Publisher’s version / Version de l'éditeur:
Cement and Concrete Research, 8, January 1, pp. 103-112, 1978-01-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1016/0008-8846(78)90063-7
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Strength development in magnesium oxysulfate cement
Beaudoin, J. J.; Ramachandran, V. S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=bfa034be-b195-4571-8d2d-fcc236ba60f4 https://publications-cnrc.canada.ca/fra/voir/objet/?id=bfa034be-b195-4571-8d2d-fcc236ba60f4NATIONAL RESEARCH COUNCIL
OF CANADA
CONSEIL NATIONAL DE RECHERCHE DU
CANADA
STRENGTH DEVELOPMENT IN
MAGNESIUM OXYSULFATE CEMENT
ANALYZED
by J. J. Beaudoin and V. S. Ramachandran
4
Reprinted from
CEMENT
AND
CONCRETE RESEARCH
Vol. 8, No. 1, January 1978
pp. 103
-
112BUILD1
NG
RESEARCI-I
-
LIBRARY
-
FEB
lrf
1978
N A T ~ N A L REHIARCH C O V N C ~ A DBR Paper No. 750 Division of Building ResearchThis publication is being distributed by the Division of Building Research of the National Research Council of Canada. It should not be reproduced in whole or in part without per- mission of the original publisher. The Division would be glad t o be of assistance in ob- taining such permission.
Publications of the Division may be obtained by mailing the appropriate remittance (a Bank. Express, or Post Office Money Order, or a cheque, made payable to the Receiver General of Canada, credit NRC) to the National Research Council of Canada. Ottawa.
KIAOR6. Stamps are not acceptable.
A list of all publications of the Division is available and may be obtained from the Publica- tions Section. Division of Building Research. National Research Council of Canada. Ottawa.
CEMENT and CONCRETE RESEARCH. Vol. 8, pp. 103-112, 1978. Pergamon Press, I n c . P r i n t e d i n t h e U n i t e d S t a t e s .
STRENGTH DEVELOPMENT I N MAGNESIUM OXYSULFATE CEMENT
J .J. Beaudoin and V.S
.
RamachandranM a t e r i a l s S e c t i o n , D i v i s i o n of B u i l d i n g R e s e a r c h , N a t i o n a l Research C o u n c i l of Canada, Ottawa
(Communicated by J. Skalny) (Received Nov. 2, 1977)
ABSTRACT: Modulus o f e l a s t i c i t y and m i c r o h a r d n e s s measurements were made on
magnesium o x y s u l f a t e s y s t e m s ( p a s t e and compacted) h a v i n g a wide
ranCe o f p o r o s i t y . Three s e t s of compacts were made; each s e t was
made w i t h p a r t i c l e s h a v i n g a d i f f e r e n t s i z e r a n g e ( 5 t o 150 pm;
150 t o 300 urn; 300 t o 600 pm). The l o g m e c h a n i c a l p r o p e r t y v e r s u s
p o r o s i t y c u r v e s f o r p a s t e samples were l i n e a r . The c u r v e s f o r
compacted samples had a change i n s l o p e a t 7.5 p e r c e n t p o r o s i t y . A t p o r o s i t i e s l e s s t h a n 7.5 p e r c e n t , t h e d a t a f o r p a s t e and compacted samples were on t h e same c u r v e . A t h i g h e r p o r o s i t i e s m i c r o h a r d n e s s v a l u e s f o r compacted samples were g r e a t e r t h a n t h o s e f o r p a s t e s a m p l e s ; modulus o f e l a s t i c i t y v a l u e s were less.
Compacted samples made w i t h f i n e s t p a r t i c l e s had h i g h e r v a l u e s f o r
mechanical p r o p e r t i e s a t a g i v e n p o r o s i t y . The mechanical
b e h a v i o u r of t h e s e s y s t e m s was e x p l a i n e d on t h e b a s i s of t h e r e l a - t i v e c o n t r i b u t i o n o f p o r o s i t y , p o r e s i z e d i s t r i b u t i o n , i n t e r - p a r t i c l e bonds and s t r e n g t h of t h e s o l i d p h a s e .
Des mesures du module d 1 6 1 a s t i c i t E e t de l a m i c r o d u r e t g o n t Et6 p r i s e s s u r de l ' o x y s u l f a t e de magnEsium ( e n p z t e e t comprim6) a y a n t
une p o r o s i t E t r S s v a r i 6 e . T r o i s ensembles de comprimEs o n t 6tE
f a b r i q u g s ; chacun a E t E f a i t de t r o i s s 6 r i e s de p a r t i c u l e s de
g r o s s e u r d i f f E r e n t e ( 5 b 100 pm; 150 b 300 um; 300 b 600 um). Les
c o u r b e s de p r o p r i 6 t E m6canique l o g a r i t h m i q u e e t de p o r o s i t 6 d e s
E c h a n t i l l o n s e n p z t e 6 t a i e n t l i n 6 a i r e s . Les c o u r b e s des E c h a n t i l l o n s
comprim6s a v a i e n t un changement d ' i n c l i n a i s o n b 7.5 pour c e n t de
p o r o s i t 6 . A des p o r o s i t E s moindres que 7.5 p o u r c e n t l e s donn6es
des E c h a n t i l l o n s e n p z t e e t comprim6s s e s i t u a i e n t s u r l a mgme c o u r b e . Les v a l e u r s de microduretE d e s 6 c h a n t i l l o n s comprim6s b d e s p o r o s i t E s p l u s 6levEes E t a i e n t p l u s grandes que c e l l e s des Echan t i l l o n s e n p z t e ; l e s v a l e u r s du m d u l e d' E l a s t i c i t 6 E t a i e n t
moindres
.
Les E c h a n t i l l o n s comprim6s f a i t s a v e c l e s p a r t i c u l e s l e sp l u s f i n e s a v a i e n t d e s p r o p r i 6 t E s m6caniques de v a l e u r s p l u s
g r a n d e s b une p o r o s i t 6 donn6e. Le comportement mEcaniqw de c e s
s y s t s m e s a 6 t E e x p l i q u 6 e n s e f o n d a n t s u r l a c o n t r i b u t i o n r e l a t i v e
de l a p o r o s i t 6 , l a d i s t r i b u t i o n de l a g r o s s e u r du p o r e , l e s l i a n t s
Vol.
8,No.
1
J. J. Beaudoin, V. S. Ramachandran
I n t r o d u c t i o n
The p o t e n t i a l o f magnesium o x y s u l f a t e a s a b i n d e r i n b u i l d i n g m a t e r i a l s has been recognized s i n c e t h e discovery of magnesia-based cements by Sore1 i n 1867 ( 1 ) . Unlike oxychloride cement, use o f o x y s u l f a t e cement does n o t
p r e s e n t a p o t e n t i a l source f o r c o r r o s i o n o f r e i n f o r c i n g s t e e l . The o x y s u l f a t e cement has good f i r e r e s i s t a n c e , low thermal c o n d u c t i v i t y and i s r e c e n t l y being considered f o r n u c l e a r a p p l i c a t i o n s . Wood wool boards can b e made using o x y s u l f a t e cement a s a b i n d e r and waste p r o d u c t s such as wood shavings o r sawdust a s f i l l e r s .
Depending on t h e temperature and c o n d i t i o n s o f formation, f o u r types o f
o x y s u l f a t e complexes a r e known t o form i n t h e Mg0-MgS04-H20 system. They a r e
5Mg (OH) 2 . MgS04. 3H20 ( o r 2H20)
,
3Mg (OH) 2 . MgS04. 8H20, Mg (OH) 2 . MgS04. 5H20 andMg(0H)2.2MgSO4.3H20 ( 2 , 3 ) . A t temperatures below 3S°C, 3Mg(OH)2.MgS04.8H20
appears t o b e t h e most s t a b l e phase ( 3 ) . However, H a l l , Read and Brandt (4)
have a l s o i d e n t i f i e d t h e presence o f SMg(OH)2 .MgS04. 3H20. Widespread use o f magnesium o x y s u l f a t e cement has been l i m i t e d because o f l o s s of s t r e n g t h on
prolonged exposure t o w a t e r . The o x y s u l f a t e cement system i s g e n e r a l l y con-
s i d e r e d t o b e weaker t h a n t h e oxychloride cement system. S t u d i e s have shown t h a t many c e m e n t i t i o u s systems t h a t normally produce weak bodies can b e made t o have h i g h e r s t r e n g t h s by modifying c e r t a i n c o n d i t i o n s of p r e p a r a t i o n ( 5 , 6 ) . Pressed t i l e s made from oxychloride cement a r e known t o have b e t t e r d u r a b i l i t y . One o b j e c t i v e o f t h i s s t u d y was t o determine i f applying p r e s s u r e t o t h e
o x y s u l f a t e cement system would produce a s t r o n g e r body t h a n would b e o b t a i n e d by normal p a s t e h y d r a t i o n .
I n p o r t l a n d cement systems, it has been shown t h a t compacted p a s t e and
t h a t formed under i n s i t u h y d r a t i o n y i e l d s i m i l a r s t r e n g t h s f o r comparable p o r o s i t i e s , s u g g e s t i n g t h a t t h e compaction technique i s a useful method t o study t h i s system (7, 8 ) . I t i s a f u r t h e r o b j e c t i v e o f t h i s s t u d y t o t e s t
whether t h i s technique i s a l s o a p p l i c a b l e t o t h e o x y s u l f a t e cement system.
I t i s a l s o hoped t h a t an i n v e s t i g a t i o n o f p r e s s e d o x y s u l f a t e systems w i l l
provide a b e t t e r understanding o f t h e mechanism of bonding i n p r e s s e d systems.
E m e r i m e n t a l M a t e r i a l s
The following m a t e r i a l s were used:
1. Magnesium Oxide. The MgO powder was s u p p l i e d by Basic Chemicals,
Cleveland, Ohio. I t had t h e following c h a r a c t e r i s t i c s : N 2 s u r f a c e
a r e a , 20 m2/g; a c t i v e CaO, 1.5 p e r c e n t ; i g n i t i o n l o s s 4 p e r c e n t ;
f r a c t i o n p a s s i n g through 200 mesh, 98 p e r c e n t . The m a t e r i a l
s a t i s f i e d t h e requirements o f ASTM C-275-61.
2 . Magnesium S u l p h a t e S o l u t i o n s . Aqueous s o l u t i o n o f s p e c i f i c g r a v i t y 1.18 was prepared by mixing MgS04. 7H20, s u p p l i e d by Anachemia, i n a dry form w i t h d i s t i l l e d w a t e r . S a t u r a t e d s o l u t i o n s ( s p e c i f i c g r a v i t y 1.303) were a l s o used f o r t h e p r e p a r a t i o n of samples.
V o l . 8, No. 1
MAGNESIUM OXYSULFATE CEMENT, STRENGTH
P r e p a r a t i o n o f Specimens S e r i e s I
Cement p a s t e samples were prepared a t MgS04. 7H20/Mg0 s o l u t i o n - s o l i d r a t i o s of 0.59, 0.72, 0.84, 0.96, 1.07 and 1 . 4 3 by weight u s i n g aqueous s o l u - t i o n having s p e c i f i c g r a v i t y 1 . 1 8 . Mixes having s o l u t i o n - s o l i d r a t i o s 0.59 and 0.72 were c a s t i n cubic moulds and cured a t 50 p e r c e n t r e l a t i v e humidity
f o r two weeks. They were t h e n cored and s l i c e d i n t o d i s c s 3.2 cm i n diameter,
1 . 3 3 mm t h i c k ; t h e d i s c s were c o n d i t i o n e d a t 11 p e r c e n t r e l a t i v e humidity f o r
periods of up t o t h r e e months. A l l o t h e r mixes were c a s t i n 3.2 cm diameter
c y l i n d r i c a l tubes which were r o t a t e d a s h y d r a t i o n proceeded t o p r e v e n t
s e g r e g a t i o n . Discs 1 . 3 mm t h i c k were c u t from t h e c y l i n d e r s and s u b j e c t e d t o
t h e same c u r i n g procedure a s t h e d i s c s o b t a i n e d from c o r e s . S e r i e s I 1
Cement p a s t e samples were prepared u s i n g a s a t u r a t e d s o l u t i o n of'
MgS04. 7H20 ( s p e c i f i c g r a v i t y 1.303) ; s o l u t i o n - s o l i d r a t i o s and c u r i n g condi-
t i o n s were t h e same a s f o r t h e mixes i n S e r i e s I .
S e r i e s I11
The cement p a s t e samples from S e r i e s I were ground i n t o powder c o n s i s t i n g
of a mixture c o n t a i n i n g equal weight f r a c t i o n s o f m a t e r i a l f o r each s o l u t i o n -
s o l i d r a t i o p r e p a r e d . One s e t o f samples were prepared with p a r t i c l e s 300 t o
600
vm;
t h e s e p a r t i c l e s were f u r t h e r ground t o 150 t o 300 pm and a second s e to f samples was p r e p a r e d . The f i n e s t p a r t i c l e s i z e range 5 t o 150 ym, o b t a i n e d by g r i n d i n g 150 t o 300 u m p a r t i c l e s , c o n s t i t u t e d t h e t h i r d s e t . Compacts 3.2 cm i n diameter, 1 . 3 mm t h i c k , were made under compaction p r e s s u r e s o f 133 MPa t o 1665 MPa t o o b t a i n a wide range o f p o r o s i t y . Measurements
A helium pycnometer was used t o measure t h e s o l i d volume of t h e cement
specimens of known geometry. P o r o s i t y was determined from t h e apparent volume
and t h e s o l i d volume. This avoids t h e problem of d i s s o l u t i o n t h a t a r i s e s when
w a t e r i s used as t h e displacement medium. Samples were c o n d i t i o n e d a t 11 p e r
c e n t r e l a t i v e humidity f o r each measurement. A p p l i c a t i o n of t h i s technique t o o t h e r i n o r g a n i c cement systems i s d e s c r i b e d elsewhere ( 9 ) .
The modulus of e l a s t i c i t y was measured on d i s c s with a diameter o f 3.2 cm and a t h i c k n e s s o f 1 . 3 mm. This procedure involves measuring t h e d e f l e c t i o n
of a specimen when i t i s loaded a t i t s c e n t r e and supported a t t h r e e p o i n t s
l o c a t e d on the circumference o f a c i r c l e 2.5 cm i n diameter. Each v a l u e given
i n t h i s paper i s t h e average o f measurements on t h r e e specimens. Microhard-
ness was measured with a L e i t z microhardness t e s t i n g machine w i t h a Vickers
i n d e n t e r . Each microhardness value recorded h e r e i n i s t h e average f o r t h r e e
d i s c s ; f i v e microhardness measurements were made on each d i s c . D i f f e r e n t i a l
thermograms were o b t a i n e d u s i n g t h e 990 DuPont thermal a n a l y s i s s y s tem.
Twenty mg o f t h e m a t e r i a l were used f o r each run. The r a t e o f h e a t i n g was
20°C/min. (T = 150 s e c / i n . ) and t h e s e n s i t i v i t y was AT = 5 m c a l / s e c / i n .
The m i c r o s t r u c t u r e o f some of t h e samples was examined u s i n g a Cambridge
Vol. 8, No.
1
J. J. Beaudoin, V. S. RamachandranX-ray examinations were c a r r i e d
1 0 0 0 9 0 0
:::
'"
4 0 0g
joO-
E 200 "? Y) u LII=
l o o < 9 0::
2 so O 70 6 0 5 0 4 0 10 20 0 I I I - I I I-
-
1
,\
-
-
7
1
-
r k r r t GIIOUIVO. L C O M P A C T E D "-!L
* A R T I C L E 5 5.b.0 p m-
-
I A R T I C L E S I ~ O - ~ U Q ~ ~ ,-
-
\\
- ~ ~ I T I C I E S 1 0 0 - b ~ ~ y m - : I : I-
.k.
-
*..,
\ ..'---\...
\<# I I I I I I 5 o u t w i t h a Debye S c h e r r e r camera. Microdensitometer t r a c e s were madefrom t h e X-ray f i l m s . S u r f a c e a r e a was o b t a i n e d by means o f a Numinco- O r r s u r f a c e area-pore volume
a n a l y s e r with N 2 a s t h e a d s o r b a t e . Powdered samples were vacuum d r i e d f o r 3 h r a t 50°C p r i o r t o measure- ments.
R e s u l t s
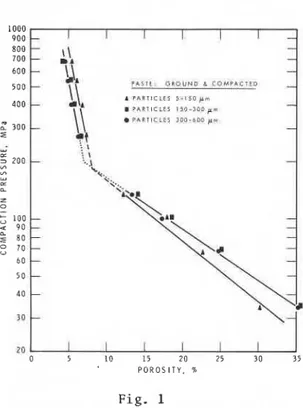
The logarithm o f compaction p r e s s u r e i s p l o t t e d a g a i n s t p o r o s i t y on Figure 1. Two l i n e a r r e g i o n s can b e seen, one up t o approximately 7.5 p e r c e n t p o r o s i t y and t h e o t h e r from 7.5 t o approximately 35 p e r c e n t p o r o s i t y . The s l o p e o f t h e l i n e up t o 7.5 p e r c e n t p o r o s i t y , . i s much s t e e p e r than t h a t f o r t h e l i n e a t h i g h e r p o r o s i t y . Other systems
.
l o P O R O S I T Y . l5 % 25 j5 such a s gypsum, h a l i t e and cementp a s t e , showed a l i n e a r r e 1 a t i o n s h i p
Fig. 1 o v e r t h e range o f p o r o s i t y s t u d i e d .
Compaction p r e s s u r e v s p o r o s i t y f o r ( 7 , 1 0 ) . magnesium o x y s u l f a t e cement compacts
The l o g a r i t h m of microhardness and modulus o f e l a s t i c i t y a r e p l o t t e d a g a i n s t p o r o s i t y i n Figures
2 and 3; t h e s e p l o t s a r e l i n e a r f o r S e r i e s I and I 1 p a s t e samples and obey t h e general r e l a t i o n s h i p H, E = (Ho, Lo) exp [ ( - b H , $ ) p ] where H and E a r e microhardness and
modulus o f e l a s t i c i t y , r e s p e c t i v e l y , and p i s p o r o s i t y .
For microhardness d a t a , however, t h e r e s u l t s f o r S e r i e s I11 compacts a r e l i n e a r o n l y when t h e p o r o s i t y i s l e s s than 7.5 p e r c e n t . This behaviour i s s i m i l a r t o t h a t f o r compaction p r e s s u r e versus p o r o s i t y
(Figure 1 ) . The log microhardness v e r s u s p o r o s i t y r e l a t i o n s h i p f o r S e r i e s I11 compacts follows more
e n - $ 0
-
-
20 + x a E ,A "7 1 0 Yz
:
I I I I I I \ P A S T E H Y D R A T E D - \ O o S E R I E S I-
O> 0 ' , t R l t l I , - \' e r 3 ' I GROVND d C O M P C C ' [ Cf4&
I t l l f - . I , - -;
\b
1 P A I T l ~ ~ ~ ~ 3 . 1 3 0 P- ~ r x r r r ~ . t : I S ~ - i o n p w \; j; 'Aqh. -, . L!; ,00-... +",Lo
lo
""''-*.
.... . Y A
-
I... 9 -'\'-.-.c
.-.-,:'.
....,,, - 8 -\
-
:-.. ,:.. , 7 - 6 - OD\ 0 Yw,C, 3 2 U\
-'\
*:..
:
o 5 :o115
210 :5 O: 35 T h e d a t a f o r S e r i e s I I I c o m p a c t s P O R O S I T Y , 70 f a b r i c a t e d from p a r t i c l e s 5 t o 150 p m l i e s l i g h t l y above t h e d a t a f o rFIG. 2 S e r i e s 111 compacts made w i t h
Microhardness v s p o r o s i t y f o r c o a r s e r s i z e p a r t i c l e s . For magnesium oxysul f a t e cement p o r o s i t y g r e a t e r t h a n 7.5 p e r c e n t
2 4
\
c l o s e l y t h e r e l a t i o n s h i p f o r S e r i e s 5 --
\
q. :.. \.:y-
-
IY
I and I 1 p a s t e s up t o 7.5 p e r c e n t p o r o s i t y than f o r h i g h e r p o r o s i t i e s .V o l . 8, N o . 1
MAGNESIUM OXYSULFATE CEMENT, STRENGTH
I I I I I a l l I H Y D P I T I O - \ P 0 S I R I I I I (r l t A l t 5 I I P R I T ~ I GROUND 4 C D M F h C l I D l l R l i l I I I
-
-
1 0 5 1 0 15 2 0 25 30 35 P O R O S I T Y . % FIG. 3 Modulus o f e l a s t i c i t y v s p o r o s i t y f o r magnesium o x y s u l f a t e cementt h e r e l a t i o n s h i p s become non l i n e a r . Microhard- P O R E D I A M E T E R . pm n e s s v a l u e s f o r a l l compacts were g r e a t e r t h a n
microhardness v a l u e s f o r p a s t e samples a t a l l FIG. 4
p o r o s i t i e s g r e a t e r t h a n 7.5 p e r c e n t . Pore s i z e d i s t r i b u t i o n s f o r p a s t e hydrated and compacted
A t t h e p o i n t where t h e r e i s a change i n magnesium o x y s u l f a t e samples s l o p e , microhardness v a l u e s o f 160, 126 and 102
MPa were o b t a i n e d f o r compacts made w i t h
p a r t i c l e s 5 t o 150 pm, 150 t o 300 pm and 300 t o
600 pm, r e s p e c t i v e l y . Microhardness v a l u e s a r e g r e a t e r f o r compacts made w i t h f i n e r p a r t i c l e s f o r p o r o s i t y up t o 30 p e r c e n t .
The l o g modulus o f e l a s t i c i t y v e r s u s p o r o s i t y r e l a t i o n s h i p s (Figure 3) f o r a l l S e r i e s I11 compacts l i e below t h e r e l a t i o n s h i p f o r p a s t e samples. The r e l a t i o n s h i p f o r compacts, p a r t i c l e s 5 t o 150 pm, i s l i n e a r up t o approxi- mately 23 p e r c e n t p o r o s i t y . The modulus d a t a f o r a l l compacts approach t h e v a l u e s f o r S e r i e s I p a s t e samples a t low p o r o s i t i e s . The r e l a t i o n s h i p s f o r t h e compacts made w i t h p a r t i c l e s 150 t o 300 pm and 300 t o 600 pm have a change i n s l o p e between 6 and 7 p e r c e n t p o r o s i t y and g e n e r a l l y t h e modulus v a l u e s a r e lower a s p a r t i c l e s i z e i n c r e a s e s . There i s a s e p a r a t e modulus of e l a s t i c i t y - p o r o s i t y r e l a t i o n s h i p f o r S e r i e s I and S e r i e s I 1 p a s t e samples
(Figure 3 ) ; however, t h e r e i s a s i n g l e microhardness-porosity r e l a t i o n s h i p f o r a l l S e r i e s I and S e r i e s I 1 samples (Figure 2 ) .
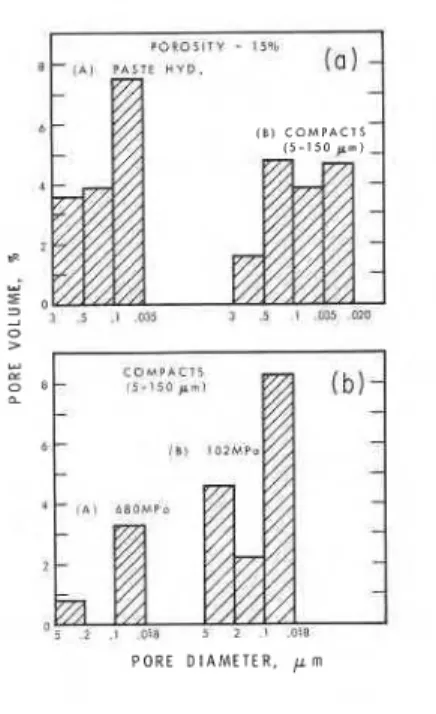
Mercury p o r e s i z e d i s t r i b u t i o n s f o r p a s t e hydrated and compacted samples a r e given i n F i g u r e s 4 ( a ) and 4 ( b ) . I t can b e seen t h a t t h e compacted samples have a l a r g e r number o f f i n e pores than do p a s t e samples a t t h e same p o r o s i t y
(15 p e r c e n t ) .
Mercury porosimetry r e s u l t s show t h a t i n compacts ( p o r o s i t y = 15 p e r c e n t ) made w i t h t h e f i n e s t p a r t i c l e s t h e f i n e r pores c o n t r i b u t e t o a l a r g e r s h a r e o f t h e t o t a l p o r o s i t y than those made w i t h p a r t i c l e s '150 pm.
Figure 5 ( a ) i s a micrograph o f o x y s u l f a t e p a s t e having s o l u t i o n / s o l i d r a t i o of 0.72. The compacted o r c o n s o l i d a t e d s t r u c t u r e a t t h i s s o l u t i o n / s o l i d r a t i o g e n e r a l l y l e a d s t o b e t t e r s t r e n g t h than t h a t prepared a t a r a t i o o f 1 . 4 3 i n
108 Vol.
3 ,
No. 1 J. J. Beaudoin, V. S. Ramachandran FIG. 5 ( a ) Magnesium o x y s u l f a t e p a s t e s o l u t i o n / s o l i d = 0 . 7 2 which p o r o s i t y i s h i g h e r and an i n c r e a s e d amount o f p l a t y m a t e r i a l i s o b s e r v e d ( F i g u r e 5 (b).
F i g u r e 5 ( c ) i l l u s t r a t e s t h e s u r - f a c e f e a t u r e s o f a n o x y s u l f a t e sample compacted a t 133 MPa. The appearance i s g e n e r a l 1 y amorphous and d e n s e r w i t h some e v i d e n c e o f p l a t y m a t e r i a l and t h e sample i s much s t r o n g e r t h a n i n 5 ( b ) , al'though t h e p o r o s i t i e s a r e a b o u t 15 p e r c e n t i n b o t h samples. F i g u r e 6 i s a p l o t o f t h e r e l a t i o n s h i p between modulus o f e l a s t i c i t y and microhardness f o r samples s t u d i e d . The r e l a t i o n - s h i p s a r e l i n e a r ; t h e l i n e f o r t h e p a s t e samples having a s t e e p e r s l o p e . Soroka and S e r e d a have o b s e r v e d s i m i l a r r e s u l t s f o r p o r t l a n d cement p a s t e and compacts ( 1 1 ) . FIG. 5(b) Magnesium o x y s u l f a t e p a s t e s o l u t i o n / s o l i d = 1 . 4 3 FIG. 5 ( c ) Magnesium o x y s u l f a t e compact; compaction p r e s s u r e = 133 MPa I n F i g u r e 7, t y p i c a l thermograms f o r S e r i e s I and I 1 magnesium o x y s u l f a t e samples a r e p r e s e n t e d . There a r e two low t e m p e r a t u r e endothermal peaks a t 105°C and 155°C. There i s a l s o a l a r g e endothermal peak a t a p p r o x i m a t e l y 4 5 0 ' ~ . The two low temperature peaks have been a t t r i b u t e d t o t h e d e h y d r a t i o n o f 3 Mg(0H) 2. MgSOt, .8H20 (3).
The l a r g e peak a t 475OC i s due t o t h e decom-p o s i t i o n o f Mg(OH)2. There i s l e s s Mg(0H) p r e s e n t i n S e r i e s I 1 samples. Also, t h e r a t i o o f t h e low t e m p e r a t u r e peak h e i g h t s t o t h e peak h e i g h t a t
Vol. 8, N o . 1
MAGNESIUM OXYSULFATE CEMENT, STRENGTH 450°C i s g r e a t e r f o r S e r i e s I 1 samples
than f o r S e r i e s I samples. The a r e a s o f
t h e two low temperature peaks a r e g r e a t e r f o r S e r i e s I 1 samples i n d i c a t i n g more
magnesium o x y s u l f a t e complexes have formed. 3 o O P A S , L H Y D R A , [ o
X-ray d i f f r a c t i o n measurements were made on a l l S e r i e s I samples and S e r i e s I 1
samples having s o l u t i o n / s o l i d = 0 . 7 2 and
1 . 0 7 . The r e s u l t s i n d i c a t e t h e p r e s e n c e of
3Mg (OH) 2 . MgS04. 8H20, l a r g e amounts o f
Mg(OH)2 and some anhydrous MgO i n S e r i e s I
samples. I n a d d i t i o n t o t h e above presence
o f 5Mg(OH) 2 .MgS04. 3H20 was d e t e c t e d i n
S e r i e s I 1 samples. However, t h e presence o f 3Mg(OH)2.MgS04.8H20 i n b o t h S e r i e s I and
I 1 was more r e a d i l y apparent from DTA
P A S T [ G R O U N D d I O M P A C T r D A P A R T I C L E S 2 - 1 5 0 p m P d R T C L E S 1 5 0 - 3 0 0 pm 0 ~ L U I I C L ! . 3 0 0 - 6 0 0 p m , . t r a c e s ; i t i s suggested t h a t f o r i d e n t i f i -
,I
.
I I c a t i o n o f o x y s u l f a t e complexes DTA i s a I ! O 1more s e n s i t i v e t o o l than XRD. DTA r e s u l t s 3 2 4 I 6 I 8 1 0 1 2
I
c l e a r l y show t h a t t h e amount o f Mg(OH)2 i s M O D U L U S O F E L A S T I C I T Y , ~ ~ 3 x 1 0 . ~
l e s s i n S e r i e s I 1 samples compared w i t h S e r i e s I samples; a l s o , t h e amount of
o x y s u l f a t e complex formed i s g r e a t e r than FIG. 6
t h a t formed f o r S e r i e s I samples. In Microhardness v s modulus of
S e r i e s I 1 samples, more MgO h a s p r e f e r e n - e l a s t i c i t y f o r magnesium
t i a l l y r e a c t e d t o form t h e o x y s G f a t e complex l e a v i n g l e s s Mg(OH)2.
oxysul f a t e cement S u r f a c e a r e a and microhardness measurements f o r p a s t e samples a r e given i n Table I . I t i s apparent t h a t t h e r e i s no d i r e c t r e l a t i o n s h i p between s u r f a c e
a r e a and s t r e n g t h development f o r t h e v a r i o u s o x y s u l f a t e p r e p a r a t i o n s , e
.
g . ,samples a t s o l u t i o n / s o l i d = 0.84 have h i g h e r s u r f a c e a r e a b u t lower microhard-
n e s s t h a n samples with s o l u t i o n / s o l i d = 0 . 5 9 .
FIG. 7
110
Vol.
8,
No.
1
J. J. Beaudoin, V. S. Ramachandran
TABLE I
Surface a r e a and microhardness d a t a f o r magnesium o x y s u l f a t e p a s t e samples S u r f a c e Area Microhardness S o l n / S o l i d Cm2/ g) IMPa) 0.59 11.5 30 3 0.72 15.6 175 0.84 24.8 101 0.96 23.8 6 2 1.07 20.4 5 9 1 . 4 3 14.6 2 2 Discussion
The change i n s l o p e o f t h e l o g compaction p r e s s u r e v e r s u s p o r o s i t y curve (Figure 1) may p o s s i b l y b e explained a s follows: The p a r t i c l e s themselves a r e porous, although i t was observed t h a t pore s i z e s l a r g e r than 1 pm were a b s e n t i n t h e f i n e s t p a r t i c l e s i z e range. P a r t i c l e s i n t h e 300 t o 600 pm s i z e range had t h e following pore s i z e d i s t r i b u t i o n : 58 t o 10 pm (4 p e r c e n t ) , 10 t o 1
pm ( 2 . 5 p e r c e n t ) , 1 t o . 1 pm (3.1 p e r c e n t ) and .1 t o . O 1 pm (9.4 p e r c e n t ) ;
p a r t i c l e s 150 t o 300 pm: 35 t o 10 pm ( 1 p e r c e n t ) , 10 t o 1 pm ( 3 p e r c e n t ) ,
1 t o .1 pm (3.4 p e r c e n t ) and .1 t o .O1 pm ( 9 . 8 p e r c e n t ) ; p a r t i c l e s 5 t o 150
pm: 1 t o .1 pm (3.4 p e r c e n t ) and .1 t o . O 1 pm (4.4 p e r c e n t ) . I n t h e high p o r o s i t y region, compaction probably o c c u r s mostly by s l i d i n g o f p a r t i c l e s p a s t one a n o t h e r w i t h some deformation and f r a c t u r e . In the region below 7.5 p e r c e n t p o r o s i t y , most of t h e compaction may involve a decrease of small p o r e s which can b e accomplished o n l y by deformation and f r a c t u r e . I t i s n o t e - worthy t h a t t h e region below 7.5 p e r c e n t covers a p o r o s i t y range o f o n l y 3.5 p e r c e n t .
Figure 4(b) shows t h a t by i n c r e a s i n g t h e compacting p r e s s u r e from 102 MPa t o 680 MPa t h e r e i s a d e c r e a s e i n t h e o v e r - a l l p o r o s i t y due t o e l i m i n a t i o n of some l a r g e r p o r e s and a 60 p e r c e n t decrease i n t h e percentage o f f i n e pores. Compaction a t low p o r o s i t i e s o c c u r s mostly by f r a c t u r e of p a r t i c l e s t h a t have a l r e a d y been f r a c t u r e d ; t h e s e crushed p a r t i c l e s a r e probably s t r o n g e r and a r e confined by t h e i n t e r f e r e n c e with t h e i r neighbours. Also a t low p o r o s i t i e s , i t i s p o s s i b l e t h a t bond s t r e n g t h may i n c r e a s e p a r t l y a s t h e r e s u l t of chemi- c a l bond formation and p o s s i b l y a l s o due t o s t r a i n hardening. Smaller
p a r t i c l e s form s t r o n g e r b o d i e s , n o t o n l y because of t h e foregoing r e a s o n s b u t because of i n c r e a s e d bond a r e a .
Generally, s t r e n g t h o f cementi t i o u s systems, f o r a p a r t i c u l a r p o r o s i t y , i n c r e a s e s a s t h e average pore r a d i u s d e c r e a s e s (12). This i s c o n s i s t e n t w i t h the r e s u l t s o f t h i s s t u d y f o r compacted and p a s t e hydrated samples.
The e x p l a n a t i o n a s t o why t h e modulus of e l a s t i c i t y v a l u e s f o r compacts a r e lower t h a n t h e v a l u e s o b t a i n e d f o r p a s t e samples, while t h e converse i s t r u e f o r microhardness, i s n o t c l e a r . However, t h e r e s u l t s may b e t e n t a t i v e l y e x p l a i n e d i f i t i s assumed t h a t f o r compacted samples t h e i n t e r p a r t i c l e bonds a r e weaker than they a r e f o r p a s t e hydrated samples. The i n t e r p a r t i c l e bonds may p l a y a dominant r o l e i n determining t h e modulus o f e l a s t i c i t y . One would expect lower v a l u e s o f modulus of e l a s t i c i t y f o r compacted samples t h a n f o r p a s t e samples if t h e bonds a r e n o t a l l remade on compaction of t h e powder. On
Vol. 8, No. 1
MAGNESIUM OXYSULFATE CEMENT, STRENGTH
t h e o t h e r hand, i f microhardness i s dependent t o a l a r g e e x t e n t on t h e
s t r e n g t h of t h e s o l i d component, i t would b e p o s s i b l e f o r p a s t e samples t o
have lower v a l u e s o f microhardness than compacted samples. P a s t e s a r e weaker
than compacts a t p o r o s i t i e s g r e a t e r than 7.5 p e r c e n t ; i n p a s t e hydrated samples p r e c i p i t a t i o n and c r y s t a l l i z a t i o n l e a d s t o formation of l a r g e r
c r y s t a l s with l e s s bond a r e a ( s e e Figure 5 ( b ) ) . For p a s t e s a t low p o r o s i t i e s , t h e r e i s more i n t i m a t e s u r f a c e - t o - s u r f a c e c o n t a c t between p a r t i c l e s of t h e
hydrated product because t h e r e i s l e s s space f o r migration o r formation o f
d i s c r e t e c r y s t a l s (Figure 5 ( a ) )
.
I n some c e m e n t i t i o u s systems, t h e i n c r e a s e i n compressive s t r e n g t h has been explained i n terms o f t h e development o f high s u r f a c e a r e a ( 1 3 ) . Recent
evidence has i n d i c a t e d t h a t t h e r e i s no c o r r e l a t i o n between s u r f a c e a r e a and
s t r e n g t h development i n such systems (14, 1 5 ) .
S u r f a c e a r e a measurements provided l i t t l e i n s i g h t i n t o t h e observed mechanical behaviour o f magnesium o x y s u l f a t e cement system a s t h e r e was no c o r r e l a t i o n between s u r f a c e a r e a and s t r e n g t h development.
Oxysulfate cement has been g e n e r a l l y considered t o b e weaker t h a n oxychlo- r i d e cement, b u t t h i s comparison h a s n o t been done on t h e b a s i s of equal
p o r o s i t y . A comparison o f t h e p r e s e n t r e s u l t s with o b s e r v a t i o n s on oxychlo-
r i d e cement (8) i n d i c a t e s t h a t f o r t h e same p o r o s i t y , oxychloride samples a r e s t r o n g e r than o x y s u l f a t e samples.
Conclusions
The o x y s u l f a t e system behaves d i f f e r e n t l y than t h e p o r t l a n d cement system
when t h e s e systems a r e formed by compaction, i . e
.,
t h e o x y s u l f a t e p a s t e , un-l i k e t h e p o r t l a n d cement system, i s weaker than t h e compacted system a t h i g h e r
p o r o s i t i e s . I t i s apparent t h a t t h e use o f compacts a s s t r u c t u r a l models f o r
t h e o x y s u l f a t e system i s l i m i t e d t o low p o r o s i t i e s , i . e . , l e s s than 7.5 p e r
c e n t . I t i s a l s o a p p a r e n t t h a t compaction p r o c e s s e s a t h i g h e r p o r o s i t i e s
a l t e r t h e morphology, pore s i z e ' d i s t r i b u t i o n and t h e e x t e n t of bonding between p a r t i c l e s r e s u l t i n g i n d i f f e r e n c e s i n s t r e n g t h of p a s t e and compacted systems.
From t h e p r a c t i c a l p o i n t of view, however, i t appears t h a t p r e s s i n g t h e oxy-
s u l f a t e sys tem can produce specimens having good s t r e n g t h .
There appears t o be no d i r e c t r e l a t i o n s h i p between s u r f a c e a r e a and s t r e n g t h development f o r t h e v a r i o u s o x y s u l f a t e p r e p a r a t i o n s s t u d i e d . The mechanical behaviour o f magnesium oxysul f a t e cement can b e explained on t h e b a s i s o f t h e r e l a t i v e c o n t r i b u t i o n o f p o r o s i t y , pore s i z e d i s t r i b u t i o n , i n t e r p a r t i c l e bonds and s t r e n g t h of t h e s o l i d phase.
This paper i s a c o n t r i b u t i o n from t h e Division o f Building Research,
National Research Council of Canada and is p u b l i s h e d w i t h t h e approval of t h e
D i r e c t o r of t h e Division.
References
1 . S . S o r e l . Compt. Rend. - 65, 102 (1867).
2 . T . Demediuk and W . Cole. Aust. J . Chem. - 10, 287 (1957).
3 . E.S. Newman. J . of Research, Nat. Bur. S t d . A, 68A, 645, (1964).
4 . G . H a l l , G . Read and R . B r a d t . Paper p r e s e n t e d a t 78th Meeting American
112 Vol. 8, No. 1
J. J. Beaudoin, V . S. Ramachandran
I
V.S. Ramachandran and R . F. Feldman. Cem. and Concrete Research
3,
729 (1973).
R.F. Feldman and V.S. Ramachandran. J . Amer. Cer. Soc. 49, 268 (1966).
-
I . Soroka and P . J . S e r e d a . J . Am. Ceram. S o c . 51, 337 (1968) -
J . J . Beaudoin and V.S. Ramachandran. Cem. and Concrete Research
-
5,617 (1975).
R.F. Feldman. J . Cem. Tech. 3,
-
5 (1972).P . J . S e r e d a , R . F . Feldman, and E . G . Swenson. Highway Research Board Sp.
Report 90, 58 (1966).
I . Soroka and P . J . S e r e d a . Proc. V I n t . Symp. Chem. Cerm., Tokyo,
P a r t 111, Vol. 111, 67 (1968).
A . T r a e t t e b e r g and V.S. Ramachandran. J . Appl. Chem. and B i o t e c h . 24,
-
169 (1974).
A. C e l a n i , M. C o l l e p a r d i and A . Rio. L 1 I n d u s t r . I t a l . Cemento,
36,
669 (1966).
V.S. Ramachandran. Matgriaux e t C o n s t r u c t i o n s - 4 , 3 (1971)