O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 13967
To link to this article :
DOI:10.1016/j.jeurceramsoc.2013.08.010
URL :
http://dx.doi.org/10.1016/j.jeurceramsoc.2013.08.010
To cite this version :
El Horr, Nahida and Guillemet-Fritsch, Sophie and Rousset, Abel
and Bordeneuve, Hélène and Tenailleau, Christophe Microstructure
of single-phase cobalt and manganese oxide spinel Mn3−xCoxO4
ceramics. (2014) Journal of the European Ceramic Society, vol. 34
(n° 2). pp. 317-326. ISSN 0955-2219
Any correspondance concerning this service should be sent to the repository
administrator: staff-oatao@listes-diff.inp-toulouse.fr
Microstructure
of
single-phase
cobalt
and
manganese
oxide
spinel
Mn
3−x
Co
x
O
4
ceramics
N.
El
Horr
∗,
S.
Guillemet-Fritsch,
A.
Rousset,
H.
Bordeneuve,
C.
Tenailleau
InstitutCarnotCIRIMAT,UniversitéPaulSabatier,UMRCNRS5085,118routedeNarbonne,31062ToulouseCedex9,France
Abstract
Thispaperreportsmicrostructuralstudiesofsingle-phaseMn3−xCoxO4(0.98≤x≤2.93)spinelceramicsusingtransmissionelectronmicroscopy
(TEM)andenergydispersiveX-rayspectroscopy(EDX).Theseceramicswereobtainedbyconventionalsinteringorbysparkplasmasintering (SPS)ofpowderspreparedbythermaldecompositionofcoprecipitatedoxalateprecursors.Forx<1.78orx≥1.78,themonophasicceramics correspondrespectivelytoquadratic(Q)orcubic(C)spinelstructure.TheferroelasticcharacterofthestructuralphasetransitionfromCtoQ ishighlightedbyspecificmicrostructuralfeatures.Theeffectofchemicalcompositionandheattreatmentconditionsonthemicrostructureand essentiallyonthepresenceandthecharacteristicsoftwinswereinvestigated.Thecoherenttwininterfacesareparallelto(112)planesintheQ cell.Twinscancorrespondto:tweeds,singlelamellae(widths:5–306nm)arrangedparalleltoeachother,largelamellae(widths:69–928nm) internallytwinnedandsometimesarrangedincyclicforms(triangularshapes).
Keywords:Cobaltmanganite;Sintering;Microstructure;Twins;Electronmicroscopy
1. Introduction
Manganites have been of great interest to materials
researchers since the 1950s.1–4 Indeed, in addition to their widerangeofchemicalcompositionsandtheirbroadvarietyof structuralphases(withahighnumberofpossiblecation distribu-tions),theycanalsoexhibitalargerangeofphysical,chemical, electric,magneticproperties,etc.
Mixed-valence transition-metal manganites with a spinel
structureMn3−xMexO4(Me=Co,Ni,Fe,Cu,Zn,CrandZr)are
knownasbasicmaterialsusedtomanufacturethermistorswith Negative Temperature Coefficient (NTC).5–9 This specificity makes themhighly attractivefor severaltechnology domains (microelectronics,optoelectronics,etc.)andtheyarethusused inmanyindustrialapplications10–17:temperaturesensors,time delayelements,infrareddetectors,voltageregulators,etc.
Apart from the numerousapplications arising from spinel manganites,itisimportanttonotethatthesematerialsarealso attractingconsiderableinterestinfundamentalresearchdueto
∗Correspondingauthor.Tel.:+33562258253;fax:+33562258277.
E-mailaddress:nahida.el-horr@iut-tlse3.fr(N.ElHorr).
the complexityof the relationshipsthat existbetween,firstly, the chemicalmethodsusedtopreparepowdersandceramics, secondly,the structures(andinparticularcationdistribution), microstructures(density,grainsize,grainmorphology,presence ofseveralphases,presenceofdefects:precipitates,twins,etc.) of the obtainedproducts andthirdly,theirmanyphysicaland chemical properties:electric,magnetic,etc. Judicious control oftheserelationshipscouldprovidenewwaystoobtainspinel manganiteswithcontrolledproperties.
The microstructureof spinelmanganiteceramics hasbeen
the subject of many studies,7–9,18–33 many of which were
performed in our laboratory. In most cases, these
mangan-ites are obtained by sintering powders prepared either by
hydrothermal methods (thermal decomposition of precursors
producedbycoprecipitation)orbytraditionalmethodsand
cor-respond tobinarysystems: Mn–Ni–O,Mn–Fe–O,Mn–Zn–O,
etc. to ternary systems: Mn–Ni–Co–O, Mn–Ni–Cu–O,
Mn–Ni–Zn–O, Mn–Ni–Zr–O, Mn–Ni–Fe–O, Mn–Ni–In–O,
etc.andsystemswithfourormorecations:Mn–Ni–Co–Al–O,
Mn–Ni–Co–Zn–O, Mn–Ni–Fe–Cr–O, Mn–Ni–Co–Cu–Si–O,
etc.
Amongstthesestudies,wenotethatthepresenceofdefects suchasprecipitatesortwins,inthesemanganites,andalsothe
characteristics oftheobservedtwins,depend onthechemical compositionofthesamplesandtheirheattreatmentconditions. Additionally,insomecases,thesedefectscanplayanimportant roleincontrollingtheelectricalpropertiesofmanganites.
Veryfewstudies34–41hadbeenperformedontheMn–Co–O systembeforetheworksconductedinourlaboratory.Theyhave
involved more detailed works on filmsand powders than on
ceramics. The recent works on Mn3−xCoxO4 ceramics done
inour laboratoryandwhichwere the subjectof several pub-lications, havefocused on structures (identificationof phases and cation distributions), along with magnetic and electrical properties.42–46Wenotethattheelectricalpropertiesstudies42,46 ofsingle-phaseMn3−xCoxO4spinelceramics,obtainedby
con-ventional sinteringor bySPS,hadshownthat theseceramics aresemiconductorsandpossessinterestingelectrical character-isticsmakingthemattractivematerialsforindustrialapplications as NTCthermistors.Also,thesestudiesshowedthat for mea-surements made at 25◦C, the minimum resistivity (ρ25◦C)
value is of about 387.cm for x=1.78 and thisρ25◦C value
increasesin amuchmoreintense manner onthe zonewhere
x<1.78(particularlyfor0.98≥x>1.54)thenonthezonewhere
x>1.78 (for x=0.98, ρ25◦C=49,552cm and for x=2.93,
ρ25◦C=5020cm).Electricalconductioncouldtakeplaceby
thepolaronshoppingbetweencations.
To ourknowledge, the workreportedinthisarticle corre-spondstothe firstmicrostructural study of cobaltmanganese oxideceramics,Mn3−xCoxO4.
2. Experimental
2.1. Samplepreparation
Ashasbeendescribedindetail byH.Bordeneuveetal.42, oxalic precursors, Mn1aCoaC2O4·nH2O,were first obtained
by the coprecipitation of an aqueous solution of ammonium
oxalateandamixtureofmanganeseandcobaltnitratesin pro-portionsvaryingaccordingtothechemicalcompositionofthe finalproduct.Thus,theresultingsolutionwasagedfor30min, thenfiltered,washedwithwateranddriedinairat90◦C.Oxide
powderswereproducedbythermaldecompositionof coprecip-itatedoxalateprecursors(inairat800◦C).Pelletsof6mmin
diameterwereobtainedbypressingoxidepowdersat500MPa. Afterthat,twotypesofsinteringwereused:conventional sinter-ingandSparkPlasmaSintering(SPS).Ashasbeenpreviously reportedindetail,42,47producingsingle-phasecobaltmanganese oxideceramicsisdifficulttoachieveandrequiresanadaptation of the sintering conditions according tothe desired chemical compositionofthesample.
Forx<1.78andtakingintoaccountthephasediagramofthe Mn3O4–Co3O4system(reportedinpreviousworks42,48),
single-phaseceramicpreparationisrestrictedbythemanystructural transformationstakingplaceatvarioustemperatures.Thus,as hasbeenpreviouslydescribed,42,47thesinteringprocessmustbe carriedoutathightemperature(between1160◦Cand1280◦C
inair,forthesampleswhicharetheobjectofthisstudy)inorder tobe placedinthe zone of thesinglecubic (C) spinelphase andto givethe ceramic sufficient densification.Additionally,
the samples must be quenched to avoid obtaining two-phase
samples.Infact,whentheyhavebeencooledatdifferentrates (i.e.thesampleshavenotbeenquenched),theobtainedceramics werebiphasic.Thetemperatureofquenchingcouldbealittle lowerthan the sintering temperature (but still in the domain ofthe singleCphase) toavoidcracking the samples.So,for
x<1.78,quadratic(Q)single-phaseceramicsweresuccessfully producedunderthesinteringconditionsreportedinTable2.
Forsamplescorrespondingtox=1.54andforwhichtheC spinelphaseexistsatlowertemperatures thanfor x<1.54(as hasbeendescribed previously),42,47 theSPSmethodhasalso beenused(Table2)inadditiontoconventionalsintering.
For x≥1.78, the preparation of single-phase ceramics is restrictedbythefact that thereduction ofthe Cspinelphase occursatlowertemperaturesthaninthedomainthatcorresponds tox<1.78,makingitdifficulttoobtainsingle-phaseceramics withhighdensities byconventional sintering.42,47 So,in this case,therearetwopossibilitiestoconductthesinteringofthe samples:
- conventional sintering with a sintering temperature higher thanthesamplereductiontemperaturebutwithacoolingrate lowenoughtoreoxidizethesample.
- SPS sintering which allowing us to decrease the optimum
sinteringtemperaturecomparedtotheconventionalmethod. Thesetwopossibilitieshaveproventobevalidforx=1.78, but,as the x value increases, the reoxidation of the samples becomesmoredifficultwiththeconventional method.So,for
x>1.78,onlytheSPSsinteringhasledtous,obtainingC single-phaseceramicswithhighdensities.42,47Allsinteringconditions arereportedinTable2.
ForSPSsintering,theapparatususedwasaSumitomo2080 (PNF2CNRSplatformavailableattheUniversityofToulouse, France).Theoxidepowderswerepre-compacted,thenplacedin agraphitedieandheatedundervacuumattemperaturesbetween 700and750◦C,dependingonsamplecompositionandundera
pressureof50MPa.Thepressurewasmaintainedconstantuntil theendofthedwelltimeat700or750◦C.Afterthat,thepressure
wasremoved,andthesamplewascooledtoroomtemperature byshuttingdownthepowersupply.Theresultingsampleswere polishedtoremovethe(Co,Mn)Othinlayerdepositedonsurface samplesduringsintering(ashasbeendescribedpreviously).42,47 Thedensificationvalues46ofobtainedsampleswerebetween 93%and94%forsamplessubjecttoconventionalsintering,and between95%and97%forthosesinteredbySPS(Table1).
2.2. Samplecharacterization
ABruckerD4powderdiffractometerwasusedtodetermine
sample X-ray diffraction (XRD) patterns. The
diffractome-ter operated with an emitting source of Cu (Ka1,a2
mean=1.5418 ˚A).
Wenoted thattheceramic samplessinteredbySPS,along withthosesinteredbyconventionalmethod,butwithlowcobalt contents, belong to brittle materials (like glass) and hence, the preparation of these samples for transmission electron
Table1
Sinteringconditions,spacegroupandcellparametersdeterminedbyXRD42,47ofMn
3xCoxO4ceramics.Therelativedensity46(dR),averagegrainsize(Dav)and
celldeformation(celldef)arealsoreported.
x Sinteringmethod dR(%) Phase Dav(mm) a′(nm) c(nm) c/a′ Celldefe(%)
0.98 conva 94 Qc 22 0.80956(3) 0.92052(4) 1.13706(9) 13.7 1.27 conv 93 Q 13.5 0.81163(5) 0.89743(7) 1.1057(2) 10.6 1.54 conv 93 Q 17.3 0.82258(3) 0.85998(4) 1.04547(9) 4.6 1.54 SPSb 95 Q 1.00 0.82250(2) 0.86020(4) 1.04584(7) 4.6 1.66 conv 94 Q 12.0 0.82481(4) 0.85081(4) 1.03152(9) 3.2 1.78 conv 94 Cd 10.0 0.83183(4) 1 1.78 SPS 95 C 0.67 0.83130(1) 1 1.99 SPS 95 C 0.56 0.82760(9) 1 2.22 SPS 97 C 0.44 0.82211(3) 1 2.93 SPS 96 C 0.34 0.80995(7) 1
aconv:conventionalmethod.
b SPS:Sparkplasmasintering.
cQ:quadraticwiththespacegroupI4
1/amd.
d C:cubicwiththespacegroupFd-3m.
eCelldef(%)=(c/a′
−1)×100.
microscopy(TEM) observations requires a lotof ability and resourcefulness.ThemethodusedforTEMsamplepreparation isasfollows:adiamondsaw(ESCIL3032-4)wasusedtoobtain asampleblockfromeachpellet,whichwasthenplacedinside abrasstube(offinediameter∼3mm)andboundwithanepoxy resin(GatanG1).Theresinwaspolymerizedat50◦Covernight,
then 500mm thick discs were collected by slicing the tube
using thesamediamondsaw.To makeeach sample
electron-transparent,i.e.,toreduceitsthicknesstoapproximately100nm,
each sample was mechanically polished (using ESCIL: ESC
300GTL),thenconcavedimplepolished(withaEA-Fishione
– model 2000 – polishing liquid: solution with diamond in
suspension)andfinallyionbeam-thinned(usingGATANPIPS).
TheceramicsampleswereobservedusingaJEOLJEM2010
electron microscope (200kV – emitter: single crystal LaB6
tip – maximum resolution: 0.23nm point–point and 0.14nm
line–line).Thechemicalcompositionofeachsamplewas ana-lyzedbothqualitativelyandquantitatively(simultaneouslywith TEMobservations)byusingaTracorVoyagerEnergy Disper-siveX-rayanalyzer(EDX).Probesizemaybereducedto7nm.
For each sample, granulometric analyses andtwin
lamel-lar thickness of TEM images were performed using imageJ
software.49
3. Results
AsisreportedinTable1,allobtainedsamplescorrespondto Cspinelstructure(Fd-3mspacegroup)forx≥1.78andtoQ spinelstructure(I41/amdspacegroup)forx<1.78.Tofacilitate
thecomparisonbetweenQandCspinelcells, theQcellwas convertedtoabiggerunitcellwitha′=a√2andc′=candthe
c/a′deformationwasevaluatedforeachsample.
3.1. Grainsizes(GSs)
BeforedescribingtheGSofceramicsamples,werecallthat theGSofinitialpowders,47forwhich0.98≤x≤2.93,presenta relativelywideGSdistributionandvaryasfollows:GSsareof
theorderofafewhundrednmforx=0.98.Theythenincrease withincreasingxfrom0.98to1.27,wheretheyreachamaximum
(without exceeding amaximum GS value of about 600nm).
Theythendecreaseasxvariesfrom1.27to2.72,thusreaching values closetoafew tensof nm.From thislattervalueof x, GSvaluesoncemoreincreaseuptox=2.93andbecomeofthe orderofafewhundrednm.
3.1.1. Sampleswithconventionalsintering (0.98≤x≤1.78)
TEM observations of the ceramic samples reveal that the
grainspossesspolyhedralshapesandaremostlyequiaxed.Each
sample presents a wide dispersion of GSs. Minimum GS is
aboutfewmicrons(1–4mm)forallsamplesbutmaximumGS is respectively40mm, 25mm, 33mmand19mmfor x=0.98,
x=1.27, 1.54 and1.78, accordingto the sintering conditions andthe GS ofthe initialpowders. Theaverage GSs(Dav) of
theseceramicsamplesarereportedinTable1.
3.1.2. SampleswithSPSsintering(1.54≤x≤2.93)
These samples alsocontain grainswith polyhedralshapes
and which are mostly equiaxed. GSs are polydispersed and
verysmallcomparedtothoseofsamplesobtainedby
conven-tional sintering. They vary between 300nm and 1.7mm for
x=1.54 anddecrease systematically withincreasing xvalue, i.e.,withincreasingCocontent.Thus,theminimumand
max-imumGSvaluesarerespectively135–1210nm,103–1016nm,
95–781nm,and76–611nmforx=1.78,x=1.99,x=2.22and
x=2.93.TheGSvariationofthefinalsamplesisduetochanges insinteringconditionsandalsointheGSsoftheinitialpowders. TheDavvaluesoftheseceramicsarereportedinTable1.
3.2. Defects
Samples with x<1.78 show grainswith high densities of two-dimensionalstructuraldefects.Dependingontheconcerned sample,theselattercorrespondtosomeofthefollowingtypes ofdefects:
Fig.1.BrightfieldTEMimageofasinglequadraticphaseMn3−xCoxO4ceramic
sampleforx=1.27showingatriplejunctionandtheintensepresenceineach
grainoflargelamellae(LIT)whichareinternallytwinned(i.e.containingfine
lamellae(l)).
- twins,intheformoflamellae(L)paralleltoeachother(each lamellacorrespondstoasingleatomicdomain).
- largelamellae(LIT)whichareinternallytwinned(i.e.,
con-tainingfinelamellae(l)).
- tweeds(veryfinelamellaearrangedintwomutually perpen-diculardirections).
3.2.1. MonophasicsampleswithQstructure
AsingleQphasewasobtainedforsampleswithx<1.78that weresinteredbytheconventionalmethodorbySPSsintering. Thelatticeparametersandthec/a′ deformationofthese
sam-plesarereportedinTable1.Quantitativechemicalanalysisby
EDXhadshownthat each ofthesesamples ishomogeneous.
TheexperimentalvaluesofxarereportedinTable2.Theyfit wellwiththeexpectedvaluesofxcorrespondingtotheoretical chemicalcompositions.
- sampleswith0.98≤x<1.54:Thesesamplescontainalmost LITlamellae(Figs.1 and2)andaresometimesarrangedin
cyclicformscorrespondingtotriangles(Fig.3).Toour knowl-edge,thistrianglearrangementshapeofLIT,correspondsto
Fig.2.BrightfieldTEMimageofasinglequadraticphaseMn3−xCoxO4ceramic
sampleforx=0.98showingthatthelamellae(LIT)aremuchlargerthanthose
ofceramicsamplewithx=1.27.
Fig.3.BrightfieldTEMimageofasinglequadraticphaseMn3−xCoxO4ceramic
sampleforx=0.98showingLITlamellaearrangedinacyclicform
correspond-ingtoatriangleobservedforthefirsttimeinmanganites.Thisarrangement
couldbeexplainedbytheexistenceofa3-foldsymmetryaxisintheCphase
(high-temperaturephase)withspacegroupFd-3m.
atypeofarrangementobservedforthefirsttimein mangan-ites. This arrangementcould beexplained bythe existence of a3-foldsymmetryaxisintheCphase(high-temperature phase)withspacegroupFd-3m.ThewidthsofLITandalso
thewidthsofthelfinelamellae(insideLIT)arereportedin
Table2.ThemaximumvalueofLITwidthisgreatlydecreased
withincreasingxvaluefrom0.98to1.27.
- sample withx=1.54sintered byconventional method:This samplecontainsonlyapproximatelyLlamellae(Fig.4).The formsoftheselamellaecorrespondtoeitherright-angledtwins (i.e., domains with practically right-angled twin walls) or needle-shaped twins (i.e.,the trajectories of the twinwalls arelikeneedles)(Fig.5).Thisisquitetypicalfora ferroelas-tictransition.50–53Thewidthsoftheselamellaearereported inTable2.
- sample withx=1.54sinteredbySPS:Thissamplecontains onlyLlamellae(Fig.6).Thelamellawidthsarereportedin Table2andaremuchfinerthanthoseofthesamplesintered bythe conventionalmethod.Thelatticeparametersandthe
c/a′deformationarealmostthesameasthoseobtainedwith
conventionalsinteringforx=1.54.Also,thetypeofstructural defectsdoesnotchangeandcorrespondstoLlamellae.Butthe SPSsinteringgeneratedasignificantdecreaseinGScompared toconventionalsintering,whichcouldbethecauseofthedrop inlamellawidths,aswasreportedintheliteratureconcerning theeffectofGSonthewidthsoftwinlamellae.54–57
Table2
Preparationconditions,structureandmicrostructuraldefectsformonophasicceramics.Thetheoretical(theor.)valuesofx(Cocontentinsamples)andtheexperimental
onesdeterminedbyenergydispersiveX-rayanalysis(EDX),arealsoreported.
x(theor.value) Sinteringmethod-sintering
temperature(◦C)
Coolingrate Phase Twintypes Lamellawidths
min–max(nm)
xvalueby
EDX
0.98 conva-1280 120◦C/handair-quenchedfromT=900◦C Qc L
ITe 77–928 0.95(3)
lf 3–13
1.27 conv-1180 120◦C/handair-quenchedfromT=800◦C Q LIT 69–409 1.25(5)
l 3–26
1.54 conv-1180 air-quenchedfromT=1180◦C Q Lg 12–306 1.54(3)
1.54 SPSb-750 cut-offofthefurnace Q L 5–45 1.55(3)
1.66 conv-1160 120◦C/handair-quenchedfromT=900◦C Q Th 2–14 1.64(3)
1.78 conv-1160 10◦C/hto400◦Cand20◦C/hto25◦C Cd Nodefects 1.75(4)
1.78 SPS-750 Cut-offofthefurnace C Nodefects 1.72(5)
1.99 SPS-750 Cut-offofthefurnace C Nodefects 1.97(4)
2.22 SPS-700 Cut-offofthefurnace C Nodefects 2.21(3)
2.93 SPS-700 Cut-offofthefurnace C Nodefects 2.91(6)
aconv:conventionalmethod.
b SPS:sparkplasmasintering.
cQ:quadraticphase.
d C:cubicphase.
eL
IT:largelamellaewhichareinternallytwinning.
fl:theinternaltwins,i.e.,twinswhichareinsideeachlargelamella.
g L:lamellaewithoutinternaltwins.
h T:tweed.
- samplewithx=1.66:Thissampleincludestweedswithhigh frequency(Fig.7).Thesetweeds are very fine withwidths varyingfrom2to14nm.
Consideringallsamples,thedomainwalls(DW)ofthe
inter-nal twins (l) and also the DW of domains with no internal
twins (L)belongtothe type-Imechanicaltwin,meaning that theatomicarrangementofonedomainisthemirrorreflection of theotherbythetwininterfaceplane, correspondingtothe (112) crystallographic plane for our samples. So,as hadbe showninapreviousstudy21,andmentionedinotherstudies,9,26 theseDW (i.e.,corresponding toLandl) constitutecoherent
Fig.4.BrightfieldTEMimageofasinglequadraticphaseMn3−xCoxO4ceramic
sampleforx=1.54(conventionallysintered)showingLlamellae(without
inter-naltwins)correspondingtoright-angledtwins(i.e.,domainswithpractically
right-angledtwinswalls).Themaximumvalueoftheirwidthsisaboutafew
hundredofnanometers.
twinboundaries.TheDW oftheLIT largelamellae,however,
formincoherenttwinboundaries.
Thetype-Imechanical112twinsareshowninthediffraction patternsreportedinFig.8.Thediagramsareindexedaccording totheQcell,andcorrespondtothezoneaxes[1¯10]and[1¯31]. The presence of twins insamplemicrostructures isshownin thesediagramsby asplitting ofthe reflectionscorresponding
Fig.5.BrightfieldTEMimageofasinglequadraticphaseMn3−xCoxO4ceramic
sampleforx=1.54(conventionallysintered)showingLlamellaecorresponding
toneedle-shapedtwins(i.e.thetrajectoriesofthetwinwallsarelikeneedles)
Fig.6.BrightfieldTEMimageofasinglequadraticphaseMn3−xCoxO4ceramic
sampleforx=1.54(sinteredbySPS)showingLlamellaethatareverymuch
finer(maximumwidthvalueisafewtensofnm)thanthoseofthesamplewith
thesamevalueofxbutconventionallysintered.
tothediffractionof zoneslocatedoneithersideofthe(112) twininterfaceplane(i.e.,thetwinboundary).Splitting magni-tudeincreaseswiththedistancefromthecenterofthepattern. Nonsplitreflectionscorrespond tocrystallographicrows per-pendiculartothe(112)twininterfaceplane.
3.2.2. MonophasicsampleswithCstructure
AsingleCphasewasobtainedforsampleswithx≥1.78and sinteredbySPSsinteringorbytheconventionalmethod(only forx=1.78).Thelatticeparametersofthesesamplesarereported inTable1.QuantitativechemicalanalysisbyEDXhadshown thateachofthesesamplesishomogeneous.AsshowninTable2, foreachsample,theexperimentalvalueofxfitswellwiththat correspondingtothetheoreticalchemicalcompositions.
These samples (i.e., x≥1.78) differ from previous sam-ples (i.e., 0.98≤x<1.78) in that they contain no defects (Figs.9and10).Wenotedthat,forx=1.78,thetwosintering methods(SPSandconventionalsintering)leadtotheproduction
Fig.7.BrightfieldTEMimageofasinglequadraticphaseMn3−xCoxO4ceramic
sampleforx=1.66showingthepresenceofdefectscorrespondingtotweeds
(veryfinelamellaewithwidthsofafewnm,andarrangedintwomutually
perpendiculardirections).
of samples free from defects (the only changes induced by
themodificationof thesintering method,correspondtoaGS variationofthefinalsamples,asmentionedabove).
4. Discussion
Single-phaseMn3−xCoxO4(0.98≤x≤2.93)spinelceramics presentavarietyofmicrostructuralcharacteristicsdependingon thesample’schemicalcomposition(i.e.,valueofx)andthetype ofheattreatmentappliedpriortoobtainingthefinalproduct.
We recall that for x<1.78 or x≥1.78, the monophasic ceramicscorrespondtoQ(I41/amdspacegroup)orC(Fd-3m
spacegroup)spinelstructuresrespectively. Qphasearisesby thestructuraltransitionofCphaseinagreementwiththephase diagram of the Mn3O4–Co3O4 system.42,48 This structural
Fig.8.Selectedareaelectrondiffraction(SAED)patternsofasinglequadraticphaseMn3xCoxO4ceramicsampleforx=1.54:(a)alongthezoneaxis[1¯31],(b)
alongthezoneaxis[1¯10],and(c)shematicreproductionofapartof(b).Thesepatternsexhibitsplittingofspots(insuchawaythattherearespotscorrespondingto
thematrixandotherspotscorrespondingtotwinswhicharedeductedfromthefirstonesbyreflectionfromaplanemirrorcoincidingwiththetwininterfaceplane).
Thereflectionsthatarenotsplitcorrespondtocrystallographicrowsperpendiculartothe(112)twininterfaceplane.Themagnitudeofsplittingincreaseswiththe
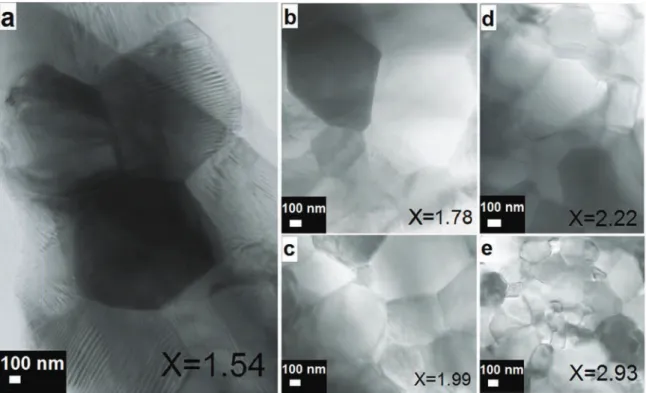
Fig.9.BrightfieldTEMimagesofallsamplessinteredbySPS:(a)forx=1.54,(b)forx=1.78,(c)forx=1.99,(d)forx=2.22,and(e)forx=2.93.Theyshowthat
forx≥1.78,notwinsdefectswereobservedinthesinglecubicphaseceramicsamples,unlikethecaseofthesinglequadraticphaseceramicsamplewithx=1.54
andwhichcontainstwinslamellae,L.
phasetransitioniscausedbythecooperativeJahn–Tellereffect asinothermanganitecompounds.21,22,26,58,59 TheCstructure isstableonlyathightemperaturesandcannotbeobservedat roomtemperatureafterquenchinginthemanganese-richoxide. Thegrainshapesofthesamplesarepolyhedralandequiaxed, inaccordancewiththeidentifiedcrystallographiccells:Ccell orQcellwithparametersclosetothoseofCcell(sincethereis verylittledifferencebetweena′(a′=a√2)andcparametersofQ
cell).GSsvarydependingonsinteringtypeandconditions,and alsoontheGSsoftheinitialpowders.Whenxvaluevariesfrom 0.98to1.27,thedropinmaximumGSfrom40to25mmcould beduetothedecrease insinteringtemperature from1280◦C
to1180◦Caswellastothedecreaseinquenchingtemperature
from900◦Cto800◦C.Whenxchangesfrom1.27to1.54,the
maximumGSincreasesfrom25to33mm,despitethedecrease ininitialpowderGSandthiscouldbeduetotheincreaseofthe
Fig.10.BrightfieldTEMimageofasinglecubicphaseMn3−xCoxO4ceramic
sampleforx=1.78(conventionallysintered)showingatriplejunctionandthe
absenceineachgrainoftwindefects.
quenchingtemperaturefrom800◦Cto1180◦C.Forx=1.78the
maximumGS isdiminished comparedtothatof x=1.54and
thusisreducedto19mm.Thiscouldbeduetothedecreasein GSof theinitialpowders,as wellas tothechange incooling conditions(quenchingforx=1.54andslowcoolingtoambient temperatureforx=1.78).
For ceramic samples obtained by SPS (i.e., x=1.54 and
x≥1.78)theGSsaremuchsmallerthanthosesinteredbythe conventional methodandarerelativelysimilartothoseof the initialpowders,whichispredictableduetothefactthatSPS sin-teringgreatlyreducesvolumediffusion(i.e.,graincoarsening) comparedtoconventionalsintering.60–63
Forx<1.78,twinsarewellpresentinallceramicsamples, buttheydifferintheircharacteristics fromsampletosample. Theexistenceoftwinsinthesesamplescouldbeduetothefact that the structural transition is ferroelastic,as it isin Mn3O4
hausmannite.21Infact,ferroelastictransition,whichbelongsto ferroic transitions,isabletoproduce structuraldomainswith different orientation states under adapted conditions.64–68 In thiscase,structuraldomainsoccurinmaterialstoaccommodate the constraints accompanying the phase transition since the latterleads,amongstothers,toareductionofcrystallographic symmetryelementsandadeformationofthe crystallographic cell evaluated by the value of c/a′ (a′ (i.e., a√2) and c
corresponds to Q cell parameters) with respect to the high-temperature phase. The twins arising in this case are called transformation twins. We can also note that the structure of the samplesstudied inthiswork, obeythefact that thepoint
group symmetryof the low-temperature phase(4/mmm) isa
thusthestructuraltransitionofthesesamplesbelongstoAizu speciesm3mF4/mmminhisnomenclatureofferroelasticphase transitions.67 The results obtained from electron microscopy revealed thatthe twins observedinthesesamplescorrespond to 112 twins (i.e.,the twin interface plane is(112)). These results are in conformity withthe theoretical predictions65,66 about the (hkl) twininterface planes that may exist when a ferroelasticphasetransition occursfromCphasewith(m3m)
pointgroupsymmetrytoQphasewith(4/mmm) pointgroup
symmetry (h, k and l are the Miller indices). Considering
changes in the characteristics of twins for x<1.78, we can mention that the mainfactorswhich couldhave an effecton thesevariationsare:
- increaseincelldeformationc/a′.
- thefactthatthechemicalcompositionofthesampleisnearor farfromthecompositioncorrespondingtothephase transi-tion.Foroursamplesthiscompositioncorrespondstox=1.78 asindicatedbythephasediagramoftheMn3O4–Co3O4
sys-temreportedinpreviousworks.48 - thevariationofGSs.
For x=1.66, TEM observations indicate the existence, in
thissample, of tweeddomains shownbya modulatedimage
contrast in two directions withwidths on the orderof a few nm.Thisresult could beattributed tothefact that the chem-ical composition of thissample is closeto that of the phase transition (i.e., x=1.78) and thus could be noted as one of thefeatureshighlightingtheferroelasticcharacterofthephase transition inMn3−xCoxO4.Thisis becausemany
experimen-talandtheoreticalstudieshaveshowntheapparitionoftweeds whenapproachingthephasetransitioninthecaseof ferroelas-tictransitions.26,55,64,68–70Amongthevariousinterpretationsin thisregardandtakingintoaccountthatthesampleusedinthis
study hasan homogeneouschemical composition(within the
resolutionoftheanalyses)andcontainsonlyonephase,wenote thattheobservedtweedscouldberelatedtothefactthat,close tothephasetransition,DWmotionleadsthemicrostructureto assumeastablearrangementcorrespondingtoaminimumfree energy value (including elastic energy) and whichis consis-tentwithstrainsinducedbythephasetransformation.Thus,this arrangementisestablishedwiththetweedmicrostructureandis compatiblewiththec/a′sampledeformationwhichisofabout
3.15%andisthelowestvaluecomparedtothoseoftheothers sampleswithx<1.78.
Whenxdecreasesfrom1.66to1.54,thechemical composi-tionofthesamplebecomesalittlebitfarfromthatofthephase transitionandtheTEMobservationsrevealthatthemodulated microstructureisentirelyreplacedbytwindomains correspond-ing toparallel Llamellae.Asmentioned above,for thesame chemical composition (i.e., x=1.54), the decrease in lamella widthsofthesamplesinteredbySPScomparedtothoseofthe sampleobtainedbyconventionalsinteringcouldbeduetothe decreaseinGSsinducedbySPSsintering,inaccordancewith that hasbeen reportedintheliterature.54–57 Forbothsamples (i.e., SPS and conventionally sintered samples), the value of thec/a′deformationisofabout4.6%,notexcessivelylargein
comparisontothoseoftheothersampleswithlowervaluesof
x.However,whenxdecreasesfrom1.54to1.27,theincreasein straincausedbytheenhancementofthec/a′valuecouldleadto
enlargementofthelamellaeaswellastheformationinsidethem ofnewfinetwinlamellael,correspondingtointernaltwinning. In fact,as has been reportedinthe literature,71 for asample microstructure, there are potential sites for the formation of twinsandwhenthedeformationincreases,twinsareformedat thesesites.Thus,theincreaseindeformationwhengoingfrom 1.54to1.27,could generateanincrease inthetwininterface energy and then the activation of the sites that give rise to internaltwins.Theoccurrenceoftheselattersispromotedby theincreaseinthewidthsoftheinitialtwinlamellae.
Then,adecreaseinxfrom1.27to0.98isalsoaccompanied byanincreaseinc/a′ deformation,whichcouldbecontribute,
on the one hand, to a large increase in width of the (LIT)
lamellae(morethantwicethe widthofthe (LIT)lamellaefor
x=1.27)andontheotherhand,tothe apparitionof newfine internaltwins(i.e.,totheincreaseininternaltwindensity)and couldultimatelyleadtointernaltwinwidthsalittlethinnerthan forx=1.27.Also forx=0.98the LITlamellaearesometimes
arrangedincyclicformscorrespondingparticularlytotriangular shapesobservedforthefirsttimeinmanganites.Intheliterature, twinsforwhicharrangementshapesarelikethree-pointedstars, nottriangles,havebeenreportedforferroelasticphase transi-tionsandhavebeenattributedtothelossofthe3-foldaxisof thehigh-temperaturephase.66
Furthermore,itiswellknownthattheactivation energyof incoherentDWismuchhigherthanthatofcoherentDW.21,26,72 Thus,asignificantincreaseinactivationenergycausedbythe presenceofincoherentDWcouldleadtoadecreaseincation siteexchange.Inlightofthefactthatalargeproportionofthe materialvolumecorrespondstoDW,theincoherentoneswhich arepresentwhenx<1.54couldcontribute,inadditiontoother factors(relatedtocationdistributionsandwhicharementioned inpreviousworks),42,46tothefactthatthevariationin electri-calresistivityoftheseceramicsshowsaverylargeincreasefor 0.98≤x<1.54.
Forx≥1.78,nodefectswereobservedintheseceramic sam-ples withC spinel structure (for x=1.78, this result remains validfor both samples, i.e., SPS andconventionally sintered samples).Thiscouldbeduetotheabsenceofphasetransition for thesechemicalcompositionsas it hasbeen shownbythe phasediagramoftheMn3O4–Co3O4system.48
5. Conclusion
Themicrostructureof cobaltmanganeseoxide ceramicsis presented, for the first time, inthis paper. The studied sam-plescorrespondtosinglephaseMn3−xCoxO4(0.98≤x≤2.93) spinelceramicsandtheirmicrostructuresweredeterminedusing
TEMandEDX.Theywereobtainedbyconventionalsintering
orbySPSofpowders(elaboratedbythermaldecompositionof coprecipitatedoxalateprecursors).SingleQphaseandsingleC phasewereobtained respectivelyforx<1.78 andx≥1.78.Q phaseoccursbythe structuraltransition of Cphasein agree-mentwiththephasediagramoftheMn3O4–Co3O4system.48
The structural transition of these samples belongs to Aizu speciesm3mF4/mmminhisnomenclatureofferroelasticphase transitions.67 Samples with x<1.78 show grains with high domaindensities(i.e.twindensities)whichcouldbeduetothe factthatthestructural transitionisferroelastic.Coherenttwin interfaceplanesareparallelto(112)planesintheQcellwhichis inaccordancewiththeoreticalpredictions65,66foraferroelastic phasetransitionoccuringfromCphasewith(m3m)pointgroup
symmetrytoQphasewith(4/mmm)pointgroupsymmetry.
The twins that are present in ceramic samples with
x<1.78,correspondto:tweeds,singlelamellae,i.e.,L(widths: 5–306nm) arranged parallelto each other(and whose forms correspondtoeitherright-angledtwinsorneedle-shapedtwins), largelamellae,i.e.,LIT(widths:69–928nm)internallytwinned
andsometimesarrangedincyclicformscorrespondingto trian-gularshapesobservedforthefirsttimeinmanganites.Variations inthecharacteristicsoftwinswereinvestigatedinrelationto:the variationofthecelldeformationc/a′,thefactthatthechemical
composition of the sample is near or far from the composi-tion corresponding tothe phase transition (i.e., x=1.78) and the GS variations. The incoherenttwin interfacespresent for
x<1.54couldhaveaneffect(inadditiontoothereffectsrelated tocationdistributions andmentioned inprevious works42,46) on the important increase in electricalresistivity for x<1.54 becauseitiswell-knownthattheactivationenergyofan inco-herenttwininterfaceisverymuchhigherthanthatofcoherent twininterface.
Nodefectswereobservedinceramicsamplescorresponding toCphase(i.e.,x≥1.78)whichcouldberelatedtotheabsence ofphasetransitionforthesechemicalcompositions.
Acknowledgments
The authors would like to thank Mr. Laurent Weingarten
(ServiceCommundeMicroscopieElectroniqueTEMSCAN–
UniversitéPaul Sabatier – Toulouse) for his kindhelpinthe preparationofsamplesforTEMobservations.
References
1.Van Santen JH, Jonker GH. Electrical conductivity of ferromag-netic compounds of manganese with perovskite structure. Physica
1950;16(7–8):599–600.
2.FinchGI,SinhaAPB,SinhaKP.Crystaldistortioninferrite-manganites.
ProRSocLondAMathPhysSci1957;242(1228):28–35.
3.Goodenough JB, Loeb AL. Theory of ionic ordering, crystal distor-tion,andmagneticexchangeduetocovalentforcesinspinels.PhysRev
1955;98(2):391–408.
4.ZenerC.Interactionsbetweenthedshellsinthetransitionmetals.PhysRev
1951;81:440–4.
5.CaffinJP,RoussetA,CarnetR,LagrangeA.ChemicalpreparationofNTC thermistorswithlowresistivityandhighstability.In:VencenziniP,editor.
HighTechCeramics.Amsterdam:Elsevier;1987.p.1743–51.
6.Guillemet-FritschS,ChanelC,SarriasJ,BayonneS,RoussetA,AlcobeX, etal.Structure,thermalstabilityandelectricalpropertiesofzincmanganites.
SolidStateIonics2000;128(1–4):233–42.
7.JabryE, BoissierG,RoussetA,CarnetR,Lagrange A.Preparation of semiconductingceramics(NTCthermistors)bychemicalmethod.JPhys Colloques1986;47:C1843–847.
8.RoussetA,LegrosR,LagrangeA.Recentprogressinthefabricationof ceramicnegativetemperature coefficientthermistors.J EurCeram Soc
1994;13(3):185–95.
9.BattaultT,LegrosR,BrieuM,CoudercJJ,BernardL,RoussetA.Correlation betweenmicrostructureandageingofironmanganitethermistors.JPhys III(France)1997;7(5):979–92.
10.MetzR. Electrical properties of N.T.C. thermistors made of mangan-iteceramicsofgeneralspinelstructure:Mn3−x−x′MxNx′O4(0≤x+x′≤1; Mand NbeingNi,CoorCu).Agingphenomenonstudy.JMaterSci
2000;35:4705–11.
11.SachseHB.Semiconductingtemperaturesensorsandtheirapplications. NewYork:Wiley;1975.
12.UmadeviP,NagendraCL.Preparationandcharacterisationoftransition metaloxidemicro-thermistorsandtheirapplicationtoimmersedthermistor bolometerinfrareddetectors.SensActuatorsAPhys2002;96(2):114–24.
13.KopiaLP,LeeIIIRB.Thermistorbolometerscanningradiometer: applica-tionsandflightexperience.OptEng1992;31(1):156–65.
14.SheftelIT.Thermistors.Moscow:Nauka;1973.
15.Aleksi´cOS,Nikoli´cPM,Simi´cMN,Pejovi´cV ˇZ,Vasiljevi´c-Radovi´cDG. Resistivityversusgeometryrelationinbulk-sinteredandthickfilm MnCoFe-oxidethermistors.In:Stojanovi´cBD,SkorokhodVV,Nikoli´cMV,editors.
Advancedscienceandtechnologyofsintering.NewYork:Kluwer Aca-demic,PlenumPublishers;1999.p.425–30.
16.Aleksi´cOS,Savi´cSM,Lukovi´cMD,Radulovi´cKT,Luki´cLS.Segmented thermistorsprintedbyNTCnanometricpasteandappliedinvolumeair-flow sensors.MaterSciForum2006;518:247–52.
17.EdwardsL,MurthyR.Versatilethermistorsforwide-rangingapplications.
Electrotechnology1987;15:89–91.
18.MetzR,BrieuM,LegrosR,RoussetA.Intergranularphasesin electro-ceramics.JPhysColloques1990;51:C11003–1008.
19.BrieuM, CoudercJJ, Rousset A, Legros R. TEM characterization of nickelandnickel–cobaltmanganiteceramics.JEurCeramSoc1993;11(2): 171–7.
20.RoussetA,LagrangeA,BrieuM,CoudercJJ,LegrosR.Influencedela microstructuresurlastabilitéélectriquedesthermistancesCTN.JPhysIII (France)1993;3:833–45.
21.CoudercJJ,FritschS,BrieuM,VanderschaeveG,FagotM,RoussetA.A transmissionelectronmicroscopystudyoflatticedefectsinMn3O4 haus-mannite.PhilMagB1974;70(5):1077–94.
22.CoudercJJ,FritschS,BrieuM,VanderschaeveG.Atransmissionelectron microscopystudyofaccomodationtwinsinBa–Ni–Mnoxides.PhilMagA
1996;74(6):1351–65.
23.FritschS,SarriasJ,BrieuM,CoudercJJ,BaudourJL,SnoeckE,etal. Corre-lationbetweenthestructure,themicrostructureandtheelectricalproperties ofnickelmanganitenegativetemperaturecoefficient(NTC)thermistors.
SolidStateIonics1998;109:229–37.
24.MartinDeVidalesJL,Garcia-ChainP,RojasRM,VilaE,Garcia-Martinez O.Preparationandcharacterizationofspinel-typeMn–Ni–Co–Onegative temperaturecoefficientceramicthermistors.JMaterSci1998;33:1491–6.
25.ChanelC.Optimisationdescaractéristiquesstructurales,microstructurales etélectriquesdesmanganitesdenickel,dezincetdenickel-zincenvuede l’applicationauxthermistancesàcoefficientdetempératurenégatif(CTN). ThèsedeDoctoratdel‘UniversitédeToulouse;1998.
26.MetzmacherC,GroenWA,ReaneyIM.Microstructureandelectrical prop-ertiesofMn–Ni–Inspinels.PhysStatSol2000;181:369–86.
27.Park K. Microstructure and electrical properties of Ni1.0Mn2−xZrxO4 (0≤x≤1.0)negativetemperaturecoefficientthermistors.MaterSciEng B2003;104:9–14.
28.ParkK,HanIH.EffectofAl2O3additiononthemicrostructureandelectrical propertiesofMn0.37Ni0.3Co0.33−xAlxO4 (0≤x≤0.03)NTCthermistors.
MaterSciEngB2005;119:55–60.
29.ParkK,LeeJK,KimSJ,SeoWS,ChoWS,LeeCW,etal.TheeffectofZnon themicrostructureandelectricalpropertiesofMn1.17−xCo0.93Co0.9ZnxO4 (0≤x≤0.075)NTCthermistors.JAlloysCompd2009;467:310–6.
30.Bodak O, Akselrud P, Demchenko P, Kotur B, Mrooz O, Hadzaman I, et al. Microstructure, crystal structure and electrical properties of Cu0.1Ni0.8Co0.2Mn1.9O4ceramicsobtainedatdifferentsinteringconditions.
31.VargheseJM,SeemaA,DayasKR.Ni–Mn–Fe–Cr–Onegative tempera-turecoefficientthermistorcompositions:correlationbetweenprocessing conditions and electrical characteristics. J Electroceram 2009;22(4): 436–41.
32.Park K. Fabrication and electrical properties of Mn–Ni–Co–Cu–Si oxides negative temperature coefficient thermistors. J Am Ceram Soc
2005;88(4):862–6.
33.VargheseJM,SeemaA,DayasKR.Microstructuralelectricalandreliability aspectsofchromiumdopedNi–Mn–Fe–ONTCthermistormaterials.Mater SciEngB2008;149:47–52.
34.BorgesFMM,MeloDMA,CâmaraMSA,MartinelliAE,SoaresJM,de AraújoJH,etal.MagneticbehaviorofnanocrystallineMnCo2O4spinels.J
MagnMagnMater2006;302:273–7.
35.Vasil’evGP,PakhomovLA,RyabovaLA.Structuralandelectrical prop-erties of d.c. sputtered MnCo2O4 films. Thin Solid Films 1980;66: 119–24.
36.RiosE,PoilleratG,KoenigJF,GautierJL,ChartierP.Preparationand char-acterizationofthinCo3O4andMnCo2O4filmspreparedonglass/SnO2:F byspraypyrolysisat150◦Cfortheoxygenelectrode.ThinSolidFilms 1995;264:18–24.
37.Martinde VidalesJL,VilaE, RojasRM,Garcia-MartinezO.Thermal behaviorinairandreactivityinacidmediumofcobaltmanganesespinels MnxCo3−xO4 (1≤x≤3) synthesized atlow temperature. Chem Mater
1995;7(9):1716–21.
38.KolomietsBT,SheftelJ,KurlinaE.Electricalpropertiesofsomecompound oxidesemiconductors.SovPhysTechPhys1957;2:40–58.
39.WickhamDG,CroftWJ.Crystallographicandmagneticpropertiesof sev-eralspinelscontainingtrivalentja-1044manganese.JPhysChemSolids
1958;7:351–60.
40.JabryEH,RoussetA,LagrangeA.Preparation andcharacterization of manganeseandcobaltbasedsemiconductingceramics.PhaseTransitions
1988;13(1–4):63–71.
41.Buhl R. Manganites spinelles purs d‘éléments de transition prépara-tionsetstructurescristallographiques.JPhysChemSolidsA1969;30(4): 805–12.
42.BordeneuveH,Guillemet-FritschS,RoussetA,ShuurmanS,PoulainV. Structureandelectricalpropertiesofsingle-phasecobaltmanganeseoxide spinelsMn3−xCoxO4 sinteredclassicallyandbysparkplasmasintering (SPS).JSolidStateChem2009;182(2):396–401.
43.BordeneuveH, Tenailleau C, Guillemet-Fritsch S, SmithR, Suard E, RoussetA.StructuralvariationsandcationdistributionsinMn3−xCoxO4 (0≤x≤3)denseceramicsusingneutrondiffractiondata.SolidStateSci
2010;12(3):379–86.
44.BordeneuveH,RoussetA,TenailleauC,Guillemet-FritschS.Cation distri-butioninmanganesecobaltitespinelsCo3−xMnxO4(0≤x≤1)determined bythermalanalysis.JThermalAnalCalorim2010;101(1):137–42.
45.Guillemet-FritschS,TenailleauC,BordeneuveH,RoussetA.Magnetic propertiesofcobaltandmanganeseoxidespinelceramics.AdvSciTech
2010;67:143–8.
46.RoussetA,TenailleauC,DufourP,BordeneuveH,PasquetI, Guillemet-FritschS,etal.ElectricalpropertiesofMn3−xCoxO4(0≤x≤3)ceramics: aninterestingsystemfornegativetemperaturecoefficientthermistors.IntJ ApplCeramTechnol2013;10(1):175–85.
47.BordeneuveH.EtudedusystèmeMn3−xCoxO4(0≤x≤3)sousformede poudresetdecéramiques.Structure,microstructure,propriétésmagnétiques etélectriques.ApplicationsauxthermistancesàCoefficientdeTempérature Négatif(C.T.N.).ThèsedeDoctoratdel‘UniversitédeToulouse;2009.
48.AukrustE,MuanA.Thermodynamicpropertiesofsolidsolutions with spinel-typestructure.I:ThesystemCo3O4–Mn3O4.TransMetSocAIME 1964;230:378–82.
49.AbramoffMD,MagelhaesPJ,RamSJ.ImageprocessingwithImageJ.
BiophotonicsInt2004;11(7):36–42.
50.Salje EKH, Ishibashi Y. Mesoscopic structures in ferroelastic crys-tals: needle twins and right-angled domains. J Phys Condens Matter
1996;8:8477–95.
51.SaljeEKH,BuckleyA,VanTendelooG,IshibashiY,NordJrG.L.Needle twinsandright-angledtwinsinminerals:comparisonbetweenexperiment andtheory.AmMineral1998;83:811–22.
52.BuckleyA,RiveraJP,SaljeEKH.TwinstructuresintetragonalSrTiO3:the ferroelasticphasetransitionandtheformationofneedledomains.JAppl Phys1999;86(3):1653–6.
53.OrlovskayaN,BrowningN,NichollsA.Ferroelasticityinmixed conduct-ingLaCoO3basedperovskites:aferroelasticphasetransition.ActaMater 2003;51:5063–71.
54.ZhuL,RuanH,LiX,DaoM,GaoH,LuJ.Modelinggrainsizedependent optimaltwinspacingforachievingultimatehighstrengthandrelatedhigh ductilityinnanotwinnedmetals.ActaMater2011;59(14):5544–57.
55.Salje EKH. Phase transitions in ferroelastic and coelastic crystals. CambridgeUniversityPress;1990.
56.CaoW,RandallCA.Grainsizeanddomainsizerelationsinbulkceramic ferroelectricmaterials.JPhysChemSolids1996;57(10):1499–505.
57.El-DanafE,KalidindiSR,DohertyRD.Influenceofgrainsizeand stacking-faultenergyondeformationtwinninginfccmetals.MetallMaterTransA
1999;30:1223–33.
58.RobbrechtGG,Henriet-IserentantCM.Onthelatticeparametersandthe tetragonaldistortionofthecopperandcadmiummanganitesystems.Phys StatusSolidi(b)1970;41(1):K43–6.
59.GautierJL,MezaE,SilvaE,LamasC,SilvaC.EffectoftheZnNiyMn2−yO4 (0≤y≤1)spinelcompositiononelectrochemicallithiuminsertion.JSolid StateElectrochem1997;1(2):126–33.
60.HungríaT,GalyJ,CastroA.Sparkplasmasinteringasausefultechnique tothenanostructurationofpiezo-ferroelectricmaterials.AdvEngMater
2009;11(8):615–31.
61.Chaim R, Shen Z. Grain sizecontrol by pressure application regime during spark plasma sintering of Nd-YAG nanopowders. J Mater Sci
2008;43:5023–7.
62.RajeswariK,HareeshUS,SubasriR,ChakravartyDibyendu,JohnsonR. Comparativeevaluationofsparkplasma(SPS),microwave(MWS),two stagesintering(TSS)andconventionalsintering(CRH)onthedensification andmicrostructuralevolutionoffullystabilizedzirconiaceramics.SciSinter
2010;42:259–67.
63.Lu K. Nanoparticulate materials: synthesis, characterization and processing.Wiley;2013.
64.SaljeEKH,HaywardSA,LeeWT.Ferroelasticphasetransitions:structure andmicrostructure.ActaCrystallogrA2005;61(1):3–18.
65.Sapriel J. Domain-wall orientations in ferroelastics. Phys Rev B
1975;12:5128–40.
66.BoulesteixC.Asurveyofdomainsanddomainwallsgeneratedby crys-tallographicphasetransitionscausingachangeofthelattice.PhysStatus Solidi(a)1984;86(1):11–42.
67.AizuK.Possiblespeciesofferromagnetic,ferroelectricandferroelastic crystals.PhysRevB1970;2:754–72.
68.Tagantsev AK, Cross TL.Domains in ferroic crystals and thin films. Springer;2010.
69.MengX,Baba-KishiKZ,PangGKH,ChanHL,ChoyCL,LuoHS.Tweed domainsin ferroelectricswith compositionsatthemorphotropic phase boundary.PhilMagLett2004;84(3):191–7.
70.TribaudinoM,BennaP,BrunoE.I1–I2/cphasetransitioninalkaline-earth feldspars:evidencefromTEMobservationsofSr-richfeldsparsalongthe CaAl2Si2O8–SrA12Si2O8join.AmMineral1995;80:907–15.
71.PutnisA.Introductiontomineralsciences.CambridgeUniversityPress; 1992.
72.PriesterL.Lesjointsdegrainsdelathéorieàl’ingénierie.EDPSciences; 2006.