Publisher’s version / Version de l'éditeur:
Journal of the American Ceramic Society, 49, 5, pp. 268-273, 1966-08-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Character of hydration of 3CaOAl2O3
Feldman, R. F.; Ramachandran, V. S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=4fbd44ca-ef99-4ed0-8399-3b5f9ded4ce0
https://publications-cnrc.canada.ca/fra/voir/objet/?id=4fbd44ca-ef99-4ed0-8399-3b5f9ded4ce0
Kepi-inted fl.om the Journal 01 T l ~ c A111crica11 Cel.:rlllic SocicLy. VoI. 4!J. S o . 5. hlay, 1iIG6. CopyrighL 1966 by the Arnericar~ Cernrnic Socicty
Character of Hydration of 3CaOaAl
20
3R . F. F E L D M A N
a n dV . S . R A M A C H A N D R A N *
Divlsion o€ Building Research, National Rcsearch Council, Occawa, Ontario, Caliada
The C3A compacts were hydrated and the reac- tion was studied by DTA, X-ray diffraction, mer- cury porosimetry, and volume change analysis. The hexagonal hydroaluminates C2AH8 and C.IAH1g formed at 2", 12O, and 23°C by a direct mechanism between C3A and H 2 0 . The hydra- tion reaction at 52" and 80°C was stopped by formation of C5A.HG around the C5A. grains. The rate of conversion of the hexagonal hydrates to cubic C3AHG increased with temperature. Vol- ume change analysis confirmed that C3AHG grows epitaxially on the surface of the C3A grain. The reaction at this surface and the passage of water through the layer of hexagonal hydroaluminates control the overall reaction rate. The conversion
of the hexagonal hydrates to C3AHG accelerates the reaction by removing the layer of products from around the C3A grain by a solution mecha- nism. At 52" and 80°C, C3AHG may form with- out the intermediate formation of the hexagonal
hydrate.
Rcceived May 14, 1963, revlscd copy reccivccl S o v c n ~ b e r 12, 1965.
At the tinic this work was done, tllc writcrs were, respectively, research otlicer and postdoctoratc fellow (on deputation frorn the Ccritral Buildilig Research I n s t ~ t u t e , Roorkcc, India), Inorganic Materials Section, Division of Buildi~lg. Research, Natioi~al Research Cou~lcil
*
Kow senior scic~~tific oflicer, Ce~itral Building. Rcsearch In- stitute, Roorkee, India.May
1966
Character
ofHydrc
I. Introduction
T
IIE aluminate phase in portland cement, although of lowproportions, is of great importance owing to its role in the early hydration reactions. Research on this phase has pro- ceeded in several directions, one of these being a study of the equilibria in the system CaO-A1203-H?O. Work by several investigators1-4 has established t h a t the only stable com- pounds in the system a t normal temperatures are gibbsite,
a n d C3AH6
*
T h e previous work provided some basis for predicting reac- tions which occur during hydrati011 of CaA. Reactions of this type, however, are complex because equilibrium is not attained for some time. T h e kinetics of these reactions are not only affected by the products forined, b u t also by the changing conditions of the reaction owing to the forination of products. These reactions occur in limited quantities of water so t h a t metastable products may exist for long periods a n d may have profound influence on the mechanism a n d rate of the wl~ole reaction.
During the hydration of portland cement, C3A hydrates along with the other constituents in the presence of CZL(OI-I)~, CaS0,.2H20, and alkalis i n solution, which will affect the reaction. T h e study of C3A hydrating in a solution of Ca- (OH)2 is aimed a t determining rates and mechanisms in the hydration of C3A in portland cement.
T h e hydration of C3A has been studied by many methods; X-ray and microscopic examination a t several temperatures led to the conclusion that the development of the cubic hexa- hydrate was preceded by formation of the hexagonal hydro- aluminates C2AH7-9 a n d C4AI-112-14.3~"6 I t was not clear whether C3AI36 could forin directly a t higher temperatures a n d lime concentrations, although Lafuma7 a n d Xssarsson5 suggested this. T h e metastable hexagonal hydrates con- verted to the stable cubic hexahydrate more rapidly a t the higher temperatures, but a more specific knowledge of the reaction was lacking. Conflicting results have been obtained and even identical preparations did not yield completely similar r e s ~ l t s . ~ T h e analysis by Brocard8 of the supernatant liquid of dilute suspensions of CaA as a function of time showed that the rate of solubility increased with teinperature although the solubility itself did not change much. Electrical conductornetric work by Solov'eva a n d SegalovaQonfirmed this. Steinlo a n d othersl1 followed the conversion of the hexagonal hydrate to the cubic by calorinletric measure- ments. The DTA, although applied to some of these systems and used for kinetic studies to some extent in the hydration of C3A by Segalova et al.12 a n d by Young13 was not used to full advantage. This technique, although not coillpletely quantitative, makes i t possible to detect minute quantities of hydrates which might be very poorly crystalline as is often the case i n the reaction in question.
A method for stopping the hydration reaction was de- veloped i n this work. A volume change method aiid po- rosilnetry were used i n conjunction with DTA for analysis and X-ray diffraction was used in some cases as confirmation
11. Experimental Procedure
C3A was prepared from reagent grade CaCOa and A1203.- 3 H 2 0 . A stoichiometric mixture containing 2% excess CaO was fired three times a t 1375' to 159O0C. X-ray diffrac- tion showed no aluminate other than C3A. Free lime, de- termined by the alcohol-glycerol 1nethod,l4 was 2.2%; C 0 2 and Ca(OI-I)2, determined by thermogravimetry, were 0.16 and 0.5%) respectively.
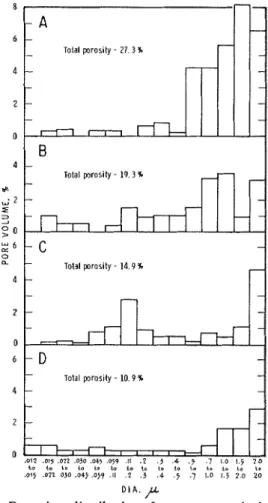
T h e C3A compacts were hydrated a t zO, 12O, 23', 5z0, a n d 80°C. Calculating from the porosity (Fig. l ( i l ) ) , the watcr/ solid ratio within the specimen was 0.12. Compaction pro-
*
Throughout the text, the following cemeilt chemistry nomen- clature is used: C = CaO, A = A1203, and H = H?O.Total p r a s i l y - 14.9 %
%
Fig. 1. Pore-size distribution for compacts hydrated at various conditions. 7-
-
- - - - - 8 6 4 2 0cedures, length change ineasurements, and hydration tech- niques have been discussed p r e v i o ~ s 1 y . l ~ ~ ~ ~
T h e reaction was stopped by placing the coinpact i n liquid air. T h e specimen was ground, washed, a n d filtered in cold absolute alcohol a n d degassed in a desiccator for 6 hr. Throughout the operations great care was taken to avoid contamination by CO?.
The D T A on all specimens was perforined in the absence of air; the heating rate xvas 10°C/min.
Pore volume a n d pore size distribution wcrc detcrinined with a mercury porosimeter. The lninimu~n pore size in which mercury could be injccted was 0.01zP in diameter.
111. Results and Discussion
T h e unhydrated C3A for all phases of this work was in compact form. This technique has some distinct advantages, as discussed p r e v i ~ u s l y , ~ ~ ~ ~ ~ n d facilitates studying very early reactions. Only low water/solid ratios, however, can be studied.
Stopping the reaction in licluid air has no effect on the hy- drates formed; this was tested o n specimens where hydration was very slow. Use of ahsolute alcohol or acetone for partial drying is well established3; i t was verified in the present work by D T A t h a t i t h a d no effect o n either the unhydrated or hydrated materials. T h e degree of drying achieved b y the degassing proccdures is open to question. I t is possible that some hydrate water is removed from the hexagonal hydro- aluminates CzAIls and CiAHz. Roberts17 reported CrAH19 as the reaction product b u t Steinlo suggested t h a t C,,AI-113 may occur a t spots with local water shortage.
The consistency of the results a t any one temperature and from temperature to temperature gives confidence in the assumption t h a t if these procedures are used throughout, valid interpretations can be made.
-
A
--
Tolal p r o s i l y - 27. 3 % - - - - --
I I 1 [ ' I> 5 DAYS
-
= ", TL'!PERATURE. 'C T E K P E R A I U R E . 'C-
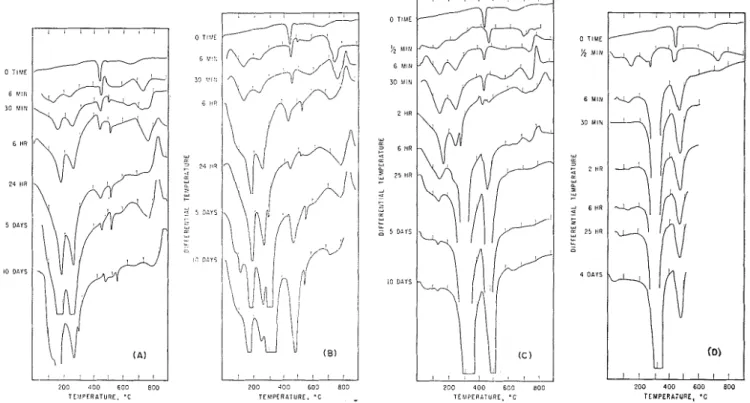
T E Y F E R L T U R E , 'C TEMPERA;URE, ' CFig. 2. Thermograms for C3A hydrated at ( A ) Z°C, (B) 12"C, (C) 23"C, and (D) 52OC.
Figures 2 and 3 show DTX thermograms for C3X hydrated a t 2O, 12", 23O, 52O, and SO°C, each for several tiines. The interpretation of these therinograms was based in part on the results of several workers. The dehydration of C3AIIc was shown to take place in two stages by Schneider and Thorvald- sonls at --27j0 and at 400° to 500°C. Other w~rkersl"~? suggested, however, that the thermogram of C ~ A H G has only one endotherm, a t 315O to 330°C. Work by Young13 and by Majumdar and Roy?3 confirined that endotherms exist a t 330° and 500°C whereas Segalova et 111.12 suggested that the thermogram includes a small endotherin near 150°C
Kalousek et aL20 have shown that the thermogram for C44H13 consists rnainly of a large endotherrn a t 240°C whereas Young13 assigns three eildothermic peaks a t 120°, 21s0, and 310°C to a mixture of C4XI113 and C2XfIa. Segalova et a1 l Y
showed large endothermic peaks at 190°, 2G0°, and 290°C, with small peaks a t 410° and 490°C representing the hex- agonal aluminates. Rey2' suggested that C?XKs is represented mainly by peaks a t 140° and 310°C. In the present study, the CZAI& hydrate is considered to be represented mainly by an endothermic peak varying from 140° to 190°C, and C4AI-113 is represented by an endother~nic peak varying from 230' to 2G0°C.
The DTA therinograms show formation of the hexagonal aluminates a t all teinperatures a t the earliest times. As- sociated with these peaks representing these compounds is an exothermic peak between SOOO and 900°C which has been attributed to the formation of CaO and A1203 from the de- hydrated C ~ A H B , ~ " but it is not clear froin this work that the exothermic effect is associated with both C2AHs and C4.$H13. This effect is absent for C ~ A H G and is useful in confirming the presence of the hexagonal hydroaluminates. From a study of the thermograms, it is clear that the reactions involved are very temperature dependent; the rate of hexagonal hydrate formation increases as the teinperature increases; at 6 ~ n i n for 52' and 80°C, only a small ainount of hexagonal hydro- aluminate is present, C ~ A H G being present at the earliest times examined (30 sec a t 52' and 5 sec at 80°C). There is evidence of a small ainount of C3AH6 after 10 days at 2OC, after 6 hr a t lZ°C, and after 30 min a t 23OC. At 12O and
0 T I M E 5 SEC 15 SEC e 2 + a Cc b, CL I w + 6 M I N 2 a -
-
z w Cc w k k 0 Fig. 3. Thermograms for C3A hydrated at80°C.
200 400 600
T E M P E R A T U R E , 'C
23OC, a t certain tiines of hydration, both the hexagonal hydrates and C3AI16 were increasing in quantity, but in general the disappearance of the hexagonal hydrates indicated that they were converting to C~AIIG, and at a faster rate as the temperature increased. The probability of C3AHG forming directly, especially a t the higher temperatures, is very real and will be discussed later. There is no evidence that the C2Xf18 converts to C4A11,3 as an intermediate phase in the conversion to C ~ X H G . ~
The DTA shows that a t 52OC little hydration occurs after 30 min whereas a t SO°C, considering the rapid rate of hydra- tion in the first 15 sec, the rate has been greatly reduced a t G mnin; for 23OC hydration the product forms steadily up to
25 hr and slowly beyond that. The degree of hydration determined by TGX after 25 hr a t 23OC is 52..5y0, whereas after 4 days a t 52OC it is 17.570.
T h e endothermic peak a t 300° to 530°C that developed with the other peaks a t -150° and 240°C when hydration occurred a t 2O and 12OC, was absent at the higher tempera- tures and thus does not seem to be characteristic of the hex- agonal or cubic hydrates. This endotherm may be due to a small amount of Ca(OH)Z formed during the hydration of C3X a t low temperatures even though the temperature of the endotherm is somewhat higher than the Ca(OI-I)2 with endo- therm a t 450°C, in the original C3X preparation The latter endotherm was probably formed, however, by vapor phase hydration of CaO a t more accessible regions, whereas the former endotherm may be the result of Ca(OI-I)2 embedded in the hydroaluminate. Alternatively, the hydration of CaO in the C3A can explain the endotherm but i t should produce a thermogram for the 23OC hydrated specimen similar to that a t 2O and 12OC.
The complete disappearance of the peak a t 140°C for hy- dration a t 3" and 80°C and almost complete a t 23OC indi- cates that the therniogram for CsXII6 has only two endo- therms, a t about 320' and 500°C. I t is evident that for systems that Inay produce several compounds, and where transformations of various types may occur, DTA thermo- grams must be interpreted from series obtained a t different stages as the reaction progresses. Also, in these reactions, where products may form on the surface of an unhydrated grain and progress inward, and where the crystal size or habit may change, the peak temperatures will shift with an in- crease in degree of hydration as was observed in these thermo- grams.
Formation of a hydration product causes a decrease in the rate of hydration, but the subsequent rate is still temperature dependent. Althot~gh this may be partly due to the dif- ference in rate of moisture flow to the unhydrated C3A, the reaction rate between C3A and 1-120 a t the surface of the un- hydrated C3A grain must be considered as is illustrated by the great increase with temperature of the reaction rate very early when little product was formed. Thus i t is considered that the reaction rate is controlled by both the reaction be- tween C3A and I-I?O a t thc surface of the unhydrated C3'4 grain and the rate of permeation of water through the hydrate procluct formed close to the surface of the C3A grain. T h e effect of this second factor is well illustrated l ~ y the results of Steinlo; he observed (through calorimetric measurements) a marlcecl increase in hydration rate clue to the conversion of the hexagonal hydroaluminates to C3AI-16, thus removing the barrier close to the grain surface.
Specimens used for X-ray diffraction patterns were those in which larger amounts of product were formed. In general the lines for C.,AI-I13 and C?AH8 were diffuse and not clear, in contrast to those for C3A and C3AH6. Consequently the results are useful only in a general sense and show the ad- vantage of using DTA for this system.
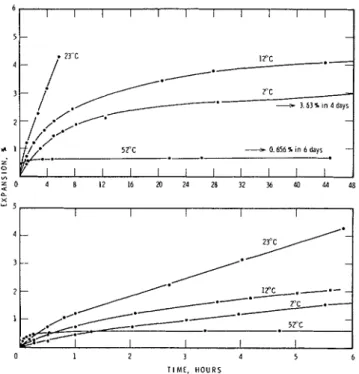
Length changes, measured a t four temperatures, are shown in Fig. 4. These results are reproclucible to *5yo except a t 23OC where the rate and degree of hydration and expansion were very great, with the result that in some cases the speci- mens broke. These results are used for qualitative and semi- quantitative analysis of the system. At each temperature, expansion began as soon as water contacted the specimen. T h e results corresponded to those obtained from the DTA.
T h e expansion rate a t 52OC a t short times is very high rela- tive to the other temperatures b u t after 5 lnin i t decreases rapidly. After 1 day the expansion ceases completely a t 0.66%, even thougl~ it was kept for 3 days a t 52OC a n d for 3 more days a t 23OC. This specimen, autoclavecl a t 214OC and 300 psi, expanded by 21% and was completely hydrated. I n results not shotvn here, C3X after hydrating for 92 clays a t 12OC, had expanded 14.1y0 and the rate of expansion was slow.
T h e pore size clistribution and total pore volume results are shown in Fig. 1. Specimen A is the unhydrated compact of C3X. Specimen B is a C3.4 compact hydrated a t 52OC for 1
.
23'C ZC + 3.631 In4days - --;r 0.6561 in 6 days - I I I I I I I $ 0 4 8 I2 16 20 24 28 32 35 40 44 48 0 1 2 3 4 5 6 T I M E . H O U R SFig. 4. Length change of CIA compacts hydrated at 2", 1 2 " 23", and 52°C.
days (1'7.5% hydrated); the volume of larger pores above 0 . 5 ~ is still about half the original. Thus hydration of C3A a t 5.)' and 80°C involves formation of C3AH6 which acts as a very impermeable barrier localized around the C3X grain, despite the fact that adecluate space is available and the reac- tion has a high potential for expansion. T h e CJAHB formed a t a different region than that from the reaction a t B ° C , the mechanisms of the respective reactions probably cliflering.
Specimen C in Fig. 1, a compact of C3X hydrated a t 23OC for 25 hr (52.570 hydrated), shows a large reduction in volume of large pores and an increase in volume of smaller pores rela- tive to specimens il and B. Specimen D hydrated a t 2OC for
1 week (26.6% hydrated by estimate) gave a total pore volume of 10.9y0. By calculation, assuming the average composition of the hexagonal hydroaluminates as CSAHIO.:, with a density of 2.04,?j the pore volunie is 9.1:3y0. Errors in the degree of hydration determination and use of C ~ A H ~ ~ . S as the formula for the mixed product may account for this difference. These results show that the increase in solid volume of 1 g of the solid, t o be accommodated in the body, is 0.1190 cm3 for specimen C and 0.1345 cm3 for specimen D. T h e approxi- mate expansions would be 6 to 8% and 40/0, respectively, suggesting that under the conditions of the reactions the hexagonal hydroaluminates can be accommodated in the voids with the development of less stress than the C3AI-Is. T h e rate of the respective reactions may also have a n in- fluence. I t is significant, however, t h a t although the forma- tion of the hexagonal hyclroaluininates is the more accom- modative, considerable hydration pressures are exerted as shown by the 4% expansion. Mikhailov2%tatecl t h a t forma- tion of plate-stnictured coinpounds like the hexagonal hydro- aluminates does not lead to expansion, but it is clear now that this is not the case. I t is indicated that the stresses created by the rapid formation of CJAHG a t 23OC a n d subse- quent rupture of bonds weaken the structure under these conditions. This is in contrast to the views of Segalova et al.,12 who consider that the conversion t o the cuhic form occurs by a solution mechanism resulting in dissolution of the bonds. I t is not likely that all the bonds would break simultaneously; during the conversion more bonds can form because of the crystallization of C3AI-16 and further hydration of the CIA.
272
Joz~rnal oj T h e A ~ n e r i c a n Ceramic Society-Feldma~z and Rnmaclznndmn
Vol.49,
No.5
Many factors operate in structure formation Ily a hydrate product and in the resultant length changes during hydra- tion; these factors include the initial particle size and degree of dispersion of the product and its crystal form a n d habit. T h e rate and mechanisn~ of the reaction, involving the degree of supersaturation t h a t may be attained, are also inlportant. For this complex systcm the le11gt11 change reflects only the degree and rate of hydration and does not give a cluantitative evaluation.
( 1 ) Mechanism of the Reactiolz
The apparent solubility of a hydrating material and the solubility of its prorlucts have long been considered the im- portant criteria governing the reaction rate; approaches to the hydration lnecl~anis~n have not considered the reactivity of the solid. Workerss~' have shown, however, that solu- b~lities of C3A and its hydrates do not increase much with temperature although the rate of clissolution increases
Defects in solids are now recognized as being closely con- nected to their cl~emical behavior 2 7 s 2 8 Experi~nental evt- dence has indicated that dislocat~ons intersecting a surface have a significant influence on reaction rates
"
Gilman?" has shown how clislocation density contributes to the rate of dissolution and dislocations intersecting the surface may also be the starting points for the hydration process.Environmental factors affect the operation of the various types 01 surface sources of clislocations ancl the emergence of dislocations throllgll the surface of the material I t has been suggestedm tllat adsorbed polar molecules on thcse sites can hinder the operation of these sources. I t is suggested here that certain ions in solution decrease the rate of reaction of C3A with water or its dissolution rate by a process similar to the foregoing. The hydration of Ca-4 is retarded in the presence of Ca(OI1)q in s o l ~ t i o n . ~
T h e exact mecllanism of the C3-4 and \\rater reaction is difficult to prove conclusively, b u t the follo~ving explains most of the experimental evidence: water a t 2O, 12', ancl 23OC reacts immediately, forming poorly crystalline hexag- onal l~yclroal~~lninates by direct reaction on tlze surface of the C3A grain. These soluble products are a source of ions i n the solution ancl also contribute to retardation of the reaction by limiting the supply of water to the CaA. This stage of the reaction is consisLent with the immediate expansion observed on first wetting. As the hydration of Car\ continues, the crystals of the hesagonal aluminates may become bigger. At a certain stage in the reaction the conversion to CoAI-IG occurs, probably by a "through-solution mechanism." This conversion removes the hexagonal l~yclroaluminates fro111 the surface of the grain and the reaction accelerates. I t is sig- nificant t h a t for a time a t these three temperatures the quantity of both C3AI-IG and tlle hexagonal hydroalunlinates increases. T h e conversion begins earlier a t the higher tein- perature l~ecause the energy conditions a t this temperature
, permit earlier nucleation. T h e rate of conversion is governed
also by the rate of dissolution.
Energy conditions a t 52O and SO°C permit CaAI-IG to form not only by direct reaction of CaA with watcr, but without going through tllc intermediate stage of the hexagonal hydro- aluminate. This may account for the complete elinlination of reaction between Ca.4 ancl I-120; the CaAI-I6 grows epi- taxially on the CaA grain completely sealing it on. X small amount of hexagonal hydroaluminate may be evidence, lzow- ever, for a very rapid conversion of most of it t o the CaAI-IG but the possibility exists of CJAI-IG forming by both mechanisms.
IV.
Conclusion'The hexagonal hydroaluminate forms a t 2O, 12O, and 23OC by a direct mecllanisln between CaX ancl H?O. The rate of the reaction a t the surface ancl the passage of water througll the layer of hexagonal l~yclroaluminates control the overall rate of the reaction. Polar ions in solution can retard the rate of hydration by adsorbing on thc sitcs of defects and
dislocations, hindering their operation. T h e conversion of the hexagonal hytlrates to C~A'IG accelerates the reaction b y removing this layer fro111 around the C3.4 grains by the through-solution rnecllanism. At 52' and SO°C, C3-41-I6 forms immediately. This reaction may also occur by a direct mecha- nism and it forms a layer of C3.11-IG on the surface of the C3A grain.
Acknowledgment
T l ~ e authors are grateful to E. G. Swenson for helpful dis- cussions ancl to H. F. Sladc, S. E. Dods, and P . J. Lefebvre for sctting up the apparatus ancl collecting the data.
References
1 L. S. Wrlls, W. F . Clarke, ancl H. F. McMurdic, "Study of
thc System CaO-AI?O:,-1-150 a t Tempcratures of 21' and 90°C,"
J . Xes. Natl. BTLY. Std., 30 [ 5 ] 367-409 (1943) RP 1539.
? F . E. Jones and M. H. Roberts, "The System Ca0-Alz03-
H?O a t 2Fi°C," Department of Science and Iilclustrial Research, Building Research Station, Notc E965, Hcr Majesty's Stationery Oflice, London.
13. H. Stcinour. "Aqucous Ccmcntitious Systen~s Containing Lime and Alu~nina?" Xes. alrd Develop. Labs. I'ortla71d Cenzelzt ilssoc. Bull., NO. 34, 100 pp. (February 1951).
R. Turriziani; Cllaptcr 6 , p. 262 in Chemistry of Ccments, Vol. 1. Eclitccl by I-I. F. W. Taylor. Academic Press, New York. 1964.
Gunnar Assarsson, "Chemistry of the Hydration of Calcium Aluminatcs a l ~ d Cements," Zenielzt, 26, 293-98, 311-15, 327-30
(19371 \ - 1
W. E. Bcsscy; pp. 158-215 in Chc~nistry of Cement, Vol. I- Procccdil~gs of the Second International Sy~nposiu~n, Stockholm,
1938.
'
Henri Lafuma, "Evolution of the Hexagonal Hydratcd Calciun~ illun~inatcs," Collzpt. Rend., 196, 1671-72 (1933).J. Brocard, "Hydration and Hydrolysis of Silicates and Alun~inatcs of Calcium as a Function of Temperature," Alzn.
Insb. T'eclz. Bdtiltlc~~t Trau. Pz~bl., New Scries, No. 12, 32 pp. (February 1948).
WE. S. Solov'eva and E. E. Segalova, "Study of thc Kinetics of Supersaturation in Aqueous Suspensions of Tricalcium Aluminate and Detcrminatio~~ of Its Metastable Solubility," Kolloid. Zh.,
23 [ 3 ] 306-14 (1961).
lo H . N. Stcin, "Mechanis~n of thc Hydrati011 of 3Ca0,AI?O3,"
J . il/<pl. Clzeltz. ( L o t ~ d o n ) , 13, 228-32 (May, 1963).
11 E. Calvet and P. Longuet, "Microcalori~~~ctric Study of Calcium Aluminatcs," Cornpt. Re~zd. Z T C Congr. Intern. Clzillz.
I d . , Brzlssels, 1954, 3 ; I,~zdt~strie Cl~irrt. Belge, 20, Spec. No.,
3 - 3 4 (1955)(in Frcnch).
E. E. Segalova, E . S. Solov'cva, anrl P. A. Icebinder, "Influ- cncc of Ternperaturc on Proccsscs of Crystallization Structure Formation in Suspensions of Tricalcium Alumillate," Kolloid.
Zh., 23 [ 2 ] 194-99 (1961).
l 3 J. F . Youns, "Hyclration of Tricalcium Aluminate with L i g ~ ~ o s u l ~ l ~ o n a t c Additives," lll(~g. Co7lcrele Xes., 14 [42] 137-43 119621.
l 4
F.
M. Lea and C. H. Dcsch, Chemistry of Cement and Con-crctc; 2d ctl., p. 103. Edward ilrnold, London, 1956.
'VZ. F. Fcltlnlan, P. J. Scrccla, and V. S. Ramachandra~~, "Stntly of Lcngth Changes of Compacts of Portlantl Ccmcnt on Esposure to H?O," Highway Research Board, Highway Research Iiecord No. 62, Publication 1246, pp. 106-18 (1964).
l G R. F. Feldman, V. S. R a n ~ a c h a ~ ~ d r a n , and P. J. Sereda, "Influence of CaCOa on the Hyclration of 3Ca0.111203," J. A I I Z .
Ceran~. Soc., 48 [ I ] 25-30 (1965).
li M. $1. Roberts, "Ncw Calciu~n Aluminate Hydrates," J.
Appl. Clzet~z. (Lolldon), 7 1101 5 4 3 4 6 (1957).
la W. G. Schncider and Thorberrur Thorvaldson. "Dehvdra- tion of Tricalcium Aluminate ~ c s i h ~ d r a t e , " Ca?z. 'J. Res&rciz,
19B [ 5 ] 123-28 (1941).
I". S. Ramachandran, R. I?. Fcldman, and P. J. Sereda, "Application of Differential Thernlal Analysis in Cc~ncnt Re- search." Hiqh~vav IZescarch Board. Hifihivav Rcscarch IZccord. No. 62, ~ub7ic:~tion 1246, pp. 40-61 (1964).
20 G. L. Kalousck, C. W. Davis, Jr., ant1 W. E. Schrncrtz, "Investigation of Hydrating Ccmcnfs and Rclated Hydrous Solids by Diffcrential Thcrmal ilnalys~s," J . A I I Z . Corzcrele Imst., 20 [ 6 ] 693-712 (1949).
21 ilr~nin Petzolcl and Ir~ngartl Gohlcrt, "Diffcrcl~tial Thermal
Analysis of Hydrated Clinker Minerals," Trni~ld.-Ztg. Keral~z.
Rztt~dscha~t, 86 [ l o ] 228-32 (1962).
9 2 M. Rcy, "Examination of Hydraulic Binders by Diffcrential Thermal Analysis," Silicates 11zd.. 22 [ l o ] 533-40 (1957).
2 3 A. J. Majutnclar and Rusturn Roy, "The System CaO- 111?0:J-13z0," J. A I I L . Ceranz. Soc., 39 [12] 4 3 6 4 2 (1956).
9.1 P. 1'. B~tdnikov a ~ ~ d 0 . M. Sologubova, "IZcaction Betwccn
Icaolin and Calciu~n Carbonate in Wllite-Ccmcnt Protluction,"
M a y 1966
Character of Hydration of 3Ca0.A120a
273
26 See Ref. 14, p. 227. 28 A. R . C. Westwood, "Sensitive Mechanical Propcrties,"
26 V. V. Mikhailov; pp. 927-55 in Cllemistry of Cement, Vol. Ind. Eng. Chem., 56 [9] 15-25 (1964).
11-Proceedings of the Fourth International Symposium, 29 J. J. Gi,l,man, "Direct Measurements of the Surface Energies
Washington, 1960. Natl. Bur. Std., ( U . S.) Motlograph, No. 43, of crystals, J . ~ ~phys,, ~ 31 1121 2208-18 (1960). 1 ,
l n c n
I Y U D .
27 A. L. G. Rees, "Significance of Solid State Defects in Chein- 30 A. R . C. Westwood, "Rebinder Effect and the Adsorption- ical Science and Technology," Australia?~ J. Sci., 26 [8] 239-46 Locking of Dislocations in Lithium Fluoride," Phil. Mag., 7 [76]