Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Paper (National Research Council of Canada. Division of Building Research); no.
DBR-P-1134, 1983
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=f7b16de3-8b03-44a0-b53a-f4d37b1bbed7 https://publications-cnrc.canada.ca/fra/voir/objet/?id=f7b16de3-8b03-44a0-b53a-f4d37b1bbed7
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001783
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Ignition of poly(methyl methacrylate) slabs using a small flame
Clark, F. R. S.
N21d
National Research Conseil national
no*
1134
'
Council Canadade
recherchesCanada
I
BLDG
IGNITION OF POLY(METHYL METHACRYLATE) SLABS USING A SMALL FLAME
by F.R.S. Clark
Reprinted from
Journal of Polymer Science
Polymer Chemistry Edition, Vol. 21, 1983 p. 2323
-
2334DBR Paper No. 1134
Division of Building Research
Price $1.25 OTTAWA N R c
-
C I S T IBLDG.
RES.
L I B R A R Y
83-
12-
0 5
B I B L I O T H ~ Q I I E
Rech.
BAiirn.
C N R C -* .
NRCC 22652Lorsqu'on e x p o s e 8 l a flamme p l a t e e t allong'ee d ' u n mdlange o x y g 3 n e h y d r o g S n e d e s p l a q u e s d e p o l y ( m 6 t h a c r y l a t e d e m d t h y l e ) , e l l e s s'enflamment a p r P s u n l a p s d e temps q u i dQpend d e l a
tempdrature du gaz e t de l e u r d i s t a n c e d e l a flamme. te l a p s d e temps p e u t s ' e x p r t m e r p a r une 10% du t p e r h e n i u s d o n n a n t u n e
P
Q n e r g f e d ' a c t i v a t i o n de 96 + 8 KJmrnole-.
k l a f i n d e l ' e s s a i l a e u r f a c e d e 1 ' 8 c h a n t i l l o n Q t a l t piqu'ee, sl le delsi d ' i n f l a m m a t i o n B t a i t l o n g , e t p r e s q u e i d e n t i q u e a 1 le d s l a i e t a i t c o u r t . La p r o p a g a t i o n d e ~ f l a m m e s m e a u r ' e e i m m S d i a t e m e n t a p t s s l ' i n f l a m m a e i o n Q t a i t p r o p o r t i o n n e l l e au d a a i d ' i n f l a m m a t i o n , l a c o n a t a n t e d e p r o p o r t i o n n a l i t ' ? v a r i a n t s e l o n l a d i a t a n c e s'eperant f a p l a q u e t t e d e l a f lamme. Ces v s l e u r s d l a i n u a i e n t a u f u r e t 3 meeure que l a t e m p k a t u r e a u g m e n t a i t ; l a p e n t e d e e courbes d 6 d u i t e s d e l a g q u a t i o n d ' A r r h e n i u s Btait ind'ependante d e l a d i s t a n c e e n t r e l a p l a q u e t t e e t l a €lamme. Avant l ' i n f l a m m a t i a n , d e L'anhydride c a r b o n i q u e s e f o r m a i t a l ' i a t ' e r i e u r d e l a p l a q u e t t e e t une l u e u r b l e u e de pr'e-infltlmmstion k t a i t visiblesur l ' e x t l ' e r i e u r d e s bards. Ces o b s e r v a t i o n s s ' e x p l i q u e n t 3 l ' a i d e d ' u n mod&le d a n s l e q u e l l e d d l a i d ' i n f l a m u e t i o n ddpend d e l a p d r i o d e d ' i n d u c t i o n d e s r h c t i o n s e n p h a s e g a z e u s e d a n s l e s c o u c h e s e x t ' e r i e u r e s ou 8 p r o x i i n i t 5 d e c e l l e s - c i . Les m o d s l e s 03 l e d d l a i d ' i n f l a m m a t i o n ddpend du temps d e r d c h a u f f e m e n t du polymere j u s q u ' 8 u n e temp'erature c r i t i q u e d ' i n f l a m m a t i o n n e c o r r e s p o n d a i e n t p a s suffisaroment a u x o b s e r v a t i o n s .
Ignition of Poly(methy1 Methacrylate) Slabs Using a
Small Flame
FERRERS R. S. CLARK, National Research Council, Division of Building Research, Ottawa, Canada K I A OR6
Synopsis
When exposed to a lean hydrogen-oxygen flat flame, slabs of poly(methy1 methacrylate) ignited to flaming combustion after a delay, the length of which depended on the gas temperature and the separation between the slab and the igniting flame. The delay obeyed an Arrhenius-type expression, giving an activation energy of 96 f 8 kJ mol-l. By the end of the delay the surface of the sample was pitted if the delay was long and almost unchanged if the delay was short. The rates of flame development measured immediately after the ignition were proportional to the ignition delay, the proportionality constant varying with the separation between slab and flame. These rates decreased as temperature increased; the slope of the linear Arrhenius plots was independent of slab-flame separation. During the delay, carbon dioxide was formed within the boundary layer and a blue preignition glow was visible at its outer edge. These data were explained by a model in which ignition delay is governed by the induction period of gas-phase reactions in or near the boundary layer. Models in which delay is governed by the time taken to heat the polymer to a critical ignition tem- perature did not satisfactorily explain the data.
INTRODUCTION
The ignition delay of a solid, defined as the time between first exposure to an ignition source and the appearance of flame from the solid, has been measured many times and models have been devised for predicting it. Hunter and Hoshalll have recently described a test for the ignitability of polymer slabs, based on ignition delays that occur upon exposure to a flat flame. This procedure has considerable appeal for the evaluation of polymers because of its simplicity. Further, the authors predicted that a simple thermal model should adequately describe the dependence of the delay on thermal properties of the polymer and on gas temperature.
As it was likely that most of the heat transferred from the flat flame would be by convection, it seemed Bhat the procedure of Hunter and Hoshall could form the basis of a method to evaluate ignition caused by convective heat transfer from a flame. This article reports such an evaluation.
In the sections to follow, the material, apparatus, and procedure used are de- scribed. Ignition delays are reported, for various conditions of temperature and proximity to a flat flame. Additional measurements are reported of events in both the gas and solid phase during and after the delay. The ability of simple thermal theory to explain the data is tested and in consequence a modified view of the ignition phenomenon under these conditions for this polymer is devel- oped.
Journal of Polymer Science: Polymer Chemistry Edition, Vol. 21,2323-2334 (1983)
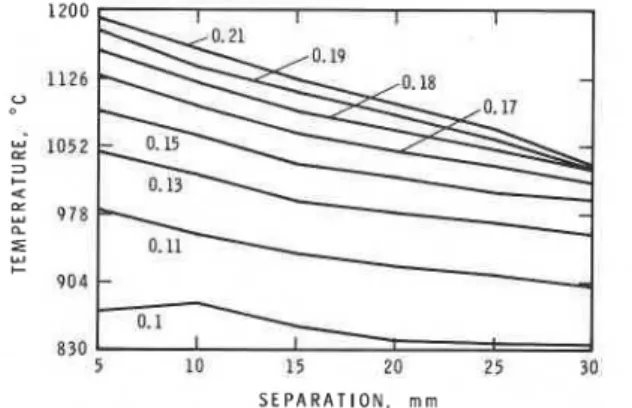
IGNITION O F PMMA SLABS 2325 of oxygen required for complete conversion of the hydrogen to water divided by the actual volume of oxygen supplied, from 0.1 to 0.21 (Fig. 2). The exit velocity of the gases from the burner sinter, assuming complete combustion a t 1000°C, was 2.4 f 0.3 m s-I over the equivalence ratio range used, implying a Reynolds number Re of about 558. The radiant heat output of the flame delivered to the sample position was determined with a water-cooled pyroheliometer (HyCal model 8400-B-lo), fitted with a quartz window and further protected from convective heating with a thin, cool air blast. The readings obtained did not exceed 3 kW m-2.
The heat flux delivered to a flat heat flux gauge 12 mm in diameter and bolted to the sample plate was measured. At an equivalence ratio of 0.1, the maximum flux measured was 19.7 kW m-2 (at 5 mm separation); a t 30 mm separation the flux measured was 11.2 kW m-2.
Ignition Delay
The reaction zone of the igniting flame appeared as a very thin, flat, blue disk about 1 mm below the sinter and was barely visible in a darkened room. I t did not change when a specimen placed on the sample plate was raised into position 5-30 mm below the sinter. Some time during the ignition delay, a pale blue glow was observed just above the specimen surface. Ignition was considered to have occurred and the timer stopped when a much brighter white or yellow light flashed across the surface. When the gas temperature was low, the delay ended with an audible report and a rapid flash of light. When the gas temperature was high, the delay ended as a lazy flame, often quite yellow, spread across the specimen surface. Polymer specimens often continued to burn after removal from the igniting flame, but not reliably enough to allow the continuation of burning to be predicted from any set of conditions used. When delays were short, the surface of the polymer was essentially unchanged from its original condition after ignition. If, however, the delay was long, the surface became progressively pitted and bubbles formed on it.
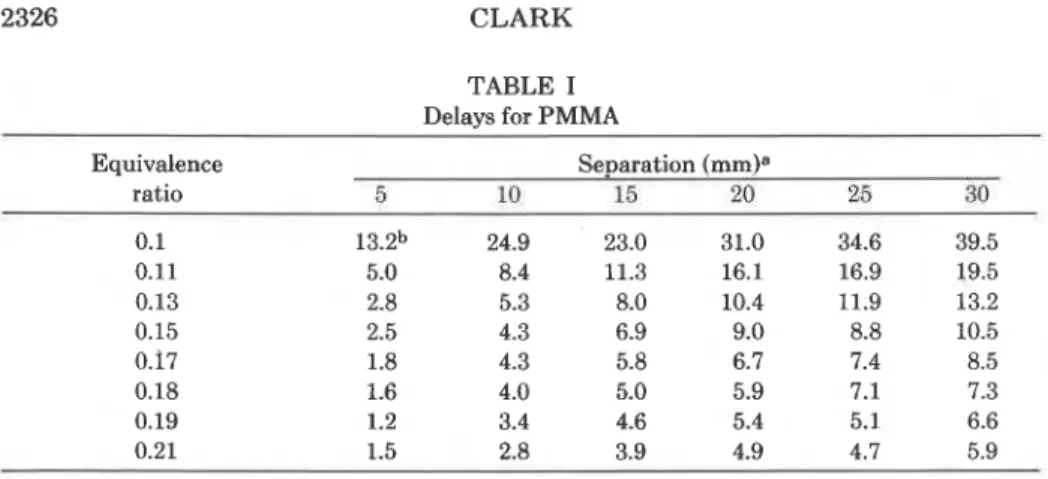
The ignition delays were measured in quadruplicate for PMMA slabs a t six separations from a hydrogen-oxygen flame a t eight equivalence ratios. The coefficient of variation for each quadruplicate set of trials was typically less than 5%. A summary of the data obtained appears in Table I.
S E P A R A T I O N , mrn
2326 CLARK TABLE I Delays for PMMA Equivalence
ratio
Separation (mm)a
5 10 15 20 25 30
a Separation between specimen and sinter.
Each entry is the mean of four determinations of ignition delay in seconds.
Models to Explain Ignition Delay
Models to explain ignition behavior of solid materials are basically of two kinds. In the first kind, termed the thermal models, the surface of the material is heated until it reaches the critical temperature at which the pyrolysis rate is sufficiently high to allow ignition. The gas-phase reactions occurring a t ignition are much faster than the process of heating the solid and thus the latter governs the du- ration of the ignition delay. Assuming that the transfer of heat from the hot gas to the sample is convective and that the conduction of heat within the sample is as for one-dimensional heat flow in an inert, semi-infinite solid, it is found that4
where T,, T,, and Tr are the temperature of the flame wake, the specimen surface a t ignition, and the room, respectively; K is the thermal conductivity of the specimen and k is its thermal diffusivity; tig is the ignition delay and h is the heat transfer coefficient. The equations are equivalent to the expression in the article by Hunter and Hosha1l.l
When ignition occurs so rapidly that heat conduction is negligibly small during the delay, alternative gas-phase reaction models are often used. Here the du- ration of the ignition delay is determined primarily by the induction period of the reactions of the pyrolysis products above the solid that precedes thermal runaway to flaming combustion. Gas-phase reaction models of ignition have often been used to explain ignition by hot gas streams5; in most cases the gas temperatures were higher than in the present study and the delays shorter. Warashima and Akita? however, studied ignition delays for PMMA a t lower temperatures than in the present study. They found that the surface temper- ature a t the time of ignition was constant a t low gas temperatures (and long de- lays) but dropped as the gas temperature was increased (shorter delays). They suggested that this indicated the importance of gas-phase processes to ignition delays a t the higher gas temperatures they used.
IGNITION OF PMMA SLABS 2327
Application to the Present Data
The extent to which the existing models explained the results of the present study was probed. A necessary prerequisite was to measure the temperature of the polymer surface a t the end of the delay. I t was predicted that the exper- imental conditions would influence the values of T, obtained, since Kashiwagi6 has reported that they are strongly influenced by orientation, and the work of Warashima and Akita5 indicated that the temperature of the gas stream may be important. The surface temperature was monitored with fine Chromel- Alumel butt-welded thermocouples (bead diameter 0.12 mm) suspended to be just in contact with the surface.
At an equivalence ratio of 0.21 the temperature of the surface a t the end of the delay varied from 227°C a t 25 mm separation to 420°C a t 5 mm separation. At an equivalence ratio of 0.10, the temperature of the surface at the end of the delay varied from 290°C a t 25 mm separation to 367OC a t 5 mm. Inconsistent with the work of Warashima and Akita? it was found that the surface temperature achieved increased as the gas temperature increased. I t is important to note that the earlier work was done a t a gas velocity lower than in the present work, and with a cylindrical sample rather than a slab; no ignition pilot is mentioned in the Japanese work but the ignition here is certainly stimulated by the burner flame. However, both studies indicate that a surface ignition temperature cannot be defined independent of the heating conditions.
T o probe the applicability of thermal models, and following Kanury? plots of X [from both eqs. (1) and (2)] against
(T,
-
T,)/(T,-
T,) were prepared fordata obtained a t 20 mm separation, both a t the value of h expected for this stagnation point flow system,7 viz. 22 W m-2 K-l, and over a wide range of other values of h. No value of h was found for which there was even remotely satis- factory agreement between the experimental data and the predictions based on eq. (1). The same was true at all separations. Similar iteration of value of T, over wide ranges, in view of the uncertainties in measurement of this quantity, failed to allow reconciliation between predicted and experimental values of the delay for constant values of h. I t was conceivable that the results could be ac- commodated if it was allowed that h would vary with the velocity of the gas stream, and indeed, it is predicted to vary as Re1/2.7 At constant separation, the velocity could only vary because of change in the gas equivalence ratio, and this variation was restricted to about lo%, far too small to explain the differences in
h. It may be concluded that the thermal model alone is not adequate to explain the results.
Alternative Models for Ignition Delay
Consider a model in which the occurrence of ignition depends on the Damk- ohler number, defined as the ratio of the time of flow of gaseous reactants through the zone where reaction is possible, to the chemical reaction time required for ignition. Ignition in this model occurs when the Damkohler number is unity, when flow is just slow enough to allow ignition to occur before the flammable gases are swept out of the heated zone. The critical quantity here is the velocity gradient, defined as the velocity divided by the burner-sample separation. The
2328 CLARK
greater the gradient, the more rapid the reaction needs to be and thus the higher the concentration of pyrolysis products in the gas phase must be in order for the ignition to occur. Thus this model would predict that high gradients should be associated with long delays. However, the velocity gradient is highest at smallest separation, yet the delay decreases as separation decreases a t constant gas temperature. The failure of the Damkohler criterion is doubtless due to the fact that ignition invariably first occurs very close to the polymer and thus the concept of velocity gradient as defined above is not applicable here. I t is interesting to contrast this result with the successful use of the Darnkohler number argument to explain extinction results on similar e q ~ i p m e n t . ~
I t is commonly assumed in ignition studies that the energetics of pyrolysis are1> neglegible.5 A major problem in assessing the importance of this factor has been
the paucity of thermochemical data obtained a t heating rates approximating those occurring in fires. Polymers that decompose endothermically a t low heating rates may do so exothermically at higher ratesg and use of data obtained a t low heating rates has led to poor predictions of ignition beha~ior.~JO
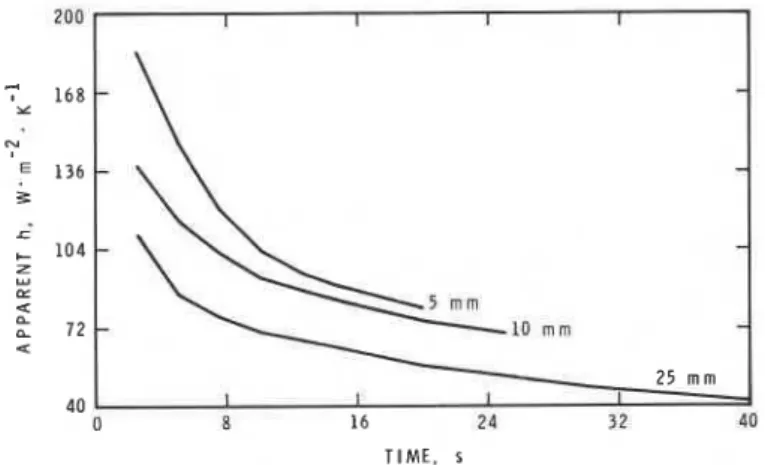
To gauge the importance of pyrolytic processes in this system, the temperature of the surface of PMMA specimens was followed during the delay. I t has been described above how the final temperature reached before ignition is sensitive to experimental conditions. The process by which this temperature is reached varies with the conditions. From the measured surface temperature, the gas temperature and known thermal properties of PMMA, plots of the apparent heat transfer coefficient versus time were prepared (Fig. 3), assuming that heating of the surface was by convection and that dissipation of heat from the surface was by conduction through the solid, assumed to be one-dimensional and semi- infinite. Heat transfer apparently becomes less effective as the surface tem- perature increases and the delay progresses.
Three phenomena could cause the decrease in h with time. Upon pyrolysis, evolution of gases from the surface occurs, in opposition to the impressed flow from the burner fuels. Transpiration cooling7 of the surface would, depending on the pyrolysis gas flow, cause an apparent drop in the heat transfer coefficient calculated from the surface temperature. The average mass pyrolysis rate during the delay is no more than 50 g m-2 s-l, from weight loss measurements. As-
TIME, S
IGNITION O F PMMA SLABS 2329 suming pyrolysis yields only methyl methacrylate that behaves as an ideal gas, the outgassing velocity Uw will be about 2.4 X m s-l. The injection pa- rameter (UW/V,)Re1l2, where V, is the flame gas velocity, will be no more than 0.03,11 indicating that transpiration cooling is negligible.
A second explanation for the decrease in h with time is that radiation from the flame gases is obscured progressively by the pyrolysis gases accumulating above the polymer surface.1° However, given the small contribution of radiation to the total heat flux incident on the surface, the decrease in h during the delay is too large to be attributed to this effect.
The remaining phenomenon capable of depressing the apparent heat transfer coefficient is the energy demand of pyrolysis itself. Even early in the delay, the temperatures a t the surface easily exceed the temperature required for pyrolysis to commence, even in the absence of oxygen.12
In the absence of methodology to measure the thermochemistry of PMMA pyrolysis a t the heating rates found in this experiment, consider the prediction that one carbon-carbon single bond is broken for each monomer unit released to the gas phase. This process will require about 1200 J g-l of polymer. Given the upper limit of the mass pyrolysis rate, 50 g m-2 s-l, pyrolysis would require an energy flux of some 60 kW m-2, three times the maximum delivered flux measured. Clearly the heat of pyrolysis is lower than 1200 J g-l. Similarly, the use of a literature value of 1610 J g-l for this quantity led Kashiwagi1° to predict surface temperatures for PMMA during ignition delays that were 100°C too low, indicating that the pyrolysis is less endothermic than supposed. I t is clear, however, that pyrolytic energy demand is sufficiently important to dominate, under certain circumstances, the surface energy balance.
The initial values of h in Figure 3 are up to nine times higher than estimated for stagnation point flow. Substantial enhancement of heat transfer has often been observed when reactive gas flows hit cool surfaces. The enhancement is believed to be caused by the release of energy in the gas phase near the cool surface by radical recombination processes.13 Note, however, that it is the change in h and not its absolute value that demonstrates the importance of py- rolysis processes to the surface temperature.
It
was of interest to determine whether the rate of pyrolysis could dominate the rate at which the polymer is brought to ignition. Since time of reaction is a linear function of the inverse of the rate constant, the conventional Arrhenius expression for the temperature dependence of reaction rate may be rearranged to accommodate ignition delay:ti, = A exp(E/RT) (3)
where A is a constant, R the universal gas constant, T the absolute gas temper- ature, and E the activation energy. Plots of logti, vs. 1/T were remarkably linear for all separations; in every case the coefficient of correlation to a linear regression line exceeded 0.97. The mean activation energy from these plots was 96 f 8 kJ mol-l. In an experiment comparable to that reported here, Kachi, Jellinek, and Hall have estimated the activation energy of the ignition delay of PMMA, ignited in an air stream of velocities somewhat lower than in the present case, but a t similar temperatures.14 Their Arrhenius plot shows considerable scatter and the value reported, 41.5 kJ mol-1, is less than half the value reported in the present work.
2330 CLARK
In the present work and in ref. 14, the Arrhenius analysis was based on gas- phase temperatures, and not the temperatures of the solid. The solid was always cooler than the gas, and its temperature varied during the delay and with depth below the surface. Use of gas temperatures in the Arrhenius analysis thus leads
to overestimation of the activation energy if indeed the rate-determining process is confined to the condensed phase. The activation energy will be more accurate if the process occurs in the gas phase.
The value of E from either the present work or that of Kachi, Jellinek, and Hall' I should be compared with that derived from vacuum pyrolysis of PMMA. Ma- I dorsky15 reports a value of 230 kJ mol-l for high-molecular-weight PMMA. I t is clear that pyrolysis is not the rate-determining process of the ignition delay of PMMA in air or under the conditions of the present experiment.
Further, the large difference in the activation energy under the present con- ditions compared with air14 indicates that delay is quite sensitive to gas-phase conditions above the heated polymer.
In summary, although pyrolysis clearly occurs and is endothermic, it is not the governing process of the delay duration.
The Gas Phase
Since ignition clearly is initiated in the gas phase, further study of events there was undertaken.
I t has been described earlier how during the delay a blue glow was restricted to a narrow zone just above the polymer, and was observed in flames of all equivalence ratios. As the flame was made richer in hydrogen, the blue zone gradually separated from the polymer. With a stoichiometric flame, the blue zone was a t a considerable distance from the polymer surface and could be held there indefinitely, since ignition did not occur under these conditions. Tracking a fine thermocouple through the flame revealed that the blue zone was situated a t the outer edge of the temperature profile of the boundary layer, where the temperature became the same as that of the hot gas from the burner.
In a second experiment 2.5-mL gas samples were taken 2 mm above the polymer during the delay (equivalence ratio 0.1, separation 5 mm) and analyzed by gas chromatography for carbon dioxide. Halfway through the delay a con- centration of 180 ppm C02 was present, while just before the ignition it was 742 ppm. Provided there was no catalytic oxidation during sampling, this is evidence that some oxidative reactions occur above the polymer well before the delay is over. The blue preignition glow is a second indication that preignition oxidation occurs. Such glow has been observed in hydrocarbon-air mixtures and is likely due to emissions of HCO, CH, and OH radicals16 but was clearly distinct in color and location from the OH radical emissions of the igniting flame.
After the specimen was raised into the wake of the burner flame and ignition had occurred, the resulting bright flame was confined to a small zone extending perhaps 10 mm from the polymer surface and covering the entire area of the polymer so long as the separation between sample and sinter did not exceed 20 mm. In a third experiment, the light emission from this flame was recorded with a United Detector Corporation model lOAP silicon photodetector of active area 100 mm2, insensitive to the blue preignition glow.
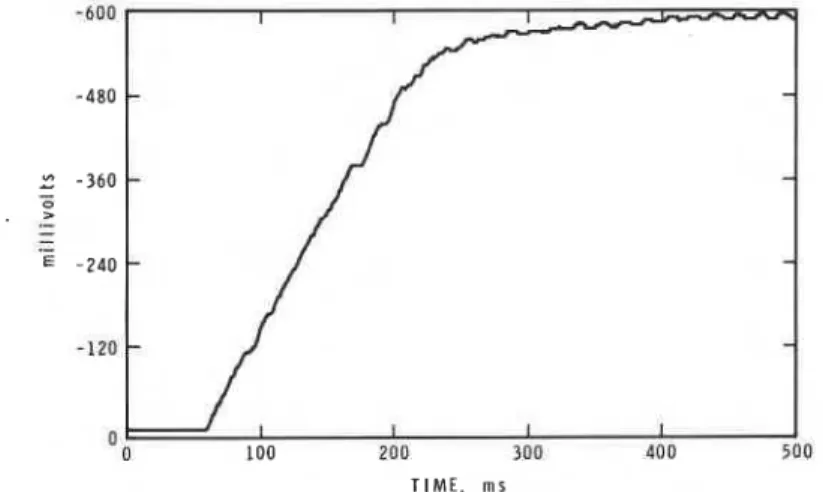
IGNITION OF PMMA SLABS 2331 length of a white line, a t a rate proportional to the velocity of the initial flame spread. Signals from the detector were amplified and fed to an 8-bit transient digitizer (LeCroy model 2256AS). Subsequently the data were transferred to a desk-top computer for analysis and display.
A typical signal output as a function of time is displayed in Figure 4. In some plots high-frequency fluctuations in the detector output were observed, partic- ularly a t large separations and high temperature, but these are not considered here. Further discussion is restricted to a consideration of the linear portion of the millivolt-time plots. The rate of rise of the output signal during the constant rate period will be looked upon as a measure of the rate of flame spread across the specimen surface.
For four separations and eight equivalence ratios, such curves were recorded five times, the tangents of the linear portions estimated from linear regression analysis using several hundred points on this portion of the curve, and the mean calculated. Fits to linear regression lines were accepted when the correlation coefficient exceeded 0.99. For most conditions the percentage standard deviation from the mean of slopes obtained was less than 10% but a few were as high as 25% of the mean value. Figure 5 shows the mean rates of flame development thus derived at various specimen-burner separations as functions of gas temperature. For all separations, the rate of development decreases as temperature increases. These data follow as exponential relationship, v = B exp(CIRT), where v is rate of development and B is a constant; C has the units of energy and has the value 71 kJ mol-l (f 3%) for separations of 5-20 mm. The fit to linear regression lines of plots of logv vs. 1/T exceeds 0.95 in all cases. Since the rate of development drops as temperature increases, C is not the activation energy of an elementary step of the governing mechanism of initial flame spread.
Such negative temperature coefficients of rates are common in combustion. For mixtures of many combustible gases in air, and in particular these hydro- carbon-air mixtures that exhibit preignition glow phenomena, a temperature region often exists in which increased temperature leads to a drop in reaction rate. I t is believed that the phenomenon is caused by competition between two chain branching agents, the more thermally stable agent propagating more slowly
TIME, rns
2332 CLARK
T E M P E R A T U R E . O C
Fig. 5. Initial rate of flame development at 5-20 mm separation.
than the less stable.17 Evidence is assembled below, however, that suggests this is not the operating mechanism in the present experiment.
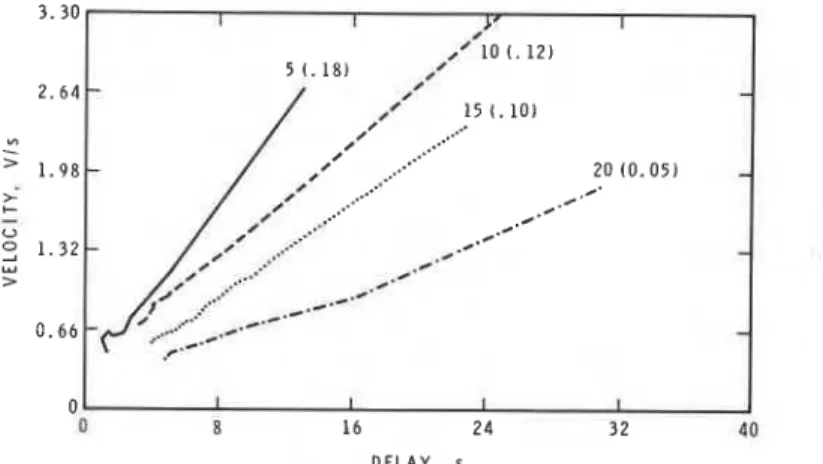
In the present experiment, changes in the gas temperatures are associated with changes in ignition delays. Plots of rate of flame development (in Vls) versus delay were thus prepared. Remarkably, the rate of development was demon- strated to be a linear function of delay, the slope steadily decreasing with in- creasing burner-specimen separation (Fig. 6). These lines are the result of linear regression analysis; in each case the correlation coefficient exceeded 0.99. The
slope is approximately a linear function of separation; the equation slope =
-0.0082(separation)
+
0.2150 is obeyed with a correlation coefficient of 0.98.Dugger, Weast, and Heimel18 found that when propane and air were stored a t high temperature before igniting, the flame velocity obtained after ignition decreased as the storage time increased, the decrease was more marked if the storage was a t higher temperature, and oxygen was consumed during storage a t rates dependent on the equivalence ratio. These workers proposed a preignition
D 8 16 24 3 2 40
D E L A Y , s
Fig. 6. Initial rate of flame development as a function of preceding delay; numbers are separation (slope).
IGNITION OF PMMA SLABS 2333
oxidation to explain their results. The dependence of flame velocity on preig- nition delay in the present experiment has precisely the reverse relationship to that observed by Dugger, Weast, and Heimel, yet experiments, viz. the blue preignition glow and the formation of carbon dioxide, have been adduced in this article to suggest that here too oxidative pyrolysis occurs before ignition is complete.
Observations similar to those in this article were made by Kashiwagi and
Summerfieldlg and Kashiwagi et aLZ0 in their studies of ignition of solid polymer
in a hot, high-speed oxidizing gas stream passing parallel to the exposed surface of the fuel. When ignition delays were short, the flame that followed moved slowly; long delays resulted in high flame velocities. This phenomenon was at- tributed to a rate-controlling step for flame propagation dominated by the flame fuel supply process.
PROPOSED MECHANISM
The results of the experiment reported above are consistent with a mechanism
of ignition of PMMA slabs under these flaming conditions that owes much to
that proposed by Kashiwagi and Summerfieldlg and Kashiwagi et a1.20 When the hot flame wake hits the sample, the polymer surface very rapidly reaches a temperature range at which pyrolysis occurs, liberating mainly methyl methacrylate to the zone above the polymer. Because of the highly reactive flame and the presence of oxygen, oxidative chain reactions commence in the gas phase, liberating carbon dioxide and showing a preignition glow. At a rate controlled by the time elapsed since first exposure to the flame and by proximity to the ignition flame, these reactions accelerate. Eventually a critical rate condition is reached and a white flame appears to signal the end of the delay. The time taken to reach this condition will not be determined by the pyrolysis gas concentration, which certainly builds up during the delay at a rate linear in time, but rather by the concentration of chain propagating species that lie on the oxidation pathway. Thus the delay is shorter when the production of chain propagating species is enhanced, by small polymer-flame separation and by high gas temperature. Further, the rate of pyrolysis itself does not determine the duration of the delay.
However, after the delay the concentration of pyrolysis gas above the polymer will be proportional to the duration of the delay. The initial rate of flame de- velopment has a rate constant first order in the concentration of pyrolysis gas. Thus after a long delay (generally because of low gas temperature), the rate of flame development is high, explaining the negative temperature coefficient of rate of flame spread. The linearity of the rate of development-delay plots occurs in consequence of the increase in pyrolysis gas concentration a t a rate propor- tional to elapsed time of exposure.
Further work is in progress to'test these concepts with other polymers and to monitor the critical chemical species during the ignition sequence.
The author gratefully acknowledges the invaluable assistance of his colleagues, and in particular, Mr. Raymond Flaviani. This article is a contribution from the Division of Building Research, Na- tional Research Council of Canada, and is published with the approval of the Director of the Divi- sion.
2334 CLARK
References
1. L. W . Hunter and C. H. Hoshall, Fire Muter., 4,201 (1980).
2. F. Rodriguez, Principles of Polymer Systems, McGraw-Hill, New York, 1970, pp. 520-525. 3. J. P. Botha and D. R. Spalding, Proc. Soc. London Ser. A, A255.71 (1954).
4. A. M. Kanury, Fire Res. Abstr. Rev., 14,24 (1972).
5. K. Akita, in Aspects of Degradation and Stabilization of Polymers, H. H. G. Jellinek, Ed., Elsevier, Amsterdam, 1978, Chap. 10, p. 501.
6. T . Kashiwagi, Combust. Flame, 44,223 (1982).
7. W . M. Kays, Convective Heat and Mass Transfer, McGraw-Hill, New Y o r k , 1966. 8. K. Seshadri and F. A. Williams, J . Polym. Sci. Polym. Chem. Ed., 16,1755 (1978). 9. L. S. Bouk, A. D. Baer, and N. W . Ryan, i n Fourteen Symposium (International) o n Com- bustion, T h e Combustion Institute, Pittsburgh, 1973, p. 1165.
10. T . Kashiwagi, Fire Safety J., 3,185 (1981).
11. J. R. Holrnan, Heat Transfer, 5 t h ed., McGraw-Hill, New Y o r k , 1981, p. 510. 12. N. Grassie and H. Melville, Proc. R. Soc. London, 199,14 (1949).
13. J. K. Kilham and M. R. I. Puwis, Combust. Sci. Technol., 18,81 (1978).
14. K . Kachi, H . H . G. Jellinek, and M. Hall, J. Polym. Sci. Polym. Phys. Ed., 19,1131 (1981). 15. S. L. Madorsky, J. Polym. Sci., 11,491 (1953).
16. A. G. G. Gaydon, T h e Spectroscopy of Flames, 2nd ed., Chapman and Hall, London, 1974, p. 173.
17. J . C. Dechaux, Oxid. Combust. Rev., 6.75 (1975).
18. G. L. Dugger, R. C. Weast, and S. Heimel, in Fifth Symposium (Znternutiorzul) on Combustion, Chapman and Hall, London, 1955, p. 589.
19. T . Kashiwagi and M. Summerfield, in Fourteenth Symposium (International) on Combustion, T h e Combustion Institute, Pittsburgh, 1973, p. 1235.
20. T . Kashiwagi, R. W . MacDonald, H. Isode, and M. Summerfield, in Thirteenth Symposium (International) on Combustion, T h e Combustion Institute, Pittsburgh, 1970, p. 1073.
Received January 12,1983
Thie publication is being distributed by the Division of Building R e s e a r c h of the National R e s e a r c h Council of Canada. I t should not b e reproduced i n whole o r in p a r t without p e r m i s s i o n of the original publisher. The Di- vision would be glad to b e of a s s i s t a n c e in obtaining s u c h permiseion.
Publications of the Division m a y be obtained by m a i l - ing the a p p r o p r i a t e r e m i t t a n c e (a Bank, E x p r e s s , o r P o s t Office Money Order, o r a cheque, m a d e payable t o thk Receiver General of Canada. c r e d i t NRC) t o t h e National R e s e a r c h Council of Canada, Ottawa. K1A OR6
.
S t a m p s a r e not acceptable.A l i s t of allpublications of the Division i s available and m a y be obtained f r o m the Publications Section, Division of Building Research, National R e s e a r c h Council of Canada, Ottawa. KIA OR 6.