READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Influence of admixtures on the structure and strength development
Sereda, P. J.; Feldman, R. F.; Ramachandran, V. S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=5334bd64-3f15-4b80-8de5-b867b215f4c9
https://publications-cnrc.canada.ca/fra/voir/objet/?id=5334bd64-3f15-4b80-8de5-b867b215f4c9
- -
Ser
T H ~
National Research
Conseil national
~ 2 1 d
I
*
Council Canada
de recherches Canada
no.
979
c . 2
BLDG'
INFLUENCE OF ADMIXTURES ON THE
STRUCTURE AND STRENGTH DEVELOPMEN7
by
P.J. Sereda, R.F. Feldman and V.S. Ramachandran
Reprinted from
7th International Congress on the
Chemistry of Cement
VoL
I, Paris
1980
p.
VI-1/32
aVI-1/44
DBR Paper No.
979
Division of Building Research
1
4
SOMMAIRE
L'introduction d'adjuvants, tels que les accGlGrateurs ou retardateurs de prise, les rEducteurs d'eau et les super- plastifiants dans le ciment hydrati5 ou les minEraux cimentaires influe selon des degrgs divers sur la prise, la rssistance, la morphologie, la porosit6, la densitE, l'aire surfacique, le volume, etc. Dans le prEsent msmoire, les changements qui s'opsrent sont examings en se r6f6rant 1 la
documentation plus rgcente qui porte sur ce sujet. La structure du ciment hydrati5 ou des minEraux cimentaires dgpend du type et de la quantitG d'adjuvant, de la composition chimique et mingralogique du ciment, de la reactivitg par unit6 de surface, du rapport eaulciment, du temps ngcessaire
3 l'hydratation, de la temperature et de l1humiditG.
IIII~III~I~~~~I~~~~~IIIIII~I
I
o9 02
07
- -
SUB-THEME V I
-
1
Structure formation and
development
in hardened cement pastes
P.J.
SEREDA
R.F. FELDMAN
V.S.
RAMACHANDRAN
Conseil National de
Recherche du Canada
Ottawa
- CANADA
4. INFLUENCE OF ADMIXTURES ON THE
STRUCTURE AND STRENGTH DEVELOPMENT
In the previous sections the nature and significance of bonds, porosity and other structural aspects of portland cement pastes have been discussed. Introduction of admixtures to the hydrating cement influences to different extents, changes in setting, strength, morphology, porosity, density, surface area, volume, etc. In this section such changes are
discussed with particular reference to more recent contributions. It must be recognized that variations in results reported in the literature for apparently similar systems are not unexpected, especially where commercial admixtures are used. In the presence of admixtures, the structure of the hydrating cement or cement mineral depends on the type and amount of admixture, chemical and mineralogical composition of
.
cement, surface area, reactivity, w/c ratio, period ofhydration, temperature and humidity.
4.1 STRENGTH
4.1.1 Cement Minerals
A direct study of the influence of admixtures on the hydration and structuration of cement, although a useful approach from the practical point of view is not easy to interpret because the admixture may act in a complex way to affect the hydration of the
individual phases and their hydration products. Hence, much attention has been directed to a study of the role of admixtures on the strength development in individual cement minerals.
Addition of CaC12 to portland cement results in a significant reduction in setting times and acceleration of strength development. Because of its readyavaila- bility, low cost and predictable performance charac- teristics and application over several decades, CaC12 is more widely studied than other accelerators (1). Compared with many other complex admixtures, CaC12 is relatively simple in terms of its chemical andphysical naturs. However, there is not only much controversy regarding the actual mechanism of its action but alsa a persistent disagreement on its effects on
concrete (2).
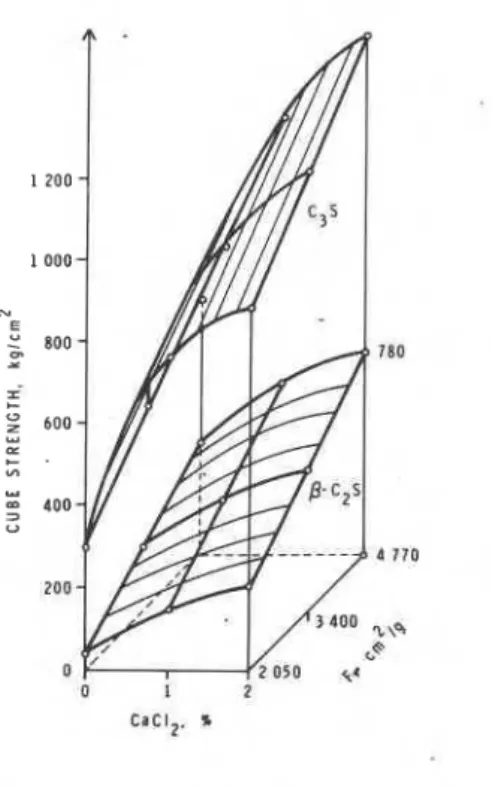
The reactivity of cement minerals C3S and C2S'to H20 may be enhanced by increasing their fineness. In an examination of the strength development in C3S andC2S ground to different fineness values, Balazs and Boros (3) found that increased dosages of CaC12 and
fineness resulted in higher strengths (Fig. 4.1). In
the presence of citric acid, the strengths decreased with increased dosages. However, as the fineness increased the strengths were enhanced. The main factors promoting strength development at increased fineness values are the initial compactness of the structure and higher reactivity.
It is generally recommended that the dosage of CaC12 in concrete should not exceed 1.5% by weight of portland cement. This does not necessarily mean that strengths are lowered at higher dosages in cement pastes or in silicate phases. It has been observed by Traetteberg and Ramachandranm(4) that in the system
C3S-CaC12-H20, at a w/s ratio = 0.5, addition of
2% CaCl2 gives better strength than that obtained by
5% CaC12. However, at a w/s = 0.3, addition of
5% CaC12 yields even higher strengths than those
obtained by 2% CaC12 at a w/s = 0.5 (Table 4.1) (4).
This indicates that in a more compacted system
proximity of the particles would promotebetterbonding
on the surfaces and therefore higher than normal amounts of CaC12 can be tolerated for achieving strengths.
- - - * - - - * - - -
TABLE 4.1
-
Microhardness for C3S pastes containingdifferent amounts of CaC1 (4)
Microhardness ( ~ / m m ~ )
Hydration Water/solid = 0.5 Water/solid = 0.3,
time
,
days C3S+2% C3S+5% C3S+2% C3S+5%
CaC12 CaC12 CaCl2 CaC12
1 113 13 132 98 2 8 3 2 3 221 155 3 9 8 2 7 271 225 7 117 41 292 284 10 150 5 7 360 346 15 186 92 311 353
Modulus of elasticity of a cementitious system can be obtained by the ultrasonic pulse velocity technique. In a study of C3S hydrated with calcium salts of thiocyanate, propionate, maleate, perchlorate and chloride, Lawrence et a1 (5) found that the velocities were higher with pastes containing admixtures. The velocity-time behaviour could partly be explained by the higher degree of hydration. Results with CaC12 were anomalous; compared to other admixtures, it gave lower velocities and lower porosity at equal degrees of hydration. Since the velocity depends on the density of the product and the numberofcontactpoints,
Fig. 4.1
-
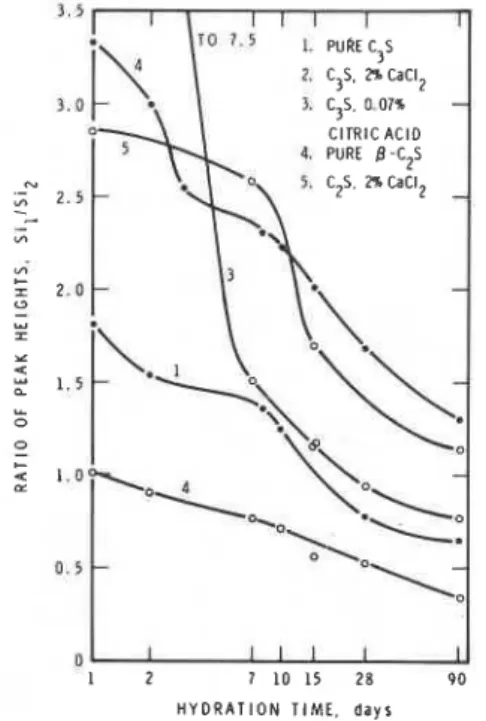
Effect of CaC12 on cubeit is possible that in the presence of CaC12 the higher density product that is formed reduces the contact points. Hence the applicability of the ultra- sonic technique for the cementitious systems contain- ing admixtures should be re-examined especially since the structure of the hydrate product is changed. The silicate structure changes occurring in the hydrated cements was followed by Lents (6) using the trimethylsilylation reaction. He found that with time of hydration the orthosilicate content gradually decreased, giving rise to disilicate and polysilicate structures. As an extension of this idea, polymeriza- tion of silicates in pastes containing C3S or C2S and admixtures CaC12, citric acid and thionyl chloride was examined by Tamas et a1 (7). The hydrated products
were found to contain mainly the dimeric ~ i ~ions 0 ~ ~ -with small amounts of trimeric chains and tetrameric rings. In both C3S and BC2S, the ratio of the mono- silicate/disilicate peak heights were lower for pastes containing CaC12 (Fig. 4.2) (7) indicating the increase in the disilicate formation withthe progress of hydration. As it is known that there is an increase in strength in the pastes containing CaC12, it is possible that strengths tend to be higher in pastes containing larger amounts of polymerized silicates. The citric acid admixture which is a retarder shows less polymerization effect at very early ages. More work should be done before it canbe stated that polymerization is directly related to strength development.
A comparison of the strengths developed in pastes containing different types of admixtures are usually compared at equal times of hydration. This method
1. 5 I I l l I 1 3.0 CITRIC A C I D 4. PURE B-C2S 5. C2S. CaC12 4 0 H Y D R A T I O N T I M E , d a y s
Fig. 4.2
-
Peak height ratios in chromatograms of silylated reaction product of C3S and C2S pastes,, with and without admixtures, as a function of hydration time (7)does not provide information on the intrinsicproperty of the product. The intrinsic property can bestudied by comparing the strengths at equal degrees of hydration. In an investigation of tensile strengths developed by BC2S containing CaC12, NaF. NaHC03, NaH2P04 and NaC1, comparison at 60% hydration showed different values (8). This would indicate that the nature of the products formed in the presence ofthese admixtures is different.
Admixtures also influence the strengths developed in C4AF and C3A pastes. In suspensions containing CaC12 concentrations of more than 3N, early strength of C4AF can increase with the formation of a high chloride complex (9). Similar observations have been reported in the C3A-CaC12-H20 system containing 16% CaC12 (10). These results would indicate that depending on the conditions of hydration and the materials, a much higher than normal amount of CaC12 prescribed in cement can be used without detrimental effects to strengths.
4.1.2 Cement Pastes
The relative importance of factors influencing strength in a cement p x t e containing CaC12 is not completely resolved. Although it is recognized that CaC12 yields high early strengths, there is no agree- ment on its influence on long-term strength develop- ment; the values may increase, decrease or remain the same as that of the reference cement paste. It isnot even clear what the optimum dosage of CaCl2 is for achieving the maximum strength. For example, contrary to general belief, Wolhutter and Morris (11) have found that the maximum strength at 28 daysis achieved with 4 to 6% anhydrous calcium chloride. This indicates the existence of many factorsthat influence the strength development in concretes. As described already, comparison of the propertiesat equal degrees of hydration forms a good basis to study the intrinsic property of pastes hydrated in the presence of dif- ferent amounts of admixtures. This approach was adop- ted by Ramachandran and Feldman (12) for an examina- tion of strength development in portland cement pastes hydrated in the presence of 0, 1, 2 and 3f% CaC12. Figure 4.3 (12) shows that at any one degree of hydration the sample with 31% CaC12 has the lowest strength; at lower degrees of hydration the sample containing 0% CaC12 is the strongest, although with the progress of hydration samples containing 1 to 2% CaC12 form stronger bodies than all others. Porosity, density and bonding are factors that affect these results. It is thus evident that addition of CaC12 not only changes the rate of hydration but also the intrinsic nature of the hydration products. The Soviet literature contains references to the use of many complex admixtures (13,14). For example, by using nitrites and nitrates in combination with ~ a ~ l i higher strengths are reported at below-freezing
temperatures. Mchedlov-Petrosyan et a1 (15) found that among the combinations used, a mixture of 1.1% CaC12 and 1.2% Ni(N03)2 developed maximum strength. The higher strengths were attributed to a more complete hydration, low basic C-S-H formation and complex formation of the salt with the hydrating cement. Strengths are not a linear function of the degree of hydration and caution should be exercised when comparison is made of pastes containing complex admixtures. For example at 50% hydration of cement, the strengths developed with the Ca(N03)2-CaC12 combination and the reference cement were,
respectively, 160 to 230 kg/cm2 (15.69 to 22.56 ~/nunZ) and 100 kg/cm2 (9.8 ~/nunZ) (16).
0 -0 4 6 8 10 1 2 14 16 . "9 NON-EVAPORABLE WATER, %
Fig. 4.3
-
Strength vs non-evaporable water relationship for cement paste containing calcium chloride (w/c 7 0.4) (12)A
number of organic and inorganic compounds. such as aiuminates, sulfates, fonnates, thiosulfates,catbonates and amines, have been suggested as alterna- tives to the CaC12 accelerator. None of them hasbeen found to be as efficient and economical as CaC12. Using chlorides of Ca, Ba, Mg and Fe, Ranga Rao (17) found fhat 1.6% BaCl2 gives 1-day strength equivalent to that obtained with 2 % CaC12. However, the 28-day strength with BaCl2 was lower than that obtained with CaC12
.
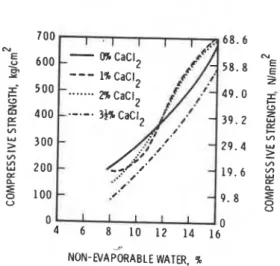
It was also found that BaC12 is not as efficient an accelerator as CaC12 at 10 to lS°C. It is likely that the differences in the solubility of BaC12 and CaCI2 play a role in the mechanism of acceleration and strength development.Triethanolamine is a constituent in certain admixture formulations in concrete and its addition is thought to reduce the excessive retarding action of a water- reducing admixture. Consequently. it is assumed that when used alone it should act as an accelerator. It may act as an accelerator for C3A, C3A + gypsum hydration (18) and as a retarder for the hydration of C3S (19). When added to portland cement,
triethanolamine decreases its strength at all agesand also alters the setting characteristics (20). Fig. 4.4 (20) shows the strength development in cement pastes containing 0, 0.1, 0.25, 0.35, 0.5 and 1%
triethanolamine. Strength decreases as the amount of triethanolamine is increased. Complex factors that may be involved for low strengths are: formation of
C-S-H with higher C/S ratio, retardation of C ~ S hydration, rapid initial setting followed by large heat developmeqt and a more porous structure. Sodium carbonate is also found to be an accelerator for the setting of cement. Strengths (1 to 28 days),however. seem to decrease by the addition of 2% N a ~ C 0 3 (21). In addition, there is an increase in the water requirement caused by the quick initial hydration of CjS and C3A components and the precipitation ofCaCOg. The slow strengths are attributed mainlyto decreased rate of subsequent hydration.
Water reducers, such as lignosulfonates, hydroxycarboxylic acids and, more recently,
superpIasticizcrs have been used widely in concrete practice either to achieve higher water reductions while maintaining the same workability or ta obtain higher workability at the same w/c ratio. Use of
superplasticizers pennits production of flowing ,
concrete without any loss in strength. They can also be used to reduce water requirements by about 30% for the develapment of high strengths. Using 1 to 4% of a sulfonated melamine formaldehyde-based admixture, strength increases of the order of 50% over the
control concrete have beeH obtained at the same slump value (Fig. 4.5) (22). There is still controversy as to whether the ultimate strength achieved, using a low W/C ratio (at nominal consistency) in thepresence of a water reducer, is equal to or higher than the reference cement paste or concrete made at the same w/c ratio but at low workability (23-25). There is also some disagreement as to the relative strength values of cement pastes made with or without water reducers made at the same normal w/c ratio but with different workability characteristics. Generally the mix containing the water reducer yields higher strengths. The cement paste containing the water
I
14 28
C U R I N G . d a y s
Fig. 4.4
-
Compressive strengths of cement pastes containing triethanolamine (20)-
--.-.---
-
-
-4 80-
~ ~ A D M I X ~ 4 % E-
E-
70-
-
-
N O M I N A L S L U M P 70 m m CEMENT CONTENT 5 0 0 t g l m 3-
-
1 3 7 14 20 30 ACE AT I E S T . d a y sFig. 4.5
-
The variation in strength development of constant initial slump concrete with a superplasticizer (22)VI
-
1/35
reducer will have higher workability and will be containing 1% CaC12, Berger et a1 (35) found that at better compacted, resulting in a better bonding of the equal degrees of hydfation the porosity of the paste hydrated products. Collepardi and Massida (26) have containing CaC12 was lower than.that of the reference observed that a reduction in porosity occurs in such paste. A plot of gel space ratio vs compressive pastes containing the water reducer. In these pastes strength indicated a linear relationship for pastes .
a higher degree of hydration has also been observed. with or without CaC12 hydrated to different degrees. It is thought that the water layer surrounding the It was assumed that the specific volume of the pastes
1
cement particles in the presence of water reducers was not changed by the addition of CaC12. Recent provides a source of efficient hydration (27). work of Ramachandran and Feldman (12) on cement pastes
has shown, however, that CaC12 influences thespecific Renewed interest has recently been shown in preparing
volume of the pastes. Since these workers (12) used cement pastes at a very low w/c ratio. This is Hg porosimetry with a maximum intrusion pressure of achieved by using a high surface area clinker
30 000 lb/sq in. (206.9 N/mm2), only pores of
(6000 to 7000 ~ m 2 / ~ ) and a mixture of lignosulfonate diameter >0.0065 pm could be registered. However, it with a carbonate or bicarbonate of K or Na. By this
is known that pastes containing CaC12 can have a method a w/c ratio of 0.20 to 0.25 is possible and substantial portion of total porosity in pores of strengths of the order of 19 000 lb/sq in. N/mm2) diameter below 0.0065 (36-38). collepardi (39) have been obtained (28-31). Typicallyat aw/c = 0.22, found that in C3S hydrated with 2% CaC12 at 1 day, at
the strengths at 15 h, 24 h and 3 days are, a w/c
= 0.5, the hydrated prod!ct had most of the respectively, 2000, 9500 and 12 500 lb/sq in. (13.8, pores in the range of 10 to 5nA radius. Skalny and 65.5 and 86.2 ~/mm2). Strength gain follows the co-workers (40) using
N2
isotherms also concluded that kinetics of hydration, unlike what was observed in the C3S hydrated in the presence of CaCIZ for 28 days has hydration of portland cement containing gypsum. It has a lower hydraulic radius (31.BA) than that of the also been reported that addition of the Na2C03 +reference paste (53.3A). Na-lignosulfonate combination to clinker or clinker +
gypsum at the same w/c ratio results in 1-daystrength Figure 4.6 (41) shows the total porosity and effective being higher and 28-day strength lower for gypsum- pore diameter relationship obtained for C3S hydrated containing clinkers (30). The mechanism responsible to different times in the presence of 1% CaC12. AS for these effects is not immediately apparent. hydration progresses, porosity decreases because the hydration products fill the pores, and at 30 days the Roy and co-workers (32) have been able to obtain
strengths as high as 95 000 lb/sq in. (655.0 N/mm2) in product contains mainly pores of smaller diameter. cement pastes by using the technique of hot-pressing.
The resultant reaction products formed using this
technique are different from those obtained in normal - 20
I I I
-
hydration reactions. Also a substantial portion of s =
-
-
the cement remains unhydrated. In an investigation of g 16
-
>
-
-the effect of various admixtures, viz.,
-
triethanolamine, CaC12, citric acid, sulfonated
-
naphthalene condensate and a polymer resin on hot
-
pressed type I and type I11 cements, CaC12 was found -
to give higher strengths (33,34). Generally strengths increased as the hydration progressed.
Citric acid did not improve early strengths and 0 . 0 1 0 . 1
.
1triethanolamine gave very low strengths. The effect
of admixture seems to be to change the rate of ,LFFECTIVE P O R E D I A M E T E R . pm
hydration and form a product that bonds the high
density matrix of varying po-rosity. The presence of Fig. 4.6 - Intrusion curves of a series CaC12 seems to result in the inhibitionof crystalline of C3S pastes hydrated with calcium
products (34) and a change in the nature of the chloride (41)
polymerization of the silicates. 4.2 PORE STRUCTURE
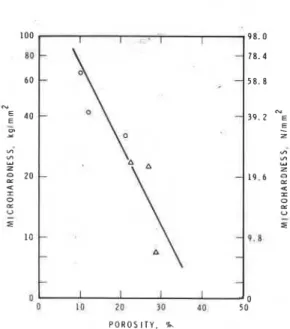
Total porosity and pore-size distribution are In a study of the effect of 2 to 5% CaC12 on the important parameters that provide information ,on the properties of C3S hydrated at w/s ratios of 0.3 and possible strength and durability characteristics of 0.5, Traetteberg and Ramachandran (4) found that an cementitious materials. A plot of strength vs approximate linear relationship exists between porosity plotted on a linear scale or as a semi-log porosity and logarithm of microhardness (Fig. 4.7). plot shows the.dependence of strength on porosity. Lack of perfect linearity may be attributed to Although such a relationship appears to exist oyer a differences in pore-size distribution, composition and range of porosity values, its use for prediction morphology.
purposes is limited because the relation is different
Some work has been carried out on porosity and pore- for different materials prepared in different ways.
size distribution characteristics of C3S pastes Some work has been carried out to determine the
hydrated in the presence of non-chloride admixtures effect of admixtures on the pore structure of hydra-
such as calcium lignosulfonate, Na2C03, polymers and ting cement and cement minerals. Porosity and pore
calcium salts of maleate, perchlorate, propionate and size distribution data for apparently similar thiocyanate. According to Collepardi (39,42), in C3S I
I materials may show significant differences depending pastes hydrated to 1 day the porosity and pore-size on what methods are adopted for their determination. distribution data for the reference paste and that
4
4.2.1 Cement Minerals containing calcium lignosulfonate are almost the same.
However, in the presence of Na2C03 the total porosity A considerable amount of work has been done on the
decreases from 0.091 to 0.06 ~ m 3 / ~ , and the volume of influence of CaC12 on the porosity and pore-size pores in the range 10 to 501 is lower than that of characteristics of C3S paste. Working on C3S
D
0 ID 20 30 40 50 P O R O S I T Y . %.
Fig. 4.7
-
Microhardness vs porosity relationship for C3S paste; w/s = 0.5 (A), w/s = 0.3 (0) (4)the reference. The lower porosity was attributed to the precipitation of CaC03 on the pore entrances due to the reaction between Na2C03 and Ca(OH)2. No data were collected for these systems to assess how the porosity and pore-size effects determine strength. Although the pore structure is similar for the reference paste and that treated with calcium lignosulfonate, the intrinsic structure may have been different if the results had been obtained at the same degree of hydration.
Young and co-workers (5,41) have measured the porosity and pore-size distribution values of C3S pastes hydrated with various calcium salts. Using mercury porosimetry, pore diameter only above 0.0065 pm could be determined. At a constant degree of hydration the intruded volume, as well as the pore-size distribu- tion, varied from one sample to the other. The dif- ferences were ascribed to variations in gel porosity, isolated large pores, air-entrainment, etc., assuming that they should have the same porosity. As already discussed, there is no reason to assume that the paste containing an admixture should have the same porosity as the reference material at the same degree of hydration.
4.2.2 Cement Pastes
In an investigation on the effect of different amounts of CaC12 (0, 1, 2 and 3t%) on the properties of cement pastes, Ramachandran and Feldman (12) found that the product shows a decrease in porosity as hydration .progresses (Fig. 4.8). Helium pycnometry was used to
determine the absolute density of discs of the pastes, and the porosity was computed from absolute and apparent densities. When cement is mixed with water it sets into a rigid body. Water in the pores slowly reacts with the unhydrated particles forming the hydrated products; these products fill the spaces originally occupied by the water molecules as well as spaces originally held by the unhydrated particles. Because the density of the hydrated cement is lower than that of the unhydrated cement a decrease in porosity results during hydration. The decrease is
steep during the-first 24 h, and at the same time
T I M E , h TIME, d a y s
Fig. 4.8 - Porosity of cement pastes containing different amounts of ' calcium chloride (w/c = 0.4) (12)
there is a rapid rate of hydration. After 28 days most samples show a 50% decrease in porosity.
In Fig. 4.9 (12) the porosity of cement pastes is expressed as a function of the non-evaporable water content. Porosity decreases as the degree of hydration increases. The main factor causing this decrease is the filling of pores by the low density hydrated cement. A plot of absolute density vs
...
2P CaCI,N O N - E V A P O R A B L E W A T E R . 9c
Fig. 4.9 - Porosity vs non-evaporable water relationship; (a) w/c = 0.25,
(b) w/c = 0.4 (12)
porosity shows a linear relation (Fig. 4.10) -(12). The relatively--higher porosity,values for pastes with a W/C = 0.4 are due to the higher initial amounts of water. A completely hydrated cement formed at w/c = 0.4 has an absolute density of 2.19 g/cm3 (43). An extrapolation of the line representing the-sample made at a w/c = 0.4 to a density of 2.19 g/cm3 corres- ponds to a porosity of 22.5%. This is the minimum porosity that can be attained with a cement paste prepared at a w/c = 0.4 (made with or without admix- tures). At the same degree of hydration for samples made at a w/c ratio of 0.4, porosity decreases as follows: Cement + 33% CaC12>Cement + 2% CaC12>Cement + 1% CaC12 >Cement + 0% CaC12. Collepardi et a1 (44) compared the porosity of cement hydrated for 7 days with and without CaC12 and found that the total porosity was higher in the paste containing CaC12. The differences in the porosity obtained at the same degree of hydration indicate that the types of hydra-
.
tion products formed in pastes containing differentpermit an evaluation of the intrinsic nature of the paste because pore volume was not determined for pore
1 sizes below 0.01 um. In another study, however,
Collepardi et a1 (23) investigated the pore volume vs pore radius (down to 10W) relationship for cement hydrated with calcium lignosulfonate. In the pore
radius range 10 to 70A, both the reference specimen 1 and that containing lignosulfonate showed a similar
relationship. The lignosulfonate-treated sam le showed a larger volume of pores of radius >7Of than the reference paste. Similar trends were obtained in C S astes containing lignosulfonate. The higher 3 .p shrinkage rate observed in pastes containing
5 0 k
-
4 0 > c-
3o v, og
0 1 0 6 0 - 1 1 1 1 ' WIC-
xop20.4
T
-
dB
-.fl
9/*d*0.25-
Z01./09-
-
-
I I I I I I0 lignosulfonate was explained by the presence of larger
2 . 3 2.4 2.5 2 . 6 2.7 2 . 8 2.9 3 . 0 amounts of pores from which water can escape more A B S O L U T E D E N S I T Y , g l c m 3 rapidly.
Sodium carbonate can be used as an accelerator of setting. Addition of this admixture to cement changes Fig. 4.10
-
The influence of absolute the intrinsic nature of the hydrated product. Total density on the porosity of cement pastes porosity and pore-size distribution values of hydrated hydrated with calcium chloride (12) cement containing Na2C03 are not the same as that ofthe reference paste. In the presence of Na2C03 smal- ler pores (r = 10 to 1001) are decreased and larger pores (r >1501() are increased slightly (21). The amounts of CaC12 are intrinsically different in reduction in small pores may be due to the precipita- nature. It can also be concluded that in this system tion of CaC03. A decrease in total porosity of the differemesin porosity are mainly due to the cement pastes from 19.2 to 8.1% was observed by Butt variation in the absolute densities of the hydration and Kolbasov (45) when NaN02 was used as an admixture.
products
.
The decrease in porosity was attended by an increaseAlthough a substantial amount of work has been in compressive strength.
carried out on the effect of superplasticizers on the Uchikawa and Tsukiyama (46) compared the porosity of physico-mechanical characteristics of concrete, only two regulated set cements A and B containing citric meagre information is available on their effect on acid and CaS04.fH20, respectively. When strength was cement pastes. Col lepardi (26) compared the porosity at different times, cement A containing and pore-size distribution characteristics of cement citric acid appeared stronger than B containing pastes (with or without a superplasticizer) made at CaS04.fH20. However, a comparison of strength at the same w/c 0.30, 0.35 and 0.44. The equal porosity values showed that cement B mix was covered only pore diameters in the range 0.01 to 1 vm. stronger than cement A mix. This implies that the Porosity values were lower in samples containing the internal structure of mix B was different from that of admixture and this was partly due to a higher degree mix A.
of hydration (Fig. 4.11) (26). The results do not
4.3 SURFACE AREA
Surface area studies of a hydrated cement may yield
PORE DIAMETER. m ~ c r o n information on the reactivity, strength, shrinkageand
1 . 0 0 . 5 0 . 2 0. 1 0 . 0 5 0 . 0 2 volume changes of the paste. The unhydrated portland
cement has a Blaine surface area of about 3000 to 4000 ~ m 2 / ~ . In a set cement (saturated condition) the 0 . 1 0
-
-
values, determined by the small-angle X-ray scatteringm
E technique (47). are as high as 600 m2/g. This figure
0 . 0 8
-
is even higher if computed for the C-S-H portion ofu
I hydrate. The specific surface area determined by Hz0
a 0 . 0 6
-
-4 vapour yields a value of about 250 m2/g and that by
0
> N2 decreases to 50% of this value or less. The large
0 . 0 4
-
P: surface area indicates that the individual particles
0
a. comprising the hydrated cement are of colloidaldimen-
0 . 0 2
-
sions. It is generally observed that the surface areaof a cement paste determined by Hz0 vapour gives a reasonably constant value independent of the methodof
500 1 0 0 0 5 000 10 000 preparation or history. Surface area determined by
PRESSURE, P S I N2 gives values that differ for different preparations
and accounts for changes occurring as a result of sample history and ageingprocesses. Addition of Fig. 4-11
-
Cumulative pore volume in admixtures influences significantly the N2 surface function of pore diameter or pressurearea of pastes; in this section these effects will be of intruded mercury. The cement discussed.
pastes were hydrated for 3 days with
the following w/c ratios: 4.3.1 Tricalcium Silicate
1
-
w/c = 0.44; 2-
w/c = 0.35; The N2 surface area of hydrated C3S or C2Sisincre'ased I 3-
w/c = 0.30; 4-
w/c = 0.44; in the presence of CaC12 (37,39,40,48-52). The extent 5-
W/C = 0.35; 6-
w/c = 0.30. of thi's increase differs, depending on factors such as1-2-3 without Rheomac; particle size and purity of the silicate, w/s ratio. 4-5-6 with Rheomac (26) amount of CaC12 added, extent of hydration,temperatt~=e
of hydration and drying conditions. If water is used as the adsorbate, surface area values are found to be much higher than N2 values. The Hz0 surface areas of C3S pastes containing calcium chloride are not much different from those hydrated without it.
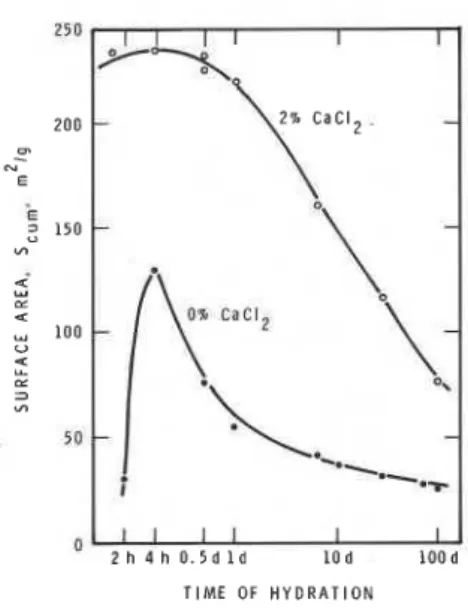
Figure 4.12 shows the N2 surfaro-area values as a function of the time of hydration'for C3S (51). In the presence of CaC12 in the first day, the surface area is about 220 m2/g, which decreases to a value of about 80 m2/g in 3 months. This value is still about three times that determined for C3S + 0% CaC12. Nitrogen area for C3S + 5% CaC12 is even higher than that for C3S containing 2% CaC12 (53,54). Though surface area decreases as the temperature of hydration isincreased, at any temperature, samples containing CaC12 show higher surface areas than those without it (54). In Fig. 4.12, C3S samples show a decrease in surfacearea as hydration progresses, and this is due to the "ageing" effect involving formation of physical and/or chemical interlayer bonds.
Surface area (by H20 vapour) of hydrated CgS carried out at a hydration degree of 64% shows a value of 324 m2/g for C3S + 0% CaC12 and a value of 261 m2/! for CjS + 2% CaC12 (40). The lower surface area is explained by the formation of C-S-H of higher c/s ratio and increased average thickness of the gel unit
(55). A higher c/s ratio in the gel need not neces- sarily mean that it would have a lower surface area. For example, the gel with 5% CaCl2 has a surface area of about 15 m2/g larger than that formed with 2% CaC12, though the c/s ratio of the gel at 5% CaC12 is higher. Shrinkage of a C3S paste is increased in the presence of CaC12
.
If the measurements are carried out at a particular time, the values may reflect the extent to which the hydration has progressed. Hence, Berger et a1 (35) compared the shrinkage of the C3S paste and C3S paste + 1% CaC12 at constant degrees of hydration.At any degree of hydration the paste containing CaC12 showed a higher shrinkage. Though the higher shrin- kage values have been thought to be due to an increase in the finer pore size range, it can be considered that a higher surface area promotes greater shrinkage.
The non-chloride admixtures may also influence the surface area of the C3S pastes. At 1 day of hydration the specific surface areas of the hydrated portion of the reference C3S paste, and that contain- ing CaC12, Na2C03 and calcium lignosulfonate, are, respectively, 45, 198, 26 and 47 m2/g (39). Since C3S is hydrated to different extents at 1 day, the results do not mean that the values will be similar if compared on this basis at all levels of hydration. Addition of triethanolamine (TEA) results in an
increase in the surface area of C3S paste. At 28days of hydration N2 surface areas of CgS + 0% TEA, C3S + 0.1% TEA and C3S + 1% TEA are, respectively, 24.8, 30.9 and 44.6 m2/g (19). It may be envisaged that the chemisorption of TEA on freshly formed C-S-H inhibits the orderly growth of plates in a tubular form and thus promotes the formation of a higher surface area product. Triethanolamine also increases the amount of non-crystalline Ca(OH)2, and this factor may partly account for the increased area.
4.3.2 Cement Pastes
The addition of CaC12 increases the surface area of hydrated C3S and hence it follows that a similar effect should also operate in portland cement paste. In the presence of CaC12, the surface area values of portland cement, pozzolanic cement and blast furnace cement increase from initial values of 43.2, 45.4 and
u -
Z h 4 h 0 . 5 d l d 10 d 100 d
T I M E O F H Y D R A T I O N
Fig. 4.12
-
Surface area of a C3S paste containing calcium chloride (51)45 m2/g to 76.3, 61.0 and 198 m2/g, respectively (44). Calcium chloride is more effective in increasing the surface area of C3S paste than that of portland cement paste. Part of the explanation is that CaC12 increases only marginally the surface area of the BC2S component of cement. The small increase in surface area of the pozzolanic cement is due to the negligible influence of CaC12 on the lime-pozzolana reaction. The activation of the slag by CaC12 may explain its significant influence on this system. The surface area of various cement pastes, determined at a particular degree of hydration, was reported by Ramachandran and Feldman (12). At a particular degree of hydration the surface area generally increases as the amount of CaCI2 is increased (Table 4.11) (12). Chemisorption of C1-ions on the
---
TABLE 4.11 - Specific surface areas of cement pastes containing CaC12 at a particular non-evaporable water content (12) Non-evaporable Water, CaC12, 8.2 14.4 Surface Area, m2/g 0 20.5 22.1 1 20.3 24.3 2 37.9 28.7 3!? 37.3 32.8
hydrating surfaces may be responsible for this effect (53). The significant differences in the sur- face area values for the same degree of hydration suggest the existence of differences in the intrinsic properties of samples. At higher degrees of
1 0 I I I I I - S H R I N K A G E v s a
-
SURFACE A R E A 0.8 - a-
ae
- a a *-
a 0.6 -a
l l a-
a a t a-
A a Y Z - 0 . 4-
l l-
u I - *a-8
:
a-
cn 0.2 --
-
- 0-
I I I I I 4 0 5 0 6 0 7 0 8 0 9 0 100 S U R F A C E A R E A ,m 2 / g
Fig. 4.13 - Shrinkage vs surface area for several admixtures (56)
containing 2 and 3t CaC12 may be due to ageing. The shrinkage of a cement paste containing CaC12 depends on its surface area and the degree of hydration (44). If the degree of hydration is constant, the shrinkage values should depend on surface area. In Table 4.11
(12), the surface areas of pastes containing CaC12 are larger than those containing no CaC12, indicating that shrinkage values should be higher in pastes containing CaC12
.
This has been verified for the system C3S-CaC12-H20 (35).The significance of surface area on shrinkage in cements hydrated with calcium lignosulfonate, hydroxycarboxylic acid and triethanolamine has been investigated by Feldman and Swenson (56). A plot of shrinkage (drying from 100 to 50% RH) vs surface area (N2) for various samples showedthat samples producing higher shrinkages had significantly higher surface areas (Fig. 4.13) (56). It appears that shrinkage is
a s s o c i a t e d m o r e w i t h t h e d e g r e e o f d i s p e r s i o n of the pastes than with the chemical composition or morphology. As stated earlier, CaC12 at normal dosages increases the surface area substantially. At normal dosages calcium lignosulfonate increases only marginally the surface area of cement paste. Collepardi et a1 (23) found that with calcium lignosulfonate the areas increased from 43-48 to 48-53 m2/e. This increase was
resulting in the publication of innumerable micro- graphs. It is beginning to be recognized that comparison of results by different workers has an inherent limitation because of the small number of micrographs usually published and the correspondingly small area represented by these micrographs, which may indicate a non-representative view of the structure. What may be selected by one researcher as the representative structure may differ from that selected by another. Even the description of apparently similar features becomes subjective (58). Consequently, speculations on the origin of strength and other properties, when based on these observa- tions, have limited validity, especially since many properties of cement paste are influenced at a much lower microlevel than can be observed by the electron microscope.
In spite of human and instrumental limitations, electron microscopic techniques have provided useful information on morphological features and an estimate of the elements contained in the microunits of various products: Depending on the starting materials and conditions of hydration, addition of admixtures to cements and cement minerals modifies to different extents the microstructure of the hydration products. 4.4.1 Tricalcium Silicate
partly attributable to a higher degree of hydration in The tricalcium silicate phase, constituting the major the presence of calcium lignosulfonate. Under similar
conditions, addition of Na2C03 decreased the surface component in portland cement, greatly influences its area of cement paste from 43-48 to 22.7
-
26.0 m2/g greater attention than other phases. A number of strength characteristics and hence has received because of the blocking of pores by precipitated investigators have studied the effect of different CaC03. The shrinkage values were also lower in thesepastes. concentrations of CaC12 on the morphological charac-
teristics of hydrated calcium silicate. There has, 4.4 MICROSTRUCTURE however. been variance in the actual descri~tion of
the morphology (1). According to Odler and sialny The microunits formed in the cement paste are too (55), hydrated C3S normally forms spicules or sheets small to be amenable to investigation by the optical rolled into cigar-shaped fibres 0.25 to 1.0 um long microscope and consequently the electron microscope and in the presence of CaC12 a spherulitic morphology capable of very high resoltuion has received recogni- is facilitated. Kurczyk and Schwiete (59) reported tion for the examination of the microstructure of
cementitious materials. Radczewski et a1 (57) were that needle-like products change to spherulites inthe probably the first to apply this technique for presence of CaC12. Young (41) found that the
investigating cementitious systems and since then morphology of hydrated C3S + 0% CaC12 was needle-like several refinements to the technique have been made, whereas that of hydrated C3S in structure. In contrast to the above, Murakami and + 2% CaC12 was lace-like
Tanaka (1) found the existence of a fibrous cross- linked structure in C3S pastes treated with CaC12. Using transmission electron microscopy, Ramachandran
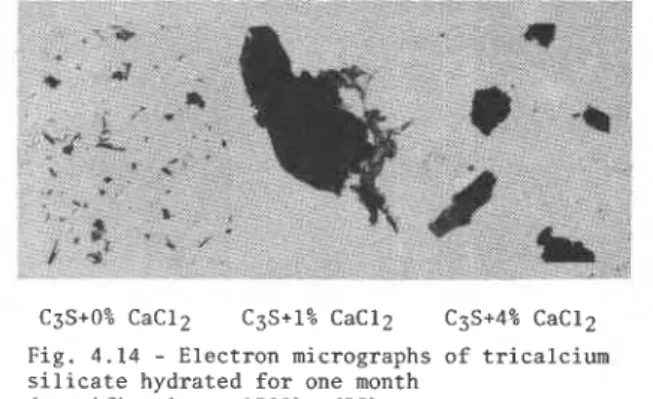
(53) found that C3S hydrated at a w/s ratio = 0.5
showed needle-like morphology, whereas that hydrated in the presence of 1% or 4% CaC12 exhibited a platy or crumpled foil-like morphology (Fig. 4.14). Collepardi and Marchese (51) and Berger et a1 (60) came to similar conclusions. These changes in morphology may have resulted from the chemisorption of C1-ions onthe C-S-H surface and introduction of these ions into the C-S-H lattice.
The morphological features become less distinct when the hydrated products are formed in a confined space, as for example, when the pastes are prepared at low w/s ratios. This is because the particles are so close to each other that there is not enough space for crystals to grow into larger dimensions. In Fig. 4.15 (12) are shown the micrographs of C3S hydrated at a w/s ratio of 0.3 and obtained with 0, 2 and 5% CaC12. A dense structure for pastes with 2 and 5% CaC12 is evident and this feature,may be responsible fora 50to 150% increase in microhardness values over the paste
containing no CaC12
.
It may, however, be arguedthata lower porosity and better contact or
bond in^
of theparticles are factors causing strength increases. Bendor and Perez (61) have ascribed the higher str.engths in C3S pastes with CaC12 to the honeycomb nature of the paste as opposed to the sponge-like feature in the reference C3S paste. Berger et a1 (35, 41) compared the microstructure of C3S hydrated with and without CaC12 at the same degree of hydration and concluded that differences in external morphology indicated the differences in pore-size distribution. Porosity and pore-size distributions are recognizedas important parameters affecting the strength develop- ment. However, in this work (35), it is not easy to assess pore sizes below 0.1 vm from the morphological features. Lawrence et a1 (5), on the other hand, have concluded that the outer morphology observed by SEM is not as important as the contact points closest to the unhydrated giains (which cannot be resolved by SEM) in assessing the mechanical properties of the C3S paste. It is important, therefore, to recognize that morphology, porosity, density and chemical composition are mutually dependent factors that determine strength characteristics.
Addition of CaC12 modifies the morphology of autoclaved C3S. Fibrous crystals and rectangular
block-shaped c r ~ t a l s normally present are not found
with CaC12. In the presence of CaC12, C3SH1.5 and a-C2SH are not formed (62).
Changes in the C3S paste.microstructure occurring in the presence of admixtures containing Ca are well documented but their relevance to strength develop- ment is still largely unknown. Young and co-workers
(5,63) studied the effect of various anions contain- ing Ca++ as the common cation. The anions included perchlorate, thiocyanate, propionate, maleate and chloride. There was a change in the C-S-H morphology from an acicular to a lacey and honeycomb structure in the presence of some admixtures. Other changes such as the number of CH crystals per square millimetre, their relative sizes and crystal axis
ratios, also occurred (Table 4.111) (63). The
relevance of these changes to strength development is not completely clear and the .results suggest that capillary porosity is the dominant factor controlling the tensile strength. The importance of the role of anions in modifying the morphology of BC2S pastes has also been examined using sodium salts of fluoride, bicarbonate, chloride and orthophosphate (8).
Berger and McGregor (64) have made an extensive study of the microstructure of C3S paste, with particular reference to the CH component. The pastes that
Fig. 4.14 - Electron micrographs of tricalcium
silicate hydrated for one month
(magnification x 1500) (53)
TABLE 4.111 - Influence of admixtures on the microstructure of C3S pastes (63)
Effect on Hydration Effect on Morphology Approximate
Admixture Kinetics number of
(24 h) CSH Phase CH CH crystals/mm2
Calcium chloride Strong accelerator Modified Large
-
c<<a
Calcium thiocyanate Accelerator Modified Large 2.5
c<<a
Calcium propionate Accelerator Unchanged Small 14.0
irregular
Calcium perchlorate Weak accelerator Unchanged Large
:.
0c<<a
Calcium sulfate dihydrate Weak accelerator Unchanged Small 4.0
c<a
Calcium maleate Retarder Modified Large 9.0
Fig. 4.15
-
Microstructural features of cement pastes containing calcium chloride and hydrated to a non-evaporable water content of 8.2% (magnification x 2400) (12)resulted by using 62 admixtures could be divided into four groups, based on the c/a axis ratios. The morphology was found to be dependent more on the type of anion than on the type of cation. There is need to investigate how these differences influence the engineering properties. It has often been reported that in C3S pastes containing various admixtures, the hexagonal phase typical of CH may or may not be present. According to Bendor and Perez (61), in pastes containing CdI2 the hexagonal CH phase formed, whereas it was absent in the presence of CaCl2 and CrC13. The mechanism responsible for these differen- ces is not clear. In some instances the CH crystals may be obscured by the C-S-H gel.
4.4.2 Cement Pastes
Addition of CaC12 to the hydrating cement affects the microstructural features of the paste. Without the admixture, the paste may consist of clusters of sheets mixed with fibres, the fibres being much more abundant than in C3S pastes. With the addition of
.
2% CaC12 the fibrous particles may be completely absent (23).Ramachandran and Feldman (12) compared the
microstructural features of cements containing 0. 1, 2, and 3f% CaC12, all hydrated to the same degree. At lower degrees of hydration (corresponding to a constant non-evaporable water content of 8.2%) pastes
containing 0% CaC12 showed well-defined needles representing C-S-H and ettringite formation. Columnar
particles of CH, identified by energy-dispersive X-ray
analysis, were also present. At 1% CaC12 addition, however, thin plates of C-S-H appeared. At 2% CaC12 the structure became more consolidated and contained plates and small particles. A sponge-like mass was evident at 34% CaC12 addition. Specific area, density, porosity and strength differences existing between samples could not be explained on the basis of microstructure. Some of these may depend on microstructural features that are not easily resolved by the SEM. Also some of the features may be
obscured by the deposition of a gel-like hydration product. Micrographs, however, showed that there were some morphological differences, especially at lower degrees of hydration. In hot pressed cements
containing admixtures such as CaC12 , triethanolamine
and citric acid, Sarkar and Roy (34) found dif- ferences in strength development but the micro- structural investigation yielded no useful informa-
tion to explain the streng:h variations.
Cement pastes containing a normal water-reducing agent, as well as a superplasticizer, and formed at w/c ratios in the range 0.30 to 0.50 may not show any marphological changes with reference to the cement paste containing no admixture (23,65,66). This would imply that the differences observed in creep and shrinkage with calcium lignosulfonate cannot be explained by the microstructural examination using the SEM technique (66).
A study of the low porosity portland cement paste ,
(containing no gypsum) prepared at w/c ratios of
0.22 to 0.24 with Na-lignosulfonate andNa-bicarbonate
admixture has shown that the admixtures modify the
microstructure (67). In place of fibrous and
reticular network-type structure, normally found in a cement paste, the ahixture-treated paste appeared as thin sheets in the first few hours and than as small equant grains. The presence of the admixtures promoted a more massive type of structure, implying improvement in mechanical properties. Odler et a1 (68) used a mixture of lignosulfonate and carbonate with clinker and portland cement and found no
distinct differences in morphological characteristics of pastes (with or without admixtures) though-there were differences in the composition of the hydrated products.
It is generally difficult to study the microstructure of pastes formed at very low w/s ratios. Further difficulty is experienced when the pastes are formed using the hot-pressing technique. Because of high pressure, high temperature and low amounts of water, the structure becomes dense and nearly 70% of portland cement remains unhydrated. Using the hot- pressing technique, Oyefesboi and Roy (33) made compacts of cebent in the presence of various admixtures. Although substantial differences in strengths were observed, SEM showed no obvious differences.
REFERENCES (SECTION 4)
1.- V.S. RAMACHANDRAN (1976), "Calcium chloride in concrete," Applied Science Publishers, U.K., 216 p.
2. - V.S. RAMACHANDW (1978), "Calcium chloride in
concrete
-
applications and ambiguities," Can. J.Civil Eng., 5, 213-221.
3.- GY. BALAZS and M. BOROS (1978). "Effect of calcium chloride and citric acid on the hydration of C3S and BC2S pastes," Periodica Polytechnica, 22, 29-35.
4.- A. TRAETTEBERG and V.S, RAMACHANDRAN (1974), "The microstructural and hardening behaviour of tricalcium silicate pastes in the presence of calcium chloride," J. Appl. Chem. Biotechnol., 24, 157-170.
5.- F.V. LAWRENCE, J.F. YOUNG and R.L. BERGER (1977), "Hydration and properties of calcium silicate pastes," Cem. Concr. Res., 7, 369-378. 6.- C.W. LENTZ (1966), "The silicate structure
analysis of hydrated portland cement paste," Symp. Structure of Portland Cement Paste and Concrete, Highway Research Board, Special Rept. 90, pp. 269-283.
7.- F.D. TAMAS, A.K. SARKAR and D.M. Roy (1976), "Effect of variables upon the silylation products of hydrated cements," Proc. Conf. Hydraulic Cement Pastes - Their Structure and Properties, Univ. Sheffield, Cem. Concr. Assoc., pp. 55-72.
8.- J.F. YOUNG and H.S. TONG (1977), "Microstructure and strength development of beta dicalcium silicate pastes with and without admiktures," Cem: Concr. Res., 7, 627-636.
9.- N.P. STUKALOVA and E.P. ANDREEVA (1977), "Structure formation and chemical reapion in aqueous suspensions of tetracalcium
aluminoferrite in the presence of calcium chloride," Kolld. Zh., 39, 508-512.
10.- A . TRAETTEBERG and P.J. SEREDA (1976), "Strength
of C3A paste containing gypsum and CaC12," Cem. Concr. Res., 6, 461-474.
11.- C.W. WOLHUTTER and R.M. MORRIS (1973),
"Strength-imposed limitations on admixed calcium chloride content of concrete," Civil ;Eng., S. Africa, 15, 129-131.
12.- V.S. RAMACHANDRAN and R.F. FELDMAN (i978), "Time-dependent and. intrinsic characteristics of portland cement hydrated in the p$esence of calcium chloride," I1 Cemento, 75, 3Z1-322.
13. - V.S. RAMACHANDRAN (1978), "AdmixtureS," Cements
Research Progress, Amer. Ceram. Soc., Chapt. VI,
pp. 119-157.
14.- V.S. RAMACHANDW (1977), "Admixtures," Cements
Research Progress, Amer. Ceram. Soc.,, Chapt. VI,
pp. 97-140.
15.- O.P. MCHEDLOV-PETROSYAN, A.G. OL'GINSKII and YU. M. DOROSHENKO (1977), "Influence of certain combined chemical additives on hydration and mechanical properties of clinker cement," Zh. Prik. Khim., 51, 1493-1498.
16.- V . RATINOV, T. ROZENBERG and V. TOKAR (1973),
"The structure of the concrete pore space with CNN and CNNC admixtures," Proc. Int. Symp. Structure and Properties of Materials, Prague, pp. 205-215.
17.- M.V. RANGA RAO (1976), "Investigation of
admixtures for high early strength development," Indian Concr. J., 50, 279-289.
18.- V.S. RAMACHANDRAN (1973), "Action of
triethanolamine on the hydration of tricalcium aluminate," Cem. Concr. Res., 3, 41-54. 19.- V.S. RAMACHANDRAN (1972), "Influence of
triethanolamine on the hydration characteristics of tricalcium silicate," J. Appl. Chem.
Biotechnol, 22, 1125-1338.
20.- V.S. RAMACHANDRAN (1976), "Hydration of cement - role of triethanolamine," Cem. Concr. Res., 6 , 623-631.
21.- M. COLLEPARDI, A. MARCIALIS and L. MASSIDA (1973), "The influence of sodium carbonate on the hydration of cements," Annali di Chimica, 63, 83-93.
22.- W.G. RYAN and R.L. MUNN (1978), "Some recent experiences in Australia with superplasticizing admixtures," Proc. Int. Symp. Superplasticizers in Concrete, Ottawa, Canada, pp. 279-293.
23.- M. COLLEPARDI, A. MARCIALIS and V. SOLINAS (1973), "The influence of calcium lignosulfonate on the hydration of cements," I1 Cemento, 70, 3-14.
24.- R.C. MIELENZ (1968), "Use of surface active agents in concrete," Proc. V Int. Symp. Chem., Cern., Tokyo, Vol. IV, pp. 1-29.
25.- W.F. PERENCHIO, D.A. WHITING and D.L. KANTRO (1978), "Water reduction, slump loss and entrained air void systems as influenced by superplasticizers," Proc. Int. Symp.
Superplasticizers in Concrete, Ottawa, Canada, pp. 295-323.
26.- M. COLLEPARDI and L. MASSIDA (1976), "The influence of water-reducing admixtures on the cement paste and concrete properties," Proc. Conf. Hydraulic Cements - Their Structure and Properties, Univ. Sheffield, Cem. Concr. Assoc., pp. 256-267.
27.- M.J. McCARTHY (1979), "Tests on set retarding admixtures," Precast Concr., 10, 128-130, 1979. 28. - S. DIAMOND and C. ' GOMEZ-TOLEDO (1978) ,
"Consistency, setting and strength gain characteristics of a 'low-porosity' portland cement paste," Cem. Concr. Res., 8, 613-622. 29.- K.M. HANNA (1977),"Application of experience
with low-porosity cement pastes and mortar," Zement-Kalk-Gips, 30, 140-142.
30.- J. SKALNY and I. ODLER, "Use of admixtures in production of low porosity pastes and concretes," Transp. Res. Rec. 564, pp. 27-38.
31.- I. ODLER, U. DUCKSTEIN and TH. BECKER (1978), "On the combined effect of water solubles, lignosulfonates and carbonates on portland cement and clinker pastes. I. Physical properties," Cem. Concr. Res., 8, .469-480.
32.- D.M. ROY and G.R. GOUDA (1973), "High strength generation in cement pastes," Cem. Concr. Res., 3, 807-820.
33.- S.O. OYEFESBOI and D.M. ROY (1977), "Effect of admixtures on hot-pressed cements," Cem. Concr. Res., 7, 165-172.
34.- A.K. SARKAR and D.M. ROY (1977), "Hot-pressed cements prepared with admixtures," h e r . Ceram. SOC. Bull., 56, 984-986.
35.- R.L. BERGER, J.H. KUNG and J.F. YOUNG (1976), "Influence of calcium chloride on th2 drying shrinkage of alite paste," J. Test. Eval., 4, 85-93.
36.- M. COLLEPARDI, G. ROSS and G. USA1 (19681, "The paste and ball mill hydration of tricalcium silicate in the presence of calcium chloride," LtIndustria Ital. Cem., 38, 657-663.
37.- J. SKALNY and I. ODLER (1972), "Pore structure of calcium silicate hydrates," Cem. Concr. Res., 2, 387-400.
38.- J.F. YOUNG (1973), "Effect of calcium chloride on the capillary porosity distribution in tricalcium silicate pastes," RILEM Proc. Int. Symp., Prague, pp. 197-204.
39.- M. COLLEPARDI (1973), "Pore structure of hydrated tricalcium silicate," Proc. Int. Symp. Pore Structure and Properties of Materials, Prague, pp. 25-49.
40.- J. SKALNY, I. ODLER and J. HAGYMASSY (1971), "Pore structure of hydrated calcium silicates. I. Influence of calcium chloride on the pore structure of hydrated tricalcium silicate,"
J. Colld. Interface, 35, 434-440. 41.- J.F. YOUNG (1973), "Capillary porosity in
hydrated tricalcium silicate pastes," Powder Technol., 9, 173-179.
42.- M. COLLEPARDI (1974), "Porous structure of .
pastes of hydrated tricalcium silicate," I1 Cemento, 71, 11-22.
43.- R.F. FELDMAN (1972), "Density and porosity studies of hydrated portland cement," Cem. -Technol. 3, 5-14.
44.- M. COLLEPARDI, A. MAKCIALIS and V. SOLINAS (1973), "The influence of calcium chloride on the properties. o£ cement pastes," I1 Cemento, 70,, B3-92.
45.- YU. M.
BUTT.^^'^
V.M. KOLBASOV (1975), "Features of structure formation during the solidification of cements in the presence of water-soluble chemical additives," Khim. Elet. Metall. Khim. Tekhnol. Silik., Mendelecusk. S'ezd. Obshch. Prikl. Khim., 11th pp. 186-187; Chem. Absts., 85, 197073, (1977).46,- H. UCHIKAWA and K. TSUKIYAMA (1973), " T ~ T
hydration of jet cement at 20°C," Cem. Concr. Res., 3, 263-277.
47.- D.N. WINSLOW and S. DIAMOND (1974); "Specific surface of hardened portland cement paste as determined by small angle X-ray scattering,"
J. Amer. Ceram. Soc., 57, 193-197.
I
48.- M. COLLEPARDI and L. MASSIDA (1971), "Hydrationof tricalcium silicate in suspension," Annali di Chimica, 61, 160-168.
49.- A. RIO (1974), "Approaching to macromolecular characterization of the C3S hydration process," VI Int. Congr. Chem. Cem., Moscow, Supp. paper, Section 11, pp. 53.
50.
-
J. SKALNY and I. ODLER (1973), "Pore structureby nitrogen and/or water vapour of calcium silicate hydrates formed under different
'conditions," XI SILICONF, Budapest, pp. 505-519.
51. - M. COLLEPARDI and B. MARCHESE (1972),
"Morphology and surface properties of hydrated tricalcium silicate pastes," Cem. Concr. Res., 2, 57-65.
I
52.-
I. ODLER and J. SKALNY (1971), "Pore structureof hydrated calcium silicates. I1 - Influence of calcium chloride on the pore structure of 6-dicalcium silicate." J. Colld. Interface Sci.,
53.- V.S. RAMACHANDRAN (1971), "Possible states of chloride in the hydration of tricalcium silicate in the presence of calcium chloride," Matgriaux et Constructions, 4, 3-12.
58.- P.J. SEREDA and V.S. RAMACHANDRAN (1975), "Predictability gap between science and technology of cements. 11. Physical and mechanical behaviour of hydrated cements," J. Amer. Ceram. Soc., 58, 249-253.
59.- H.G. KURCZYK and H.E. SCHWIETE (1960), "Electron microscopic and thermochemical investigations on the hydration of calcium silicates 3CaO.Si02 and B2Ca0.Si02 and the effects of calcium chloride and gypsum on the process of hydration," Tonind. Ztg., 84, 585-598.
60.- R.L. BERGER, J.F. YOUNG and F.V. LAWRENCE
(1972), Discussion of Ref. 51, Cem. Concr. Res., 2, 633-636.
61. - L. BENDOR and D. PEREZ (1976) , "Influence of
admixtures on strength'development of portland cement and on the microstructure of tricalcium
silicate," J. Mater. Sci. 11, 239-245.
62.- G. CHIOCCHIO and M. COLLEPARDI (1974), "Autoclave hydration of the constituents of portland cement in the presence of calcium chloride," I1 Cemento, 71, 57-66.
63.- J.F. YOUNG, R.L. BERGER and F.V. LAWRENCE
(1973), "Studies on the hydration of tricalcium silicate pastes. 111. Influence of admixtures on hydration and strength development," Cem. Concr. Res., 3, 689-700.
64.- R.L. BERGER and J.D. McGREGOR (1972), "Influence of admixtures on the morphology of calcium hydroxide formed duriilg tricalcium silicate hydration," Cem. Concr. Res.. 2. 43-55.
54.- M. COLLEPARDI, L. MASSIDA and G. USA1 (1971), 65.- S.M. KHALIL and M.A. WARD (1977),
"The kinetics and the mechanism of ageing of "Microst~cture of cement hydrates containing
tobermorite gel," I1 Cemento, 68, 3-8. Ca-lignosulfonate admixture," Mater. Struct.,
10, 67-72.
55.- I. ODLER and J. SKALNY (1971), "Influence of 66.- S.M. KHALIL and M.A. WARD (1977), "Effect of.
calcium chloride on paste hydration of degree of hydration upon creep of mortars
tricalcium silicate," J. Amer. Ceram. Soc., 54, containing calcium lignosulfonate," Mag. Concr.
362-363. Res., 29 (98), 19-25, 1977.
67. - S. DIAMOND and C. GOMEZ-TOLEDO (1978), "The
56.- R.F. FELDMAN and E.G. SWENSON (1975), "Volume microst~cture of low porosity portland cement
change on first drying of hydrated portland paste," I1 Cemento, 75, 189-194.
cement with and without admixtures," Cem. Concr.
Res., 5, 25-35. 68.- I. ODLER, R. SCHONFELD and H. DORR (1978), "On
the combined effect of water soluble
lignosulfonates and carbonates on the portland
57.- O.E. RADCZEWSKI, H.O. MULLER and W. EITEL (19391, cement and clinker pastes. 11. Mode of action
"On the hydration of tricalcium silicate," and structure of the hydration products," Cem.
T h i s publication is being d i s t r i b u t e d b y the D i v ~ a i o n of Building R e s e a r c h of t h e National R e s e a r c h Cou.is:il of Canada. I t should nor b e r e p r o d u c e d in whole o r ln p a r t without p e r m i s s i o n of the o r i g i n a l p u b l i s h e r . The Di- v i s i o n would b e glad t o b e of a s s i e t a n c e i n obtaining s u c h p e r m i s s i o n .
P u b l i c a t i o n s of the Division m a y b e obtained by m a i l - ing t h e a p p r o p r i a t e r e m i t t a n c e ( a Bank, E x p r e s s , o r P o s t Office Money O r d e r , o r a cheque, m a d e p a y a b l e t o t h e R e c e i v e r G e n e r a l of Canada, c r e d i t NRC) t o t h e National R e s e a r c h Council of Canada, Ottawa. K1A OR6
.
S t a m p s a r e n o t a c c e p t a b l e .A l i s t of a l l publications of the Division i s a v a i l a b l e and m a y b e obtained f r o m the P u b l i c a t i o n s Section, Division of Building R e s e a r c h , National R e s e a r c h Council of Canada, Ottawa. KIA OR 6.