PHARMACOLOGY AND CELL METABOLISM
Alteration of Glutamate/GABA Balance During Acute Alcohol Withdrawal in Emergency Department:
A Prospective Analysis
G. Brousse1,2,3,*, B. Arnaud1, F. Vorspan4, D. Richard2,5,6, A. Dissard1, M. Dubois1, D. Pic1, J. Geneste1, L. Xavier5, N. Authier2,5,6, V. Sapin2,7, P.-M. Llorca2,3,8, I. De Chazeron3,8, R. Minet-Quinard2,7and J. Schmidt1,2
1
CHU Clermont Ferrand, Urgences Adultes, 28 Place Henri Dunant BP 69, 63003 Clermont-Ferrand Cedex 01, France,2Université Clermont 1, UFR
Médecine, Place Henri Dunant, Clermont-Ferrand F-63001, France,3Université Clermont 1, UFR Médecine, EA 3845, Clermont-Ferrand F63001, France,
4
Inserm U705, UMR CNRS 8206, Neuropsychopharmacologie des Addictions, Université Paris Diderot, Hôpital Fernand Widal Assistance Publique des
Hôpitaux de Paris, 200 rue du Fg Saint-Denis, 75 475 Paris Cedex 10, France,5CHU Clermont-Ferrand, Service de Pharmacologie, Hôpital G. Montpied,
F-63003 Clermont-Ferrand, France,6INSERM 1107, Neurodol, Université d’Auvergne, BP38, 63001 Clermont-Ferrand, France,7Department of Biochemistry
and Molecular Biology, Laboratoire de biochimie, CHU Gabriel Montpied, Rue Montalembert. BP69, 63003 Clermont Ferrand CD1, France and8CHU
Clermont Ferrand, Service Psychiatrie de l’adulte CMP B rue Montalembert 63003 Clermont-Ferrand Cedex 01, France
*Corresponding author. Tel.: +33-04-73-754-785; Fax: +33-04-73-754-781; E-mail: gbrousse@chu-clermontferrand.fr (Received 1 December 2011; first review notified 13 January 2012; in revised form 4 June 2012; accepted 12 June 2012) Abstract — Aims: Animal studies suggest that in alcohol withdrawal the balance of neurotransmitters gamma aminobutyric acid (GABA) and glutamate is altered. To test this in humans, we aimed to measure plasma levels of glutamate, GABA and glutamate/ GABA ratio in alcoholic patients presenting with complicated AWS with the same values in non-alcohol abuser/dependent controls and to determine prognostic factors for severe withdrawal. Methods: 88 patients admitted to the emergency room for acute alcohol intoxication (DSM-IV) were prospectively included. Measurements of GABA and glutamate were performed on admission (Time 1, T1) and after 12 ± 2 h (T2). The experimental group (EG) was composed of 23 patients who presented at T2 with a severe AWS. The control group (CG) consisted of healthy subjects paired with the EG (gender and age). Logistic regression was performed in order to compare associated clinical and biological variables that could predict severe withdrawal. Results: The concentration of GABA in the EG at T1 was significantly lower than that in the CG. The concentration of glutamate in the EG at T1 was significantly higher than that in the CG. The glutamate/GABA ratio in the EG at T1 was significantly higher than the ratio in the CG. With a multivariate logistic regression model, glutamate level at admission remained the only criterion identified as a predictor of AWS at 12 h. Conclusion: Decreased synthesis of GABA and increased synthesis of glutamate might be related to withdrawal symptoms experienced on brutal cessation of chronic alcohol intake.
INTRODUCTION
About 50% of alcohol-dependent patients develop clinically relevant symptoms of withdrawal (Schuckit, 2009). Clinical features of alcohol withdrawal syndrome (AWS), which follow the cessation of regular high-dose alcohol ingestion as soon as the blood level decreases significantly, are quite common in emergency rooms (Etherington, 1996a,b; Hall and Zador, 1997; Holbrook et al., 1999a). AWS occurs in 8% of hospitalized patients in general hospitals and between 10 and 15% of patients during a programmed detoxification (Foy and Kay, 1995;Monte et al., 2009).
Commonly, AWS is divided into three sets of symptoms (Hall and Zador, 1997): The first consists of symptoms of autonomic hyperactivity, which appear within hours of the last drink and usually peak within 24 h. The most common features are trembling, sweating, nausea, vomiting, anxiety and agitation. The second set of symptoms concerns neuron-al excitation and includes epileptiform seizures and globneuron-al confusion, usually occurring within 24–48 h of abstinence. The third set of symptoms comprises delirium tremens or alcohol withdrawal delirium (AWD) with auditory and visual hallucinations, confusion and disorientation, clouding of con-sciousness, impaired attention and pronounced autonomic hyperactivity (Knott et al., 1981; Kosten and O’Connor, 2003;Mayo-Smith et al., 2004). AWD would occur in ~5– 20% of alcohol-dependent individuals who present in inner city hospitals for detoxification (Ferguson et al., 1996). If AWD is untreated, death may occur from respiratory or
cardiovascular collapse (Cushman, 1987). Treatment today consists of good supportive care, including intravenous fluids, thiamine, correction of electrolyte disorders and ben-zodiazepines therapy delivered according to a symptom-triggered approach (Holbrook et al., 1999b). The aim of the treatment is to prevent fatal progression and to relieve un-comfortable symptoms (Holbrook et al., 1999b;Kosten and O’Connor, 2003; Mayo-Smith et al., 2004). Indeed, during alcohol withdrawal, the symptoms that are perceived nega-tively by the patients play an important role in the continu-ation of alcohol dependence, alcohol craving and relapse (Koob, 2003,Heilig et al., 2010).
AWS in emergency rooms ranges from uncomplicated withdrawal to delirium tremens (Etherington, 1996b). Diagnosing acute alcohol-related conditions is challenging for emergency-room physicians who have to treat discomfort and to prevent complications of AWS, particularly a prob-lematic evolution to AWD (Etherington, 1996b). Several factors have been associated with severe withdrawal. These include the number of days since the last drink (Ferguson et al., 1996), patients referred from emergency room to de-toxification unit (Mennecier et al., 2008), age (Liskow et al., 1989), prior complicated alcohol withdrawal, medical serious co-morbidity, elevated blood pressure (Ferguson et al., 1996;
Fiellin et al., 2002) and amino transferase level (Mennecier et al., 2008).
Most of the symptoms observed during the withdrawal period seem to be related to the adaptive changes in central inhibitory and excitatory systems that result from continuous
Alcohol and Alcoholism Vol. 47, No. 5, pp. 501–508, 2012 doi: 10.1093/alcalc/ags078 Advance Access Publication 11 July 2012
© The Author 2012. Medical Council on Alcohol and Oxford University Press. All rights reserved
alcohol intake (Tsai, 1998; Koob, 2003; Esel, 2006;
Vengeliene et al., 2008). A down- regulation of gamma ami-nobutyric acid (GABA) central inhibition confirmed in animal (Faingold et al., 2000; Cagetti et al., 2003) and human (Gomez et al., 2012) studies appears commonly as one of the main causes of insufficient central inhibition during alcohol withdrawal, which leads to the symptoms of hyperexcitation and to relapse (Dahchour and De Witte, 2003a; De Witte et al., 2003; De Witte, 2004). Otherwise, raised extracellular glutamate levels during alcohol withdraw-al have been observed in animwithdraw-als (Rossetti and Carboni, 1995). Thus, Dahchour et al. (1998)reported an augmenta-tion of the extra cellular level of glutamate by 300% in animal models during acute withdrawal. The increase in glu-tamatergic activity could be responsible for many significant symptoms of withdrawal such as hyperexcitation, anxiety and epileptic seizures. This increased activity can be also re-sponsible for the neurodegeneration that develops during withdrawal periods (Kretschmer et al., 2002; Nagy et al., 2003).
Few human studies have partially confirmed dysregulation in glutamate/GABA ratio during withdrawal syndrome. Recently, Hermann et al. (2012) have measured brain glu-tamate levels during detoxification in 47 alcohol-dependent patients and in 57 healthy control subjects, and found signifi-cantly increased glutamate levels during acute withdrawal in prefrontocortical regions. Monitoring for increase in intracer-ebral glutamate concentration and decrease in GABA con-centration could have diagnostic and predictive value. However, in clinical conditions (emergency department), it is impractical to employ the invasive instrumentation required to determine directly central nervous system glutamate or GABA levels. To that end, some researchers have quantified systemic glutamate and GABA concentration (most often in plasma) as an indirect measure of brain glutamate efflux. The first study in emergency room was carried out byAliyev et al. (1994). They studied plasma levels of glutamate and GABA in 20 male patients following alcohol withdrawal and in 20 normal controls. The levels of glutamate were higher and the value of GABA was lower in the patients than in the controls. The correlation between the plasma level of excita-tory amino acid and the rating of subjective discomfort was positive. Otherwise, in 2002, Aliyev and Aliyev (2002)
reported comparisons of plasma levels of GABA and glu-tamate in 106 men. The subjects for this study were 46 patients hospitalized for delirium tremens, 20 patients with an AWS, 20 alcohol-dependent patients and 20 non-alcoholic controls. In this analysis, subjects with delirium had signifi-cantly lower serum values for GABA and higher values for glutamate. Moreover, Tsai et al. (1998) have published data showing an augmentation of glutamate in cerebrospinal fluid (CSF) and a diminution of GABA in alcohol-dependent patients compared with non-alcoholics 1 week and 1 month after cessation of ethanol ingestion. All theses studies confirm a possible plasma dysregulation of glutamate/GABA balance during withdrawal, reflecting brain dysregulation.
To our knowledge, there are no data on prospective mea-sures of plasma concentration of GABA and glutamate during the first hours of alcohol cessation and the physio-pathology of AWS remains poorly understood, particularly during this acute phase. We wanted to focus on these changes occurring during the withdrawal syndrome,
particularly concerning inhibitory and excitatory systems, which interact with each other and are purported to play an important role in the development of AWS. We have hypothesized that alterations to glutamate and GABA plasma levels are present during the first hours of the acute phase of AWS and that this dysregulation could be a prognostic factor for severe withdrawal. To confirm this, we have prospective-ly assessed plasma levels of glutamate, GABA and glutam-ate/GABA balance in alcoholic patients presenting with AWS in the emergency department.
MATERIALS AND METHODS Subjects
Eighty-eight adult patients hospitalized for alcohol acute in-toxication in the emergency room of the Centre Hospitalier Universitaire of Clermont-Ferrand (France), between 1 February and 30 June 2008, were included in the study. The experimental protocol was approved by the Committee for the Protection of Individuals (Comité de Protection des Personnes, CPP, opinion of November 2007, ID RCB: 2007-A00920-53). The inclusion criterion was diagnosis of alcohol acute intoxication according to the DSM-IV criteria [(303.00),American Psychiatric Association, 1994)]. The ex-clusion criterion was the patients’ refusal to participate in the study (<5%) or the presence of serious somatic disability (<2%). Among the 88 participants, 79 were presented with AWS [diagnosed on the basis of DSM-IV, (291.8)]. Among this 79 participants, an experimental group (EG, n = 23) was constituted with patients presenting with severe AWS accord-ing to the Cushman score in the first 12 ± 2 h after admission (score≥8). The control group (CG) was matched by age and gender to the EG. The CG consisted of healthy subjects. The inclusion criteria for the CG concerned the negative screen-ing for abuse and dependence accordscreen-ing to the CAGE Questionnaire (Ewing, 1984) and the MINI (Lecrubier et al., 1997).
Measures of AWS and alcohol problems
AWS was assessed by the French adaptation of the Cushman clinical score (Cushman et al., 1985;Cushman and Sowers, 1989;Mennecier et al., 2008), which has been validated for this purpose (Société Française d’Alcoologie, 2006). This scale, which is classically used in emergency departments for assessing the severity of the withdrawal syndrome, takes into consideration pulse, systolic blood pressure, respiratory rate, tremor, sweating, agitation and sensorial disorders (Table1) (Mennecier et al., 2008).
A withdrawal syndrome with a Cushman score of ≥8 is considered severe and that with a Cushman score of ≥12 and/or the presence of criticized hallucinations (Mennecier et al., 2008) is considered complicated. The MINI is a semi-structured questionnaire used for diagnostic purposes (Lecrubier et al., 1997). We used the‘Alcohol abuse/Alcohol dependence’ module of this questionnaire to identify the type of alcohol misuse in our sample. This module and the CAGE questionnaire were used to verify the absence of alcohol problems into the CG. The CAGE is a short ques-tionnaire developed to detect life-time alcohol dependence with a threshold score of >1 (Ewing, 1984).
Biochemical measures
Blood alcohol levels were measured by means of the auto-mated alcohol dehydrogenase enzyme method (Modular, Roche®, Meylan, France), which was a routine part of the examination for these patients. Data on the following bio-logical variables were also recorded: mean corpuscular volume (MCV), serum levels of aspartate amino transferase (AST), alanine amino transferase (ALT), gamma glutamyl transferase (GGT) and carbohydrate deficient transferring (CDT). In parallel, a sample of blood was taken to measure the plasma concentration of GABA and glutamate. Separated amino analysis with a specific procedure was necessary to analyse for the two acids. Concerning GABA, each sample was transferred quickly on ice before centrifugation. Then the serum was stored at −80°C before analysis. A specific protocol of extraction for GABA was practised: a solid-phase extraction with cation-exchange polymeric groups was used (OASIS®MCX, Waters), and a specific derivation by silyla-tion (MTBSTFA/ACN, 35/15, v/v) at 70°C for 30 min. Quantification of GABA was undertaken with an internal standard (6-aminocaproic acid, 6-ACA) and different calibra-tion standards. The analytical protocol used gas chromatog-raphy (HP 6890, Agilent) coupled with a mass spectrometry detector (HP 5973, Agilent). Measurement of the area under the curves for specific fragments for quantification and quali-fication of the GABA (m/z = 274, 258, 316) and its internal standard (6-ACA, m/z = 302, 344, 170) allows a highly spe-cific and sensitive quantitative analysis. Concerning glutam-ate, the blood sample was centrifuged (+ 4°C, 4500g, 10 min) and the plasma was deproteinized with sulfosalicylic acid (50 mg/ml). The supernatant was stored at−80°C before the plasma amino analysis which was performed by ion ex-change chromatography with an amino acid autoanalyser (JLC-500/V AminoTac; JEOL). The results of our participa-tion in ERNDIM (the European Quality Control Scheme) in-dicate the accuracy of our amino acid determinations. All results are expressed in micromoles per litre (µmol/l). Procedure
Inclusion was scheduled at the time of admission to the emergency room. The medical team used a standard chart to achieve blood test and determine the Cushman score a few minutes after admission (T1) and 12 ± 2 h after (T2). All the patients underwent the same therapeutic approach according to standard recommendations based on the Cushman score (Société Française d’Alcoologie, 2006). Retrospective signed consent to take part in this study was obtained directly from the patients, as soon as the patient’s mental state allowed
free and informed consent (generally, the day after admis-sion). Only after collecting this consent were data on the fol-lowing variables recorded: gender, age, regular medical treatment, age at onset of excessive alcohol intake (>60 g per day), type of alcohol consumed, number of prior alcohol withdrawal attempts and history of convulsion or delirium tremens.
Statistical analysis
The SPSS software (SPSS Inc., Chicago, IL, version 15.0) was used for statistical analyses. Numerical data were expressed as frequency and proportions (%). Measured data were expressed as means with standard deviations. First, data were characterized using descriptive methods. The normality of dependent variables was assessed by skewness and kurto-sis statistics (the chosen criterion was that the dividend of the coefficient and the standard error did not exceed ± 2.0). Analysis of variance F-test was used to conduct between-and within-group comparisons. Because EG between-and CG were paired, plasma concentrations of EG (T1 and T2) and of CG were defined in the same within-subject factor (three levels). Simple contrast type was chosen to compare the mean of each level with the mean of a specified level (in General Linear Model repeated measures procedure).
In order to identify prognostic factors of severe withdrawal syndrome, logistic regression (backward stepwise) was used to examine a multivariate prediction model that included all potentially useful variables for discriminating patients who progressed with severe AWS at T2 from patients with non-severe AWS at T2. Because these predictive variables are unknown, stepwise procedure was selected in this explora-tory research context (Agresti and Finlay, 1997). Then, back-ward elimination rather than forback-ward inclusion was chosen as the method of stepwise regression. Indeed, backward step-wise elimination has the advantage of keeping in the equa-tion variables that increase the predictive validity of another variable, or a set of variables (i.e. a suppressor effect). With backward elimination, because all variables are already in the model, there is less risk of failing to find a relationship when one exists (Menard, 2001).
All hypotheses were tested by using a two-sided test and a significance level ofα = 0.05.
RESULTS
Among the 88 patients included in our study, 79 were diag-nosed as presenting with AWS [19 females (24%), 60 males Table 1. Cushman score
Score 0 1 2 3
Pulsea(bpm) ≤80 81–100 101–120 >120
Systolic bloodb(mmHg) ≤135 136–145 146–155 >155
Respiratory ratea(cycles per minute) ≤16 16–25 26–35 >35
Tremor 0 Extended hand Entire upper limb Affecting whole body
Perspirationa 0 Palms Palms and forehead Affecting whole body
Agitation 0 Discrete Generalized/controllable Generalized/uncontrollable
Sensorial disorders 0 Retreat from noise or light, pruritis Hallucinations (criticized) Hallucinations (non-criticized)
a
Criteria if corporal temperature <38°C.
b
Criteria between 31 and 50 years old; if age >50 add 10 mmHg.
(76%)]. A history of treatment for alcohol-related disorders was present in 85% of these participants and 45% had a history of AWS. All were alcohol dependent. They were pre-dominantly excess consumers of wine. In this sample, serum levels of AST, ALT, GGT and CDT were disturbed (Table2).
Among these 79 AWS cases, 23 had a Cushman score su-perior or equal to 8 at T2 (severe or complicated AWS). This experimental sample was composed by 22 males and 1 female (mean age: 45.04 years, SD: 8.51). All these patients had a history of receiving treatment for alcohol-related disor-ders and 73% had a history of AWS. The body mass index of the EG was into the normal World Health Organization range (M: 23, 34; SD: 5.08) and did not differ from the BMI of the CG (M: 24.52; SD: 6.01).
The total CAGE mean score of the EG was 3.43 (± 0.59). This score was greater than that of the CG (0.04 ± 0.21), F (1,44) = 676.000, P < 0.001, which highlighted the lack of a problem of alcohol in this population (≤1). The total Cushman mean score at T1 (6.43 ± 2.97) was lower than at T2 (8.78 ± 2.11), F (1,22) = 17.970, P < 0.001.
The mean plasma concentrations of GABA at T1 (8.17 µmol/l ± 2.95) and at T2 (8.05 µmol/l ± 2.65) were not sig-nificantly different, F(1,21) = 0.153, P > 0.05. By contrast, the mean plasma GABA concentration for the CG (27.66 µmol/l ± 3.12) was greater than the mean plasma GABA concentration at T1 (8.17 µmol/l ± 2.95), F(1,21) = 368.362, P < 0.001, and at T2 (8.05 µmol/l ± 2.65), F(1,21) = 406.787, P < 0.001 (Fig.1).
The mean plasma concentration of glutamate in T1 (88.06 µmol/l, SD: 59.92) was higher than at T2 (69.78 µmol/l ± 44.91), F(1,22) = 9.790, P < 0.01, and higher than in the CG (48.42 µmol/l ± 15.59), F(1,22) = 10.527, P = 0.004. In the same way, the mean plasma concentration of glutamate at T2 (69.78 µmol/l ± 44.91) was greater than in the CG (48.42 µmol/l ± 15.59), F(1,22) = 5.872, P < 0.05 (Fig.2).
The mean glutamate/GABA ratio at T1 (11.39, SD: 6.73) was higher than at T2 (9.38 ± 5.30), F(1,21) = 5.094, P < 0.05, and than in the CG (1.76 ± 0.54), F(1,21) = 44.729, P < 0.001 (cf. Fig. 2). The mean glutamate/GABA ratio at T2 (9.38, SD: 5.30) was greater than in the CG (1.76 ± 0.54), F(1,21) = 46.637, P < 0.001 (Fig.3).
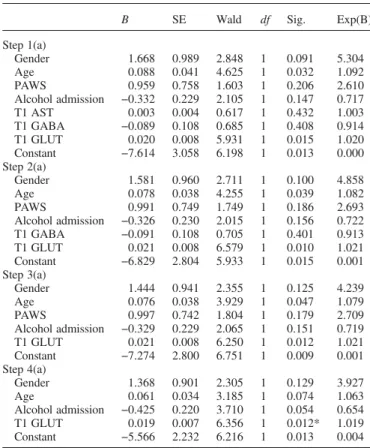
Concerning logistic regression analysis, 10 variables were examined. Seven were the perturbed variables documented in the literature as associated with withdrawal (gender, age, personal antecedents of withdrawal syndrome, serum levels of AST and ALT, serum levels of GGT and alcohol blood level at admission). There were variables supporting our
hypothesis of acute glutamate/GABA dysregulation [mean plasma concentrations of GABA and glutamate at admission (T1), and ‘glutamate/GABA ratio’ at T1]. Because of strong co-linearity with the mean plasma concentration of glutamate (T1), the variables ‘GGT’, ‘MCV’ and ‘glutamate/GABA Table 2. Biological parameters of the 79 alcohol-dependent patients with
AWS
Normal value Mean SD Mini Maxi
Alcohol blood level T1 (g/l) 0 2.81 1.44 0.8 5.11
MCV (fl) 80–100 95.68 6.82 80.3 119
AST (IU/l) 10–40 101.6 85.4 20 419
ALT (IU/l) 10–50 63.3 63.5 8 460
GGT (IU/l) 7–32 284.9 451.2 10 2271
CDT (%) <1.7 5.2 5.0 0.3 25.8
MCV, mean corpuscular volume; AST, aspartate amino transferase; ALT, alanine amino transferase; GGT, gamma glutamyl transferase; CDT, carbohydrate deficient transfering.
Fig. 1. Mean plasma concentrations of GABA at Time 1, Time 2 in experimental group and in control group.
Fig. 2. Mean plasma concentrations of glutamate at Time 1, Time 2 in experimental group and in the control group.
Fig. 3. Mean of glutamate/GABA balances at Time 1, Time 2 in experimental group and in the control group.
ratio’ were excluded from the regression model in accord-ance with classical procedure (Menard, 2001) (r = 0.843, P < 0.01, r = 0.347, P < 0.01 and r = 0.728, P < 0.01, respectively). Likewise, serum levels of ALT, which were highly correlated with the serum level of AST (r = 0.783, P < 0.01), were excluded from the regression analysis. Despite a significant positive correlation between glutamate level and the serum levels of AST (r = 0.362, P < 0.01), ‘AST’ (which reflects hepatic enzyme activity) was kept in the regression analysis to evaluate the involvement of liver status in predicting severe AWS. In addition, because CDT is a marker of recent alcohol intake, it does not constitute a relevant biochemical variable for our predictive model. The logistical regression was performed using the data resulting from the 79 partici-pants who presented with AWS. Among the seven remaining variables, only the mean plasma concentration of glutamate at T1 is a significant predictor of AWS at T2, χ2= 6.356, P = 0.012. The model’s statistical results yield a satisfactory, but not optimal, response predictability (χ2= 12.516; df = 4; P = 0.014; Nagelkerke R2= 0.254).
DISCUSSION
We have shown in this prospective study the dysregulation of glutamate/GABA plasma ratio during acute withdrawal syndrome. Hyper-glutamatergic activity (>200% compared with non-alcoholics) in this human study could confirm the results of animal studies that showed a similar increase in the brain [00% forDahchour et al. (1998)]. It is also compatible with early clinical manifestations of withdrawal, particularly those such as hyper-excitation and anxiety. Glutamate plasma levels seem to decrease during the acute period, unlike hypo-GABA activity, which seems stable during the first 12 h of withdrawal. These results are consistent with other human studies (Aliyev et al., 1994;Aliyev and Aliyev, 2002). Moreover, we have emphasized the precocity of this dysregulation.
In our CG, the mean plasma concentration of glutamate is in the normal range for plasma concentration (Hediger and Welbourne, 1999; Rainesalo et al., 2004). In our EG, the mean plasma concentration of glutamate is lower than the serum levels found by Aliyev et al. (1994) for 20 male patients following AWS (160 + 3 µmol/l). Concerning GABA plasma levels, our measurements for the control and EGs are not in line with previous published works, which are themselves heterogeneous. Thus, Petty et al. (1997)
reported GABA plasma levels in alcoholic males of about 0.100 µmol/l and Aliyev and Aliyev (2002)found about 3.5 µmol/l for patients with AWS, while in our study, levels of about 25 µmol/l were reported. However, the coefficient of variation intra-assay was around 11% in our study while it was reported to be around 6.4% by Petty et al. (1997), testi-fying to the quality of the two analyses. We can assume that these discrepancies are linked with differences in method-ology between studies rather than with techniques and meas-urement equipment used. Indeed, the time of collection of plasma for measurement of amino acids was not the same between these studies. In our work, plasma was collected a few minutes after admission while it was collected later in other studies. In addition there is an important variability in the GABA plasma levels reported in specialized articles
(Rainesalo et al., 2004;Vaiva et al., 2006;Küçükibrahimolu et al., 2009), testifying to the instability of this amino acid and the necessity of a rapid analytical procedure like the one we used. As regards techniques and equipment, various tech-niques have been used to determine plasma amino acid con-centrations. For Reynolds et al. (2002), it may also account for the varying conclusions reached by different research groups.
The most important question posed by this study concerns the difficulty in correlating plasma and brain glutamate and GABA levels. Several data support the following hypothesis: First of all, elevated plasma and brain glutamate levels have been reported for many pathophysiological conditions asso-ciated with neurologic injury (including stroke, motor neuron disease, olivopontocerebellar atrophy and preterm birth as-phyxia) (Castillo et al., 1997;Babu et al., 1998). In the same way, variation of glutamate and GABA plasma levels have been reported to be prognostic factors or biomarkers during psychiatric disorders (Vaiva et al., 2006; Küçükibrahimolu et al., 2009) or human epilepsy (Rainesalo et al., 2004). Then animal data support the hypothesis of a correlation between the time course changes of glutamate and GABA in plasma and of those of CSF (Apud et al., 1981;
Küçükibrahimoglu et al., 2009). In this way, glutamate and GABA plasma dysregulation could reflect brain activity, which presents similar changes during withdrawal (Aliyev et al., 1994;Tsai et al., 1998;Hermann et al., 2012).
In our study, hyperglutamatergic activity appears as a prognostic factor in the evolution of withdrawal; that is, the higher the plasma concentration of glutamate in the acute phase, the greater the risk of serious immediate withdrawal. These results could support the hypothesis of the neurotox-icity of elevated glutamate concentration. Tsai et al. (1998)
have suggested that augmentation of excitatory neurotrans-mission in CSF during withdrawal may lead to enhanced oxidative stress (Tsai et al., 1998). This augmentation could promote acute neurological damage (Delirium, Gayet Wernicke) or chronic sensitization of the brain as a kindling phenomenon (Gass and Olive, 2008).
In our study, GABA was not a prognostic factor for com-plicated withdrawal. Adinoff et al. (1995) have measured GABA in both plasma and CSF from 14 male alcohol-dependent patients at day one of acute alcohol withdrawal and 21 days after inpatient treatment. They did not find sig-nificant correlations between the indices of alcohol with-drawal and plasma or CSF GABA levels, suggesting that the involvement of other neurobiological factors should be investigated. Adinoff et al. (1995) also suggested that there would be major variation of GABA during the acute with-drawal phase but they did not assess variation of GABA during the first hours. It was thus impossible to compare their findings with our results that show stability of GABA during the first 12 h (Adinoff et al., 1995). In the same way, according to Roy et al. (1990), CSF GABA was not asso-ciated with the antecedents of severe AWS. According to
Petty et al. (1997), GABA could be an indicator of brain sensitization and relapse: Thus, they followed 49 alcohol-dependent patients for up to 18 months of continuous abstin-ence following inpatient treatment. Alcohol-dependent patients with low-plasma GABA had significantly better out-comes than patients with GABA plasma values in the normal control range.
Knowledge of the dysregulation phenomenon of glutam-ate/GABA balance during the acute phase of withdrawal, with a prognostic role for glutamatergic hyperactivity, can help to supplement the classical acute therapeutic response to the withdrawal syndrome in the emergency room. Indeed, the classical approaches consist of GABA reinforcement (Hall and Zador, 1997;Mayo-Smith et al., 2004) by benzo-diazepines, but concerns about their abuse potential drive the search for new treatments. It could be interesting to prevent rapid hyper-glutamatergic activity during the acute phase of withdrawal by using antiglutamatergics treatment (Carroll, 2008). Acamprosate seems to be a protective agent during hyper-glutamatergic syndrome occurring during alcohol withdrawal (Dahchour and De Witte, 2003b) by reducing calcium-related neurotoxicity (Al Qatari et al., 2001,Koob et al., 2002). This protective effect appears in animal models when acamprosate is chronically and preventively adminis-tered before withdrawal but an acute administration during AWS could not stem the massif influx of glutamate (Mann et al., 2008). High doses could be tested, especially since this product has low toxicity and seems to reduce central brain glutamate activity during withdrawal in human studies (Umhau et al., 2010). Moreover, for a significant number of patients, chronic evolution of alcohol dependence is marked by alternation of consumption and withdrawal periods. For them, long-term treatment by acamprosate could prevent it-erative discharges of hyper-glutamatergic activity and protect the brain from long-term damage (Kiefer and Mann, 2010). Other new antiglutmategic therapeutic approaches using drugs such as topiramate, memantine or lamotrigine (Krupitsky et al., 2007; Amato et al., 2011) could be investigated.
Our study has other limitations. Firstly, the sample was of a limited size, especially the severe withdrawal group, al-though other studies published in this area have similar sample sizes. These include the only three prospective studies assessing predictive factors of delirium tremens and severe withdrawal (Thiercelin et al., 2012). Secondly, metab-olism and plasma levels of glutamate and GABA (derived from glutamate, Buddhala et al., 2009) are classically deter-mined and influenced by liver status and, to a lesser extent, by dietary intake (Hediger and Welbourne, 1999). In this study, the experimental and CGs did not differ in nutritional status. We found an absence of correlation between body mass index of the EG and levels of glutamate and liver enzymes, but significant positive correlation between glutam-ate levels and AST and GGT. These correlations could impli-cate liver status in the glutamate plasma level. However, results of backward logistic regression highlight the role of glutamate in predicting risk of AWS when hepatic variables are excluded from the equation (Table 3). Thirdly, the two selected time points for the study (at admission and 12 ± 2 h later) only reflect the acute phase and are insufficient for ex-haustive evaluation of the kinetics of amino acid plasma level evolution. Fourthly, we have not studied exhaustively all the potential factors that could be implicated in the occur-rence of severe withdrawal. In fact, we have focused our at-tention on glutamate and GABA dysregulation and found that glutamate plasma concentration could predict the gravity of AWS. It is true that the model’s predictive capacity is not high but consistent with previous work (Aliyev et al., 1994;
Aliyev and Aliyev, 2002). A larger study should investigate
exhaustively the implication of other amino acids such as homocysteine and taurine (Bleich et al., 2000a;Bleich et al., 2000b; Olive, 2002; Bayerlein et al., 2005; Hillemacher et al., 2007). Otherwise, meta-analysis should be done in order to determine definitively the implication of clinical and biological factors. Genetic factors should also be studied (Van Munster et al., 2007). Further work is required, but without doubt, therapeutic brain protection should be insti-gated as early as possible during alcohol cessation therapy in alcoholic patients.
CONCLUSION
In this study, we have confirmed a change in the glutamate/ GABA balance in alcohol-dependent subjects during with-drawal. The decreased synthesis of GABA and increased synthesis of glutamate might be related to chronic alcohol intake and may be made apparent by brutal cessation. Withdrawal symptoms are directly caused by this acute dis-equilibrium. It seems that hyper-glutamatergic activity pro-duces an oxidative stress which inpro-duces acute brain symptoms such as seizures and may lead to chronic altera-tions, especially since withdrawals are iterative and there is no therapeutic protection. Alteration of GABA could
Table 3. Logistic regression analysis (backward) of selected variables to distinguish patients with severe AWS at 12 ± 2 h from patients with
non-severe AWS
B SE Wald df Sig. Exp(B)
Step 1(a) Gender 1.668 0.989 2.848 1 0.091 5.304 Age 0.088 0.041 4.625 1 0.032 1.092 PAWS 0.959 0.758 1.603 1 0.206 2.610 Alcohol admission −0.332 0.229 2.105 1 0.147 0.717 T1 AST 0.003 0.004 0.617 1 0.432 1.003 T1 GABA −0.089 0.108 0.685 1 0.408 0.914 T1 GLUT 0.020 0.008 5.931 1 0.015 1.020 Constant −7.614 3.058 6.198 1 0.013 0.000 Step 2(a) Gender 1.581 0.960 2.711 1 0.100 4.858 Age 0.078 0.038 4.255 1 0.039 1.082 PAWS 0.991 0.749 1.749 1 0.186 2.693 Alcohol admission −0.326 0.230 2.015 1 0.156 0.722 T1 GABA −0.091 0.108 0.705 1 0.401 0.913 T1 GLUT 0.021 0.008 6.579 1 0.010 1.021 Constant −6.829 2.804 5.933 1 0.015 0.001 Step 3(a) Gender 1.444 0.941 2.355 1 0.125 4.239 Age 0.076 0.038 3.929 1 0.047 1.079 PAWS 0.997 0.742 1.804 1 0.179 2.709 Alcohol admission −0.329 0.229 2.065 1 0.151 0.719 T1 GLUT 0.021 0.008 6.250 1 0.012 1.021 Constant −7.274 2.800 6.751 1 0.009 0.001 Step 4(a) Gender 1.368 0.901 2.305 1 0.129 3.927 Age 0.061 0.034 3.185 1 0.074 1.063 Alcohol admission −0.425 0.220 3.710 1 0.054 0.654 T1 GLUT 0.019 0.007 6.356 1 0.012* 1.019 Constant −5.566 2.232 6.216 1 0.013 0.004
Variables included in the model: gender, age, Personal Antecedents of Withdrawal Syndrome (PAWS), serum levels of AST, alcohol blood level at admission, mean plasma concentrations of GABA and glutamate (GLUT) at admission (T1).
Intercept and covariates: −2Log = 62.791; R2 (Nagelkerke) = 0.254; Max
rescaled R2= 0,173;χ2= 12.516; df = 4; P = 0.014; *P < 0.05.
strengthen this phenomenon. The implication of withdrawal symptoms in craving and relapse must be taken into account. AWS is ‘a bad old companion’ to patients and clinicians. It is surprising that it is still so poorly understood and treated as difficult.
Funding— This study was supported by the University Hospital of Clermont-Ferrand and financed by a grant from the French Society of Emergency Medicine.
REFERENCES
Adinoff B, Kramer GL, Petty F. (1995) Levels of gamma-aminobutyric acid in cerebrospinal fluid and plasma during alcohol withdrawal. Psychiatry Res 59:137–44.
Agresti A, Finlay B. (1997) Statistical Methods for the Social Sciences, 3rd edn. Upper Saddle River, NJ: Prentice-Hall. Al Qatari M, Khan S, Harris B et al. (2001) Acamprosate is
neuro-protective against glutamate-induced excitotoxicity when enhanced by ethanol withdrawal in neocortical cultures of fetal rat brain. Alcohol Clin Exp Res 25:1276–83.
Aliyev NN, Aliyev ZN. (2002) The role of amino-acid transmitters in the pathogenesis of delirium tremens: a brief report. J Stud Alcohol 63:531–3.
Aliyev NA, Aliyev ZN, Aliguliyev AR. (1994) Amino acid neuro-transmitters in alcohol withdrawal. Alcohol Alcohol 29:643–7. Amato L, Minozzi S, Davoli M. (2011) Efficacy and safety of
pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database Syst Rev 15: CD008537.
American Psychiatric Association. (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th edn. (DSM-IV). Washington, DC: APA.
Apud JA, Racagni G, Iuliano E et al. (1981) Role of central nervous system-derived or circulating gamma-aminobutyric acid on prolactin secretion in the rat. Endocrinology 108:1505–10. Babu GN, Bawari M, Mathur VN et al. (1998) Blood glutamate
levels inpatients with motor neuron disease. Clin Chim Acta 273:195–200.
Bayerlein K, Hillemacher T, Reulbach U et al. (2005) Alcoholism-associated hyperhomocysteinemia and previous withdrawal seizures. Biol Psychiatry 57:1590–3.
Bleich S, Degner D, Bandelow B et al. (2000a) Plasma homocyst-eine is a predictor of alcohol withdrawal seizures. Neuroreport 11:2749–52.
Bleich S, Degner D, Wiltfang J et al. (2000b) Elevated homocyst-eine levels in alcohol withdrawal. Alcohol Alcohol 35:351–4. Buddhala C, Hsu CC, Wu JY. (2009) A novel mechanism for
GABA synthesis and packaging into synaptic vesicles. Neurochem Int 55:9–12.
Cagetti E, Liang J, Spigelman I et al. (2003) Withdrawal from chronic intermittent ethanol treatment changes subunit compos-ition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol 63:53–64.
Carroll FI. (2008) Antagonists at metabotropic glutamate receptor subtype 5: structure activity relationships and therapeutic poten-tial for addiction. Ann N Y Acad Sci 1141:221–32.
Castillo J, Davalos A, Noya M. (1997) Progression of ischaemic stroke and excitotoxic amino acids. Lancet 349:79–83.
Cushman P, Jr. (1987) Delirium tremens. Update on an old dis-order. Postgrad Med 82:117–22.
Cushman P, Jr, Sowers JR. (1989) Alcohol withdrawal syndrome: clinical and hormonal responses to alpha 2-adrenergic agonist treatment. Alcohol Clin Exp Res 13:361–4.
Cushman P, Jr, Forbes R, Lerner W et al. (1985) Alcohol withdraw-al syndromes: clinicwithdraw-al management with lofexidine. Alcohol Clin Exp Res 9:103–8.
Dahchour A, De Witte P. (2003a) Excitatory and inhibitory amino acids changes during repeated episodes of ethanol with-drawal: an in vivo microdialysis study. Eur J Pharmacol 459:171–8.
Dahchour A, De Witte P. (2003b) Effects of acamprosate on excita-tory amino acids during multiple ethanol withdrawal periods. Alcohol Clin Exp Res 27:465–70.
Dahchour A, De Witte P, Bolo N et al. (1998) Central effects of acamprosate: part 1. Acamprosate blocks the glutamate increase in the nucleus accumbens microdialysate in ethanol withdrawn rats. Psychiatry Res 82:107–14.
De Witte P. (2004) Imbalance between neuroexcitatory and neuroin-hibitory amino acids causes craving for ethanol. Addict Behav 29:1325–39.
De Witte P, Pinto E, Ansseau M et al. (2003) Alcohol and with-drawal: from animal research to clinical issues. Neurosci Biobehav Rev 27:189–97.
Esel E. (2006) Neurobiology of alcohol withdrawal: inhibitory and excitatory neurotransmitters. Turk Psy Der 17:1–9.
Etherington JM. (1996a) Emergency management of acute alcohol problems. Part 1: Uncomplicated withdrawal. Can Fam Physician 42:2186–90.
Etherington JM. (1996b) Emergency management of acute alcohol problems. Part 2: Alcohol-related seizures, delirium tremens, and toxic alcohol ingestion. Can Fam Physician 42:2423–31. Ewing JA. (1984) Detecting alcoholism: the CAGE questionnaire.
J Am Med Assoc 252:1905–7.
Faingold C, Li Y, Evans MS. (2000) Decreased GABA and increased glutamate receptor-mediated activity on inferior col-liculus neurons in vitro are associated with susceptibility to ethanol withdrawal seizures. Brain Res 868:287–95.
Ferguson JA, Suelzer CJ, Eckert GJ et al. (1996) Risk factors for delirium tremens development. J Gen Intern Med 11:410–4. Fiellin DA, O’Connor PG, Holmboe ES et al. (2002) Risk for
delir-ium tremens in patients with alcohol withdrawal syndrome. Subst Abus 23:83–94.
Foy A, Kay J. (1995) The incidence of alcohol-related problems and the risk of alcohol withdrawal in a general hospital popula-tion. Drug Alcohol Rev 14:49–54.
Gass JT, Olive MF. (2008) Glutamatergic substrates of drug addic-tion and alcoholism. Biochem Pharmacol 75:218–65.
Gomez R, Behar KL, Watzl J et al. (2012) Intravenous ethanol infu-sion decreases human cortical γ-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry 71:239–46.
Hall W, Zador D. (1997) The alcohol withdrawal syndrome. Lancet 349:1897–900.
Hediger MA, Welbourne TC. (1999) Introduction: glutamate trans-port, metabolism, and physiological responses. Am J Physiol 277:F477–80.
Heilig M, Egli M, Crabbe JC et al. (2010) Acute withdrawal, pro-tracted abstinence and negative affect in alcoholism: are they linked? Addict Biol 15:169–84.
Hermann D, Weber-Fahr W, Sartorius A et al. (2012) Translational magnetic resonance spectroscopy reveals excessive central glu-tamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry 71:1015–21.
Hillemacher T, Frieling H, Bayerlein K et al. (2007) Biological markers to predict previous alcohol withdrawal seizures: a risk assessment. J Neural Transm 114:151–4.
Holbrook AM, Crowther R, Lotter A et al. (1999a) Diagnosis and management of acute alcohol withdrawal. CMAJ 160:675–80. Holbrook AM, Crowther R, Lotter A et al. (1999b) Meta-analysis
of benzodiazepine use in the treatment of acute alcohol with-drawal. CMAJ 160:649–55.
Kiefer F, Mann K. (2010) Acamprosate: how, where, and for whom does it work? Mechanism of action, treatment targets, and indi-vidualized therapy. Curr Pharm Des 16:2098–102.
Knott DH, Lerner WD, Davis-Knott T et al. (1981) Decision for alcohol detoxication: a method to standardize patient evaluation. Postgrad Med 69:65–76.
Koob GF. (2003) Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 27:232–43.
Koob GF, Mason BJ, De Witte P et al. (2002) Potential neuropro-tective effects of acamprosate. Alcohol Clin Exp Res 26:586–92. Kosten TR, O’Connor PG. (2003) Management of drug and alcohol
withdrawal. Engl J Med 348:1786–95.
Kretschmer BD, Schmidt WJ, Kostrzewa RM et al. (2002) Amino acids in neurobiology: neuroprotective and neurotoxic aspects of
amino acids involved in neurotransmission and neuromodulation —general introduction. Amino Acids 23:1–7.
Krupitsky EM, Rudenko AA, Burakov AM et al. (2007) Antiglutamatergic strategies for ethanol detoxification: compari-son with placebo and diazepam. Alcohol Clin Exp Res 31:604–11.
Küçükibrahimolu E, Saygin MZ, Calikan M et al. (2009) The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur J Clin Pharmacol 65:571–7.
Lecrubier Y, Sheehan DV, Weiller E et al. (1997) The Mini International Neuropsychiatric Interview (MINI). A short diag-nostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry 12:224–31.
Liskow BI, Rinck C, Campbell J et al. (1989) Alcohol withdrawal in the elderly. J Stud Alcohol 50:414–21.
Mann K, Kiefer F, Spanagel R et al. (2008) Acamprosate: recent findings and future research directions. Alcohol Clin Exp Res 32:1105–10.
Mayo-Smith MF, Beecher LH, Fischer TL et al. (2004) Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 164:1405–12.
Menard S. (2001) Applied Logistic Regression Analysis. Sage University Papers Series on Quantitative Applications in the Social Sciences. Series no. 07-106. Thousand Oaks, CA: Sage.
Mennecier D, Thomas M, Arvers P et al. (2008) Factors predictive of complicated or severe alcohol withdrawal in alcohol depend-ent inpatidepend-ents. Gastrodepend-enterol Clin Biol 32:792–7.
Monte R, Rabuñal R, Casariego E et al. (2009) Risk factors for de-lirium tremens in patients with alcohol withdrawal syndrome in a hospital setting. Eur J Intern Med 20:690–4.
Nagy J, Kolok S, Dezso P et al. (2003) Differential alterations in the expression of NMDA receptor subunits following chronic ethanol treatment in primary cultures of rat cortical and hippo-campal neurones. Neurochem Int 42:35–43.
Olive MF. (2002) Interactions between taurine and ethanol in the central nervous system. Amino Acids 23:345–57.
Petty F, Kramer GL, Davis LL et al. (1997) Plasma gamma-aminobutyric acid (GABA) predicts outcome in patients with
alcohol dependence. Prog Neuropsychopharmacol Biol Psychiatry 21:809–16.
Rainesalo S, Keränen T, Palmio J et al. (2004) Plasma and cerebro-spinal fluid amino acids in epileptic patients. Neurochem Res 29:319–24.
Reynolds JD, Amory DW, Grocott HP et al. (2002) Change in plasma glutamate concentration during cardiac surgery is a poor predictor of cognitive outcome. J Cardiothorac Vasc Anesth 16:431–6.
Rossetti ZL, Carboni S. (1995) Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol 283:177–83.
Roy A, Dejong J, Ferraro T et al. (1990) CSF gamma-aminobutyric acid in alcoholics and control subjects. Am J Psychiatry 147:1294–6.
Schuckit MA. (2009) Alcohol-use disorders. Lancet 373:492–501. Société Française d’Alcoologie (2006) Evaluation des pratiques
pro-fessionnelles en alcoologie. Sevrage en alcool. http://www. sfalcoologie.asso.fr.
Thiercelin N, Rabiah Lechevallier Z, Rusch E et al. (2012) Risk factors for delirium tremens: A literature review. Rev Med Interne 33:18–22.
Tsai G. (1998) Glutamatergic neurotransmission in alcoholism. J Biomed Sci 5:309–20.
Tsai GE, Ragan P, Chang R et al. (1998) Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am J Psychiatry 6:726–32.
Umhau JC, Momenan R, Schwandt ML et al. (2010) Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry 67:1069–77.
Vaiva G, Boss V, Ducrocq F et al. (2006) Relationship between posttrauma GABA plasma levels and PTSD at 1-year follow-up. Am J Psychiatry 163:1446–8.
Van Munster BC, Korevaar JC, de Rooij SE et al. (2007) Genetic polymorphisms related to delirium tremens: a systematic review. Alcohol Clin Exp Res 31:177–84.
Vengeliene V, Bilbao A, Molander A et al. (2008) Neuropharmacology of alcohol addiction. Br J Pharmacol 154:299–315.