HAL Id: hal-02960337
https://hal.archives-ouvertes.fr/hal-02960337
Submitted on 17 Dec 2020HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Pharmacologic Approach to Sinoatrial Node Dysfunction
Pietro Mesirca, Vadim Fedorov, Thomas Hund, Angelo Torrente, Isabelle
Bidaud, Peter Mohler, Matteo E. Mangoni
To cite this version:
Pietro Mesirca, Vadim Fedorov, Thomas Hund, Angelo Torrente, Isabelle Bidaud, et al.. Pharma-cologic Approach to Sinoatrial Node Dysfunction. Annual Review of Pharmacology and Toxicology, Annual Reviews, 2021, 61 (1), �10.1146/annurev-pharmtox-031120-115815�. �hal-02960337�
1
Pharmacologic Approach to SAN Dysfunction
Running title: SAN Dysfunction Pharmacology
Pietro Mesirca1,2 Vadim V. Fedorov3,4, Thomas J. Hund3,5, Angelo G. Torrente1,2, Isabelle
Bidaud1,2, Peter J. Mohler3,4, Matteo E. Mangoni1,2
1Institut de Génomique Fonctionnelle, Univ. Montpellier, CNRS, INSERM, Montpellier, France. 2LabEx Ion Channels Science and Therapeutics (ICST).
3Frick Center for Heart Failure and Arrhythmia at the Davis Heart and Lung Research Institute,
The Ohio State University, Columbus, OH, USA
4Departments of Medicine and Physiology and Cell Biology, The Ohio State University College
of Medicine, Wexner Medical Center
5Department of Biomedical Engineering, The Ohio State University, Columbus, OH, USA
ORCiD: Mesirca: https://orcid.org/0000-0002-9538-3096 Fedorov: https://orcid.org/0000-0003-1035-4569 Hund: https://orcid.org/0000-0002-9957-7306 Torrente: https://orcid.org/0000-0003-1721-6564 Bidaud: https://orcid.org/0000-0002-1812-1447 Mohler: https://orcid.org/0000-0002-7977-8717 Mangoni: https://orcid.org/0000-0002-8892-3373 Correspondence: Matteo E. Mangoni, PhD
Institut de Génomique Fonctionnelle CNRS UMR 5203, Inserm U 1191 Université de Montpellier 141, rue de la Cardonille 34094 Montpellier, France Phone +33 (0)4 34 35 92 46 Fax +33 (0)4 67 54 24 32 Email: matteo.mangoni@igf.cnrs.fr
2
Abstract
The spontaneous activity of the sinus node initiates the heartbeat. Sinus node dysfunction (SND), also referred to as ‘sick-sinus-syndrome’, is caused by failure to generate a normal sinus node action potential. In the clinical practice, SND is generally considered as an age-related pathology, secondary to degenerative fibrosis of the heart pacemaker tissue. However, other forms of SND exist, including idiopathic primary SND showing genetic legacy and forms that are secondary to cardiovascular or systemic disease. The incidence of SND in the general population is expected to increase over the next sixty years, boosting the needs for implantation of electronic pacemakers. During the last two decades, our knowledge of sinus node physiology and of the pathophysiological mechanisms underlying SND has advanced considerably. This review will summarize the current knowledge about SND mechanisms and discuss the possibility of introducing new pharmacologic therapies for handling SND.
Keywords: Sinus Node Dysfunction; SAN; G protein-activated K+ channels; Cav1.3; Ankyrin-B;
Tertiapine-Q;
3
Introduction
The cardiac impulse is generated in the sinus node (SAN) by a highly-integrated mechanism involving ion channels, intracellular Ca2+ dynamics, membrane receptors and connexins (1; 2).
Despite the intrinsic robustness of the pacemaker mechanism, SAN dysfunction (SND) constitutes a relatively common clinical condition, especially among the population over 65 (1/600, (3)). SND generally manifests as an insufficiency of the heart rate to meet the needs of the organism. SND may be differentiated into reversible (acute), or chronic symptomatic forms (4).
Acute SND is generally handled pharmacologically or by temporary transvenous or transaesophageal pacing (4; 5). In contrast, only a few options are currently available to treat chronic symptomatic SND, and to date permanent electronic pacing (PPM) by an implanted electronic pacemaker remains the primary and definitive therapy (4; 5). In this regard, symptomatic SND and heart block account for about half of the total pacemaker implantations in the U.S (6) and this is predicted to double over the next half century (7). In addition, clinical studies indicate an increasing necessity for implantation of complex electronic pacemakers (8).
In this review, we will discuss some of the most documented forms of primary and secondary SND, especially in relation to current pharmacologic management. Research over the last 20 years has considerably advanced our knowledge of SND aetiology. Several genes coding for ion channels (9-21), scaffolding proteins (22), cytoskeleton proteins (23), as well as connexins and proteins involved in cardiac development have been linked to previously unexplained forms of primary familial SND (24). Furthermore, development of animal models of primary and secondary SND, as well as ex vivo exploration of SANs from human hearts with history of SND have shed new light
in the mechanisms of secondary forms of SND. These approaches have identified a host of novel targets for managing SND, including cardiac G protein-activated IKACh/IAdo (GIRK1/GIRK4)
(25-29) and Ca2+-activated small conductance K+ (SK) channels (30-32). The identification of GIRK
channels as potential molecular targets for SND may extend the indication of pharmacologic approaches to chronic forms of SND. More generally, it is possible that innovative molecules targeting specific SND mechanism will help manage chronic SND, which is now handled only by PPM.
Clinical Definition of SAN Dysfunction
The diagnosis of SND is based on the correlation between the patient’s symptoms and ECG hallmarks (see also Supplementary Appendix 1), which provide important criteria for PPM (4; 5; 33). Historically, SND patients have been identified as having one or more of the following ECG findings (34): (i) persistent, unexpected sinus bradycardia (Figure 1), (ii) short periods of
sinus arrest during which atrial or junctional rhythms replace normal sinus rhythm, (iii) long periods of sinus arrest in the absence of junctional rhythms, resulting in cardiac standstill, and finally (iv) episodes of sinus exit block not related to drug therapy (34; 35). This early definition of SND remains in use in current clinical guidelines (4; 5; 36). Sinus bradycardia is generally defined as a heart rate below 50 bpm (4). Sinus pauses or sinus arrest are included in the current definition of SND, particularly when manifest as ‘tachycardia-bradycardia syndromes’, in which sinus bradycardia, pauses or arrest follow periods of abnormal atrial tachycardia, atrial fibrillation or flutter (37). In tachycardia-bradycardia syndromes, SND can manifest as poor or sluggish return of sinus rhythm following cardioversion (38) (Supplementary Figure 1). Another hallmark of SND
is chronotropic incompetence, defined as the inability of heart rate to attain 80% of the expected heart rate during exercise (39). Symptomatic SND carries invalidating symptoms that can impact quality of life (36). One of the most common symptoms of SND is syncope, which is present in
4
about half of SND patients (5; 40). While asymptomatic bradycardia is not associated with adverse outcomes, patients with untreated symptomatic SND have high risk of deterioration to cardiovascular events including atrial fibrillation (41), heart failure (42) and systemic thromboembolism (5; 40). Age-dependent SND and chronotropic incompetence are associated with an increased risk of cardiovascular death and overall mortality (4).
SAN Pacemaking: general overview
The SAN is a highly-complex, heterogeneous tissue (1). Surprisingly, pacemaker cells do not constitute the predominant cell type in the SAN. Early studies of SAN tissue indicated that atrial myocytes (43) and fibroblasts (44) are important constituents of SAN structure and integrative properties. More recently, RNA sequencing indicates that the SAN is composed of atrial myocytes, adipocytes, epithelial cells, fibroblasts, vascular endothelial cells, macrophages and neurons (45). In addition to heterogeneous cellular composition, pacemaker cells within the SAN are poorly-coupled electrically (46). This low inter-cellular conductance is due to high expression of Cx45 and low or absent expression of Cx43 in pacemaker cells (46-48). Finally, the SAN region includes specific non-conductive structures and redundant impulse propagation pathways (29; 49) that help ensure proper intra-sinus and sinus-to-atria conduction (50; 51).
Normal pacemaking depends on a unique action potential (AP) profile of SAN pacemaker cells. Importantly, the SAN AP undergoes a spontaneous diastolic depolarization phase driving the membrane voltage from the end of the repolarization to the threshold of the following action potential (Figure 2). Catecholamines positively regulate the slope of the diastolic depolarization
via activation of ß-adrenergic receptors (ßARs). The adrenergic activation stimulates the synthesis of cAMP, that positively regulates the activity of several ion channels of the plasma membrane, and the intracellular ryanodine receptors/Ca2+-release channels (RyR2) embedded in the
sarcoplasmic reticulum (SR) (2; 52) (Figure 2). In antagonism with ßARs, muscarinic type 2
receptors (M2Rs) and adenosine type 1 (A1Rs) receptors decrease pacemaking by promoting down-regulation of intracellular cAMP and by inducing opening of G protein activated K+ channels
(GIRK1/GIRK4) underlying the IKACh / IKAdo current (53). Current understanding of the generation
of the heartbeat is best summarized by the “coupled-clock” model of pacemaking (52), stating that generation of diastolic depolarization is the result of a functional interplay between activity of ion channels at the plasma membrane and local diastolic RyR2–dependent Ca2+ release, which is
coupled to the diastolic depolarization via activation of the Na+/Ca2+ exchanger (NCX1) (2; 52;
54). Different ion channels of the plasma membrane contribute to the generation and the regulation of the diastolic depolarization and will be discussed here briefly.
The hyperpolarization-activated “funny” current If, is activated at the end of the
repolarization phase of the action potential and supplies inward current throughout the range of the diastolic depolarization (55). Catecholamines shift If activation curve to more positive voltages,
while acetylcholine induces a negative shift. These opposing effects are explained by the direct sensitivity of f-channels to cAMP (56), which increases the probability of channel opening at a given voltage (57). F- channels are encoded by the hyperpolarization activated cyclic nucleotide gated channel (HCN) family, which comprises four distinct isoforms HCN1-HCN4. However, in the SAN, the predominant isoform is HCN4, accounting for 80% of the total HCN mRNAs (58). Moreover, HCN1 protein is almost exclusively expressed in the human SAN rather than in atrial myocardium (59). Both T- and L-type Ca2+ channels are expressed in SAN pacemaker cells. Cav3.1
and Cav3.2 mRNAs are expressed in the SAN however, the predominant functional T-type isoform in the adult SAN is Cav3.1 (60). In spite of this low availability, Cav3.1 knockout mice (Cav3.1-/-) present with moderate SAN rate reduction (-10%) and prolonged atrioventricular (PR)
5
channel isoforms, Cav1.3 and Cav1.2 (61). Cav1.3-mediated ICaL is characterized by a more negative
threshold for activation than Cav1.2-mediated ICaL (-45 mV for Cav1.3- vs -25 mV for
Cav1.2-mediated ICaL, respectively) (61). Under basal conditions, Cav1.3-/- pacemaker cells show erratic
generation of automaticity, a lack of the linear phase of the diastolic depolarization (62) and a strong reduction of the total inward diastolic current compared to control littermates (26; 62).
Voltage-gated “neuronal” (n)Nav Na+ channels underling the sino-atrial TTX-sensitive Na+
current (INaTTX) have been shown to contribute to pacemaking and intra-nodal conduction in the
mouse, rabbit and human SAN (63-66). Several nNav1 isoforms have been proposed to contribute to sino-atrial INaTTX.. While nNav1.1 and nNav1.3 have been proposed to contribute to INaTTX in
mouse and rabbit (63), nNav1.6 appears to play a dominant role in human intra-nodal conduction and SND (66). The “cardiac” (c)Nav1.5 isoform underlies the TTX-resistant INa. in the mouse SAN,
but its contribution to excitability may be limited to intra-nodal or nodal-atrial impulse conduction. Consistent with this hypothesis, mice haplo-insufficient for Nav1.5 present with atrio-ventricular and intra-ventricular conduction defects, rather than with SND.
Ion channels of the transient receptor potential channels family (TRP) contribute to SAN activity (67-69). TRPC channels contribute to store-operated Ca2+ entry in pacemaker cells (69).
TRPM7 channels contribute to pacemaking by regulating HCN4 channels in the sinus and atrioventricular node (70). TRPM4 channels contribute to the basal beating rate of SAN pacemaker cells (67).
More recently, members of Ca2+ activated K+ channel family (K
Ca) have been linked to
pacemaking in mouse and rabbit SAN. KCa have been subdivided into Big (71), Intermediate (31)
and Small (32; 72) conductance K+ channels. These channels have differential sensitivity to Ca2+.
Big KCa (BK) are primarily voltage-dependent with positive regulation by Ca2+ (73). In contrast,
Intermediate and Small KCa (IK and SK, respectively) are voltage-independent and highly sensitive to Ca2+(31). Such sensitivity to Ca2+ is mediated by the association with calmodulin (CaM) (73) and
at least for SK channels coupling with L-type Ca2+ channels has been demonstrated (74; 75). SK
isoforms have been identified with different sensitivity to the specific inhibitor apamin (SK1, SK2 and SK3) (73). All three SK isoforms are expressed in the SAN (32).
Familial Primary SAN Disease and Associated Conduction Defects
While SAN disease and SND are often associated with aging or with different cardiovascular pathologies such as heart failure (76; 77), work over the past two decades has identified familial forms of SND (24; 78). In fact, congenital forms of SAN disease have been instrumental in providing molecular insights into the critical and non-redundant molecular pathways underlying SAN automaticity and signaling. Multiple ion channels and ion channel subunits are now associated with SAN disease.
HCN4 gene (HCN4) variants are linked with human asymptomatic bradycardia or SAN
disease (9-12). Mechanistically, variants have been associated with altered channel membrane targeting, aberrant ion channel activation (10) or conductance (12), as well as dysfunction in channel regulation by cyclic nucleotides (9; 13). Of note, human HCN4 variants are linked with
other forms of cardiovascular disease beyond the SAN demonstrating the key role of this ion channel (11). Beyond HCN4, human variants in SCN5A are linked with SND (79).
Loss-of-function of the SCN5A gene encoding for Nav1.5 accounts for 5% of total incidence of primary
conduction system dysfunction including SND in humans (21; 80). SCN5A variants are linked with
familial SND, bradycardia, conduction disorders, as well as atrioventricular block (24; 78). However, similar to variants in other key cardiac ion channels, SCN5A variants are linked with
other forms of atrial and ventricular arrhythmia.
While not as robustly expressed in the working myocardium as Cav1.2, Cav1.3 channels (CACNA1D) are highly expressed in the SAN (61; 81-83) (see also preceding section). While rare,
6
human CACNA1D variants that alter channel activity are linked with human SND (14; 15). Cav1.3
channels have been linked also to neonatal complete heart block related to lupus (84). Mechanistically, heart block has been explained by the presence of maternal auto-antibodies against Cav1.3 channels (85; 86). In addition to Cav1.3, T-type Cav3.1 channels (encoded by CACNA1G)
have also been linked with human bradycardia and heart block related to neonatal lupus (84; 87). TRPM4 (encoded by TRPM4), also called transient receptor potential cation channel
subfamily M member 4, is a nonselective calcium-regulated channel expressed in the SAN and atria.
TRPM4 variants have been widely linked with a number of human cardiac phenotypes including
sinus bradycardia likely through modulation of the SAN cell membrane potential (88; 89).
Alterations in a number of SAN and atrial accessory and calcium regulatory proteins have been associated with human SND. Ankyrin-B, encoded by ANK2, is a cytoskeletal adaptor protein
that associates with a host of cardiac ion channels, transporters, signaling molecules, and structural proteins. Consistent with animal models lacking ankyrin-B, humans harboring specific variants in
ANK2 may display bradycardia and conduction defects (22). Mechanistically, ankyrin-B
dysfunction alters multiple critical SAN proteins, including Cav1.3 and NCX1, resulting in aberrant diastolic depolarization. It is likely that specific genetic and environmental factors influence SND penetrance and severity in the human population based on the degree of ankyrin-B loss-of-function and potential secondary variants or environmental factors. Similar to ankyrin-B, cardiac caveolin-3 (CAV3) variants, while linked with ventricular arrhythmia, have also shown to be associated with
bradycardia (90). Mechanistically, caveolin-3 variants may impact multiple ionic currents to influence SAN node automaticity.
More recently, an exciting set of papers have linked human variants in proteins that regulate heterotrimeric G proteins to SND. To date, both GNB5 and GBN2 variants are linked with sinus
bradycardia/SND (17; 18). Similar to other genes outlined in this review, individuals harboring variants may display additional non-cardiac phenotypes including cognitive disorders. Mechanistically, these variants may impact the activity of IKACh, inducing current gain-of-function
and consequent sinus bradycardia and atrioventricular block. Consistent with this hypothesis, mutation in KCNJ3 and KCNJ5 inducing gain-of-function of GIRK1 and GIRK4 were shown to
be linked with familial SAN disease (KCNJ3 encodes GIRK1; KCNJ5 encodes GIRK4) (16; 91).
Dysfunction in cardiac calcium regulatory proteins are linked with familial SND. Calsequestrin 2 (CASQ2) is a calcium-binding protein that also serves a critical role in regulating
local calcium release and automaticity. The cardiac ryanodine receptor (RYR2) is a central
regulatory node responsible for excitation-contraction coupling. Variants in either RYR2 or CASQ2 are associated with bradycardia and potentially fatal arrhythmias in response to
catecholamines (92-94). In particular, catecholaminergic polymorphic ventricular tachycardia (CPVT) is a life threatening familial ventricular arrhythmia associated with mutations in CASQ2 or RYR2 (95). RYR2-associated CPVT is characterized by increased Ca2+ release from the SR at rest
and under adrenergic activation (95). However, some CPVT patients carrying mutations in RYR2
present also with moderate sinus bradycardia (96; 97). Bradycardia has been explained by tonic Ca2+-dependent inactivation of I
CaL and reduction of basal SR Ca2+ load in SAN pacemaker cells
(96; 97).
Additional forms of inherited SND involve a host of cardiac proteins with a range of functions in the myocyte. For example, human variants in MYH6 that encodes cardiac muscle
myosin are linked with SND (as well as other non-SAN electrical and structural phenotypes) (98). Like myosin, lamins A and C have multiple roles for cardiac function. Encoded by LMNA, lamin
A variants are associated with sinus bradycardia and conduction system disorders (99). Finally, variants in a number of other genes including SHOX2 (100), a transcription factor involved in
differentiation of the SAN, have been linked with cardiac SAN and/or atrioventricular block phenotypes.
It is important to note that like other forms of familial human disease, penetrance and disease severity will depend on both secondary genetic, environment, and social factors. Thus,
7
caution should always be utilized when interpreting genetic information from individual variant carriers.
Genetic mouse models of primary SND
A wide variety of genetically modified mouse models have been generated to study SAN disease. Among the first mouse models of SND were those involving knockout of ion channels important for SAN membrane excitability. Early studies found that Cav1.3-/- mice showed
congenital deafness, bradycardia and irregular heart rate (101). Further work has shown that Cav1.3 -/- mice constitute a model of the SAN Dysfunction and Deafness (SANDD) syndrome, which is
characterized by sinus bradycardia and atrioventricular conduction dysfunction (14). Indeed,
Cav1.3-/- mice have pronounced sinus bradycardia, associated with sinus pauses, atrial fibrillation
and flutter, and 2nd –degree atrioventricular block (26; 61; 102). Global knockout of Hcn4 prevents
proper development of the SAN and conduction system resulting in embryonic lethality (103). Inducible Hcn4 knockout mice have also been generated and found to have either mild SND with
sinus pauses (104) or severe bradycardia and conduction system defects incompatible with life (105). Knockout of Hcn1 induces bradycardia and SND in the mouse (106). Mice carrying
genetically silenced If conductance show sinus bradycardia and SND associated with 2nd-degree
atrioventricular block and ventricular arrhythmia (25). On the other hand, mice lacking cAMP-dependent regulation of HCN4 show moderate sinus bradycardia, consistently to what observed in individuals carrying cAMP regulation defective HCN4 (9; 13).
Consistent with the clinical findings, mice heterozygous for Nav1.5 display bradycardia, sinus exit block, and reduced excitability of pacemaker cells (107). Proteins important for intracellular ion homeostasis have also been explored using genetic mouse models. Atrial-specific knockout of the NCX1 (sarcolipin-Cre crossbred with NCX1 floxed mouse) display sinus arrest, burst pacemaking and junctional escape rhythm (108). In a similar vein, knockout of the SR Ca2+
buffer calsequestrin disrupted SAN Ca2+ handling leading to irregular pacemaking and SND,
consistent with observations in patients (109).
There is a growing number of mouse models with SND and defects in unconventional ion channels and accessory proteins not typically invoked when discussing SAN pacemaking. For example, the background K+ channel TREK-1 has recently emerged as a novel determinant of
SAN excitability and pacemaking. Specifically, mice with cardiac specific ablation of TREK-1 show bradycardia with frequent sinus pause (110). At the cell level, TREK-1-deficient SAN pacemaker cells show a depolarized maximum diastolic potential and altered firing rate. Similarly, several TREK-1 interacting partners have been linked to SND in mice, including the cytoskeletal protein βIV-spectrin (111) and members of the Popeye-domain containing (POPDC) family (112). Mouse models of TRPM4 related SND have been generated. Although the TRPM4 knockout (TRPM4-/-)
mouse shows no difference in heart rhythm compared to wild-type counterparts, pharmacological inhibition of TRPM4 slows spontaneous beating in wild-type but not TRPM4-/- atria (113).
Furthermore, TRPM4 mice show more frequent episodes of sinus pauses and prominent conduction block compared to wild-type counterparts (114). TRPM7 is a divalent channel-kinase abundantly expressed in mouse and human heart. Global as well as sinus/atrioventricular node-restricted knockout of TRPM7 slows the diastolic depolarization in pacemaker cells and induces bouts of sinus pause and conduction block (70). As discussed above, Ankyrin-B is an adapter protein important for proper membrane localization of multiple ion channels and transporters important for normal SAN excitability and pacemaking. Human variants in ANK2 have been linked to a complex arrhythmia syndrome including severe SND requiring pacemaker implantation (22). Consistent with the human phenotype, mice heterozygous for ankyrin-B display severe bradycardia and enhanced resting heart rate variability compared to wild-type littermates (22).
8
Mouse models have been instrumental in studying the link between dysregulation of gene expression and SND. Notably, mice deficient for the transcription factor Tbx3 show defects in development of the SAN and of the conduction system. They show also SND characterized by bradycardia and sinus pause presenting in the embryonic stage (115). The cardiac homeobox transcription factor Nkx2-5 has been studied using an atrial-specific knockout model, which shows hyperplasia of the working myocardium and conduction system, resulting in a broad spectrum of arrhythmias including bradycardia and conduction block and perinatal lethality (116). Mechanistically, Nkx2-5 deficiency was shown to activate Notch signaling and enhance myocyte proliferation early in development. Interestingly, Notch signaling is also activated with injury and a genetic mouse model has been used to study effects of transient Notch activation in the atria with observations of reduced SCN5A expression and structural remodeling of the SAN, resulting in
sinus bradycardia and sinus pause (117). Genetic mouse models have also elucidated a role for signaling through the multifunctional Ca2+/calmodulin-dependent kinase II in SND in the setting
of neurohumoral dysregulation. Specifically, transgenic mice overexpressing a CaMKII inhibitory peptide (AC3-I) were found to be resistant to development of fibrosis and sinus pause following chronic angiotensin II infusion (118). As new experimental models have been developed and applied to study SAN function and disease, so too have a host of computational models of pacemaker activity (See Supplementary Appendix 2). These models will prove important for understanding SND as the discovery of new mechanisms progresses.
Secondary forms of SND in humans and animal models
Secondary forms of SND account for the majority of patients. Indeed, the incidence of SND correlates with age, co-morbidities such as hypertension, diabetes, presence of cardiovascular disease and elevated plasma concentrations of cystatin-c and natriuretic peptide (7). However, current guidelines generally make distinction between secondary forms of SND associated with systemic disease, cardiovascular disease and drug intoxication (4). In relation to systemic conditions, SND can be a secondary manifestation of endocrine disease such as hypothyroidism and diabetes, inflammatory or rheumatologic disorders, plasma ionic imbalance and infectious disorders (4; 76).
Abnormal input of the autonomic nervous system is a potential cause underlying SND. In some patients, intrinsic SND can be worsened by autonomic nervous system imbalance (119). In addition, increased vagal input (hypervagotonia) has been proposed to constitute the direct cause of SND manifestations (119). Clinical studies have established a correlation between endurance athletic training and an increased risk of bradyarrhythmia, atrioventricular block (120) and atrial fibrillation (121), requiring PPM (122). Both hypervagotonia and intrinsic remodelling of SAN ion channels expression have been proposed as mechanisms of SND in endurance athletes. If current
and HCN4 are downregulated in animal models of athletic training (123; 124). In addition, the heart rate of endurance athletes shows reduced sensitivity to the If blocker ivabradine, suggesting
reduced HCN expression (123). It is thus possible that both changes in the sympathovagal balance and intrinsic remodeling of ion channels in SAN pacemaker cells contribute to bradyarrhythmia induced by athletic training.
Often SND secondary to cardiovascular disease is associated with atrial tachyarrhythmia and atrial fibrillation. In early studies, SND was present in 5% of patients affected by atrial fibrillation for less than one year. However, SND incidence increased up to 45% in patients suffering from atrial fibrillation for 10 years or more (38). Interestingly, in a canine model of atrial fibrillation induced by atrial tachypacing, SND was explained by a reduction in the expression of If
-channels (125). This mechanism can account for SND associated with chronic or persistent atrial fibrillation. However, since a significant number of patients present with progressively worsening
9
SND, in which association with atrial fibrillation and conduction dysfunction constitute late co-morbidities, it is an attractive hypothesis that early pharmacologic handling of SND may prevent associated arrhythmias. Ischemic disease can also lead to SND. Typical examples of ischemic conditions favoring SND are acute stenosis or thrombosis of the SAN artery (126) and myocardial infarction (127). As discussed above, heart failure is a major provider of secondary SND. Several mechanistic aspects of how myocardial heart failure leads to SND are still to be identified. However, in rabbit (128), canine (129) and mouse (130) models of heart failure, reduction of pacemaker activity has been attributed to down-regulation of If current and its predominant SAN
isoform HCN4, with consequent SND.
Myocardial infarction can often degenerate into heart failure and consequently, bring secondary SND forms. A study combining myocardial infarction with diabetic condition showed an increase in oxidized CaMKII, apoptosis of pacemaker cells, SND and mortality in wild-type mice. These effects were prevented in knockin mice expressing oxidation-resistant CaMKII, in which paired methionines in the CaMKII regulatory domain mutated to valines (131). Oxydation of CaMKII could thus constitute an important mechanism in SND secondary to myocardial infarction and neurohumoral dysregulation potentially leading to heart failure (118).
To date, several mechanisms have been proposed to account for age-related SND. The reader can find a detailed discussion about age related SND in a recent review by Peters et al. (132). Interestingly, similar to other secondary forms of SND, decline of pacemaker activity with ageing is attributed to intrinsic remodeling of the SAN structure and expression of ion channels involved in automaticity. Decrease in the intrinsic electrical coupling due to progressive tissue fibrosis has been proposed as a primary factor in age-related SND (see Csepe et al. for review (133)). However, some individuals having high degree of SAN fibrosis can be under normal sinus rhythm (134). In addition, recent work demonstrated slowed intrinsic pacemaker activity and reduced densities in If, ICaL and ICaT in SAN pacemaker cells of bradycardic aged mice (135). It is thus possible that both
structural and ionic factors contribute to age-related SND in a patient’s dependent way.
Pharmacological approaches to SND in the current clinical practice
There is a wide panel of potentially usable drugs for acute bradycardia and SND however, the most widely used are catecholaminergic agonists like isoproterenol, atropine, aminophylline and theophylline. Isoproterenol is a β-selective agonist devoid of vasoconstrictional effects. Isoproterenol has shown some positive effects in ameliorating heart rate in patients with bradycardia. However, because isoproterenol administration can induce also supraventricular tachycardia (136), its use is recommended only for intra-hospital management of acute SND or electrophysiological evaluation of SND (4). Since β-adrenergic activation augments the myocardial oxygen demand accompanied by coronary vasoconstriction, its use is not indicated in SND associated with cardiac ischemic disease (4). Other catecholaminergic agonists such as epinephrine or dopamine present more complex effects because of their mixed α− and β− receptor activation (137). Use of catecholamines is thus indicated only under intra-hospital and haemodynamic monitoring conditions (4). Atropine is a well-known inhibitor of muscarinic receptors. Clinical studies have showed heart rate improvement in patients with acute bradycardia and SND, including secondary to myocardial infarction (138; 139). Atropine is also used for diagnostic evaluation of SND (140; 141). However, some adverse effects have been reported with atropine, including tachycardia and psychotic states (138; 141). Theophylline and aminophylline belong to the pharmaceutical class of methylxanthines. Theophylline in particular, is probably the most widely used drug to handle SND under out of hospital settings. The beneficial effect of methylxanthines
10
in improving heart rate is attributed to their blocking action on adenosine receptors (142), which could make these drugs suitable in handling bradycardia in post-transplantation hearts (143; 144) and following spinal cord injury (145). In a limited clinical study, theophylline has also showed improvement of heart rate, without significant adverse effects, which prevented PPM (146).
Drug intoxication constitutes an additional challenge for pharmacologic handling of bradycardia attributable to SND (4). Frontline treatment of SND following intoxication with β-blockers, Ca2+ channels blockers and digitalis (digoxin) exist. Intoxication with β-blockers can be
handled by administration of glucagon, which stimulates hepatic adenylate cyclase leading to increased glycolysis. No systematic clinical studies including cohorts of patients is available to validate the use of glucagon for SND secondary to intoxication with β-blockers (147). However, available data support the concept of antagonizing the effect of β-blockers and Ca2+ channels
blockers using glucagon or insulin to improve heart rate in patients’ SND symptoms (148). Finally, intravenous bolus of calcium-gluconate can be used to oppose to the effects of Ca2+ channels
blockers such as verapamil or amlodipine (4; 148). Digoxin intoxication induces complex arrhythmia pattern and bradyarhythmia (149). Digoxin is a poorly dialyzable drug consequently, treatment of SND secondary to glycoside intoxication is applied using specific anti-digoxin antibodies (150). Immunotherapy against digoxin intoxication is effective in improving heart rate, with relative low associated mortality (151).
New pharmacologic targets to SND in animal models
Recently, it has also been possible to test new potential pharmacologic approaches to SND by targeting GIRK channels. Our group has tested this hypothesis by crossing Girk4-/- mice with
mice in which If conductance has been genetically silenced (25). Genetic ablation of IKACh effectively
rescued SND and atrioventricular dysfunction in these double-mutant mice and prevented also SND-associated ventricular arrhythmia (25). Interestingly, isoproterenol was effective in elevating the heart rate of these mice, but failed to correct SND hallmarks such as sinus pauses and atrioventricular dysfunction (25), suggesting that direct inhibition of IKACh could be a more effective
strategy than catecholaminergic stimulation. Furthermore, genetic ablation of IKACh was effective in
rescuing SND and atrioventricular dysfunction also in SANDD Cav1.3-/- mice (26). Rescuing of
SND is observed also by direct inhibition of IKACh by tertiapine-Q (Figure 3). This phenomenon
of “compensatory” genetic or pharmacologic targeting of IKACh can provide new therapeutic option
for SND in the future (26; 152) (see also Supplementary Appendix 3).
While no primary forms of SND linked to KCa channels have been described to date, evidence
indicating that the activity of these channels can contribute to arrhythmia, atrioventricular dysfunction and SND exists. Global deletion of SK2 channels in mice is pro-arrhythmic and induces atrioventricular block (74; 153), thus suggesting that the alteration of SK current could lead to SND. Consistent with this hypothesis, it was shown that pharmacological inhibition of SK current recovers tachycardia-bradycardia syndrome in a model of Ca2+ overload caused by NCX1
deletion (32). Similarly, selective inhibition of the IK isoform SK4 was sufficient to reduce delayed afterdepolarizations and arrhythmic Ca2+ transients in a CPVT model (31). Mechanistically,
hyperactivation of SK or IK channels in these models could lead to the hyperpolarization of SAN cells, as it has been shown in a model of neurons (154). Consistent with this hypothesis, selective inhibition of SK4 channels rescued SAN arrhythmia and SND, suggesting that the family of KCa channels could be an additional pharmacologic target to treat SND.
11
Human SAN as model of SND
The human SAN complex is a specialized and heterogeneous intramural 3D structure with multiple intranodal pacemakers and several specialized sinoatrial conduction pathways (SACPs), which are responsible for the transmission of electrical impulses to the right atrium (155; 156). The human SAN pacemaker-conduction complex relies heavily on a sophisticated machinery of multiple molecular pathways that communicate within the 3D structure in order to efficiently maintain physiologically relevant heart rate (Figure 4)(49; 66). However, there is a paucity of
studies addressing mechanisms that contribute to automaticity and intranodal conduction directly in the human SAN complex at the molecular, cellular, and tissue levels (29).
Disease-induced remodeling of many of the molecular components critical to SAN function can lead to SND in humans (27; 156; 157). However, the majority of these molecular components critical to SAN function have been studied only in animal models, which may have significantly different functional and anatomical features compared to the human SAN, especially when studying aged and/or diseased human SAN with SND. The development of optimal treatments for SND will require in-depth knowledge of the mechanisms involved in robust human SAN rhythm regulation. However, substantial gaps exist in data on SAN functions obtained directly from the human SAN (157; 158), and considerable understanding is inferred from animal models, which may not reproduce the human clinical SND phenomena (156), or from clinical electrogram recordings that are restricted only to the atrial surface (155; 159).
Recent studies of the ex vivo human heart (49; 66; 160) directly address these limitations
(Figure 4 and Supplementary Figure 4). These studies of the ex-vivo human heart (49; 161)
provide a unique opportunity to reinvent the translational study of human cardiac disease by applying state-of-the-art intramural mapping techniques consisting of near-infrared optical mapping (27; 29; 66; 161), 3D structural imaging (49; 160), and molecular mapping (59) to resolve mechanisms of human SAN function in normal and diseased hearts which are not possible in-vivo.
To establish the efficient use of explanted human hearts with intact SANs, a rigorous and robust state-of-the-art approach has been developed to investigate e human hearts (with and without arrhythmia including SND) with 3D integrative approaches under ex vivo physiological conditions.
Explanted human chronic failing hearts are acquired alive from the OSU cardiac transplantation program. Donor hearts with and without cardiac diseases andcomorbidities (e.g. hypertension, diabetes and history of chronic smoke and alcohol consumption/abuse) are collected from LifeLine of Ohio, local organ donation organization (161).
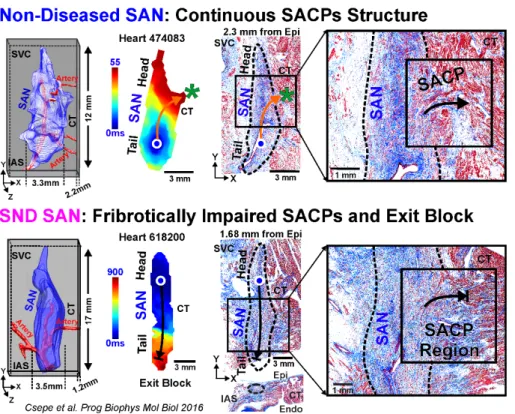
These multidisciplinary integrated approaches have identified that SND may be orchestrated by (i) heterogeneous fibrotic structural remodeling in SACPs (49; 133; 160), (ii) compartment specific molecular remodeling in If pacemaker channels (HCN1/HCN2/HCN4)(59)
and/or (iii) adenosine A1 receptor and IKAdo (GIRK1/GIRK4) (Supplementary Figure 4) (27; 29;
161) and/or (iv) neuronal (n)Nav (nNav1.6) and cNav1.5 isoforms in human SAN (66). Recent, optical mapping studies revealed that unlike cNav, nNav may predominantly contribute to SAN intranodal conduction, rather than atrial conduction. On the other hand, cNav play important roles in both SAN pacemaking and conduction, especially during adenosine or atrial pacing challenges to prevent intranodal conduction failure. Impairment of Nav can lead to SAN exit block, disorganized intranodal pacemakers, and SAN micro- and macro-reentry. Furthermore, these functional observations are supported by higher expression of nNav (Nav1.1 and Nav1.6) and lower expression of cNav1.5 in human SAN pacemaker cells versus the surrounding atrial myocardium. Importantly, several nNav transcripts were vulnerable to cardiac remodeling associated with heart failure, cardiac hypertrophy and modifying risk factors including history of chronic alcohol consumption, which could promote a substrate for SAN arrhythmias (66).
12
From a clinical perspective, ex-vivo human SAN studies (66) highlight limiting the use of
drugs that may block nNav channels especially when vagal tone is high, or in heart failure (162) and atrial fibrillation (163) patients with high plasma levels of adenosine. Furthermore, the ex-vivo
human studies also suggest that region-specific SAN disease remodeling, such as fibrotic infiltration (133), significantly contribute to the intrinsic region-specific SAN conduction abnormalities, and arrhythmias (Figure 4). Disease-induced structural remodeling may exacerbate region-specific
conduction abnormalities induced by both Nav blockers (66) and adenosine through GIRK channels and predispose to SND (Supplementary Figure 4). As such specific GIRK4 channel
blockers (e.g. Tertiapin-Q) can prevent SAN pacemaker arrest and exit block induced by adenosine and parasympathetic hyperactivity and eventually be used in patients with atrial fibrillation and SND (29).
In summary, integrative ex-vivo human SAN studies revealed that the availability of multiple
redundant structural compartments (intranodal pacemakers and SACPs) and molecular components in the human SAN are important backup mechanisms to robustly protect SAN conduction and pacemaking, and prevent rhythm failure and SND, in the context of multiple disease-induced conduction impairments (29; 133; 160).
Conclusions and perspectives
The development of new pharmacologic and molecular strategies to handle chronic primary and secondary forms of SND has been complicated by a lack of knowledge about the mechanisms underlying this complex pathology. However, new and unexpected mechanisms of SND have been described during the last years using human genetics, animal models of SND, as well as ex vivo SAN
from human hearts with history of secondary SND. These investigations have indicated potentially innovative pharmacologic targets to manage SND such as GIRK and SK channels. Furthermore, exploring animal models of SND and human SAN may help redirect the application of antiarrhythmic drugs and create innovative therapies for concomitant control of SND and associated arrhythmias. We expect that additional new SND mechanisms will be unraveled in the coming years with extensive functional genomics exploration of primary familial SND and development of new cellular and animal models of secondary forms of SND.
13
Acknowledgments
The IGF group is a member of the Laboratory of Excellence “Ion Channel Science and Therapeutics” supported by a grant from ANR (ANR-11-LABX-0015). Research supported by the
Fondation pour la Recherche Medicale "Physiopathologie Cardiovasculaire" (DPC20171138970,
M.E.M.), by the Agence Nationale de la Recherche (ANR-15-CE14-0004-01, M.E.M.). Work in Ohio
State was supported by NIH HL115580 and HL135109 (VVF), HL135096 (TJH), HL134824 (TJH and PJM), HL135754 (PJM) and by funding from the Bob and Corrine Frick Center for Heart Failure and Arrhythmia Research. We thank the Lifeline of Ohio Organ Procurement Organization and the Division of Cardiac Surgery at The OSU Wexner Medical Center for providing the explanted hearts. We also thank the Fondation Leducq (TNE 19CVD03 to M.E.M and P.J.M.) for
supporting the “Fighting Against Sinus Node Dysfunction and Associated Arrhythmias” (FANTASY) network.
14
Literature Cited
1. Boyett MR, Honjo H, Kodama I. 2000. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res 47:658-87
2. Mangoni ME, Nargeot J. 2008. Genesis and regulation of the heart automaticity. Physiol Rev 88:919-82
3. Lamas GA, Lee K, Sweeney M, Leon A, Yee R, et al. 2000. The mode selection trial (MOST) in sinus node dysfunction: design, rationale, and baseline characteristics of the first 1000 patients. Am Heart J 140:541-51
4. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, et al. 2018. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. J Am Coll Cardiol
5. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, et al. 2013. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 34:2281-329 6. Rodriguez RD, Schocken DD. 1990. Update on sick sinus syndrome, a cardiac disorder of aging.
Geriatrics 45:26-30, 3-6
7. Jensen PN, Gronroos NN, Chen LY, Folsom AR, deFilippi C, et al. 2014. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol 64:531-8
8. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, et al. 2012. Trends in permanent
pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol 60:1540-5
9. Schulze-Bahr E, Neu A, Friederich P, Kaupp UB, Breithardt G, et al. 2003. Pacemaker channel dysfunction in a patient with sinus node disease. J Clin Invest 111:1537-45
10. Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D. 2006. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med 354:151-7
11. Milano A, Vermeer AM, Lodder EM, Barc J, Verkerk AO, et al. 2014. HCN4 mutations in multiple families with bradycardia and left ventricular noncompaction cardiomyopathy. J Am Coll Cardiol 64:745-56
12. Schweizer PA, Schroter J, Greiner S, Haas J, Yampolsky P, et al. 2014. The symptom complex of familial sinus node dysfunction and myocardial noncompaction is associated with mutations in the HCN4 channel. J Am Coll Cardiol 64:757-67
13. Schweizer PA, Duhme N, Thomas D, Becker R, Zehelein J, et al. 2010. cAMP sensitivity of HCN pacemaker channels determines basal heart rate but is not critical for autonomic rate control. Circ Arrhythm Electrophysiol 3:542-52
14. Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, et al. 2011. Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci 14:77-84
15. Liaqat K, Schrauwen I, Raza SI, Lee K, Hussain S, et al. 2019. Identification of CACNA1D variants associated with sinoatrial node dysfunction and deafness in additional Pakistani families reveals a clinical significance. J Hum Genet 64:153-60
16. Kuss J, Stallmeyer B, Goldstein M, Rinne S, Pees C, et al. 2019. Familial Sinus Node Disease Caused by a Gain of GIRK (G-Protein Activated Inwardly Rectifying K(+) Channel) Channel Function. Circ Genom Precis Med 12:e002238
15
17. Stallmeyer B, Kuss J, Kotthoff S, Zumhagen S, Vowinkel K, et al. 2017. A Mutation in the G-Protein Gene GNB2 Causes Familial Sinus Node and Atrioventricular Conduction Dysfunction. Circ Res 120:e33-e44
18. Lodder EM, De Nittis P, Koopman CD, Wiszniewski W, Moura de Souza CF, et al. 2016. GNB5 Mutations Cause an Autosomal-Recessive Multisystem Syndrome with Sinus Bradycardia and Cognitive Disability. Am J Hum Genet 99:704-10
19. Veldkamp MW, Wilders R, Baartscheer A, Zegers JG, Bezzina CR, Wilde AA. 2003. Contribution of sodium channel mutations to bradycardia and sinus node dysfunction in LQT3 families. Circ Res 92:976-83
20. Smits JP, Koopmann TT, Wilders R, Veldkamp MW, Opthof T, et al. 2005. A mutation in the human cardiac sodium channel (E161K) contributes to sick sinus syndrome, conduction disease and Brugada syndrome in two families. J Mol Cell Cardiol 38:969-81
21. Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, et al. 2011. HRS/EHRA expert
consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Europace 13:1077-109
22. Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, et al. 2008. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A 105:15617-22
23. Holm H, Gudbjartsson DF, Arnar DO, Thorleifsson G, Thorgeirsson G, et al. 2010. Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet 42:117-22 24. Ishikawa T, Tsuji Y, Makita N. 2016. Inherited bradyarrhythmia: A diverse genetic background. J
Arrhythm 32:352-8
25. Mesirca P, Alig J, Torrente AG, Muller JC, Marger L, et al. 2014. Cardiac arrhythmia induced by genetic silencing of 'funny' (f) channels is rescued by GIRK4 inactivation. Nat Commun 5:4664 26. Mesirca P, Bidaud I, Briec F, Evain S, Torrente AG, et al. 2016. G protein-gated IKACh channels
as therapeutic targets for treatment of sick sinus syndrome and heart block. Proc Natl Acad Sci U S A 113:E932-41
27. Lou Q, Hansen BJ, Fedorenko O, Csepe TA, Kalyanasundaram A, et al. 2014. Upregulation of adenosine A1 receptors facilitates sinoatrial node dysfunction in chronic canine heart failure by exacerbating nodal conduction abnormalities revealed by novel dual-sided intramural optical mapping. Circulation 130:315-24
28. Long VP, 3rd, Bonilla IM, Baine S, Glynn P, Kumar S, et al. 2020. Chronic heart failure increases negative chronotropic effects of adenosine in canine sinoatrial cells via A1R stimulation and GIRK-mediated IKado. Life Sci 240:117068
29. Li N, Hansen BJ, Csepe TA, Zhao J, Ignozzi AJ, et al. 2017. Redundant and diverse intranodal pacemakers and conduction pathways protect the human sinoatrial node from failure. Sci Transl Med 9:eaam 5607
30. Weisbrod D, Peretz A, Ziskind A, Menaker N, Oz S, et al. 2013. SK4 Ca2+ activated K+ channel is a critical player in cardiac pacemaker derived from human embryonic stem cells. Proc Natl Acad Sci U S A 110:E1685-94
31. Haron-Khun S, Weisbrod D, Bueno H, Yadin D, Behar J, et al. 2017. SK4 K(+) channels are therapeutic targets for the treatment of cardiac arrhythmias. EMBO Mol Med 9:415-29
32. Torrente AG, Zhang R, Wang H, Zaini A, Kim B, et al. 2017. Contribution of small conductance K+ channels to sinoatrial node pacemaker activity: insights from atrial-specific Na+ /Ca2+ exchange knockout mice. J Physiol
33. Adan V, Crown LA. 2003. Diagnosis and treatment of sick sinus syndrome. Am Fam Physician 67:1725-32
34. Ferrer MI. 1968. The sick sinus syndrome in atrial disease. JAMA 206:645-6 35. Bigger JT, Jr., Reiffel JA. 1979. Sick sinus syndrome. Annu Rev Med 30:91-118
36. Mangrum JM, DiMarco JP. 2000. The evaluation and management of bradycardia. N Engl J Med 342:703-9
37. Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, et al. 2016. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 133:e506-74
16
38. Lown B. 1967. Electrical reversion of cardiac arrhythmias. Br Heart J 29:469-89
39. Brubaker PH, Kitzman DW. 2011. Chronotropic incompetence: causes, consequences, and management. Circulation 123:1010-20
40. Menozzi C, Brignole M, Alboni P, Boni L, Paparella N, et al. 1998. The natural course of untreated sick sinus syndrome and identification of the variables predictive of unfavorable outcome. Am J Cardiol 82:1205-9
41. John RM, Kumar S. 2016. Sinus Node and Atrial Arrhythmias. Circulation 133:1892-900 42. Alonso A, Jensen PN, Lopez FL, Chen LY, Psaty BM, et al. 2014. Association of sick sinus
syndrome with incident cardiovascular disease and mortality: the Atherosclerosis Risk in Communities study and Cardiovascular Health Study. PLoS One 9:e109662
43. Verheijck EE, Wessels A, van Ginneken AC, Bourier J, Markman MW, et al. 1998. Distribution of atrial and nodal cells within the rabbit sinoatrial node: models of sinoatrial transition. Circulation 97:1623-31
44. De Maziere AM, van Ginneken AC, Wilders R, Jongsma HJ, Bouman LN. 1992. Spatial and functional relationship between myocytes and fibroblasts in the rabbit sinoatrial node. J Mol Cell Cardiol 24:567-78
45. Linscheid N, Logantha S, Poulsen PC, Zhang S, Schrolkamp M, et al. 2019. Quantitative proteomics and single-nucleus transcriptomics of the sinus node elucidates the foundation of cardiac pacemaking. Nat Commun 10:2889
46. ten Velde I, de Jonge B, Verheijck EE, van Kempen MJ, Analbers L, et al. 1995. Spatial
distribution of connexin43, the major cardiac gap junction protein, visualizes the cellular network for impulse propagation from sinoatrial node to atrium. Circ Res 76:802-11
47. Verheijck EE, van Kempen MJ, Veereschild M, Lurvink J, Jongsma HJ, Bouman LN. 2001. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc Res 52:40-50
48. Boyett MR, Inada S, Yoo S, Li J, Liu J, et al. 2006. Connexins in the sinoatrial and atrioventricular nodes. Adv Cardiol 42:175-97
49. Csepe TA, Zhao J, Hansen BJ, Li N, Sul LV, et al. 2016. Human sinoatrial node structure: 3D microanatomy of sinoatrial conduction pathways. Prog Biophys Mol Biol 120:164-78
50. Bouman LN, Duivenvoorden JJ, Bukauskas FF, Jongsma HJ. 1989. Anisotropy of electrotonus in the sinoatrial node of the rabbit heart. J Mol Cell Cardiol 21:407-18
51. Dobrzynski H, Li J, Tellez J, Greener ID, Nikolski VP, et al. 2005. Computer Three-Dimensional Reconstruction of the Sinoatrial Node. Circulation
52. Lakatta EG, Maltsev VA, Vinogradova TM. 2010. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res 106:659-73
53. Wickman K, Nemec J, Gendler SJ, Clapham DE. 1998. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron 20:103-14
54. Monfredi O, Maltsev VA, Lakatta EG. 2013. Modern concepts concerning the origin of the heartbeat. Physiology (Bethesda) 28:74-92
55. DiFrancesco D. 2010. The role of the funny current in pacemaker activity. Circ Res 106:434-46 56. DiFrancesco D, Tortora P. 1991. Direct activation of cardiac pacemaker channels by intracellular
cyclic AMP. Nature 351:145-7
57. DiFrancesco D, Mangoni M. 1994. Modulation of single hyperpolarization-activated channels (i(f)) by cAMP in the rabbit sino-atrial node. J Physiol 474:473-82
58. Shi W, Wymore R, Yu H, Wu J, Wymore RT, et al. 1999. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ Res 85:e1-6
59. Li N, Csepe TA, Hansen BJ, Dobrzynski H, Higgins RS, et al. 2015. Molecular Mapping of Sinoatrial Node HCN Channel Expression in the Human Heart. Circ Arrhythm Electrophysiol 8:1219-27
60. Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, et al. 2006. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ Res 98:1422-30
61. Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, et al. 2003. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci U S A 100:5543-8
17
62. Torrente AG, Mesirca P, Neco P, Rizzetto R, Dubel S, et al. 2016. L-type Cav1.3 channels regulate ryanodine receptor-dependent Ca2+ release during sino-atrial node pacemaker activity. Cardiovasc Res 109:451-61
63. Maier SK, Westenbroek RE, Yamanushi TT, Dobrzynski H, Boyett MR, et al. 2003. An unexpected requirement for brain-type sodium channels for control of heart rate in the mouse sinoatrial node. Proc Natl Acad Sci U S A 100:3507-12
64. Baruscotti M, DiFrancesco D, Robinson RB. 1996. A TTX-sensitive inward sodium current contributes to spontaneous activity in newborn rabbit sino-atrial node cells. J Physiol 492 ( Pt 1):21-30
65. Lei M, Jones SA, Liu J, Lancaster MK, Fung SS, et al. 2004. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J Physiol 559:835-48 66. Li N, Kalyanasundaram A, Hansen BJ, Artiga EJ, Sharma R, et al. 2020. Impaired neuronal
sodium channels cause intranodal conduction failure and reentrant arrhythmias in human sinoatrial node. Nat Commun 11:512
67. Demion M, Bois P, Launay P, Guinamard R. 2007. TRPM4, a Ca(2+)-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res 73:531-8
68. Sah R, Mesirca P, Van den Boogert M, Rosen J, Mably J, et al. 2013. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc Natl Acad Sci U S A 110:E3037-46
69. Ju YK, Chu Y, Chaulet H, Lai D, Gervasio OL, et al. 2007. Store-operated Ca2+ influx and expression of TRPC genes in mouse sinoatrial node. Circ Res 100:1605-14
70. Sah R, Mesirca P, Mason X, Gibson W, Bates-Withers C, et al. 2013. Timing of myocardial trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and
repolarization. Circulation 128:101-14
71. Lai MH, Wu Y, Gao Z, Anderson ME, Dalziel JE, Meredith AL. 2014. BK channels regulate sinoatrial node firing rate and cardiac pacing in vivo. Am J Physiol Heart Circ Physiol 307:H1327-38 72. Chen WT, Chen YC, Lu YY, Kao YH, Huang JH, et al. 2013. Apamin modulates
electrophysiological characteristics of the pulmonary vein and the Sinoatrial Node. Eur J Clin Invest 43:957-63
73. Gueguinou M, Chantome A, Fromont G, Bougnoux P, Vandier C, Potier-Cartereau M. 2014. KCa and Ca(2+) channels: the complex thought. Biochim Biophys Acta 1843:2322-33
74. Zhang Q, Timofeyev V, Lu L, Li N, Singapuri A, et al. 2008. Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circ Res 102:465-71
75. Zhang XD, Coulibaly ZA, Chen WC, Ledford HA, Lee JH, et al. 2018. Coupling of SK channels, L-type Ca(2+) channels, and ryanodine receptors in cardiomyocytes. Sci Rep 8:4670
76. Monfredi O, Boyett MR. 2015. Sick sinus syndrome and atrial fibrillation in older persons - A view from the sinoatrial nodal myocyte. J Mol Cell Cardiol
77. Monfredi O, Dobrzynski H, Mondal T, Boyett MR, Morris GM. 2010. The anatomy and physiology of the sinoatrial node--a contemporary review. Pacing Clin Electrophysiol 33:1392-406 78. Rezazadeh S, Duff HJ. 2017. Genetic Determinants of Hereditary Bradyarrhythmias: A
Contemporary Review of a Diverse Group of Disorders. Can J Cardiol 33:758-67
79. Benson DW, Wang DW, Dyment M, Knilans TK, Fish FA, et al. 2003. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). J Clin Invest 112:1019-28
80. Wolf CM, Berul CI. 2006. Inherited conduction system abnormalities--one group of diseases, many genes. J Cardiovasc Electrophysiol 17:446-55
81. Zhang Q, Timofeyev V, Qiu H, Lu L, Li N, et al. 2011. Expression and roles of Cav1.3 (alpha1D) L-type Ca(2)+ channel in atrioventricular node automaticity. J Mol Cell Cardiol
50:194-202
82. Marger L, Mesirca P, Alig J, Torrente A, Dubel S, et al. 2011. Functional roles of Ca(v)1.3, Ca(v)3.1 and HCN channels in automaticity of mouse atrioventricular cells: insights into the atrioventricular pacemaker mechanism. Channels (Austin) 5:251-61
83. Zhang Z, He Y, Tuteja D, Xu D, Timofeyev V, et al. 2005. Functional roles of Cav1.3(alpha1D) calcium channels in atria: insights gained from gene-targeted null mutant mice. Circulation 112:1936-44
84. Boutjdir M. 2000. Molecular and ionic basis of congenital complete heart block. Trends Cardiovasc Med 10:114-22
18
85. Qu Y, Xiao GQ, Chen L, Boutjdir M. 2001. Autoantibodies from mothers of children with congenital heart block downregulate cardiac L-type Ca channels. J Mol Cell Cardiol 33:1153-63 86. Qu Y, Baroudi G, Yue Y, Boutjdir M. 2005. Novel molecular mechanism involving alpha1D
(Cav1.3) L-type calcium channel in autoimmune-associated sinus bradycardia. Circulation 111:3034-41
87. Strandberg LS, Cui X, Rath A, Liu J, Silverman ED, et al. 2013. Congenital heart block maternal sera autoantibodies target an extracellular epitope on the alpha1G T-type calcium channel in human fetal hearts. PLoS One 8:e72668
88. Liu H, El Zein L, Kruse M, Guinamard R, Beckmann A, et al. 2010. Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet 3:374-85
89. den Hoed M, Eijgelsheim M, Esko T, Brundel BJ, Peal DS, et al. 2013. Identification of heart rate-associated loci and their effects on cardiac conduction and rhythm disorders. Nat Genet 45:621-31
90. Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, et al. 2006. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 114:2104-12 91. Yamada N, Asano Y, Fujita M, Yamazaki S, Inanobe A, et al. 2019. Mutant KCNJ3 and KCNJ5
Potassium Channels as Novel Molecular Targets in Bradyarrhythmias and Atrial Fibrillation. Circulation 139:2157-69
92. Bhuiyan ZA, van den Berg MP, van Tintelen JP, Bink-Boelkens MT, Wiesfeld AC, et al. 2007. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation 116:1569-76
93. Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, et al. 2002. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res 91:e21-6
94. Postma AV, Denjoy I, Kamblock J, Alders M, Lupoglazoff JM, et al. 2005. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet 42:863-70
95. Bezzina CR, Lahrouchi N, Priori SG. 2015. Genetics of sudden cardiac death. Circ Res 116:1919-36
96. Neco P, Torrente AG, Mesirca P, Zorio E, Liu N, et al. 2012. Paradoxical effect of increased diastolic Ca2+ release and decreased sinoatrial node activity in a mouse model of
catecholaminergic polymorphic ventricular tachycardia. Circulation 126:392-401
97. Wang YY, Mesirca P, Marques-Sule E, Zahradnikova A, Jr., Villejoubert O, et al. 2017.
RyR2R420Q catecholaminergic polymorphic ventricular tachycardia mutation induces bradycardia by disturbing the coupled clock pacemaker mechanism. JCI Insight 2
98. Holm H, Gudbjartsson DF, Sulem P, Masson G, Helgadottir HT, et al. 2011. A rare variant in MYH6 is associated with high risk of sick sinus syndrome. Nat Genet 43:316-20
99. van Berlo JH, de Voogt WG, van der Kooi AJ, van Tintelen JP, Bonne G, et al. 2005. Meta-analysis of clinical characteristics of 299 carriers of LMNA gene mutations: do lamin A/C mutations portend a high risk of sudden death? J Mol Med (Berl) 83:79-83
100. Hoffmann S, Paone C, Sumer SA, Diebold S, Weiss B, et al. 2019. Functional Characterization of Rare Variants in the SHOX2 Gene Identified in Sinus Node Dysfunction and Atrial Fibrillation. Front Genet 10:648
101. Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, et al. 2000. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102:89-97 102. Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, et al. 2002. Functional Roles of Ca(v)1.3
(alpha(1D)) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ Res 90:981-7.
103. Stieber J, Herrmann S, Feil S, Loster J, Feil R, et al. 2003. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci U S A 100:15235-40
104. Herrmann S, Stieber J, Stockl G, Hofmann F, Ludwig A. 2007. HCN4 provides a 'depolarization reserve' and is not required for heart rate acceleration in mice. Embo J 26:4423-32
19
105. Baruscotti M, Bucchi A, Viscomi C, Mandelli G, Consalez G, et al. 2011. Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4. Proc Natl Acad Sci U S A 108:1705-10
106. Fenske S, Krause SC, Hassan SI, Becirovic E, Auer F, et al. 2013. Sick sinus syndrome in HCN1-deficient mice. Circulation 128:2585-94
107. Lei M, Goddard C, Liu J, Leoni AL, Royer A, et al. 2005. Sinus node dysfunction following targeted disruption of the murine cardiac sodium channel gene Scn5a. J Physiol 567:387-400 108. Torrente AG, Zhang R, Zaini A, Giani JF, Kang J, et al. 2015. Burst pacemaker activity of the
sinoatrial node in sodium-calcium exchanger knockout mice. Proc Natl Acad Sci U S A 112:9769-74
109. Glukhov AV, Kalyanasundaram A, Lou Q, Hage LT, Hansen BJ, et al. 2015. Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex1. Eur Heart J 36:686-97
110. Unudurthi SD, Wu X, Qian L, Amari F, Onal B, et al. 2016. Two-Pore K+ Channel TREK-1 Regulates Sinoatrial Node Membrane Excitability. J Am Heart Assoc 5:e002865
111. Hund TJ, Snyder JS, Wu X, Glynn P, Koval OM, et al. 2014. beta(IV)-Spectrin regulates TREK-1 membrane targeting in the heart. Cardiovasc Res 102:166-75
112. Froese A, Breher SS, Waldeyer C, Schindler RF, Nikolaev VO, et al. 2012. Popeye domain containing proteins are essential for stress-mediated modulation of cardiac pacemaking in mice. J Clin Invest 122:1119-30
113. Hof T, Simard C, Rouet R, Salle L, Guinamard R. 2013. Implication of the TRPM4 nonselective cation channel in mammalian sinus rhythm. Heart Rhythm 10:1683-9
114. Demion M, Thireau J, Gueffier M, Finan A, Khoueiry Z, et al. 2014. Trpm4 gene invalidation leads to cardiac hypertrophy and electrophysiological alterations. PLoS One 9:e115256
115. Frank DU, Carter KL, Thomas KR, Burr RM, Bakker ML, et al. 2012. Lethal arrhythmias in Tbx3-deficient mice reveal extreme dosage sensitivity of cardiac conduction system function and homeostasis. Proc Natl Acad Sci U S A 109:E154-63
116. Nakashima Y, Yanez DA, Touma M, Nakano H, Jaroszewicz A, et al. 2014. Nkx2-5 suppresses the proliferation of atrial myocytes and conduction system. Circ Res 114:1103-13
117. Qiao Y, Lipovsky C, Hicks S, Bhatnagar S, Li G, et al. 2017. Transient Notch Activation Induces Long-Term Gene Expression Changes Leading to Sick Sinus Syndrome in Mice. Circ Res 121:549-63
118. Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, et al. 2011. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest 121:3277-88
119. Desai JM, Scheinman MM, Strauss HC, Massie B, O'Young J. 1981. Electrophysiologic effects on combined autonomic blockade in patients with sinus node disease. Circulation 63:953-60
120. Stein R, Medeiros CM, Rosito GA, Zimerman LI, Ribeiro JP. 2002. Intrinsic sinus and atrioventricular node electrophysiologic adaptations in endurance athletes. J Am Coll Cardiol 39:1033-8
121. Andersen K, Farahmand B, Ahlbom A, Held C, Ljunghall S, et al. 2013. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J 34:3624-31
122. Northcote RJ, Canning GP, Ballantyne D. 1989. Electrocardiographic findings in male veteran endurance athletes. Br Heart J 61:155-60
123. D'Souza A, Pearman CM, Wang Y, Nakao S, Logantha S, et al. 2017. Targeting miR-423-5p Reverses Exercise Training-Induced HCN4 Channel Remodeling and Sinus Bradycardia. Circ Res 121:1058-68
124. D'Souza A, Bucchi A, Johnsen AB, Logantha SJ, Monfredi O, et al. 2014. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat Commun 5:3775 125. Yeh YH, Burstein B, Qi XY, Sakabe M, Chartier D, et al. 2009. Funny current downregulation
and sinus node dysfunction associated with atrial tachyarrhythmia: a molecular basis for tachycardia-bradycardia syndrome. Circulation 119:1576-85
126. Alboni P, Baggioni GF, Scarfo S, Cappato R, Percoco GF, et al. 1991. Role of sinus node artery disease in sick sinus syndrome in inferior wall acute myocardial infarction. Am J Cardiol 67:1180-4 127. Rokseth R, Hatle L. 1971. Sinus arrest in acute myocardial infarction. Br Heart J 33:639-42