
shows the effect of oxidantlfuel equivalence ratio. The equivalentYosl~io Tsuchiya
The sharp drop of HCN at equivalence ratio of 0.4 is related to the material balance given by the stoichiometric equation,
The result shows that increasing the oxidantlfuel ratio decreases HCN generation after reaching a maximum value, in agreement with experimental observations.
SIGNIFICANCE OF HCN GENERATION SHOWN BY TOXICITY FACTOR There are two types of approaches for evaluating the toxicity of gases produced from the combustion of materials. One involves exposure of laboratory animals to combustion gases t o determine the biological response of the animals (the so-called bioassay experiment). The other involves the chemical analysis of component gases followed by a toxicity evaluation using known biological data; this is sometimes called analytical toxicology. For the latter, the present author and his colleague, K. Sumi, proposed the toxicity factor [26,27]. It takes into account both the amount of toxic gases, determined by chemical analyses, and the effect of the gases on humans, estimated by animal experiments. I t i s defined as
where: T = toxicity factor
Ce = experimentally determined concentration of volatile or gaseous products
Cf = concentration o f the same product that is dangerous t o man in 30 minutes
V = volume into which the products are dispersed
W = weight of original material.
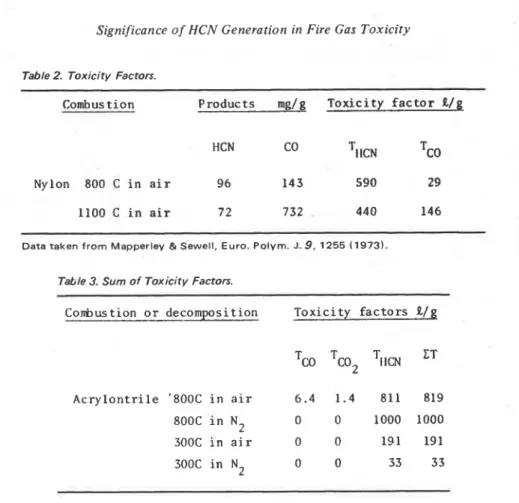
The two major toxic components in the products from the combustion of nitro- gen-containing polymers are HCN and CO. The toxicity factor provides a basis for judging which of the two is more significant from the toxicity point of view. It can be seen in Table 2, on the basis of the toxicity factor, that HCN is the major toxic component in the combustion products of nylon. Toxicity evaluation of combus- tion products has been published for five different nitrogen containing polymers
[28]
; the main toxic components were HCN and CO. The toxicity factors indicatedthat HCN was most significant for the combustion products of acrylic fibre, nylon, wool and urea resin.
Significance o f HCN Generation in Fire Gas Toxicity
Table 2 Toxicity Factors.
Comhus t i o n Products mg/g
Toxicity
f a c t o rIl/g
HCN
Nylon 800 C i n a i r 96 143 590 29
1100 C
in
a i r 7 2 732 440 146D a t a taken from Mapperley & Sewell, Euro. Polyrn. J. 9 , 1255 (1973).
Table 3. Sum of Toxicity Factors.
Conbustion o r decomposition T o x i c i t y f a c t o r s
Il/g
A c r y l o n t r i l e '800C i n a i r 6 . 4 1 . 4 811 819
800C i n N 2 0 0 1000 1000
300C i n a i r 0 0 191 191
300C i n N2 0 0 33 33
I n Table 3, toxicity factors of three major components produced from polyacry- lonitrile at different sets of conditions are shown as well as CT, the sum of the toxicity factors. C T i s the total toxicity of the gases produced from the original material and is considered to be a measure of the toxicity of the material. Only three components are considered in Table 3 on the assumption that the contribu- tion of the toxicity factors of other components t o CT i s not significant. When more analytical data and toxicity data for other components become available, additional toxicity factors could be considered in order t o improve the toxicity evaluation. As shown in Table 3, CT of a material for different conditions of combustion varies widely, because, in this case, generation of HCN varied widely. When Z T of this polymer and of other materials, such as cellulosic materials, are compared, the ranking of toxicity based on CT will depend on the condition under which combustion took place.
I n ranking the toxicity of materials by bioassay experiments the dependence of HCN generation on the conditions of combustion should also be evident. Cornish et
al [29] determined the comparative toxicity of combustion products of a variety of
Yoshio
Tsuchiyaexperiment wool was found most toxic; in the static experiment i t was found the least toxic. The present author believes this was due t o the dependence of HCN generation on the conditions of combustion.
I n selecting conditions for combustion in bioassay experiments, i t is recom- mended that as much analytical information as possible be obtained, and preferably the toxicity factor be determined to provide more insight into the test results. The toxicity factor approach, however, has i t s limitations, as has been discussed else- where [30, 311. As the toxicity factor does not predict combustion toxicity a priori, i t has t o make use of knowledge obtained from bioassay experiments. The reliability of 2 T should be confirmed by comparison with animal exposure data. Toxicity factors determined at the Division of Building Research for acrylic fibre, nylon, wool, cotton, and polyester [31] were found t o be in good agreement with the values obtained by Sakai et al
[I51
for L D S 0 using mice.SUMMARY AND CONCLUSIONS
HCN is one of the significantly harmful components in fire gases. Generation of HCN from the combustion of polymers has been known since the 1930s. Although combustion gases always contain a quantity of CO, in some animal tests HCN was found to be a more significant toxicant than CO. Analytical data for HCN genera- tion from nitrogen-containing polymers vary widely. Recent investigators have found more HCN in combustion gases than had been evident in earlier tests. Tem- perature and amount of oxygen are the two main factors that affect HCN genera- tion. I n non-oxidizing atmospheres higher temperatures favour greater formation of HCN. I n an oxidizing atmosphere HCN increases t o a maximum value and decreases with increasing temperature or increasing air supply. Results of theoretical calcula- tions of HCN concentration at chemical equilibria agree with the experimental findings. Static combustion produces less HCN than dynamic combustion. I n the latter, HCN is swept away from a hot zone and prevented from further decomposi- tion. Most nitrogen-containing materials generate HCN; the amount of HCN de- pends largely on the nitrogen content of the original material. One way t o evaluate toxicity of combustion products is t o determine the toxicity factor. This factor does not predict combustion toxicity a priori. It has t o make use of knowledge obtained from bioassay experiments, and the validity of prediction based on this approach has t o be checked with these experiments. The significance of the toxicity of HCN in fire gases has been shown by the toxicity factors. Ranking of nitrogen- containing materials based on the propensity of toxic gas generation can be variable because the quantity of HCN generated varies widely depending on the conditions of combustion.
ACKNOWLEDGEMENT
The author wishes to thank Mr. G. Williams-Leir for advice and assistance in the computation of chemical equilibria.
Significance of HCN Generation in Fire Gas Toxicity
REFERENCES
I. J. C. Olsen, A. S. Brunjes & V. J. Sabetta, Indust. Eng. Chem.22.860 (1930). 2. J. C. Olsen, G. E. Ferguson & L. Scheflan, Indust. Eng. Chem. 25, 599 (1933). 3. W. H. Easton, Indust. Medicine 11, 466 (1942).
4. L. B. Berger, H. H. Schrenk, J. A. Gale, R. W. Stewart & L. E. Sieffert, U. S. Bureau of Mines, Report of Invest. # 41 34, Oct. 1947.
5. H. A. Watson, H. J. Stark, R. L. Beatty, H. W. Busch & L. B. Berger, U. S. Bureau of Mines, Report of Invest. #4777, Jan. 1951.
6. H. Nagao, M. Uchida & A. Yamaguchi, Kogyo Kagaku Zasshi (in Japanese) 59, 940 (1956). 7. R. E. Dufour, Bulletin of Research No. 53, Underwriters' Laboratories, July 1963. 8. W. H. McDermott & F. E. Critchfield, Unpublished report, Feb. 1961. cited i n Dufour (5). 9. A. P. Hobbs & G. A. Patten, Unpublished report, March 1962, cited in Dufour (5). 10. P. W. Smith, C. R. Crane, D. C. Sanders, J. K. Abbott & 8. Endecott, lnternational
Symposium on Physiological and Toxicological Aspects of Combustion Products, Univer- sity of Utah, March 1974.
11. K. Yamamoto & T. Kato, KASAl (Fire Prevention Soc. Japan) (in Japanese) 19, 77 (1969). 12. H. Th. Hofmann & Oettel, Kunststoff Rundschau 15, 261 (19681.
13. H. Th. Hofmann & H. Sand, Kunststoff Rundschau 21, 413 (1974).
14. K. Kishitani, J. Faculty of Engineering, University of Tokyo (B) 31, 1 (1971).
15. T. Sakai, H. Tsukamoto & M. Ohashi, Medicine and Biology (in Japanese) 90, 11 1 (1975). 16. C. J. Hilado, W. H. Marcussen, A. Furst & H. A. Leon, J. Comb. Toxicol.3, 125 (1976). 17. K. Yamamoto, 2 . Rechtsmedizin 76, 11 (1975).
18. H. H. G. Jellinek & K.Takada, J. Polym. Sci.: Polym. Chem. Ed. 13, 2709 (1975). 19. M. Chaigneau & C. LeMoan, Annal. pharmaceutiques francaises30, 409 (1972). 20. B. M. Bulygin, E. A. Bulygina & A. A. Karnishin, Soviet Plastics p. 45, No. 8 (1973). 21. B. M. Bulygin, Plasticheskie Massy 5, 24 (1975). Chem. Abst. 83, 1 3 6 4 4 4 ~ (1975). 22. K. Ashida, F. Yamauchi, M. Katoh & T. Harada, J. Cellular Plastics 10, 181, (1974). 23. T. Morikawa, Bull. Japanese Assoc. Fire Sci. & Eng. (in Japanese) 22, 1 (1972).
24. L. V. Ramachandran, T. Saran, P. N. Mukherjee & A. Lahiri, Proceedings of Symposium on Chemicals and Oil from Coal, Dhanbad, India (1972). Chem. Abst.81, 66022n (1974). 25. S. Gordon & B. J. McBride, NASA SP-273, National Aeronautics and Space Administra-
tion, Washington (1971 ).
26. Y. Tsuchiya & K. Sumi, J. Appl. Chem. 17, 364 (1967). 27. Y. Tsuchiya & K. Sumi, J. Fire and Flammability 3, 46 (19721. 28. K. Sumi & Y. Tsuchiya, J. Fire and Flammability 4, 15 (1973).
29. H. H. Cornish, M. L. Barth & K. L. Hahn, lnternational Symposium on Toxicity and Physiology of Combustion Products, University of Utah, March 1976.
30. K. Sumi & Y. Tsuchiya, lnternational Symposium on Toxicity and Physiology of Com- bustion Products, University of Utah, March 1976.
31. K. Sumi & Y. Tsuchiya, lnternational Symposium on Flammability and Fire Retardants, Toronto, May 1976.
32. Anon., Chem. Metallurgical Eng. 36, 334 (1929).
33. W. J. Burlant & J. L. Parsons, J. Polym. Sci. 22, 249 (1956).
34. S. L. Madorsky, Thermal Degradation of Organic Polymers, p. 194, lnterscience Pub.. New York (1964).
35. S. Ausobsky, 2. VFDB 2/67, 58 11967).
36. B. W. Mapperley & P. R. Sewell, Euro. Polym. J. 9, 1255 (1973). 37. T. Morimoto, High Polymer Japan (in Japanese) 22, 190 (1973).
38. M. Chaigneau & G. LeMoan, Annal, pharmaceutiques francaises31, 495 (1973).
Yoshio Tsuchi),a
40. D. R. Paulson & G. F. Moran, Environ. Science & Tech.8, 11 17 (1974). 41. G . Ball & E. A. Boettner, Nuova Chimica 50, 76 (1974).
42. M. Galin & M. LeRoy, Euro. Polym. J. 12, 25 (1975).
43. D. A. Chatfield, I. N. Einhorn, R. W. Mickelson & J. H Futrell. Polymer Conference Series, Flammability of Materials, University of Utah, July 1975.
44. M. Chaigneau & G. LeMoan, Analysis 4, 28 (1976).