READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Elucidation of the role of calcium lignosulfonate in the hydraton of C3A
= Eclaircissement du rôle du lignosulfonate de calcium dans l'hydration
du C3A
Ramachandran, V. S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=e74837c9-540d-484c-a8d5-ca549219fc87
https://publications-cnrc.canada.ca/fra/voir/objet/?id=e74837c9-540d-484c-a8d5-ca549219fc87
Ser
IT H ~
National Research
Conseil national
~ 2 1 d
I
*
Council Canada
de recherches Canada
ELUCIDATION O F THE ROLE O F CALCIUM
LIGNOSULFONATE IN THE HYDRATION O F C3A
by V. S. Ramachandran
ANALYZED
Reprinted f r o m
Vol. IV, Proceedings, 7th International
Congress on the Chemistry of Cement
P a r i s 1980
DBR P a p e r No. 1024
Division of Building R e s e a r c h
This publication i e being d i s t r i b u t e d by the Divieion of Building R e s e a r c h of the National R e s e a r c h C o u ~ c i l of Canada. I t should not b e reproduced i n whole o r in p a r t without p e r m i e e i o n of the o r i g i n a l publieher. The Di- vision would b e glad to b e of a s s i s t a n c e in obtaining such p e r m i s s i o n .
Publications of the Divieion m a y b e obtained by m a i l - ing the a p p r o p r i a t e r e m i t t a n c e ( a Bank, E x p r e s s , o r P o e t Office Money O r d e r , o r a cheque, m a d e payable
to the R e c e i v e r G e n e r a l of Canada, c r e d i t NRC) to the
National R e s e a r c h Council of Canada, Ottawa. K I A OR6.
Stamps a r e not acceptable.
A l i s t of a l l p u b l i c a t i o n s of the Division i s available and m a y be obtained f r o m the Publications Section, Division of Building R e s e a r c h , National R e s e a r c h Council of
S E M I N A I R E A
Rble du
C,A sous toutes ses formes dans
Elucidation of the role of calcium lignosulfonate
in the hydration of
C,A
Eclaircissement du rdle du lignosulnonate de calcium
dans l'h ydratation du
C,A
V.S. RAMACHANDRAN, Senior Research Officer of Building Research, National Research Council o f Canada, Ottawa. Ontario KIA OR6 Canada.
RESUME: L'auteur obtient des isothermes d'adsorption et de desorption en exposant de l'aluminate tricalcique (C3A), de l'aluminate calcique hexagonal ( C ~ A H I ~ - C ~ A H ~ ) et de l'hydrate d'aluminate cubique (C3AH6) 3 des solutions de lignosulfonate de calcium (CLS) dans de l'eau ou du dim6thylsulfoxyde. Dans le milieu non aqueux, le C3A et le C3AH6 absorbent peu ou pas de lignosulfonate; la hexagonale adsorbe 5 peu prss 2.2% de CLS. Dans une solution aqueuse contenant une grande quantitg de CLS, le C3A forme un complexe de CLS basique qui est insoluble dans l'eau. Les boucles de balayage dans les isothermes pour le systsme aluminate hexagonal-CLS-Hz0 montrent une complste irrgversibilitg avec une adsorption de 10% 3 une concentration d'gquilibre de 0.10% de CLS. Une adsorption rapide et irrgversible de 2.05% de CLS se produit dans le systgme C3AHb-CLS-H20. Les phases C3A, CqAH13-C2AHg et C3AH6 agissent cozxne des absorbants pour le CLS dans l'hydratation du C3S contenant du CLS; l'hydratation du C3S qui est complstement inhibge en prPsence de 0.8% de CLS peut stre retablie par l'addition de ces phases d'aluminate. L'efficacitg relative avec laquelle l'addition de 5% de ces additifs annule l'influence inhibitrice du CLS est de l'ordre de C3A>C4AH13-CZAH~>C~AH~.
Le Ca et le Na-lignosulfonate ne contenant aucun sucre agissent de faqon similaire au lignosulfonate commercial et retardent l'hydratation'du C3A.
SUb(M.ZKY: Adsorption-desorption isotherms were obtained by exposing tricalcium aluminate (C3A), hexagonal calcium aluminate (C4AH13-C?AH8) and cubic aluminate hydrate (C3AH6) to calcium lignosulfonate (CLS) ~ o l u t i 0 n ~ in Water or dimethyl sulfoxide. In the non-aqueous medium C3A and C3AH6 adsorb little, if any, lignosulfonate; the hexagonal phase adsorbs about 2.2% CLS. In an aqueous medium containing large amounts of CLS, C3A forms a basic CLS complex that is insoluble in water. Scanning loops in the isotherms for the hexagonal aluminate-CLS-Hz0 system show complete irreversibility with an adsorption of 10% at an equilibrium concentration of 0.10% CLS. A rapid irreversible adsorption of 2.05% CLS occurs in the C3AH6-CLS-H20 system. The C3A, C ~ A H ~ ~ - C ~ A H S and C3AH6 phases act as sinks for CLS in the hydration of C3S containing CLS; the hydration of C3S that is completely inhibited in the presence of 0.8% CLS can be restored by the addition of these aluminate phases. The relative effectiveness with which the addition of 5% of these additives counteracts the inhibitive influence of CLS is in the order of C3A>C4AHl;-C,AH8>C3AHh.
Both Ca and Na-lignosulfonates containing no sugar behave in a manner similar to the commercial lignosulfonate in retarding the hydration of C3A.
INTRODUCTION
Lignosulfonic acid and its salts are widely used as water-reducing and retarding admixtures in concrete. They are known to reduce water requirements by about 5 - 10: and setting times by 30 - 60%.
Since only small amounts of lignosulfonate are needed to influence the water-reducing and retarding proper- ties of concrete, it has been concluded that the
action of this admixture involves the phenomenon of ,
adsorption. Some attempts have been made to study the adsorption of admixtures on portland cement and the individual cement minerals, viz., C3S, C2S, C3A
and C@F. Since the C3A phase plays an i?portant
role in the early stages of hydration, considerable work has been directed to a study of the adsorption of lignosulfonate on this phase. In most studies, the amount of the admixture adsorbed by C3A under so- called equilibrium conditions has been determined by exposure to an aqueous solution. In thi-5 method the hydration of C3A could not have been avoided and, consequently, conclusions drarm from such experiments are questionable.
Another unresolved question concerns the role of lignosulfonate in the rerardation of cement hydration. Commercial lignosulfonates invariably contain sugars and it is generally thought that they are, in fact, responsible for the retarding action (1-3). There is strong evidence, however, that lignosulfonate is adsorbed by the hydrating minerals (4-12). This would indicate that the lignosulfonate molecule in a commercial sample should play an important role in the dispersion and retardation of hydration of C3A.
In view of these questions, it was thought that a more realistic approach to study the adsorption isotherms of lignosulfonates on the aluminate phases should involve measurements under conditions ,in which the adsorbent does not undergo hydration.
This condition could be achieved hy using a non- aqueous solvent, viz, dimethyl sulfoxide and
adsorbents C3A, C J A H ~ ~ . - C ~ A I ~ ~ and C3AH6 or using
aqueous solutions of lignosulfonate with
C4AH13-C2AHa and C3AH6. Thus, adsoi-ption-desorption scanning branches emanating at different points on the main adsorption curve would enable a study of the type of surface interaction occurring in the system. For a further evaluation of the role of ligno- sulfonate, purified sugar-free lignosulfonate salt of Ca or Ka was prepared from the corresponding salts of commercial products and its effect on the relative rates of hydration of C3A was followed by differen- tial thermal technique.
EXPERIMLNTAL
Sraterials - Tricalcium aluminate was obtained by calcining a mixture of CaC03 and ,11203; it had a Blaine surface area of 4350 m2/g. Tricalcium silicate was prepared by calcining a mixture of CaC03 and Si02; it had a Blaine surface area of 3310 m2/g. The hexagonal calcium alwninates comprising C4AH13 and C2AHg (referred to as the hexagonal phase) were prepared by the hydration of C3A at 2OC for 5 days. The cubic calcium aluminate hydrate C3AH6 (referred
to as the cubic phase) was pre ared by steam curing
4
C3A at a pressure of 0.69 blh'/m for 24 h. Calcium lignosulfonate sample in the form'of powder was supplied by Lignosol Chemical Itd., Quebec. The sample had 4.5% reducing bodies. Sugar-free Ca or Na lignosulfonates were obtained hy fractionation of commercial lignosulfonates by continuous diffusion using a Dowex 50XX2 resin.
blethods - Amounts of lignosulfonate adsorbed by the
aluminates were determined by estimation of ligno- sulfonate, before and after adsorption, by a Perkin Elmer double beam UV spectrophotometer at a
wavelength of 275
urn.
Details of the method aredescribed in another publication (13).
Differential thermal analysis (DTA) was carried out in N2 using a Du,Pont 900 Thermal Analyser. X-,ray diffractograms (XRD) were obtained by a Hilger and Watts unit using CuKa source. Electron microscopic examination was carried out by a Cambridge
Stereoscan Mark 2A instrument. RESULTS AXD DISCUSSION
Tricalcium Aluminate-Calcium Lignosulfonate-Water Systrn
Tricalcium aluininate liydrntcs to the metastable
hexagonal hydrate and final 1)- to the cubic form.
In the presence of calcium 1 ~gnosulfonate (C1.S)
conversion to the hexagonal . ~ n d cuhic hydrate
proceeds more slowly, depending on the dosage of
CI,S (Table I). In port1:ind ccment containing 1Ono
CjA, incorporation of 3" C1.S I S equivalent to 30";
with respect to the C3A phase and hence it iias of interest to study the effect of the addition of 15,
30, 50, 100 and 200% CLS on the hydration of C j . \ .
r - - _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ - - - _ - _ - - - _ - - - + - - - .
'1'AUl.li I - T~~fll~cncc of C:~lcium I,ignosulfon;~tc on thc
llydration of C3A
Ligno- Products Formed
sulfonate,
1 day 7 days 14 days 28 days
-
-
-
-
-0 cubic cubic cuhic cu!)ic
0.5 cubic cubic cubic cubic
1.0 hexagonal hexagonal cubic cubic
+ cubic
3.0 hexagonal hexagonal hexagonal hexagonal
+ cubic
A gelatinous mass formed at concentrations equivalent to and greater than 50% CLS. Electron mic~oscopic examination revealed the gel to be a fluffy mass typical of a non-crystalline material, which appeared
different from CLS or C3A (Figs. la, b, d) (13). In
samples treated with an aqueous solution containing 30% CLS with respect to C3A, the resultant material
exhibited a honeycomb structure (Figure lc). Using
surface area, chemical analysis, DTA and X-ray methods, it was concluded that the gel formed at higher concentrations of CLS was a basic CLS containing some Al+++. Very high adsorption values reported in the C3A-CLS-Hz0 system by Rehbinder and Segalova can be explained by the formation of such a
complex (14). In the presence of <30% CLS, a non-
crystalline complex results, and this is related to that formed between CLS and the hexagonal phase. Hexaeonal Aluminate Hydrate-Calcium
Lignosulfonate-Water System
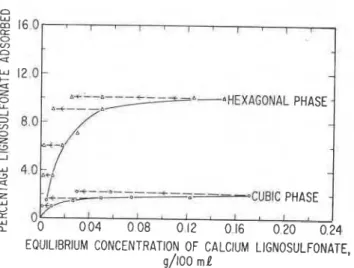
Adsorption-desorption isotherms o f CLS on the
hexagonal phase are shown in Figure 2 (15). After a
rapid adsorption, the curve tapers off between
equilibrium cor~centrations of 0.05 and 0.15% CLS.
The scanning isotherms obtained from vdrious points show complete irreversibility.
Fig. la
-
Tricalcium aluninater i g . lc - C3A treated with 302 calcium lignosulfonate
Figure 3 represents X-ray diffractograms of the hexagonal phase and CLS-treated samples on the desorption branch corres~~onding to a net adsorption value of 10% CLS (1.5). l'he hexagonal phase shows a peak at 7.908 f u r C q \ l i l i and t ~ ~ o characterist ic peaks for C2Ati8 at 10.5,; and 5.238. The sample containing loon C1.S exhibits a sharp peak of larger magnitude at 10.54 but one of reduced intensity at 7.90x. The surface area measurements indicated that whereas the hexagonal phase had a value of 11.1 m2/g, that which had adsorbed 10% CLS had an area of 15.3 m2/g. The hexagonal phase containing the irreversibly adsorbed CLS was found to be unmistakably different from the normal phase on examination by DTA, thermogravimetric analysis and electron microscopy. These results could be interpreted as follows. A complete irrever- sibility up to an adsorption value of 10% CLS was due to the formation of a complex between CLS and the hexagonal phase. The increase in the intensity of the 10.5A eak and a decrease in the intensity of that at 7.98) in XRD may be due to an increase in the C-axis spacing, as a consequence of CLS entering the interlayer position. Thus, it seems possible that retardation of conversion of the hexagonal to the cubic phase is due to CLS existing as a complex with the hexagonal phase. The interlayer complex may
Fig. Ih - Calcium lignosulfonate
Fig. Id - Gel-like material from C3A + 100% calcium lignosulfonate
a
z A+--&---+---C -"-AHEXAGONAL PHASE
0 Z I- Z W CUBIC PHASE U '
%
'0 0.04 0 0 8 012
0.16 0.20 0.24, -EQUILIBRIUM CONCENTRATION OF CALCIUM LIGNOSULFONATE, g/100 m e
Fig. 2
-
Adsorption-desorption isotherms of calcium lignosulfonate on the hexagonal and cubic phases o f calcium aluminate hydratesrestrict the free movement of the interlayer ions for conversion to the cubic form.
Tricalcium Aluminate Hexahydrate-Calcium Lignosulfonate-Water System
Adsorption-desorption scanning isotherms of this system are presented in Figure 2. After a rapid, initial adsorption the amount remains almost constant at 2.05%. Scanning isotherms from different points show irreversibility. Surface areas of C3AH6 before and after adsorption of CLS were 4.71 and 9.38 m2/g, respectively. It appears that in the presence of CLS, dispersion of C3AH6 and chemi-sorption of CLS are promoted.
Adsorption of Calcium Lignosulfonate on C3A and its Hydrates in a Son-aqueous Medium
Figure 4 glves the typical adsorption isotherms of CLS (using dimethyl sulfoxide (DMSO) as a non-aqueous
solvent) on the hexagonal phase (16). After a rapid
adsorption, the value tapers off to a value of 2.29. The irreversibility, even at low concentrations of
CLS, indicates that CLS is strongly complexed with
the hexagonal phase. The amount of adsorption of
CLS in an aqueous medium is much higher than in DMSO
because of the possibility of water with a higher dipole moment entering the interlayer positions and enabling more CLS to enter. In a non-aqueous medium, practically no adsorption of CLS occurs on C3A or C 3 M 6 phase. The relatively lower surface areas of C3A and C3AH6, the nature of the surfaces and absence of interlayer spaces are probably responsible for the lack of adsorption.
Tricalcium Silicate-Calcium Lignosulfonate-
Waper System in the Presence of Tricalcium 3.0
*
Aluminate and its Hydrates
-
sThe adsorption-desorption experiments have m 4
shown that the relative amounts of adsorp- 0 I
. tlon of CLS in the aluminate phases are in 2 4
the decreasing order C3A> C4AH13-C2AHg>
5
2.0C3AH6. Ceteris Paribus C3A hydrates faster 4 x
than C3S and, therefore, in cements the
-
Ihydrating aluminate may act as a sink for
-
ICLS. A few experiments were performed to Z +
investigate the effect of 5% of these 0
:
1.0aluminate phases on the hydration of C3S
-
containing 0.8% CLS. Addition of 0.8% CLS E 0 m
to C3S completely inhiblts its hydration,
-
4as evidenced by the absence of Ca(OH)2 w 2
formation (Figure 5, A-2) (17). Addition u
of 5% C3A is sufficient to promote hydra-
a
tion slowly at first and normally after o
3 days because C3A has the capacity to
react with large quantities of CLS
(Figure 5, B-2). The hexagonal phase,
adsorbing less CLS than the C3A phase, is relatively less efficient in counteracting the inhibitive action of CLS; the cubic
phase is practically ineffective (Figure 5,
C-1, C-2, D-1 and D-2). The greater inhibitive action of CLS (on the hydration of C3S) when added to cements after 5 min of hydration may be explained on
the basis of the hydrated products of C3A consuming
less CLS than C3A itself.
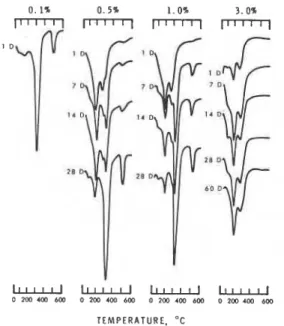
Hydration of Tricalcium Aluminate in the Presence of Sugar-free Calcium and Sodium Lignosulfonates
Figures 6, 7 and 8 compare the thermograms of CJA
hydrated in the presence of 0.1, 0.5, 1.0 or 3%
H E X A G O N A L P H A S E + l O l t l S H E X A G O N A L P H A S E 1 2 4 . 2 5 S C L S
M::
18 16 1 4 12 1 0 8 bFig. 3 - X-ray diffractogram of hexagonal phase and that treated with calcium lignosulfonate
0. 04 0. 08 0.12 0.16 0. 20
E Q U I L I B R I U M C O N C E N T R A T I O N OF CLS. l Fi'g. 4 - Adsorption-desorption isotherms of calcium lignosulfonate on the hexagonal phase
commercial Ca-lignosulfoaluminate (CLS), sugar-free
Ca-lignosulfonate (Sf-CLS] and sugar-free Na-
lignosulfonate (Sf-NLS), for periods ranging from 1 to
60 days (18). The thermal behaviour of C3A hydrated
in the absence of admixtures (not shown in the figure]
indicates, even at 15 min, a large endothermal effect
at 300'~ denoting the presence OF the cubic phase.
Slight retardation in the early stages of hydration
occurs in the presence of 0.1% admixtures but at 1 day
the cubic hydrate is the only dominant phase present.
H Y D R A T I O N . D A Y S
Fig. 5 - Kinetics of the hydration of C3S in the presence of calcium lignosulfonate, C3A or its hydrates.
A-l = C3S+H; A-2 = CsS+CLS+H; B-1 = C3S+C3A+H; B-2 = C3S+C3A+CLS+H; C-l = C3S+hexagonal phase+H; C-2 = CgS+hexagonal phase+CLS+H; D-1 = CSS+C~AH~+H; 0 - 2 = C3S+C3AH6+CLS+H.
u u u u
0 200 400 600 0 200 4 W 600 0 2 W 4 0 600 0 200 400 600 T E M P E R A T U R E . " CFig. 7 - DTA of C3A hydrated in the presence of sugar-free Ca lignosulfonate.
T E M P E R A T U R E . " C
Fig. 6 - DTA of C3A hydrated in the presence of commercial Ca lignosulfonate.
u u u u
0 200 400 600 0 200 400 600 0 200 400 &m 0 200 4Ca 6W T E M P E R A T U R E . " C
Fig. 8 - DTA of C3A hydrated in the presence of sugar-free Na lignosulfonate.
as evidenced by the presence of only the hexagonal phase at 1 day (Figure 8). In the presence of 1.0% lignosulfonate, the conversion of the hexagonal to the cubic phase is retarded in all samples. A much more efficient retardation occurs at an addition of 3% admixture. These results demonstrate that sugar- free lignosulfonate, which is presumably adsorbed irreversibly by C3A and its hydrates, is as efficient as the commercial lignosulfonate in retarding the hydration and interconversions in the C3A-Hz0 system. The XRD and TGA data on these samples were in fair agreement with those obtained by DTA.
CONCLUSIONS
In the CjA-calcium lignosulfonate (CLS)-H2O system, at low concentrations of CLS, a surface complex containing C3A, CLS and H20 may form, but at higher concentrations of CLS a highly basic CLS compound
containing some ~ 1 ~ ' results. In the hexagonal
aluminate-CLS-Hz0 system, scanning loops in the isotherms indicate complete irreversibility due to the formation of an interlayer complex that may be responsible for the retardation of the conversion of the hexagonal to the cubic phase. In the
C3AH(,-CLS-H20 system, a rapid irreversible adsorption is followed by dispersion.
In the non-aqueous medium, in contrast to the aqueous medium the C3A does not adsorb CLS. Thus the present tendency of explaining the retarding action on the basis of adsorption of CLS on C3A should be altered in favour of the concept "the consumption of CLS by the hydrating C3A."
The inhibitive action of CLS on the hydration of C3S
is modified by incorporation of C3A, the hexagonal
phase or the cubic phase. The phase consuming larger amounts of CLS lowers the inhibitive action of CLS on the hydration of C 3 S Thus, when prehydrated C3A is added to the C3S-CLS-Hz0 system there is less adsorption of CLS on aluminate phases, and hydration of C3S is inhibited or retarded more than by the addition of C3A.
Calcium and sodium lignosulfonates, deplete of sugars, are as effective a5 the commercial
lignosulfonates in retarding the hydration of C3A, indicating thereby that adsorption of lignosulfonate by the cement compounds is an important mechanism by which hydration is retarded.
The author thanks G.M. Polomark for the experimental assistance. This paper is a contribution from the Division of Building Research, National Research Council of Canada, and is published with the approval of the Director of the Division.
REFERENCES
1.- S. CHATTERJI (1967), "E1ectror.-optical and X-ray diffraction investigation of the effects of lignosulphonates on the hydration of C3AW, Ind. Concr. J., 41, 151-160.
2.- R.A. KESNERLEY, A.L. WILLIIL'lS and D.A. ST. JOHN
(1960), "Water reducing retarders for concrete", Dominion Laboratory Rept. 2026, Dept. Sci. Ind. Res., Lower Hutt, New Zealand, p. 66.
3.- N.B. MILESTOSE (1976), "The effect of
lignosulphonate fractions on the hydration of tricalcium aluminate", Cem. Concr. Res., 6, 89-102.
4.- F.M. ERNSBERGER and W.G. FRANCE (1945), "Portland cement dispersion by adsorption of calcium lignosulfonate", Ind. Eng. Chem., 37, 598-600.
5. - J.F. YOUNG (1969), "Influence of tricalcium
aluminate on the hydration of'calcium silicates",
J. Am. Ceram. Soc., 52, 44-46.
7.- D.R. ROSSINGTON and E.J. RUNK (1966).
"Adsorption of admixtures on portland cement
hydration products", J. Am. Ceram. Soc. 51,
46-50.
8. T. MANABE and N. KAWADA (1960), "Actions of
calcium lignosulfonate upon portland cement
clinker co~npounds'~, Rev. 14th Gen. Meeting,
Japan Cem. Eng. Assoc., Tokyo, p. 25-26.
9.- N. KAWADA and M. NISHIYAMA (1960), "Actions of
calcium lignosulfonate upon portland cement clinker compounds", Rev. 14th Gcn. Neeting, Japan Cem. Eng. Assoc., Tokyo, p. 25-26. 10.- W.C. HANSEN (1959), "Action of calcium sulfate
and admixtures in portland cement pastes", Symp.
Effect of Lister-Reducing 2dnixtures and
Set-Retarding Adrnlxtures o? I'ropereies 'of
Concrete, 4STN Spec. Tech. P u b l . So. 266, p.'3-37.
11. - W.C. Ili\SSEN (1970) , "Interdction of organic compounds in portland cement pnptes", J. >later. .5, 842-855.
12.- S. DIAMOND (1971), "Interactions between cement
minerals and hydroxycarl~oxylic acid retarders",
J. Am. Ceram. Soc. 54, 273-276.
13.- V.S. R A M A C H A N D W (1972), "Effect of calcium lignosulfonate on tricalcium aluminate and its hydration products", Mater. et Constr., 5, 67-76. 14.- P. REHBINDER and E. SEGALOVA (1957), "Structure
formation in the hardening binding materials", Proc. Int. Congr. Surface Activity, London, p. 492-505.
IS. - V.S. Rhf1ACHANDR.V (1973), "Differential thermal
investigation of the system tricalcium. silicate-calcium lignosulfonate-water in the presence of tricalcium aluminate and its
hydrates", Proc. 111 Int. Conf. Thermal Analysis, DavoS, Switzerland, Vol. 2, p. 255-267.
16.- V.S. RASWCHANDRAN (1971), "Adsorption of calcium
lignosulfonate on tricalcium aluminate a ~ d its
hydrates in a non-aqueous medium", Cem. Technol., 2, 121-129.
17.- V.S. RAMACHANDRAN (1972), "Elucidation of the role of chemical admixtures in hydrating cements by DTA technique", Thermochemica Acta, 3, 343-366.
18.- V.S. RAklACHANDRAN (19781, "Effect of sugar-free lignosulfonate on cement hydration", Zement Kalk Gips, 31, 206-210.
6.- B. BLANK, D.R. ROSSINGTON and L.A. HEINLAND
(1963)