HAL Id: hal-02610687
https://hal.archives-ouvertes.fr/hal-02610687
Submitted on 17 May 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0
International License
Embryo-derived and induced pluripotent stem cells:
Towards naive pluripotency and chimeric competency in
rabbits
Marielle Afanassieff, Florence Perold, Wilhelm Bouchereau, Antoine Cadiou,
Nathalie Beaujean
To cite this version:
Marielle Afanassieff, Florence Perold, Wilhelm Bouchereau, Antoine Cadiou, Nathalie
Beau-jean.
Embryo-derived and induced pluripotent stem cells:
Towards naive pluripotency and
chimeric competency in rabbits.
Experimental Cell Research, Elsevier, 2020, 389 (2), pp.1-8.
Contents lists available atScienceDirect
Experimental Cell Research
journal homepage:www.elsevier.com/locate/yexcr
Embryo-derived and induced pluripotent stem cells: Towards naive
pluripotency and chimeric competency in rabbits
Marielle Afanassieff
∗, Florence Perold, Wilhelm Bouchereau, Antoine Cadiou, Nathalie Beaujean
Univ Lyon, Université Lyon 1, Inserm, INRAE, Stem Cell and Brain Research Institute, U1208, USC1361, F-69500, Bron, FranceA R T I C L E I N F O Keywords: Stem cells Embryo Pluripotency Chimaera Rabbit A B S T R A C T
Both embryo-derived (ESC) and induced pluripotent stem cell (iPSC) lines have been established in rabbit. They exhibit the essential characteristics of primed pluripotency. In this review, we described their characteristic features at both molecular and functional levels. We also described the attempts to reprogram rabbit pluripotent stem cells (rbPSCs) toward the naive state of pluripotency using methods established previously to capture this state in rodents and primates. In the last section, we described and discussed our current knowledge of rabbit embryo development pertaining to the mechanisms of early lineage segregation. We argued that the molecular signature of naive-state pluripotency differs between mice and rabbits. We finally discussed some of the key issues to be addressed for capturing the naive state in rbPSCs, including the generation of embryo/PSC chimeras.
1. Introduction
Pluripotency refers to the ability of a stem cell to give rise to all cell types in mature organisms. During normal development, pluripotency is transient and restricted to epiblast cells. Under suitable culture condi-tions, cells derived from the epiblast acquire unlimited self-renewal while maintaining pluripotent characteristics in vitro [1]. In mice, pluripotent stem cells (PSCs) comprise the following two main types: (i) embryonic stem cells (ESCs), derived from the inner cell mass (ICM) or the early epiblast of the blastocyst (pre-implantation), which epitomise the naive (or ground) state of pluripotency and (ii) epiblast stem cells (EpiSCs), derived from the late epiblast of the egg-cylinder-stage em-bryo (post-implantation), which epitomise the primed state of plur-ipotency [2]. Only naive mouse ESCs can colonise the epiblast of the blastocyst, contribute to the development of all tissue types and gen-erate chimeras [3,4].
Marked differences with mice in the pluripotency status of pre-im-plantation embryo-derived PSCs in lagomorphs and primates have been reported. Indeed, PSC lines established from rabbit, human and non-human primate (NHP) blastocysts display nearly all the characteristic features of primed pluripotency, even though they are generated from the early epiblast of the blastocyst, as is the case with rodent ESCs [5]. All attempts to propagate rabbit and primate PSC lines using the culture conditions established previously to capture the naive or ground state (hereafter referred to as ‘naive’ state) of pluripotency as defined in rodents have been unsuccessful. Why lagomorphs and primates differ
from rodents in the molecular properties of PSC lines is unclear. The molecular mechanisms of the naive state of pluripotency in pre-im-plantation lagomorph and primate embryos may differ from those identified in rodent embryos. Recent studies on the pre-implantation embryo transcriptome in humans and NHPs suggest that not all the transcription factors that define the essential programme for naive pluripotency in mice are expressed in primate epiblasts [6–8]. More-over, the signalling pathways that activate these genes and the timing of their upregulation during pre-implantation development differ [9,10].
Capturing the original state of pluripotency in PSCs is challenging; the scarcity of human and NHP embryos makes this issue difficult to resolve. In contrast, rabbit embryos are readily available in large numbers. Moreover, the developmental features of early rabbit embryos resemble those of primates [11]. In contrast to the three-dimensional egg-cylinder shape of gastrulating rodent embryos, primate and rabbit embryos develop into a flattened disk at the surface of the conceptus. Primate and rabbit embryos are also much the same regarding the timing of zygotic genome activation and the timing and regulation of X-chromosome inactivation [12]. Moreover, unlike gastrulation in ro-dents and primates, which takes place in the implanted embryo buried deep within the uterine wall, in rabbits, it begins shortly before im-plantation, which allows easy access to a much wider window of de-velopment. As a surrogate model, the rabbit is suited to exploring the nature and mechanisms of acquisition and maintenance of pluripotency in epiblast cells and embryo-derived stem cells for a wide range of
non-https://doi.org/10.1016/j.yexcr.2020.111908
Received 11 October 2019; Received in revised form 8 January 2020; Accepted 10 February 2020
∗Corresponding author.
E-mail address:marielle.afanassieff@inserm.fr(M. Afanassieff).
Experimental Cell Research 389 (2020) 111908
Available online 11 February 2020
0014-4827/ © 2020 The Authors. Published by Elsevier Inc. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/).
rodent mammals, including primates.
In this chapter, we reviewed our knowledge of rabbit PSCs, focusing on their pluripotent state, naive or primed. We also reviewed recent observations of epiblast specification in rabbit embryos. Finally, we discussed the issues to address for capturing the naive state in rabbit PSCs and generate embryo/PSC chimeras.
2. Pluripotent state of rbESCs
Embryo-derived rabbit PSCs (rbESCs) were first reported in 2007 [13,14] by culturing isolated ICMs of early blastocysts (E3.5-E4.0) on feeder cells, on which they formed large flattened colonies. Only ac-tivin/transforming growth factor-β (TGFβ) and fibroblast growth factor 2 (FGF2) were found to sustain self-renewal [15–17] (Fig. 1A), sug-gesting that rbESCs thrive in the primed state of pluripotency. FGF2 induces PI3K/AKT, MAPK and Wnt/beta-catenin pathways [15,18,19]. Although leukaemia inhibitory factor (LIF), LIF receptor (LIFR) and GP130 are expressed in rabbit peri-implantation embryos (i.e. between E6 and E9) [20], and LIF has been used for rbESC derivation and maintenance [18,19,21], indisputable evidence supporting the role of LIF in rbESC self-renewal is lacking [22]. Attempts to derive rbESCs in a naive state using protocols originally developed in rodents and humans have been unsuccessful. Unlike the situation described in rodents, rabbit ICMs do not grow when cultured in media supplemented only with MEK and GSK3β inhibitors [22], a hallmark of naive pluripotency in rodents [23]. Moreover, as recently shown by Liu and colleagues,
FGF2 and feeder cells are both required for the derivation of dome-shaped naive-like rbESCs [24].
Besides strict FGF2-dependency, rbESCs express cell-surface mar-kers such as SSEA-3/4 and N-cadherin [17,21], they fail to activate the distal enhancer of mouse Pou5f1/Oct4 gene, and the second X-chro-mosome is covered with H2AK119ubi repressive marks and is inactive in female lines [25] (personal unpublished data) (Table 1,Fig. 1B), all of which are markers of primed pluripotency in mice. In addition, X-chromosome inactivation has been shown to occur as late as gastrula-tion in the epiblast of rabbit embryos [12,26], further suggesting that rbESCs are in vitro counterparts of the late epiblast.
PSCs generated in mice, monkeys and humans, either primed or naive, have an unusual cell cycle regulation, characterised by the lack of the DNA damage checkpoint in the G1 phase. When submitted to low-dose irradiation or DNA-damaging molecules, growth arrest takes place only at the G2 checkpoint [27–29]. Differentiation restores the G1 checkpoint that characterises somatic cells. Interestingly, the derivation method was found to influence the cell cycle regulation of rbESCs. rbESCs derived by single-cell dissociation with Accutase loosed the G1 checkpoint like their mouse and primate counterparts [30], while the conventional rbESCs exhibited both G1 and G2 checkpoints [17], sug-gesting that they are further advanced in development compared with mouse EpiSCs. This also suggests that restoration of the G1 checkpoint takes place before commitment to differentiation during gastrulation. Together, these observations indicate that rbESCs exhibit the essential features of primed pluripotency.
Fig. 1. Rabbit pluripotent stem cells. A)
Morphology, derivation or reprogramming methods, growth factor used, and
plur-ipotency state of rabbit ESCs [17], rabbit
iPSCs [17] and rbEKA cells [40].
MEFs = mitomycin-treated mouse em-bryonic fibroblasts; KOSR = knock out serum replacement; FGF2 = fibroblast growth factor 2; FBS = foetal bovine serum; LIF = leukaemia inhibitory factor; hOKSM = human factors OCT4, KLF4, SOX2 and C-MYC; Scale bar = 50 μm. B) Immunodetection of inactive X chromosome (H2AK119 ubiquitination in red) in rbESCs with DNA counterstaining (blue) (personal unpublished data). Cells analyzed here were derived by unicellular dissociation with Ac-cutase and cultured on MEFs with KOSR/
FBS + FGF2 [30]. Confocal single z-section,
Scale bar = 10 μm.
M. Afanassieff, et al. Experimental Cell Research 389 (2020) 111908
Table 1 Comparison of characteristics and properties of mouse and rabbit pluripotent stem cells. PSC type EpiSCs rbESCs rbiPSCs mESCs Lines Primed standard Conventional rbESCs rbESCs derived in presence of LIF Conventional rbiPSCs Reprogrammed rbiPSCs Naive standard References [ 2 , 4 ] [ 13 , 14 , 17 ] [ 18 , 19 , 30 ] [ 17 , 31 , 32 ] [ 25 , 40 ] [ 1 , 2 ] Colony morphology Flattened Flattened Flattened Flattened Flattened Dome-shapped Serum and growth factors KOSR + FGF2 KOSR + FGF2 KOSR/FBS + LIF KOSR + FGF2 FBS + LIF FBS + LIF Signalling pathways FGF + Activin FGF + Activin FGF + Activin FGF + Activin FGF + Activin LIF + BMP4 Cell surface markers SSEA1 E-Cadherin SSEA4 E−/N-Cadherin SSEA1/4 E-Cadherin SSEA1/4 E-Cadherin ND SSEA1 Unicellular dissociation Possible No No Yes Yes Yes Yes Enzyme Collagenase Collagenase Accutase Trypsin Trypsin Trypsin Clonogenic self-renewal Very low No Low Intermediate a Intermediate a High Pluripotency genes Oct4, Nanog, Fgf 5, T-Bra, Otx2 Eomes, Sox17, OCT4, NANOG, CDH2, GBX2, DAX1, HOX, PAX OCT4, NANOG, CDH1, PRMT14 OCT4, NANOG, CLDN6, OTX2, PITX2, GBX2, DAX1, CDX2 OCT4, NANOG, CDH1, KLF4, DPPA5, ZFP42, ESRRB, TFCP2L1, DAZL Oct4, Nanog, Fgf4, NrOb1, Zfp42, Klf4, Klf2, Esrrb, Tbx3, Tfcp2l1 Short G1 phase Yes No Yes b Yes b ND Yes DNA damage G1 checkpoint No Yes No b No b ND No Embryo colonisation Method Micro-injection Micro-injection Micro-injection Aggregation Micro-injection Micro-injection Tetraploid complementation Rate Null Null Very low at E4 Low at E4 Hight at E3.5/E4 Low at E6.5 Very High 2nd X chromosome activation No ND No c No c Yes Yes EOS vector expression d No No No Yes Yes Yes ND = not determined. aEstimation by comparison with those of mPSCs. bOnly in SSEA1-positive sub-populations. cPersonal unpublished data (see Fig. 1 B). dVector expressing GFP from ETn promoter and mouse Oct4 distal enhancer [ 68 ].
3. Pluripotent state of rbiPSCs
Rabbit induced PSCs (rbiPSCs) have been obtained after repro-gramming somatic cells from the stomach, liver and skin using retro-viral vectors expressing human OCT4, KLF4, SOX2 and C-MYC genes [17,31,32]. Several rbiPSC lines in which the four transgenes were completely turned off were described. They display the same mor-phology as rbESCs (Fig. 1A) and self-renew with FGF2 and activin (Table 1). In our laboratory, we derived both conventional rbESC and rbiPSC lines from the same breed of New Zealand white rabbits and observed differences pertaining to the pluripotency state [17] (Table 1). The rbiPSCs were more resistant to single-cell dissociation and per-formed better in a clonogenic self-renewal assay than did the rbESCs. Moreover, they displayed a stronger activity of the distal enhancer of murine Pou5f1/Oct4 than their embryo-derived counterparts. In addi-tion, the rbiPSCs exhibited a global gene expression profile closer to that of rabbit ICM cells [17]. These results strongly suggest that rbiPSCs spontaneously exhibit some naive-like features. However, as noted above, rbiPSCs, like rbESCs, depend on FGF2 and activin for the dif-ferentiation blockade. Moreover, the global transcriptome of rbiPSCs remains distinct from that of ICM cells originating from E4 rabbit em-bryos [33]. rbiPSCs are therefore not in a naive state of pluripotency.
4. Reprogramming conventional rbESCs and rbiPSCs to naive-like pluripotency
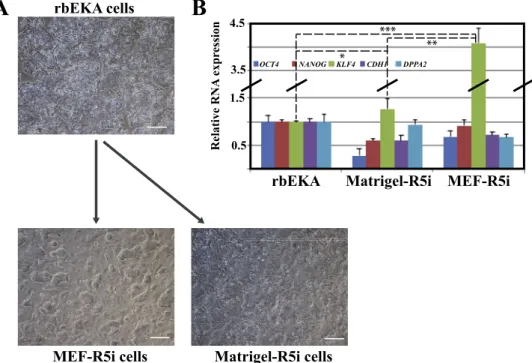
We used rbiPSCs as a cell platform to delineate culture conditions promoting a naive state. All the methods originally developed to re-program human and monkey PSCs to naive pluripotency, including NHSM [34], 5iLA [35], t2iLGöY [36] and LCDM [37], were applied following authors' protocols. None of them allowed for the stable self-renewal of rbiPSCs. Reprogrammed cells failed to sustain rapid growth and quickly differentiate [38]. Moreover, neither the gradual shift from original to new media composition nor alterations in inhibitor con-centrations allowed rbiPSCs to acquire naive markers (data not shown). We identified a new small molecule cocktail, called R5i, which allowed rbiPSCs to form dome-shaped colonies on both feeder cells (mitomycin-treated mouse embryonic fibroblasts or MEFs) and Matrigel (a synthetic extra-cellular matrix) (Fig. 2A) (personal unpublished data). R5i is made from N2B27 medium supplemented with knockout serum
replacement (KOSR), LIF, activin A, FGF2 and chemical inhibitors of MEK, GSK3β, BRAF, SRC and JNK kinases. However, the pluripotency genes OCT4, NANOG, DPPA2 and CDH1 were not up-regulated in the self-renewing colonies, except for the naive gene KLF4 in cells cultured on MEFs (Fig. 2B). Overall, these results suggest that the signalling pathways sustaining naive pluripotency in rabbits, primates and mice are different.
Transcription factors have been used to reset mouse EpiSCs and human PSCs to naive-like pluripotency. A similar paradigm was applied to rbiPSCs. The first study used a cocktail of human transcription fac-tors OCT4, KLF4, SOX2 and C-MYC in combination with LIF, KOSR, MEK and GSK3β inhibitors (2i) and Forskolin to reprogram rabbit fi-broblasts to a naive-like state [25]. Forskolin, a PKC agonist, has been shown to reinforce KLF2 and KLF4 expression in human PSCs [39]. This
OCT4+SOX2+KLF4+C-MYC+KOSR+2i+LIF+Forskolin cocktail
reactivated the second X-chromosome in female rbiPSC lines [25]. These lines formed dome-shaped colonies, exhibited an increased growth rate and improved clonogenic self-renewal, and depended on both LIF/STAT3 and PI3K/AKT pathways to inhibit differentiation. However, the naive-like state was maintained only in the presence of doxycycline to keep the four transgenes expressed, indicating in-complete resetting of the cells. We developed a second strategy based on the combination of KLF2 and KLF4 overexpression associated with LIF and foetal bovine serum (FBS) [40]. The resulting cells, named rbEKAs, were characterised by both transcriptome and epigenome re-configuration (Table 1). They expressed transcription factor genes as-sociated with naive pluripotency including DPPA2, DPPA5, ZFP42,
DAZL and PIWIL. They also exhibited more H3K9 and H3K14
acetyla-tion and heterochromatin cluster decompacacetyla-tion, suggesting a more open chromatin configuration (Fig. 3). As described in detail below, the rbEKA cells exhibited improved chimeric competency compared with conventional rbiPSCs. Nevertheless, the rbEKA cells remained depen-dent on FGF2 and not on LIF/JAK signalling for self-renewal, indicating that they failed to acquire the signalling hallmarks of naive plur-ipotency defined in rodents.
Together, these studies indicate that rbPSCs fail to respond to naive state pluripotency reprogramming factors associated with their mouse and human counterparts, which suggests that the signalling pathways sustaining naive pluripotency in rabbits differ from those described previously in both rodents and primates.
Fig. 2. Reprogramming of rbEKA cells toward
the naive state of pluripotency (personal un-published data). A) Dome-shaped morphology of R5i colonies cultured on MEFs (mitomycin-treated mouse embryonic fibroblasts) or Matrigel. R5i cells were derived by culture of rbEKA cells in N2B27-based medium supple-mented with KOSR, LIF, activin A, FGF2 and chemical inhibitors of MEK, GSK3β, BRAF, SRC and JNK kinases. Scale bar = 100 μm. B) Expression of pluripotency genes (OCT4 and NANOG) and naive markers (KLF4, CDH1 and DPPA2) in rbEKA and R5i cells. Statistical analysis (κ2 test): ***p < 0.000001; **p < 0.00001; *p < 0.01.
M. Afanassieff, et al. Experimental Cell Research 389 (2020) 111908
5. Engraftment of rbiPSCs and rbESCs into rabbit pre-implantation embryos and chimeric competency
Chimeric competency defines the capacity of PSCs to massively colonise a pre-implantation embryo and participate in the formation of the three germ layers during gastrulation [2]. Chimeric competency is considered a hallmark of naive pluripotency. In mice, embryo coloni-sation is routinely achieved by injecting mouse ESCs or iPSCs into early-cleavage-stage embryos or blastocysts, and by aggregation with morula [41,42], often resulting in high rates of chimerism (>50%). The si-tuation in rabbits is markedly different. Two-coat-colour chimeras were obtained, the first after injecting rbESCs derived from a pigmented strain, Dutch belted, into New Zealand white embryos [43], and the second after performing nuclear cell transfer into black Alaska oocytes with mesenchymal stem cells (MSCs) derived from the albino Zika strain [44]. These early results were not confirmed by later reports. On the contrary, we have recently reported that rbESCs did not colonise the ICM and were eliminated rapidly after injection into rabbit embryos [17]. Only rare chimeric embryos were obtained after aggregating rbiPSCs with morula. However, the long-term colonisation of the three germ layers and their derivatives was not observed [17]. The failure of both rbESCs and rbiPSCs to efficiently colonise rabbit embryos is likely a result of a primed nature acquired during long-term culture. Adult chimeras have indeed been obtained after the injection of freshly iso-lated ICM cells into blastocysts [45,46], and after the injection of blastomeres and male foetal gonadal cells into an 8-cell embryo or a morula [47–49].
The naive-like rbEKA cells described above exhibited much im-proved colonisation competency (Fig. 4). Indeed, the injection of rbEKA cells into 8-cell rabbit embryos resulted in extensive colonisation of the ICM in 9% early blastocysts, epiblasts in 10% mid-blastocysts, and embryonic disks in 1.4% pre-gastrulae [40]. Thus, the rate of success-fully colonised rabbit embryos decreased from the early-blastocyst stage to the early-gastrula stage. It is possible that rbEKA cells observed in the E4 rabbit blastocysts were not truly incorporated into the ICM and only a few of them successfully colonised the epiblast and con-tributed to its expansion, which is in line with previous observations in mice [50]. Another possible explanation is a weak competitive edge of rbEKA cells and their progeny against the host cells in contributing to late epiblast expansion and differentiation. Nevertheless, rbEKA cells are dependent on FGF2 and not on LIF/JAK signalling for self-renewal, which indicates that primed-type cell signalling is compatible with embryo colonisation and chimeric competency.
6. Lessons from rabbit embryo development
We currently lack a detailed description of pluripotency in rabbits at
the molecular level. The characterisation of rabbit PSCs is based largely on molecular markers of naive pluripotency defined in mice. However, substantial differences exist between rodents and lagomorphs, which could lead to confusion when assessing the naive versus the primed state. The time gap between the onset of cavitation (day 3 of devel-opment) and implantation (day 7) is extended considerably in rabbits (Fig. 5). At the time of implantation, the embryo reaches around 5 mm in diameter and has approximately 5000 cells, a stage that is sig-nificantly more advanced than that in the mouse [51]. During this period, embryonic development progresses with trophoblast differ-entiation, hypoblast migration and the onset of gastrulation at day 6.6 shortly before implantation [52]. In contrast to the mouse, OCT4/
POU5F1 is expressed in all lineages between days 3 and 6 and its
ex-pression becomes restricted to the epiblast only at day 7 [53,54]. Owing to this prolonged OCT4/POU5F1 expression in the trophectoderm, co-localisation with trophectoderm-specific markers such as CDX2 can be observed in rabbit blastocysts. Similarly, SOX2 expression increases gradually in the ICM and epiblast compartments during this time-window and it becomes specific to the epiblast only at the late-blas-tocyst stage [55]. Expression patterns of naive- and primed state-spe-cific genes also exhibit noticeable differences between mouse and rabbit. For instance, the naive-state markers Gbx2, Dax, Rex1 and Klf2, and the primed-state markers Sox17, Dkk1, Lefty1 and Nodal, expressed in the mouse ICM/early epiblast and late epiblast, respectively, do not exhibit differential expression patterns in the rabbit embryo [33,56,57]. Thus, although some other mouse genes specific of naive or primed-states, such as Klf4, Klf5, Tbx3, Gp130, Esrrb, Dppa3, Dazl and Otx2,
Pitx2, Eomes, respectively, are expressed in rabbit embryos at presumed
corresponding pluripotency states, there are substantial differences in the molecular signature of these states between mouse and rabbit (Fig. 5), complicating the characterisation of rbESCs and rbiPSCs [33]. The underlying mechanism of epiblast (EPI)/primitive endoderm (PrE) segregation also seems to differ with that of rodents. As described in mice, the expression of NANOG increases at the blastocyst stage and becomes specific to the epiblast compartment at day 4 [55,58]. How-ever, unlike the situation described in mice, GATA6 is not con-comitantly down-regulated and GATA6/NANOG double-positive cells can be observed in the rabbit epiblast. This suggests that the well-de-scribed murine mechanism of the mutual repression of Gata6 and Nanog does not operate in rabbits [55]. Furthermore, blocking the FGF4 sig-nalling pathway by culturing rabbit embryos with an inhibitor of MEK kinase does not bias epiblast segregation at the expense of PrE, as it is observed in mice [59,60]. It decreases the number of SOX17-positive cells (PrE) and increases that of SOX2/SOX17 double-negative cells that enter apoptosis, but it does not increase the number of SOX2-positive cells (EPI) [55]. This may explain why rabbit ICMs do not grow when cultured in media supplemented with MEK and GSK3β inhibitors [30].
Fig. 3. Epigenetic status of rabbit induced
plur-ipotent stem cells. Analysis of conventional (rbiPSC-B19) and reprogrammed (rbEKA) rbiPSCs expressing the GFP from ETn promoter and mOCT4 distal
en-hancer (EOS lentiviral vector [68]) [40]. Detection of
GFP (in green) and both permissive (H3K9ac) and repressive (H3K9me3) histone marks (in red), with DNA counterstaining (blue). Permissive marks are more abundant in rbEKA cells, while repressive mark accumulations are more frequent in rbiPSC-B19 nu-clei, consequently showing that the naivest cells are the reprogrammed rbEKAs. Confocal single z-sec-tions, Scale bar = 10 μm.
Thus, although FGF4 is involved in the formation of PrE in rabbits, another pathway should be playing a key role in promoting EPI.
Histone post-translational modifications (PTMs) regulate gene ex-pression in the epiblast vs. trophoblast compartments and therefore participate in the regulation of naive pluripotency [61]. In mouse blastocysts, the histone PTMs H4K8ac and H3K4me3 are enriched on the promoters of Nanog and Oct4/Pou5f1 in the ICM, correlating with their expression; in the trophectoderm, where Nanog and Oct4/Pou5f1 are repressed, their promoters are enriched in H3K9me2 [62,63]. A similar regulation by the bivalent H3K4me3 and H3K27me3 marks has been observed for the Sox2 promoter [64]. Furthermore, the depletion of lysine-specific demethylase 6 B (KDM6B) in pre-implantation mouse
embryos alters the incorporation of H3K27me3, leading to the silencing of Cdx2 and Gata3 in the trophectoderm-lineage and implantation failure [65,66].
In rabbits, a detailed analysis of histone PTMs and their role in lineage-specific gene expression are lacking. Only the global levels of PTMs have been investigated by immunofluorescence, showing a cor-relation between the segregation of the ICM vs. trophectoderm upon the formation of the blastocyst and the dynamics of H3K4me3/H3K27me3 bivalent marks [53,58]. The sole direct link between gene expression and epigenetic modifications was recently demonstrated for OCT4/
POU5F1. Interestingly, the progressive methylation of the 5′ regulatory
region of OCT4/POU5F1 accompanies progressive repression of this
Fig. 4. Embryonic colonisation capacity of
rabbit pluripotent stem cells. Chimeric blastocysts were obtained after injecting rabbit or mouse GFP-, LacZ- or dsRed-ex-pressing PSCs into 4/8-cell stage rabbit or
mouse embryos [17,30,40]. rbEKA cells are
able to colonise rabbit blastocysts but the grafted cells are lost after gastrulation. mESCs are able to efficiently colonise both mouse and rabbit blastocysts. Scale bar = 50 μm.
Fig. 5. Early rabbit embryonic development and corresponding putative pluripotent states in vivo. E1 to E7 correspond to days of development in vivo. * specific gene
signatures of in vivo rabbit epiblasts at stage E3.5/E4 (putative naive state) and E6/E7 (putative primed state) according to Schmaltz-Panneau and colleagues [33].
M. Afanassieff, et al. Experimental Cell Research 389 (2020) 111908
gene in the trophoblast [67]. This emphasises the importance of epi-genetic modifications upon lineage segregation and specification in mice and rabbits alike. However, further studies are required to deci-pher the precise role of epigenetic modifications and their regulating enzymes in rabbit pluripotency. As a result, those studies could lead to the identification of epigenetic modifiers that can trigger reprogram-ming and stabilise the naive state of pluripotency in this species.
7. Conclusion
All studies to date used paradigms and strategies developed ori-ginally for rodents and primates to capture and propagate rbPSCs into more immature states of pluripotency. However, the engineered cells did not exhibit all the cardinal features of naive pluripotency defined in rodents. In particular, they remained dependent on FGF2 signalling, thought to be a hallmark of primed pluripotency, and retained part of the transcriptome and epigenome characteristics of conventional, primed PSCs. Furthermore, the efficacy of embryo colonisation after injection into pre-implantation rabbit embryos was low, suggesting that only a small fraction of the rbPSCs in the population were capable of colonisation. New strategies must therefore be developed to reset con-ventional rbPSCs to bona fide naive pluripotency. The implementation of these strategies requires a thorough characterisation of the tran-scriptome and epigenome of the rabbit epiblast from early-blastocyst to gastrula stages to benchmark the position of PSC lines along the nai-ve–primed pluripotency gradient. It also requires an in-depth explora-tion of the genes and signalling pathways that control the segregaexplora-tion of trophoblast and PrE lineages in the morula and blastocysts, respec-tively. The inhibition of these pathways is essential for capturing the naive state of pluripotency in vitro. Efficient engraftment of rbPSCs into the host epiblast is another critical issue in the prospect of generating somatic and germline chimeras in rabbits. In order to give PSCs a stronger competitive edge over the host epiblast cells, accelerating the cell cycle of PSCs, or temporarily slowing down that of host epiblast cells is a line of research that needs to be explored.
CRediT authorship contribution statement
Marielle Afanassieff: Conceptualization, Supervision, Writing
-original draft, Writing - review & editing. Florence Perold: Methodology, Investigation, Formal analysis. Wilhelm Bouchereau: Methodology, Investigation, Formal analysis. Antoine Cadiou: Investigation. Nathalie Beaujean: Conceptualization, Supervision, Writing - original draft, Writing - review & editing.
Acknowledgements
The authors acknowledge financial support of their work by the National Agency for Research (ANR), France, as part of Laboratoire d'Excellence Revive (ANR-10-LABX-73), Laboratoire d'Excellence DEVweCAN (ANR-10-LABX-0061), Laboratoire d'Excellence CORTEX
(ANR-11-LABX-0042), IHU-B CESAME (ANR-10-IBHU-003),
Infrastructure Nationale en Biologie et Santé INGESTEM (ANR-11-INBS-0009), Infrastructure Nationale en Biologie et Santé CRB-Anim (ANR-11-INBS-0003), and the program ‘Investissements d’Avenir’ (ANR-11-IDEX-0007), by the Fondation pour la Recherche Médicale, France (Equipe FRM 2017, DEQ20170336757) and by INRAE Animal Physiology and Livestock Systems Division (PhD fellowship, CR 4000.17 Loft 112).
References
[1] A.G. Smith, Embryo-derived stem cells: of mice and men, Annu. Rev. Cell Dev. Biol.
17 (2001) 435–462.
[2] J. Nichols, A. Smith, Naive and primed pluripotent states, Cell Stem Cell 4 (6)
(2009) 487–492.
[3] A. Bradley, et al., Formation of germ-line chimaeras from embryo-derived
ter-atocarcinoma cell lines, Nature 309 (5965) (1984) 255–256.
[4] P.J. Tesar, et al., New cell lines from mouse epiblast share defining features with
human embryonic stem cells, Nature 448 (7150) (2007) 196–199.
[5] P. Savatier, P. Osteil, P.P. Tam, Pluripotency of embryo-derived stem cells from
rodents, lagomorphs, and primates: slippery slope, terrace and cliff, Stem Cell Res.
19 (2017) 104–112.
[6] T. Nakamura, et al., Single-cell transcriptome of early embryos and cultured
em-bryonic stem cells of cynomolgus monkeys, Sci Data 4 (2017) 170067.
[7] S. Petropoulos, et al., Single-cell RNA-seq reveals lineage and X chromosome
dy-namics in human preimplantation embryos, Cell 165 (4) (2016) 1012–1026.
[8] T. Boroviak, J. Nichols, Primate embryogenesis predicts the hallmarks of human
naive pluripotency, Development 144 (2) (2017) 175–186.
[9] T. Boroviak, et al., Lineage-specific profiling delineates the emergence and
pro-gression of naive pluripotency in mammalian embryogenesis, Dev. Cell 35 (3)
(2015) 366–382.
[10] A. Piliszek, Z.E. Madeja, Pre-implantation development of domestic animals, Curr.
Top. Dev. Biol. 128 (2018) 267–294.
[11] V. Duranthon, et al., On the emerging role of rabbit as human disease model and the
instrumental role of novel transgenic tools, Transgenic Res. 21 (4) (2012) 699–713.
[12] I. Okamoto, et al., Eutherian mammals use diverse strategies to initiate
X-chro-mosome inactivation during development, Nature 472 (7343) (2011) 370–374.
[13] S. Wang, et al., Generation and characterization of rabbit embryonic stem cells,
Stem Cell. 25 (2) (2007) 481–489.
[14] A. Honda, et al., Stable embryonic stem cell lines in rabbits: potential small animal
models for human research, Reprod. Biomed. Online 17 (5) (2008) 706–715.
[15] S. Wang, et al., Dissecting signaling pathways that govern self-renewal of rabbit
embryonic stem cells, J. Biol. Chem. 283 (51) (2008) 35929–35940.
[16] A. Honda, M. Hirose, A. Ogura, Basic FGF and Activin/Nodal but not LIF signaling
sustain undifferentiated status of rabbit embryonic stem cells, Exp. Cell Res. 315
(12) (2009) 2033–2042.
[17] P. Osteil, et al., Induced pluripotent stem cells derived from rabbits exhibit some
characteristics of naive pluripotency, Biol Open 2 (6) (2013) 613–628.
[18] Y.C. Hsieh, et al., LIF and FGF cooperatively support stemness of rabbit embryonic
stem cells derived from parthenogenetically activated embryos, Cell. Reprogr. 13
(3) (2011) 241–255.
[19] N.W. Lo, et al., Leukemia inhibitory factor and fibroblast growth factor 2 critically
and mutually sustain pluripotency of rabbit embryonic stem cells, Cell Transplant.
24 (3) (2015) 319–338.
[20] A.P. Catunda, et al., Characterization, chromosomal assignment, and role of LIFR in
early embryogenesis and stem cell establishment of rabbits, Clon Stem Cell 10 (4)
(2008) 523–534.
[21] P. Intawicha, et al., Characterization of embryonic stem cell lines derived from New
Zealand white rabbit embryos, Clon Stem Cell 11 (1) (2009) 27–38.
[22] P. Osteil, et al., Generation of genome-edited mouse epiblast stem cells via a detour
through ES cell-chimeras, Differentiation 91 (4–5) (2016) 119–125.
[23] Q.L. Ying, et al., The ground state of embryonic stem cell self-renewal, Nature 453
(7194) (2008) 519–523.
[24] J. Liu, et al., Deriving rabbit embryonic stem cells by small molecule inhibitors, Am
J Transl Res 11 (8) (2019) 5122–5133.
[25] Y. Jiang, et al., Xist deficiency and disorders of X-inactivation in rabbit embryonic
stem cells can be rescued by transcription-factor-mediated conversion, Stem Cell.
Dev. 23 (19) (2014) 2283–2296.
[26] P. Bermejo-Alvarez, et al., Sex determines the expression level of one third of the
actively expressed genes in bovine blastocysts, Proc. Natl. Acad. Sci. U. S. A. 107 (8)
(2010) 3394–3399.
[27] M.I. Aladjem, et al., ES cells do not activate p53-dependent stress responses and
undergo p53-independent apoptosis in response to DNA damage, Curr. Biol. 8 (3)
(1998) 145–155.
[28] A.C. Fluckiger, et al., Cell cycle features of primate embryonic stem cells, Stem Cell.
24 (3) (2006) 547–556.
[29] A.A. Filipczyk, et al., Differentiation is coupled to changes in the cell cycle
reg-ulatory apparatus of human embryonic stem cells, Stem Cell Res. 1 (1) (2007)
45–60.
[30] P. Osteil, et al., A panel of embryonic stem cell lines reveals the variety and dynamic
of pluripotent states in rabbits, Stem Cell Reports 7 (3) (2016) 383–398.
[31] A. Honda, et al., Generation of induced pluripotent stem cells in rabbits: potential
experimental models for human regenerative medicine, J. Biol. Chem. 285 (41)
(2010) 31362–31369.
[32] Z. Tancos, et al., Establishment of a rabbit induced pluripotent stem cell (RbiPSC)
line using lentiviral delivery of human pluripotency factors, Stem Cell Res. 21
(2017) 16–18.
[33] B. Schmaltz-Panneau, et al., Contrasting transcriptome landscapes of rabbit
plur-ipotent stem cells in vitro and in vivo, Anim. Reprod. Sci. 149 (1–2) (2014) 67–79.
[34] O. Gafni, et al., Derivation of novel human ground state naive pluripotent stem
cells, Nature 504 (7479) (2013) 282–286.
[35] T.W. Theunissen, et al., Systematic identification of culture conditions for induction
and maintenance of naive human pluripotency, Cell Stem Cell 15 (4) (2014)
471–487.
[36] Y. Takashima, et al., Resetting transcription factor control circuitry toward
ground-state pluripotency in human, Cell 158 (6) (2014) 1254–1269.
[37] Y. Yang, et al., Derivation of pluripotent stem cells with in vivo embryonic and
extraembryonic potency, Cell 169 (2) (2017) 243–257 e25.
[38] M. Afanassieff, et al., Interest of rabbit induced pluripotent stem cells for species
pre-servation. Proceedings of the 14th Asian Reproductive Biotechnology Congress, Thaï
[39] J.H. Hanna, K. Saha, R. Jaenisch, Pluripotency and cellular reprogramming: facts,
hypotheses, unresolved issues, Cell 143 (4) (2010) 508–525.
[40] Y. Tapponnier, et al., Reprogramming of rabbit induced pluripotent stem cells
to-ward epiblast and chimeric competency using Kruppel-like factors, Stem Cell Res.
24 (2017) 106–117.
[41] A. Nagy, et al., Embryonic stem cells alone are able to support fetal development in
the mouse, Development 110 (3) (1990) 815–821.
[42] P. Kraus, et al., A more cost effective and rapid high percentage germ-line
trans-mitting chimeric mouse generation procedure via microinjection of 2-cell, 4-cell,
and 8-cell embryos with ES and iPS cells, Genesis 48 (6) (2010) 394–399.
[43] L. Schoonjans, et al., Pluripotential rabbit embryonic stem (ES) cells are capable of
forming overt coat color chimeras following injection into blastocysts, Mol. Reprod.
Dev. 45 (4) (1996) 439–443.
[44] V. Zakhartchenko, et al., Cell-Mediated transgenesis in rabbits: chimeric and
nu-clear transfer animals, Biol. Reprod. 84 (2) (2011) 229–237.
[45] S. Bodo, et al., Production of transgenic chimeric rabbits and transmission of the
transgene through the germline, Mol. Reprod. Dev. 68 (4) (2004) 435–440.
[46] P. Chrenek, et al., Developmental rate and allocation of transgenic cells in rabbit
chimeric embryos, Zygote 16 (1) (2008) 87–91.
[47] J.R. Giles, et al., Pluripotency of cultured rabbit inner cell mass cells detected by
isozyme analysis and eye pigmentation of fetuses following injection into
blas-tocysts or morulae, Mol. Reprod. Dev. 36 (2) (1993) 130–138.
[48] K.H. Graves, R.W. Moreadith, Derivation and characterization of putative
plur-ipotential embryonic stem cells from preimplantation rabbit embryos, Mol. Reprod.
Dev. 36 (4) (1993) 424–433.
[49] A. Moens, et al., Low levels of chimerism in rabbit fetuses produced from
pre-implantation embryos microinjected with fetal gonadal cells, Mol. Reprod. Dev. 43
(1) (1996) 38–46.
[50] Z. Wang, R. Jaenisch, At most three ES cells contribute to the somatic lineages of
chimeric mice and of mice produced by ES-tetraploid complementation, Dev. Biol.
275 (1) (2004) 192–201.
[51] C. Viebahn, The anterior margin of the mammalian gastrula: comparative and
phylogenetic aspects of its role in axis formation and head induction, Curr. Top.
Dev. Biol. 46 (1999) 63–103.
[52] L. Blomberg, K. Hashizume, C. Viebahn, Blastocyst elongation, trophoblastic
dif-ferentiation, and embryonic pattern formation, Reproduction 135 (2) (2008)
181–195.
[53] C.H. Chen, et al., Spatial and temporal distribution of Oct-4 and acetylated H4K5 in
rabbit embryos, Reprod. Biomed. Online 24 (4) (2012) 433–442.
[54] M. Yin, et al., The Oct4 promoter-EGFP transgenic rabbit: a new model for
mon-itoring the pluripotency of rabbit stem cells, Int. J. Dev. Biol. 57 (11–12) (2013)
845–852.
[55] A. Piliszek, Z.E. Madeja, B. Plusa, Suppression of ERK signalling abolishes primitive
endoderm formation but does not promote pluripotency in rabbit embryo,
Development 144 (20) (2017) 3719–3730.
[56] G.R. Henderson, et al., Candidate gene expression patterns in rabbit
preimplanta-tion embryos developed in vivo and in vitro, J. Assist. Reprod. Genet. 31 (7) (2014)
899–911.
[57] Z. Tancos, et al., Cloning and characterization of rabbit POU5F1, SOX2, KLF4,
C-MYC and NANOG pluripotency-associated genes, Gene 566 (2) (2015) 148–157.
[58] Y. Liu, et al., Structural insights into trans-histone regulation of H3K4 methylation
by unique histone H4 binding of MLL3/4, Nat. Commun. 10 (1) (2019) 36.
[59] J. Nichols, et al., Suppression of Erk signalling promotes ground state pluripotency
in the mouse embryo, Development 136 (19) (2009) 3215–3222.
[60] J. Nichols, T. Boroviak, Maximizing clonal embryonic stem cell derivation by ERK
pathway inhibition, Methods Mol. Biol. 1341 (2016) 1–13.
[61] F. Li, et al., Bivalent histone modifications and development, Curr. Stem Cell Res.
Ther. 13 (2) (2018) 83–90.
[62] K.P. Nightingale, L.P. O'Neill, B.M. Turner, Histone modifications: signalling
re-ceptors and potential elements of a heritable epigenetic code, Curr. Opin. Genet.
Dev. 16 (2) (2006) 125–136.
[63] M.D. VerMilyea, L.P. O'Neill, B.M. Turner, Transcription-independent heritability of
induced histone modifications in the mouse preimplantation embryo, PloS One 4
(6) (2009) e6086.
[64] J.A. Dahl, et al., Histone H3 lysine 27 methylation asymmetry on
developmentally-regulated promoters distinguish the first two lineages in mouse preimplantation
embryos, PloS One 5 (2) (2010) e9150.
[65] B. Saha, et al., EED and KDM6B coordinate the first mammalian cell lineage
com-mitment to ensure embryo implantation, Mol. Cell Biol. 33 (14) (2013) 2691–2705.
[66] S. Chen, et al., The histone H3 Lys 27 demethylase JMJD3 regulates gene expression
by impacting transcriptional elongation, Genes Dev. 26 (12) (2012) 1364–1375.
[67] E. Canon, et al., Progressive methylation of POU5F1 regulatory regions during
blastocyst development, Reproduction 156 (2) (2018) 145–161.
[68] A. Hotta, et al., EOS lentiviral vector selection system for human induced
plur-ipotent stem cells, Nat. Protoc. 4 (12) (2009) 1828–1844.
M. Afanassieff, et al. Experimental Cell Research 389 (2020) 111908
![Fig. 1. Rabbit pluripotent stem cells. A) Morphology, derivation or reprogramming methods, growth factor used, and plur-ipotency state of rabbit ESCs [17], rabbit iPSCs [17] and rbEKA cells [40].](https://thumb-eu.123doks.com/thumbv2/123doknet/14460974.520443/3.892.54.616.83.679/rabbit-pluripotent-morphology-derivation-reprogramming-methods-growth-ipotency.webp)
![Fig. 3. Epigenetic status of rabbit induced plur- plur-ipotent stem cells. Analysis of conventional (rbiPSC-B19) and reprogrammed (rbEKA) rbiPSCs expressing the GFP from ETn promoter and mOCT4 distal en-hancer (EOS lentiviral vector [68]) [40]](https://thumb-eu.123doks.com/thumbv2/123doknet/14460974.520443/6.892.56.570.81.344/epigenetic-induced-analysis-conventional-reprogrammed-expressing-promoter-lentiviral.webp)
