Publisher’s version / Version de l'éditeur:
ACI Materials Journal, 94, May/June 3, pp. 220-226, 1997-05-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Effect of different zeolites on conversion-prevention in high alumina
cement products
Ding, Jian Dept.; Fu, Y.; Beaudoin, J. J.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=120c3bcf-889c-496f-abc2-92deb5b9e2ef https://publications-cnrc.canada.ca/fra/voir/objet/?id=120c3bcf-889c-496f-abc2-92deb5b9e2efhttp://www.nrc-cnrc.gc.ca/irc
Effe c t of diffe re nt ze olit e s on c onve rsion-pre ve nt ion in high a lum ina
c e m e nt produc t s
N R C C - 3 9 2 7 0
D i n g , J i a n D e p t . ; F u , Y . ; B e a u d o i n , J . J .
M a y 1 9 9 7
A version of this document is published in / Une version de ce document se trouve dans:
ACI Materials Journal, 94, (3), May/June, pp. 220-226, May 01, 1997
The material in this document is covered by the provisions of the Copyright Act, by Canadian laws, policies, regulations and international agreements. Such provisions serve to identify the information source and, in specific instances, to prohibit reproduction of materials without written permission. For more information visit http://laws.justice.gc.ca/en/showtdm/cs/C-42
Les renseignements dans ce document sont protégés par la Loi sur le droit d'auteur, par les lois, les politiques et les règlements du Canada et des accords internationaux. Ces dispositions permettent d'identifier la source de l'information et, dans certains cas, d'interdire la copie de documents sans permission écrite. Pour obtenir de plus amples renseignements : http://lois.justice.gc.ca/fr/showtdm/cs/C-42
Effect of Different Zeolites on Conversion-Prevention in
High Alumina Cement Products
(J
LLセセ
Lセ{'
,
",by Jian Ding, Van Fu, and
J.
J. Beaudoin
Hydration and strength del'elopment characteristics. of high alumina cemellt (HAC) containing sodium sulfate and a variety of different zeolites were studiul. The zeolites obtained from different sources included differ-ent types containing clinoptilolite, chabazite, sti/bite, and natrolite. The one-day compressive strength of HAC mortars containing a commercially available zeolite alld sodium sulfate was as high as 60 MPa. No strength reduction occurred ill the HAC mortars water-cured at 38 Cfor 330 days. Hydrogamet formatiOlI was significantly inhibited. Clinoptilolite or chaba-zite-based zeolites in combination with sodiulli sulfate were more effective in preventing the formation of hydrogal'lletillHAC paste than sti/bite or natmlite-based zeolites. Zeolite alone was IlOt able to prevent the hydrog-arnetformationiltthe HAC paste. Chabazite was the lIIost effective zeolite in promoting str{irlingite formation in the HAC paste.
Keywords: compressive strength; conversion; high alumina cement (HAC); hydrogarnet; temperature; sodium sulfate; stratlingite; zeolite(s).
RESEARCH SIGNIFICANCE
This research addresses utilization of natural zeolites in the production of a conversion-preventing additive for high alumina cement products. Zeolites are among the most plen-tiful minerals in the world. These new additives make it pos-sible to reconsider the use of high alumina cement in concrete structural members.
INTRODUCTION
The predominant phase in high alumina cement (HAC) is
calcium monoaluminate (CA*).Hydration of HAC
eventual-ly results in formation of crystalline hydrogarnet (C3AH6),a
thermodynamically stable aluminate hydrate. High early strength, good chemical resistance, and high temperature re-sistance of HAC products has encouraged the use of high alumina cement concrete in certain engineering applications.
However, the first products of hydration are usually CAHIO
or C2AHs which later convert to cubic hydrogarnet
(C3AH6).The conversion reaction which occurs in hydrated
HAC concrete under certain environmental conditions has been one of the major problems limiting its use because it is usually associated with loss of strength. The conversion
pro-'Mineralogical notation」オウエッセ。イケ in cement science is used throughout the paper: C=CaO; A=A1203 H=H20; 5=5'02'
220
cess in HAC systems .has been extensively studied for de-cades. An HAC-based blended cement containing about equal masses of HAC and ground granulated blast-furnace slag (ggbs) was commercialized by the Building Research Establishment (BRE), UK. The incorporation of the ggbs was suggested as a way of preventing the conversion
reac-tion.I-? The prevention of the reaction was attributed to
stratlingite formation in preference to the hydrogarnet. The early strength of this material was significantly less than that of HAC alone. Other siliceous additives such as silica fume have been reported to favor the formation of stratlingite
(C2ASHs).s The use of a siliceous material in combination
with a sodium salt in HAC was recently reported to be more effective than the siliceous material alone in promoting
for-mation of stratlingite instead of hydrogarnet. 9-15Siliceous
materials, including natural zeolites, fly ash, ggbs, and silica fume, were all effective in inhibiting hydrogamet foonation and preventing strength reduction in HAC products when added in combination with a sodium salt. A new conversion-preventing additive for HAC has been recently developed at
the National Research Council, Canada.16The one-day
com-pressive strength of HAC mortars containing 10-30 percent
additive, with a water/solid (HAC
+
additive) ratio of0040,was up to 60 MPa. The modified HAC products did not un-dergo strength reduction at later ages when water-cured at 38 C. A large amount of striitlingite was formed in the products. Naturally-occurring zeolites, but not by-product zeolites, have consistent pozzolanic properties. They are suitable ma-terials for industrial production of stable, high quality, con-version-inhibited HAC products, because zeolites are relatively inexpensive and the cost of HAC/zeolite blended cements should be less than that of HAC. Zeolites are crys-talline hydrated aluminosilicates of the alkalis and alkaline
ACI Materials Journal,V. 94, No.3, May-June 1997.
Received July 21. 1995, and reviewed under Institute publication policies. Copy-right© 1997. American Concrete Institute. All rights reserved. inclUding the making of copies unless permission is obtained from the copynght proprietors. Pertinent dis-cussionwill be published in the March-April 1998 ACI Materials Joumal if received by December 1, 1997.
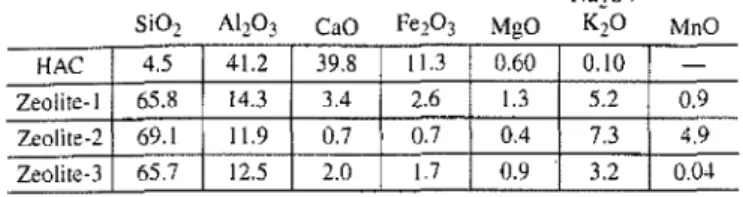
Ox ide compositions, mass percent -HAC 4.5 41.2 39.8 11.3 0.60 0.10 -Zeolite-I 65.8 14.3 3.4 2.6 1.3 5.2 0.9 Zeolite-2 69.[ 11.9 0.7 0.7 0.4 7.3 4.9 Zeolite-3 65.7 12.5 2.0 1.7 0.9 3.2 0.04
oZeolite-to: a synthetic molecular sieve (Type 13X),
hav-ing a formula (mol ratio): 1 I Na20: 1 A1203:2.8±0.2 Si02:
X H20
oAnhydrous sodium sulfate (NaZS04): a reagent grade chemical
The oxide analysis of HAC and some zeolites
(clinoptiIo-lite group) are listed in Table 1.
Na20+
Si02 AI20 3 CaO Fe,03 MgO K20 MnO
ACI member Yan Fu is a PhD calldidateillthe Departmellt of Civil Ellgilleel'illg. UIl!-veniry of OUawa. He is rhe author of rhree
us
pntem applicatiollS and,,11111(1"011.'rese(lrch pilpers o}/concrete dlirabillty andeXPQns/l'Cconctele.
Jame. }. Beaudoin is Prillcipal Research Officer and Head. Marerials LtdJOraror\,. Institllte for ReseCII'CiliiiConstructioll. Natilmal Research COllncil of Canada. He is
the author of numerous research papers andfwobooks on concrere m{l/eria[s, His
currentresearch/ocl/usonュゥN」イッセjゥ「・イ reinforced Cement systems and the application of G. c. impedance spectroscopy in ceme/U and concrete science.
earths; their crystals consist of a three-dimensional frame-work of Si04 and AI04 tetrahedra. Compositionally, zeolites may be given the general f01111ula:
jian Ding is a )'esearch engineer at the Institute for Researchin Construction,
Natiollal Research COimci! of Canada. She reeeil'ed he" SSeillchemislr"litFlldtllJ
Ulliver.,ity, Shallghai, in 1983, and her MaSc in civil ellgineering at the Ulliwl'sil\' of Ottawa ill1995.She is Ihe IIIlIhor oftwo
us
pntetll appllcatiollS and several research papers. Her researchinterests includeadmixWrej'andlIdditivesfor portlcmd cemem and lIiRh alumina cement concretes.M+ is usually Na+ or K+; M2+ is MgZ+, Caz+, or Fez+. More
than 30 distinct species of zeolites occur in nature. Most zeo-lites have a specific gravity of 2.0-2.3 and refractive indices of 1.44-1.52, Natural zeolites are supplied by several companies in North America. Two types of natural zeolite, clinoptilolite and chabazite, are available for industrial application.
The objective of this paper is to report the effect of differ-ent types of zeolite, from differdiffer-ent sources, on conversion re-actions involving stratlingite and hydrogarnet formation in HAC systems. Their influence on prevention of strength re-duction resulting from conversion reactions in HAC pastes or mortars containing sodium sulphate is evaluated.
EXPERIMENTAL Materials
The following materials were used in this study: oHigh alumina cement (HAC)
oZeolite-l: a natural mineral from Texas, USA, containing mainly c1inoptilolite, (Na4,K4)AlgSi40096024H20
oZeolite-2: a natural mineral from Nevada, USA; contain-ing mainly clinoptilolite and gismondine
oZeolite-3: a natural mineral from Western Canada, con-taining mainly clinoptilolite and gismondine
oZeolite-4: a natural mineral from Washington, USA; con-taining mainly clinoptilolite
oZeolite-5: a natural mineral from Western Canada, con-taining mainly clinoptilolite
oZeolite-6: a natural mineral from Arizona, USA, contain-ing mainly chabazite, CazAI4SisOZ4012H20
oZeolite-7: a natural mineral from Nova Scotia, Canada, containing mainly stilbite, NaCa2AI15Si13036014H20
oZeolite-8: a natural mineral from Oregon, USA, contain-ing mainly natrolite, Na2AI2Si30lOo2H20
oZeolite-9: a synthetic molecular sieve (Type 4A), having a fonnula (mol ratio); 1 Na20: I INa20: IAI 20 3:2.0±0.1 Si02: X H20
Test methods and analysis
Compressive Strength Test: HAC/zeolite cement mortars were prepared for dete1111ination of compressive strength. Ze-olites-I, 2, 3, 4, and 5 were used in this test. These zeolites, comprising mainly clinoptilolite, are all available for industri-al use in North America. Clinoptilolite is the most plentiful natural zeolite mineral in the world. The other zeolites, (i.e. Zeolites-6-10), may not be suitable for use in concrete because of their relatively high cost or limited availability. The sandi HAC ratio in the mortars was 2.75 and the water/solid (HAC
+zeolite) ratio was 0.40. The cement mortar was mixed for 3
minutes and then cast in 50.8x50.8x50.8 mm cube molds. The specimens were demolded after 24 hours of moist-curing at 23 C. Five specimens were used for each dete1111ination. The one-day compressive strength was determined after demolding. Companion specimens were placed in water at 38 C after demolding. The compositions of the HAC/zeolite mortars are given in Table 2.
X-ray diffraction analysis: X-ray diffraction analyses were carried out on the hardened HAC pastes containing 4.7 per-cent sodium sulfate and 20 perper-cent zeolite by mass of HAC. Five natural zeolites comprising mainly clinoptilolites, a chabazite, a stilbite, a natrolite, and two synthetic zeolites were used. The cement paste samples were prepared with a
water/solid (HAC+zeolite) ratio of 0.60. The paste samples
Table 2-Content of additives in HAC/zeolite mortars containing sodium sulfate
Zeolite Content. Sodium sulfate. mass percent of Samples mass percent of HAC Type HAC 1 (control) - - -2 1.0 .[ 20.0 3 3.0 -I 47.0 4 1.6 -2 10.0 5 I.S -3 18.2 6 1.0 -4 15.0 7 1.8 -5 18.2
•
•
•
•
(b)
HAC/sodium sulfate
r,',',',UI"i"Pi'r"t""'I"'i'YSFf'FiU""r""'i"r'i'ai',it"
eel',,',.,'''''!
5 10 15 20 25 3D 35 40 45 50Degrees (28)
RESULTS AND DISCUSSION
The effect of different zeolites on strength development of the HAC/zeolite mortars is shown in Table 3, The relative er-ror of compressive strength was less than 3 percent.
. The one-day strength was obtained from the specimens moist-cured at 23 C. Companion specimens were then placed in water at 38 C. The plain HAC mortar (the control) had the highest compressive strength (65/9427 MPa/psi) at one day. Its strength decreased dramatically to 34 MPa (4931 psi) at 14 days due to the conversion reaction. The strength remained at this level until 330 days. The HAC mortar con-taining 20 percent zeolite-A and 1.0 percent sodium sulfate by mass of HAC also had a relatively high one-day strength (62/8992 MPa/psi). The strength continuously increased to 72 MPa (10442 psi) at 150 days and maintained this value at 330 days. A high volume addition ofzeolite-l(47 mass. per-cent of HAC) resulted in a relatively low one-day strength (40/5801 MPa/psi) of the HAC/zeolite mortar. Its strength increased significantly at early ages and reached 64 MPa (9282 psi) at 14 days and 74 MPa (10,732 psi) at 28 days. A relatively small content of zeolite-2 was used in the HAC/ zeolite mortar sample 4. The one-day compressive strength was 60 MPa (8702 psi) and decreased slightly to 57 MPa (8267 psi) at 28 days. Further hydration increased the strength to 72 MPa (10,442 psi) at 150 days. The HAC mor-tar containing zeolite-3 had a one-day strength of 49 MPa (7107 psi). A large strength gain (to 61/8847 MPa/psi) oc-curred during the first 14 days of hydration. The strength then increased gradually to 70 MPa (10,152 psi) at 330 days, Use of Zeolite-4 and -5 resulted in a relatively low early compressive strength value of less than 50 MPa (7252 psi) before 28 days. The ultimate strength of the HAC mortar containing zeolite-D reached 62 MPa (8992 psi) at 330 days. The one-day compressive strength of the HAC/zeolite-sodi-um sulfate mortar can be as high as that of the plain HAC mortar. It is apparent that high early strength, one of the ad-vantages of HAC, can be obtained with an HAC/zeolite
28 days
o Stritllngite
• Hydrogarnet
•
Degrees
(28)(a) Plain HAC
l"i,II"'I'I'''''''I''''''.''''''''''''''''''''''''''''''.'1"',L,Lii,"iiiifji""iiprf
3 day!
5 10 15 20 25 30 35 40
4s
50
Fig. l-XRD spectra ofIIplain HAC paste and an HAC paste containing sodium sulfate
'Moist-curedat100 r.h. and 23 C (73 F)
tWater-cured at38 C(J00 F) after the first 24 hours moist-curing at 23 C (73 F)
were cast in 25 mm diameter bottles and rotated on a roller machine for 24 hours at 38 C to prevent segregation and bleeding. Samples were water-cured at 38 C after demold-ing. X-ray diffraction analysis was carried out on wet sam-ples after wet grinding in an agate mortar. The test was designed for the comparison of companion samples prepared by an identical process. The comparison is based on signifi-cant differences between parallel samples. Some samples were repeated three times. The relative error of the peak
height (e.g. CzASH8 at d=1.07 nm, calculated by Rigaku
Standard Data Processing software) was 10 percent. A Rigaku X-ray (CuKa radiation) Diffractometer System Gei-gerflex D/Max-B was used for x-ray studies.
222 ACI Materials Journal/May-June 1997
Compressive strength, MPaipsi
150 330 Sample I day* 14 dayst 28 dayst dayst dayst
I 6519427 34/4931 3615221 34/4931 34/4731 (control) 2 62/8992 6519427 69/10,007 72110,442 72110,442 3 40/5801 64/9282 74110,732 75/10,877
-4 60/8702 59/8557 57/8267 72110,442 72110,442 5 4917107 61/8847 64/9282 69/10,007 70/10,152 6 47/6817 47/6817 46/6672 61/8847 62/8992 7 38/5511 34/4931 42/6091 --Table 3-Compressive strength of HAC/zeolite mortars
o
StratUngite
• Hydrogamet
•
Zeolites
E•
•
o
c
B
5 10 15 20 25 30 35 40Degrees (29)
oI.
It.1
j
.1...&1- (
1
aセセセ
45 50 5 10 15 20 25 30 35 40 45 50Degrees (29)
o(a) HAC/zeolite pastes
without sodium sulfate
at
7 days
Zeolites
E(b) HAC/zeolite
pastes
without
sodium
sulfate
at 330
days
o
c
B
1'1'
lit'
''1'""
I'
''t''
,'I'
"p'
BセiG
''1','1'.
tT""'il
'I""'I'I'f" ,"'IT
,'''1''''
A
イセiセNLコセゥGセゥGiセlB[QTセGc
ゥセオiQ[ゥ[ZG LZエセGゥZZゥᆬGセB]GGG]GDセゥGゥZTGZエGセiGGセG
QGセゥLZBLセオBセGZセBゥZG{G[ゥGャセゥゥセGQGセiGLp[BGセゥGセャゥエ
5 10 15 20 25 30 35 40 45 50 5 10 15 20 25 30 35 40 45 50
Degrees
(29)
Degrees (29)
(c)
HAC/zeolite
pastes
with sodium sulfate
at
7
days
(d)
HAC/zeolite pastes
with sodium sulfate
at
330
days
Fig. 2-XRD spectra of HAC pastes containing zeolites from different sources, with or without sodium sulfate
o Stratlinglte • Hydrogarnet
•
Zeolites•
40 45 50Degrees
(28)
5 10 15 20 StilbiteNatrolite
r
t'i'i'"r'c
'f""!,,'.'..,')','\
Ie" riC 14,',,1'
I'.' I'"I'''
""i'
r
I'i'i'"I'
ris iii'I
5 10 15 20 25 30 35 40 45 50
Degrees (28)
•
•
•
ACI Materials Journal/May-June 1997
Degrees
(28)
(d)
HAC/zeolite pastes
with sodium sulfate at 330 days
5 10 15 20 25 30 35 40 45 50
""'t"1
I'
"1'F4'1'"iSis"I',I';""r"
"""t'
,4iii'"I'
I'is1"'1'
ii iiil"1 'i'Ii,'i'1
(b) HAC/zeolite
pastes
without sodium sulfate at 330 days
Stilbite Zeolites
(c)
HAC/zeolite pastes
with sodium sulfate at 7 days
(a) HAC/zeolite pastes
without sodium sulfate at 7 days
224
•
•
Fig. 3-XRD spectra of HAC pastes containing different types of zeolites, with or without sodium sulfate
5 10 15 20 25 30 35 40 45 50
Degrees
(20)
o Stratlingite
• Hydrogarnet
o Stratllngite • Hydrogarnet
•
Molecular Sieves 4A o 0セャセGGGゥセ
13 Xi""'I"''f''''''i':)''''i'i'T''''''''I''i'''i''''j'ji
ji''l''i'"""1" .•
'.1"1',•••,1"1
5 10 15 20 25 30 35 40 45 50 5 10 15 20 25 30 35 40 45 50Degrees
(26)
(a)
HAC/zeolite
pastes
without sodium sulfate at
7
days
o Stratllngite • Hydrogamet Molecular SIeves
Degrees (29)
(b)HAC/zeolite pastes
without sodium sulfate at 330 days
t' c' i"'"
I'••"",'
I'
i't'i"'r"i'"
.,,.lill"tAr"
iii'IiI'
iLt'I'itr'·'4
1"'1"'''''''1
5 10 15 20 25 30 35 40 45 50
Degrees
(28)
(c)
HAC/zeolite pastes
with sodium sulfate at 7 days
aセH
5 10 15 20 25 30 35 40 45 50
Degrees
(29)
Cd)
HAClzeolite pastes
with sodium sulfate at 330 days
Fig. 4-XRD spectra of HAC pastes containing different synthetic zeolites, with or without sodium sulfate
blended cement. Little or no strength reduction occurred in the HAC/zeolite mortars containing sodium sulfate.
The x-ray diffraction (XRD) spectra of a plain HAC paste and an HAC paste containing 4.7 percent sodium sulfate by
mass of HAC are shown in Fig. 1. Hydrogarnet (C3AH6)was
detected in both plain HAC and HAC/sodium sulfate pastes at all ages. Small peaks representing stratlingite could be found in the sample containing sodium sulfate at 3 days (Fig. Ib). Sodium sulfate appeared to accelerate the stratlingite formation at 38 C and early ages. Sodium sulfate alone is not able to inhibit hydrogarnet formation.
The XRD spectra of HAC/zeolite pastes with or without sodium sulfate are shown in Fig. 2. Hydrogarnet was pro-duced in all the HAC pastes containing zeolites (without so-dium sulfate) from the different sources (Fig. 2a and b). The major component of these zeolites was clinoptilolite.
ACI Materials JournalIMay-June 1997
Stratlingite was detected in these samples at 7 days. It was
depleted or disappeared at 330 days.Itis evident that
clinop-tilolite-based zeolite alone is not effective in reducing the hydrogamet formation. Hydrogarnet formation was appar-ently inhibited in the HAC pastes when sodium sulfate was added in combination with the zeolites (Fig. 2c and d). Only a trace of hydrogarnet was found in the pastes containing zeolite- I, 2, 3 or 5 at 7 days. Little or no hydrogarnet was detected in zeolite- I and 3 samples at 330 days. Zeolite-4 specimens appeared to be less effective in preventing hydro-garnet formation than the other zeolites. Stratlingite was present in the HAC/zeolite pastes containing sodium sulfate. The effect of zeolite types, e.g. zeolite- I, clinoptilolite; zeoIite-6, chabazite; zeolite-7, stilbite; zeolite-8, natrolite, on hydrogamet and stratlingite formation in the HAC paste with or without sodium sulfate was shown by XRD analysis
, (Fig. 3). Zeolite addition alone irrespective of type did not limit the hydrogarnet fonnation (Fig. 3a and b). Chabazite appeared to be more favorable for striitlingite formation in the HAC paste. Addition of sodium sulfate in the HAClzeo-lite pastes apparently inhibited the hydrogamet formation (Fig. 3c and d). Clinoptilolite and chabazite were evidently more effective in suppressing the hydrogamet formation than stilbite and natrolite. No hydrogamet peak was detected in the clinoptilolite and chabazite samples at 7 days. Only very small peaks of hydrogamet were traced in these sam-ples at 330 days. Hydrogamet was detected in stilbite and
na-trolite samples at 7 days. It is apparent that chabazite in
combination with sodium sulfate produced more str1itlingite than the other zeolites.
XRD spectra of HAC pastes containing a synthetic zeolite, Type 4A molecular sieve or Type 13X molecular sieve, with or without sodium sulfate, are shown in Fig. 4. Addition of molecular sieve alone in the HAC paste could not effectively inhibit the fonnation ofhydrogamet (Fig. 4a and b). Stratling-ite fonned in the HAC/molecular sieve pastes. Much smaller peaks of hydrogamet were detected in the HAC/molecular sieve pastes containing sodium sulfate than in the samples without sodium sulfate (Fig. 4c and d). Molecular sieves pro-mote stratlingite fonnation in the HAC paste.
Zeolites are a group of minerals containing aluminosili-cates. Substitution of different metal ions, e.g. Na+, K+, and Ca++, by ion exchange can occur. The cation present may in-fluence the activity of a zeolite in reacting with HAC and forming stratlingite. Highly effective zeolites such as syn-thetic zeolites Type 4A and Type 13X and natural chabazite will release a sufficient amount of dissolved silicate to pro-duce stratlingite in the hydrating HAC system. These zeo-lites alone can reduce hydrogarnet fonnation. However, most of the other zeolites studied were not as effective. Their ability to release the dissolved silicate required for stratling-ite formation may be more limstratling-ited. Addition of sodium salt in the HAC/zeolite system to activate the reaction of zeolite is necessary in these cases. Prevention of strength reduction of HAC products due to the conversion reactions depends primarily on the silicate release rate of the added siliceous material. Some zeolites are apparently more effective than other siliceous materials such as fly ash and silica fume. A high degree of effectiveness makes it possible to use a rela-tively low addition of zeolite and still effecrela-tively inhibit the strength reduction in HAC products. Low additions (Le. less than 20 percent) of zeolite result in HAC products with high early strength.
CONCLUSIONS
1. The one-day compressive strength of high alumina cement (HAC) mortars containing certain commercially available zeo-lites and sodium sulfate. with a water/solid ratio of 0.4. moist-cured at 23 C. can be greater than 60 MPa (8700 psi).
2. No strength reduction occurred in HAC mortars con-taining certain commercially available zeolites and sodium sulfate, with a water/solid ratio of 0.4, water-cured at 38 C for 330 days.
226
3. Hydrogamet fonnation. is significantly inhibited in HAC pastes containing certain commercially available zeo-lites and sodium sulfate. with water/solid ratio 0.6, water-cured at 38 C for 330 days.
4. Clinoptilolite or chabazite-rich zeolites in combination with sodium sulfate are more effective in preventing the for-mation of hydrogamet than stilbite or natrolite-rich zeolites in HAC paste.
5. In these experiments, zeolite additions alone were not able to prevent hydrogamet fonnation in HAC paste. The addition of sodium sulfate in combination with zeolite was required.
6. Chabazite was the most effective natural zeolite of those studied in promoting stratlingite fonnation in HAC paste.
ACKNOWLEDGMENT
Financial support from NSERC and the Network of Centers of Excel-. lence on High PeIfonnance Concrete is gratefully acknowledged.
REFERENCES
I. Majumdar A. 1.; Edmonds R. N.; and Singh. B., "Hydration of Cal-cium Aluminates in Presence of Granulated B1astfumace Slag," CalCal-cium Aluminate Cement, Chapman and Hall, London, 1990, pp. 259-271.
2. Majumdar, A. J., and Singh, B.• "Gehlenite Octahydrate-Fonning Cement Blends," Eur. Pal. Appl., No. 88309527.5, 1989,9 pp.
3. Majumdar, A. J.; Singh, B.; Tech, M.; and Edmonds,R.N., "Blended High-Alumina Cements," Cerarn. Trans.,V. 16 (Adv. Cern. Mater.), 1991, pp.661-678.
4. Majumdar, A.J.,and Singh, B., "Properties of Some Blended High-Alumina Cements," Cern. Caner. Res.,V. 22, 1992, pp. 1101·1114.
5. Majumdar, A. J.; Edmonds,R.N.; and Singh, B., "Hydration of Secar 71 Aluminous Cement in Presence of Granulated BlaSI Furnace Slag," Cern. Coner. Res.,V. 20, No.I,1990, pp.7-14.
6. Edmonds,R.N., and Majumdar, A. J., "The Hydration of Mixtures of Monocalcium Aluminate and BIaSI-Furnace Slag," Cern. Concr. Res., V. 19, No.5, 1989, pp. 779-782.
7. Singh, B.. and Majumdar, A. J., "The Hydration of Calcium Dialumi-nate and' Its Mixtures Containing Slag" Cern, Concr. Res.,V.22, No.6, 1992, pp. 1019-1026.
8. Bentsen, S., arid Sellveit, A.. "Effect of Microsilica on Conversion of High Alumina Cement," Calcium Aluminate Cements, Chapman and Hall, London, 1990, pp. 294-319.
9. Ding, J.; Fu,Y;and Beaudoin,J.J., "Study of Hydration Mechanisms in the High Alumina Cement·Sodium Silicate System," Accepted, Cern. Caner. Res., 1996.
10. Ding, J.; Fu,Y; and Beaudoin, J. J., "Stratlingite Fonnation in High Alumina Cement-Silica Fume Systems: Significance of Sodium Ions," Cem. Caner. Res.,V.25,No.6,1995,pp. 1311-1319.
II. Fu,Y;Ding, J.; and Beaudoin, J. J., "Mechanisms of Stratlingite Fonnation in High Alumina Cement-Siliceous Material Systems," Proc. 2nd CANMET/ACI IntI. Sym., Las Vegas, June 11-14,1995, ACI, SP-154, pp.461-47I.
12. Ding,J.;Fu,Y;and Beaudoin, 1.J.,"Stratlingite Formation in High Alumina Cement-Zeolite Systems;' Adv. in Cem. Res., V. 7, No. 28, 1995, pp. 171·17S.
13. Fu, Y.; Ding, J.; and Beaudoin, J. J., "Zeolite-Based Additives for High Alumina Cement Products," accepted for publicalion in Adv. Cern. Based Marerials, 1995.
14. Ding, J.;Fu, Y; and Beaudoin,J.J., "Effect of Different Inorganic Salts on Conversion-Prevention in High Alumina Cement Products," sub-mitted10J.Adv. Cern. Based MatIs., 1995.
15. Ding, J.; Fu, Y.; and Beaudoin, J. J., "Effect of Different Pozzolans on Conversion-Prevention in High Alumina Cement Products," accepted for publication in L'Industria [taUana del Cemenra, 1995.
16. Fu, Y; Ding, J.; and Beaudoin,J.J. "Conversion-PreventingAdditive for High Alumina Cement Products," U.S. Patent Application, No. 081 377.109, filed on Jan. 23, 1995.