CO
2CAPTURE USING
ALKANOLAMINE/ROOM-TEMPERATURE
IONIC LIQUID BLENDS
Absorption, Regeneration, and Corrosion Aspects
Thèse
Muhammad Hasib-ur-Rahman
Doctorat en génie chimique
Philosophiae Doctor (Ph.D.)
Québec, Canada
iii
Résumé
Le réchauffement climatique, résultant essentiellement des émissions anthropiques de dioxyde de carbone, demeure un sujet de grande préoccupation. Le captage et la séquestration du dioxyde de carbone est une solution viable permettant de prévoir une baisse des émissions de CO2 issues des importantes sources ponctuelles qui impliquent la
combustion des carburants fossiles. Dans cette perspective, les systèmes aqueux d‟alcanolamines offrent une solution prometteuse à court terme pour la capture du CO2
dans les installations de production d'électricité. Cependant, ces systèmes sont confrontés à divers accrocs opératoires tels que les limitations d‟équilibre, les grandes quantités d‟énergie requises pour la régénération, les pertes en solvant et la corrosion prononcée des installations, pour ne citer que ces quelques inconvénients. L‟eau étant la principale cause de ces complications, une mesure à prendre pourrait être le remplacement de la phase aqueuse par un solvant plus stable.
Les liquides ioniques à température ambiante, dotés d‟une haute stabilité thermique et pratiquement non-volatils émergent en tant que candidats prometteurs. De plus, grâce à leur nature ajustable, ils peuvent être apprêtés conformément aux exigences du procédé. La substitution de la phase aqueuse dans les processus utilisant l‟alcanolamine par les liquides ioniques à température ambiante ouvre une opportunité potentielle pour une capture efficace du CO2. Un aspect remarquable de ces systèmes serait la cristallisation du produit
résultant de la capture du CO2 (c-à-d, le carbamate) au sein même du liquide ionique qui
non seulement déjouerait les contraintes d‟équilibre mais également pourvoirait une opportunité intéressante pour la séparation des produits.
Étant donné le peu d‟information disponible dans la littérature sur la viabilité des systèmes utilisant la combinaison d‟amine et de liquide ionique, l‟étude proposée ici a pour but d‟apporter une meilleure compréhension sur l‟efficacité à séparer le CO2 d‟un mélange de
type postcombustion à travers une approche plus systématique. À cet effet, des liquides ioniques à base d‟imidazolium ([Cnmim][Tf2N], [Cnmim][BF4], [Cnmim][Otf]) ont été
iv
diéthanolamine (DEA) ont été examinées en détail afin d‟explorer la capture du CO2 et les
possibilités de régénération qu‟offre un système amine-liquide ionique. Les résultats ont révélé l‟intérêt de la combinaison DEA-liquide ionique étant donné que ce système pourrait aider à réduire de manière significative l‟écart entre les températures d‟absorption et de régénération, promettant ainsi une perspective attrayante en termes d‟économie d‟énergie. En outre, les liquides ioniques ont également été scrutés du point de vue de leur nature hydrophobe/hydrophile afin d‟étudier le comportement corrosif du mélange amine-liquide ionique au contact d‟échantillons d‟acier au carbone. Bien que l‟utilisation des liquides ioniques hydrophiles ait aidé à abaisser la vitesse de corrosion jusqu‟à concurrence de 72%, l‟emploi de liquides ioniques hydrophobes s‟avère plus efficace, car annulant quasiment le phénomène de corrosion même dans un environnement riche en CO2.
Dans le cas des mélanges immiscibles comme DEA-[hmim][Tf2N], une agitation continue
s‟avère nécessaire afin d‟assurer une dispersion prolongée des gouttelettes d‟amine émulsifiées au sein de liquides ioniques et ainsi atteindre une vitesse de capture optimale.
v
Abstract
Global warming, largely resulting from anthropogenic emissions of carbon dioxide, continues to remain a matter of great concern. Carbon capture and storage (CCS) is a viable solution to ensure a prevised fall in CO2 emissions from large point sources involving fossil
fuel combustion. In this context, aqueous alkanolamine systems offer a promising near-term solution for CO2 capture from power generation facilities. However, these face several
operational hitches such as equilibrium limitations, high regeneration energy requirement, solvent loss, and soaring corrosion occurrence. The main culprit in this respect is water and, accordingly, one feasible practice may be the replacement of aqueous phase with some stable solvent.
Room-temperature ionic liquids (RTILs), with high thermal stability and practically no volatility, are emerging as promising aspirants. Moreover, owing to the tunable nature of ionic liquids, RTIL phase can be adapted in accordance with the process requirements. Replacing aqueous phase with RTIL in case of alkanolamine based processes provided a potential opportunity for efficient CO2 capture. The most striking aspect of these schemes
was the crystallization of CO2-captured product (carbamate) inside the RTIL phase that not
only helped evade equilibrium constraints but also rendered a worthy opportunity of product separation.
Since there is little information available in the literature about the viability of amine-RTIL systems, the proposed research was aimed at better understanding CO2 separation
proficiency of these fluids through a more systematic approach. Imidazolium RTILs ([Cnmim][Tf2N], [Cnmim][BF4], [Cnmim][Otf]) were chosen for this purpose. Two
alkanolamines, 2-amino-2-methyl-1-propanol (AMP) and diethanolamine (DEA) were examined in detail to explore CO2 capture and regeneration capabilities of amine-RTIL
systems. The results revealed the superiority of DEA-RTIL combination as this scheme could help significantly narrow the gap between absorption and regeneration temperatures thus promising a sparkling prospect of attenuating energy needs. Furthermore, ionic liquids were scrutinized in reference to their hydrophobic/hydrophilic nature to study the corrosion behaviour of carbon steel in amine-RTIL media. Though hydrophilic ionic liquids helped
vi
decrease corrosion occurrence up to 72%, hydrophobic RTIL appeared to be the most effective in this regard, virtually negating the corrosion phenomenon under CO2 rich
environment.
In case of immiscible blends like DEA-[hmim][Tf2N], continual agitation appeared to be a
necessity to ensure a prolonged dispersion of amine in the RTIL phase and, thereby, to attain an optimal capture rate.
vii
Foreword
This PhD thesis has been divided into five chapters. The first chapter comprises the introductory portion and it also contains a short published review [1], merged and modified in accordance with the context of the “INTRODUCTION and OBJECTIVES” section. Immediately after, four research articles (listed below) follow, each presented as a separate chapter (Chapters 2-5). Out of these, three research articles ([2] to [4]) were already published while the last one [5] is under review for publication in Separation and Purification Technology journal. At the end, as „Appendix D‟, a short essay about corrosion perspective regarding amine-based CO2 capture systems (i.e. aqueous amines and
amine-ionic liquid blends) has been attached that we published in „Carbon Capture Journal‟ [6]. [1] M. Hasib-ur-Rahman, M. Siaj, F. Larachi, Ionic Liquids for CO2 Capture -
Development and Progress, Chem. Eng. Process. 49 (2010) 313-322.
[2] M. Hasib-ur-Rahman, M. Siaj, F. Larachi, CO2 Capture in
Alkanolamine/Room-Temperature Ionic Liquid Emulsions: A Viable Approach with Carbamate Crystallization and Curbed Corrosion Behavior, Int. J. Greenhouse Gas Control 6 (2012) 246-252. [3] M. Hasib-ur-Rahman, H. Bouteldja, P. Fongarland, M. Siaj, F. Larachi, Corrosion Behavior of Carbon Steel in Alkanolamine/Room-Temperature Ionic Liquid based CO2
Capture Systems, Ind. Eng. Chem. Res. 51 (2012) 8711-8718.
[4] M. Hasib-ur-Rahman, F. Larachi, CO2 Capture in Alkanolamine-RTIL Blends via
Carbamate Crystallization: Route to Efficient Regeneration, Environ. Sci. Technol. 46 (2012) 11443-11450.
[5] M. Hasib-ur-Rahman, F. Larachi, Kinetic Behavior of Carbon Dioxide Absorption in Diethanolamine/Ionic-Liquid Emulsions, Sep. Purif. Technol. Submitted February 2013. [6] M. Hasib-ur-Rahman, F. Larachi, Corrosion in amine systems – a review, Carbon Capture Journal, Sept - Oct 2012, 22-24.
viii
The research papers were prepared on my own and revised by my director, Prof. Faïçal Larachi. Prof. Larachi guided and provided expertise in designing experiments and managing data analysis during the entire research work.
Prof. Mohamed Siaj, my co-director from Department of Chemistry at Université du Québec à Montréal, facilitated through his productive recommendations on the characterization of CO2-captured products (carbamates) and helped correct the manuscripts
of first three publications.
Ms. Hana Bouteldja, co-author of the paper entitled, “Corrosion Behavior of Carbon Steel in Alkanolamine/Room-Temperature Ionic Liquid based CO2 Capture Systems”, helped
perform the corrosion experiments and was involved in configuring the Chittick technique for CO2 loading measurements. In this regard, Prof. Pascal Fongarland (supervisor of Ms.
Hana Bouteldja, and also co-author of the published work) from Ecole Centrale de Lille, Unité de Catalyse et Chimie du Solide, France, provided some fruitful suggestions.
Some of the research outcomes were presented in the following conferences:
M. Hasib-ur-Rahman, H. Bouteldja, A.N. Khan Wardag, A. Sarvaramini, G.P. Assima, M. Siaj, F. Larachi, Advances towards adept biomass gasification and efficient carbon dioxide capture processes, CQMF 4th Annual Symposium at Duchesnay, Quebec, 2011.
M. Hasib-ur-Rahman, M. Siaj, F. Larachi, CO2 Capture in
Alkanolamine/Room-Temperature Ionic Liquid Emulsion System, 61st Canadian Chemical Engineering Conference, London ON, 2011.
M. Hasib-ur-Rahman, M. Siaj, F. Larachi, Corrosion inhibition in alkanolamine/room-temperature ionic liquid based CO2 capture systems, CAMURE
8 & ISMR 7, Naantali, Finland, 2011.
M. Hasib-ur-Rahman, M. Siaj, F. Larachi, Alkanolamine/Ionic Liquid Microemulsions for Efficient CO2 Capture with Diminished Corrosion
ix Phenomenon”, CQMF 3rd Annual Symposium at Centre d'arts Orford, Orford QC, 2010.
M. Hasib-ur-Rahman, M. Siaj, F. Larachi, Alkanolamine/Ionic Liquid Microemulsions: Enhanced CO2 Capture Ability with Curbed Corrosion Behaviour,
CIGR World Congress, Québec QC, 2010.
M. Hasib-ur-Rahman, M. Siaj, F. Larachi, CO2 capture by alkanolamine/ionic liquid
microemulsion system equipped with micro-fluidic channels detector for in-situ screening of the process, CQMF 2nd Annual Symposium at UQÀM, Montréal QC, 2009.
xi
Acknowledgements
Firstly my sincere appreciation goes to my affectionate parents for their kind support and encouragement during the whole of my life. Also, I am overwhelmed with gratitude for the consistent support of my loving wife during the challenging times of my Ph.D. studies. Thank you so much.
This thesis would not be in good shape without the inspirational attitude of my siblings who wisely advised in the final stretch of my education.
I would like to express my heartfelt gratitude to my supervisor, Prof. Faïçal Larachi, for his ample guidance and help during the course of my Ph.D. through his unique thought-provoking, supportive, and composed approach. I hope that I can pass on the research values that he has given to me.
My co-director, Prof. Mohamed Siaj, is gratefully acknowledged for his support and many insightful suggestions during the project progression.
I express my gratitude to Prof. Denis Rodrigue and Prof. Maria-Cornélia Iliuta for letting use their analytical facilities.
I would also like to thank my examiners, Dr. Sylvie Fradette, Prof. Alain Garnier, and Prof. Louis Fradette, who provided constructive feedback. It is no easy task, reviewing a thesis, and I am grateful for their thoughtful comments.
It had been a great privilege to spend some fruitful years of my M.Phil. studies under the guidance of Prof. Muhammad Mazhar and Dr. Syed Tajammul Hussain, at Quaid-i-Azam University in Pakistan, who enabled me to contemplate this road. I could not have asked for better role models, each inspirational and supportive.
Thank you my friends and colleagues. You were the sources of laughter, joy, and encouragement, bearing the brunt of frustrations and sharing the joy of successes. The help of the chemical engineering department technical staff, Jérome Noël, Marc Lavoie, Yann Giroux, and Jean-Nicolas Ouellet during this research project is also appreciated.
xii
Finally, I acknowledge the financial support of Fonds de recherche du Québec – Nature et technologies (FRQNT), F. Larachi Canada Research Chair “Green processes for cleaner and sustainable energy”, the Centre québécois sur les matériaux fonctionnels (CQMF), and the Discovery Grants to F. Larachi and M. Siaj from the Natural Sciences and Engineering Research Council (NSERC).
xiii
Table of contents
Résumé ... iii Abstract ... v Foreword ... vii Acknowledgements ... xiList of figures ... xvii
List of tables ... xxi
Chapter 1: Introduction and Objectives ... 1
1.1. Background ... 1
1.2. Carbon dioxide capture through solvent scrubbing ... 2
1.2.1. Chemical solvents ... 2
1.2.2. Degradation of amines ... 4
1.2.3. Corrosion of equipment ... 8
1.2.4. Corrosion inhibition ... 9
1.3. Physical Solvents... 10
1.4. Ionic Liquid Solvents ... 11
1.5. Ionic liquids for CO2 capture - Development and progress ... 11
1.5.1. Introduction ... 13
1.5.2. CO2 capture by room-temperature ionic liquids (RTILs) ... 13
1.5.3. CO2 capture by task-specific ionic liquids (TSILs) ... 21
1.5.4. CO2 capture by supported ionic-liquid membranes (SILMs) ... 23
1.5.5. CO2 capture by polymerized ionic liquids ... 27
1.5.6. Toxicity of ILs... 28
1.5.7. Current and future developments ... 29
1.6. Research Objectives ... 32
1.7. References ... 35
Chapter 2: CO2 capture in alkanolamine/room-temperature ionic liquid emulsions: A viable approach with carbamate crystallization and curbed corrosion behavior ... 45
2.1. Introduction ... 45
2.2. Experimental ... 47
2.2.1. Materials and techniques ... 47
xiv
2.2.3. Electrochemical corrosion tests ... 48
2.3. Results and discussion ... 49
2.3.1. Fate of CO2-captured product (carbamate) ... 49
2.3.2. CO2 absorption ... 50
2.3.3. Characterization of crystalline product ... 53
2.3.4. Corrosion studies ... 57
2.4. Conclusions ... 61
2.5. References... 61
Chapter 3: Corrosion behaviour of carbon steel in alkanolamine/room-temperature ionic liquid based CO2 capture systems ... 65
3.1. Introduction ... 66
3.2. Experimental ... 67
3.2.1. Materials ... 67
3.2.2. Experimental techniques and procedure ... 68
3.3. Results and Discussion ... 70
3.3.1. Effect of amine type on corrosion of steel ... 73
3.3.2. Effect of RTIL type on corrosion behaviour ... 76
3.3.3. Effect of process temperature ... 79
3.3.4. Effect of gas loading ... 81
3.3.5. Presence of oxygen ... 82
3.3.6. Influence of water ... 84
3.4. Conclusion ... 85
3.5. References... 86
Chapter 4: CO2 capture in alkanolamine-RTIL blends via carbamate crystallization: route to efficient regeneration ... 89
4.1. Introduction ... 90
4.2. Experimental ... 93
4.2.1. Materials ... 93
4.2.2. Procedures and techniques ... 94
4.3. Results and Discussion ... 95
4.3.1. Maximum gas capture capacity ... 95
4.3.2. Nature of CO2-captured products ... 97
4.3.3. Regeneration ability ... 98
xv
4.4. Implications ... 106
4.5. References ... 107
Chapter 5: Kinetic behavior of carbon dioxide absorption in diethanolamine/ionic-liquid emulsions ... 111
5.1. Introduction ... 112
5.2. Reaction mechanism in non-aqueous amines ... 113
5.3. Experimental ... 114
5.3.1. Materials ... 114
5.3.2. Setup ... 114
5.3.3. Procedure ... 115
5.4. Results and Discussion ... 116
5.4.1. Impact of variation in amine concentration ... 117
5.4.2. CO2 volume ratio in the gaseous mixture ... 119
5.4.3. Influence of agitation speed ... 121
5.4.4. Effect of temperature variation ... 122
5.5. Conclusion ... 123
5.6. References ... 124
Chapter 6: Conclusions and recommendations ... 129
6.1. General conclusions ... 129
6.2. Future work recommendations ... 131
Appendix A: Supporting Information (Chapter 2) ... 133
Appendix B: Supporting Information (Chapter 3) ... 143
Appendix C: Supporting Information (Chapter 4) ... 149
xvii
List of figures
Figure 1. 1. Reaction scheme including reactions between amine, CO2/carbonate and protons. ... 3
Figure 1. 2. Oxidative degradation mechanism of aqueous MEA. ... 4
Figure 1. 3. Amine degradation: ■ thermal; ■ CO2 induced. ... 6
Figure 1. 4. Effect of SO2 on MEA degradation. ... 7
Figure 1. 5. Effect of corrosion inhibitor (NaVO3) on MEA degradation. ... 7
Figure 1. 6. Effect of various parameters on the corrosion rate of carbon steel C1020 in aqueous MEA (basal conditions: MEA conc. 5 kmol/m3; gas loading 0.4 mol CO 2/mol MEA; 80 °C temperature). ... 9
Figure 1. 7. Some cations and anions constituting ionic liquids (ILs). ... 13
Figure 1. 8. Solubilities of CO2, C2H4, C2H6, CH4, Ar and O2 in [bmim][PF6] at 25 °C... 14
Figure 1. 9. CO2 solubility in [emim][Tf2N] and [emim][PF6]. ... 17
Figure 1. 10. Proposed mechanism for chemical absorption of CO2 by the TSIL. ... 19
Figure 1. 11. [hmim][Tf2N]-MEA solution: (a) fresh sample; (b) on CO2 exposure; showing precipitated MEA-carbamate. ... 20
Figure 1. 12. Proposed mechanism for CO2 capture by [pabim][BF4]. ... 21
Figure 1. 13. Molar CO2 loads in solvent volume (for MEA/MDEA, consider aqueous solution volume): data for ionic liquids at 30 °C [50]; data for MEA and MDEA at 40 °C... 23
Figure 1. 14. Proposed setup for CO2 separation by SILM in a coal-fired power plant. ... 24
Figure 1. 15. Proposed mechanisms of CO2 capture: (a, b) without water; (c) with water. ... 25
Figure 2. 1. DEA/RTIL system: (a-c) (without surfactant) after CO2 capture; d) (with surfactant) before and after CO2 capture. ... 50
Figure 2. 2. CO2 capture capacity profiles of DEA/RTIL system (surfactant stabilized emulsions; 30% w/w) at atmospheric pressure and 25 °C. ... 51
Figure 2. 3. CO2 absorption isotherms for DEA/[hmim][Tf2N] surfactant stabilized emulsions obtained at 25°C. ... 52
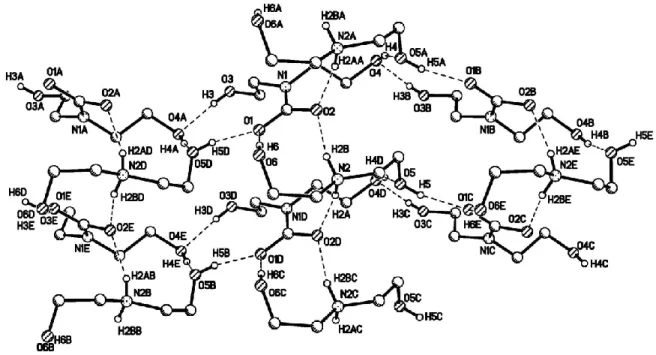
Figure 2. 4. Basic structural unit in DEA-carbamate (C9H22N2O6) crystal. ... 54
Figure 2. 5. Hydrogen bonding pattern in the compound (DEA-carbamate). H atoms not participating in hydrogen bonding are omitted for clarity. ... 55
Figure 2. 6. 13C NMR spectrum of crystalline carbamate (retaining traces of [hmim][Tf 2N]) taken in DMSO-d6 solvent. ... 56
xviii
Figure 2. 8. Tafel plots for carbon steel electrode in aqueous DEA under different environments: a)
CO2 bubbling at 25 °C, b) CO2+O2 bubbling at 25 °C, c) CO2 bubbling at 60 °C, d) CO2+O2
bubbling at 60 °C. ... 58
Figure 2. 9. Corrosion rate of carbon steel 1020 in: a) RTIL pure, CO2+O2+H2O(vap.) bubbling at 60 °C, b) DEA/RTIL emulsion, CO2+O2+H2O(vap.) bubbling at 60 °C, c) DEA(aq), CO2+O2 bubbling at 60 °C. ... 60
Figure 2. 10. SEM micrographs of working electrode specimen. In DEA/RTIL emulsion (15%
w/w): a) Fresh surface; b) after electrochemical corrosion test. In DEAaq. (15% w/w): c) Fresh surface; d) after electrochemical corrosion test. ... 60
Figure 3. 1. Experimental setup for electrochemical corrosion tests. ... 69 Figure 3. 2. MEA-RTIL fluid showing solid carbamate, after CO2 bubbling: 1) MEA+[bmim][BF4]; 2) MEA+[emim][BF4]; 3) MEA+[emim][Otf]. ... 71
Figure 3. 3. Thermogravimetric evolution of CO2 absorption for MEA-RTIL mixtures (MEA: 5 kmol/m3) at 25 °C. ... 72
Figure 3. 4. SEM micrographs of steel electrode surface before and after electrochemical
polarization runs at 25 °C under CO2(15%)+O2(5%)+N2 atmosphere in: a) MEA (aqueous); b) MEA+[bmim][BF4]; c) MEA+Water+[bmim][BF4]. ... 73
Figure 3. 5. Linear polarization curves of carbon steel 1020 at 25 °C: a) in aqueous alkanolamines;
b) in alkanolamine+[bmim][BF4] mixtures. ... 74
Figure 3. 6. Effect of RTIL type on polarization behavior of carbon steel 1020 at 25 °C. ... 76 Figure 3. 7. EDX analysis of steel electrode surface: a) freshly polished surface; b,c,d) after
electrochemical corrosion tests in MEA+[bmim][BF4], MEA+[emim][BF4], MEA+[emim][Otf] blends respectively. ... 79
Figure 3. 8. Comparison of temperature effect on steel corrosion in aqueous as well as RTIL based
media. ... 80
Figure 3. 9. EDX scan of steel electrode surface after electrochemical corrosion test in
MEA+[bmim][BF4] at 60 °C. ... 80
Figure 3. 10. CO2 loading effect on steel corrosion at 25 °C in a) aqueous MEA b)
MEA+[bmim][BF4] mixture. ... 82
Figure 3. 11. Effect of O2 concentration in flue gas on corrosion of steel a) in aqueous MEA; b) in MEA+[bmim][BF4] mixture. ... 83
Figure 3. 12. Effect of water content in CO2 capture medium on corrosion of steel. ... 84
Figure 4. 1. The simplified process flow diagram of alkanolamine-RTIL based CO2 capture
xix
Figure 4. 2. CO2 absorption isotherm for alkanolamine-[hmim][Tf2N] systems obtained at
atmospheric pressure and 35 °C temperature. ... 96
Figure 4. 3. Evaporation profiles of amines (in amine-RTIL blends) at 35 °C under N2. ... 96
Figure 4. 4. Packing diagrams: a) AMP-carbamate; b) DEA-carbamate. ... 97 Figure 4. 5. a) FTIR spectra, and b) 13C NMR spectra: AMP (fresh amine), AMPC
(AMP-carbamate) and RAMP (regenerated AMP). ... 100
Figure 4. 6. a) FTIR spectra, and b) 13C NMR spectra of DEA (fresh amine), DEAC
(DEA-carbamate) and RDEA (regenerated DEA). ... 101
Figure 4. 7. DSC/TG profiles of AMP-carbamate: Thermal behavior observed under N2 atmosphere at heating rate of 5 °C/min. ... 102
Figure 4. 8. DSC/TG curves of DEA-carbamate: Thermal behavior under N2 atmosphere, using heating rate of 5 °C/min. ... 103
Figure 4. 9. QMS monitoring of carbamates‟ decomposition by measuring positive ion current m/z
= 44 (CO2) under N2 atmosphere (100 mL/min. flow rate) at 5 °C/min heating rate. ... 104
Figure 4. 10. TG profiles of carbamates: Thermal behavior under CO2 atmosphere, using heating rate of 5 °C/min. ... 106
Figure 5. 1. Experimental set-up scheme: A) Gas inlet; B) Gas outlet (A & B connect to a gas
reservoir via closed loop system); C) CO2 probe; D) Injection port; E) Thermocouple; F) Rotor-stator homogeniser; G) Absorption cell; Hi) Heating bath inlet; Ho) Heating bath outlet. ... 115
Figure 5. 2. CO2-captured product (carbamate) precipitation in DEA-[hmim][Tf2N]: a) immediately after CO2 bubbling; b) 24 hours later. ... 116
Figure 5. 3. Influence of [DEA] molar concentration on absorption rate with respect to initial CO2 vol% in the gaseous mixture, at 33 °C and 3000 rpm agitation speed: a) 2M DEA in [hmim][Tf2N]; b) 1M DEA in [hmim][Tf2N]; c) 0.5M DEA in [hmim][Tf2N]. Smoothed lines show trends. ... 119
Figure 5. 4. Influence of initial CO2 volume ratio (in gaseous mixture) on absorption rate w.r.t. [DEA], at 33 °C and 3000 rpm agitation speed: a) 10 vol% CO2; b) 5 vol% CO2; c) 2.5 vol% CO2. Smoothed lines show trends. ... 121
Figure 5. 5. Influence of agitation on CO2 absorption rate (2M DEA in [hmim][Tf2N]; 10 vol% CO2; 33 °C). Smoothed lines show trends. ... 122
Figure 5. 6. Effect of temperature on CO2 capture rate (1M DEA in [hmim][Tf2N]; 10 vol% CO2; 3000 rpm). Smoothed lines show trends. ... 123
xxi
List of tables
Table 1. 1. Initial rates of oxidative degradation of MEA under different operating conditions. ... 5
Table 1. 2. Toxicity of absorption solvents and conventional inhibitors. ... 10
Table 1. 3. Physical solvent processes. ... 11
Table 1. 4. Henry‟s constants (bar, at 25 °C) for gases in different organic solvents. ... 15
Table 1. 5. Henry‟s constants for CO2 in different ionic liquids. ... 15
Table 1. 6. Henry‟s law constants of CO2 in ionic liquids. ... 16
Table 1. 7. CO2 solubility data in [emim][MDEGSO4]. ... 18
Table 1. 8. Viscosity values for different compositions of tri-iso-butyl(methyl)phosphonium tosylate/water mixtures. ... 19
Table 1. 9. Viscosities and water content of the ionic liquids, at 25 °C. ... 26
Table 1. 10. Summary of gas absorption capacities (at 592.3 mmHg & 22 °C) and glass transition temperatures of poly(ionic liquid)s. ... 27
Table 1. 11. Permeability, solubility and diffusivity values in: a) styrene-based poly(ionic liquid)s; b) acrylate-based poly(ionic liquid)s, at 20 °C. ... 28
Table 1. 12. Lethal concentrations (LC50) of different ionic liquids to fresh water snail (Physa acuta) in 96-hour acute toxicity exposures. ... 29
Table 1. 13. Summary of CO2 capture by ionic liquids. ... 31
Table 2. 1. Density (ρ) and viscosity (η) values measured at 25 °C. ... 50
Table 2. 2. Crystallographic data ... 53
Table 2. 3. Relevant hydrogen bonding parameters [bond distances (Å) and angles (°)]. ... 54
Table 2. 4. Corrosion rates of carbon steel 1020 ... 59
Table 3. 1. Summary of process parameters/conditions. ... 67
Table 3. 2. Viscosity values of the ionic liquids used. ... 72
Table 3. 3. Effect of amine type on corrosion parameters at 25 °C. ... 75
Table 3. 4. Effect of RTIL type on corrosion rate of carbon steel 1020 at 25 °C. ... 76
Table 3. 5. Effect of process temperature on corrosion rate of carbon steel 1020. ... 81
Table 3. 6. Corrosion rate of steel in aqueous MEA and MEA+[bmim][BF4] blends at different CO2 loadings and 25 °C. ... 82
Table 3. 7. Effect of oxygen presence/absence on corrosion rate of carbon steel 1020 at 25 °C. .... 84
Table 3. 8. Influence of water content in the gas capture fluid on corrosion of steel at 25 °C. ... 85
1
Introduction and Objectives
1.1. Background
In the energy era driven by greenhouse gas (predominantly CO2) constraints, there are
mounting concerns over the alarming situation of global warming phenomenon, being intensified brusquely by anthropogenic activities. Fossil fuel based power plants are the largest among the stationary sources, accounting for approximately 78.6% whereas refineries and oil & gas processing facilities share about 6.34% of total carbon dioxide emissions. The Intergovernmental Panel on Climate Change (IPCC) perceives that by the year 2100 there may be a rise of 1.9 °C in the global temperature [1,2]. This has turned carbon dioxide capture and sequestration into an extensively investigated topic nowadays. By and large, there are three major approaches for CO2 capture: chemical/physical
absorption/adsorption; membrane separation, and cryogenic distillation [3]. Cryogenic distillation, being exceedingly expensive, is not considered feasible regarding the flue gas purification. Though absorption processes involving chemical solvents (often aqueous alkanolamines) are being used widely, these put forth a number of limitations that include insufficient capture capacity, evaporation/degradation of costly reagents and thermal stability problems, equipment corrosion and high energy consumption during regeneration. Likewise, physical solvents such as methanol, poly(ethylene glycol) dimethyl ether, (as well as membrane technology), require higher concentrations of the acid gas in the feed stream at elevated pressures and lower temperatures. For natural gas sweetening and post-combustion capture, physical solvents and membranes may not be the efficient tools due to small concentrations of CO2 and ambient pressures [1,4]. All these discrepancies have to be
overcome and replaced by more efficient and less costly systems.
Ionic liquids (ILs) are being proposed as an alternative for CO2 capture with special
emphasis on their stability, tunabe chemistry, and negligible volatility with considerable CO2 solubility [5]. Just like common physical solvents, these necessitate feed gas at high
pressure. To surpass the efficiency of industrially well-established alkanolamines systems, researchers are investigating the abilities of functionalized ionic liquids in bulk form or
2
through supportive membranes. Nevertheless, to take advantage of CO2 capture capabilities
of both ionic liquids and alkanolamines, combinations of these two might be a better option. However, to cope with the problem of high viscosities of these ionic liquid fluids, supported ionic liquid membranes or polymerized ionic liquids are also being probed as an alternative mechanism.
1.2. Carbon dioxide capture through solvent scrubbing 1.2.1. Chemical solvents
Large point sources of CO2 include fossil fuel-based power/hydrogen production plants,
synthetic fuel industries and natural gas production facilities. Use of a gas capture process depends on the concentration/partial pressure of CO2 in the feed gas. Natural gas
processing and post combustion capture, where CO2 concentrations are in the range of 2 -
65 vol% and 3 - 15 vol% respectively, mainly involves well established amine based systems [1,6]. While pre-combustion capture employ physical solvent scrubbers where CO2
is present in proportions greater than 15% under appreciably high pressure. Amines that gained much consideration in CO2 capture include monoethanolamine (MEA),
diethanolamine (DEA), and methyldiethanolamine (MDEA). In addition to the above stated alkanolamines, there are certain propriety formulations composed of aqueous solutions of blended amines along with certain additives like corrosion inhibitors, buffers, foam depressants, etc [7,8].
Primary/secondary alkanolamines capture CO2 through carbamate formation at lower
temperatures (~ 40 °C) and stripped at higher temperature (≥100 °C). The most accepted reaction mechanism (1.1) was proposed by Caplow [9].
2 2 2 2 2 2 2 CO RNH RNH CO RNH CO B RNHCO BH (1.1)
Since amides are very weak bases, their protonation in aqueous media is not considered favourable. So the zwitterions concentration should be insignificant.
A more rational mechanism (given below) considering direct interaction of amine with CO2
3 2 2 2 2 2 CO RNH RNHCO H RNHCO H RNHCO H (1.2)
For aqueous amine solutions used in gas capture systems, the above mechanisms alone are not sufficient. Interactions of amine with carbonic acid, bicarbonate/carbonate species (Figure 1.1) have to be considered while evaluating the process efficiency.
Figure 1. 1. Reaction scheme including reactions between amine, CO2/carbonate and
protons (reproduced from [10]).
One of the negative aspects of primary/secondary alkanolamines is their low CO2 loading
capacity (~50 mol%). Tertiary amines like MDEA possess double the loading capacity (~100 mol%). However, as tertiary alkanolamines have no labile hydrogen, carbamate formation is not possible and the feasible hydrolytic mechanism (1.3) is less favourable kinetically. Most propriety industrial chemical solvents include both primary/secondary and tertiary alkanolamines (blended amines) in order to enhance the capture capacity [11, 12].
2 2 3 1 2 3 1 2 3 ( ) CO aq H O HCO H R R R N H R R R NH (1.3) RNHCO2H RNHCO2ˉ H2CO3 HCO3ˉ CO2 (aq) +H 2 N R -H2 O + H2 O -H2 N R +H 2 N R -H2 O + H2 O -H2 N R H+ H+ +H2NR -H2NR +H2O -H2O +OHˉ -OHˉ CO32ˉ H+ pH RNH3+ H RNH2 +
4
In spite of the industrial importance of aqueous amine systems in acid gas capture processes; these pose a number of drawbacks including:
Equilibrium limitations
Amine evaporation/degradation Corrosion of equipment
High regeneration costs
CO2 capture facility increases the energy consumption up to 50%, mostly consumed in
solvent regeneration, thus greatly reducing the power plant efficiency [1].
1.2.2. Degradation of amines
During recycling of amine-based CO2 absorption systems, one of the major causes of amine
losses is degradation phenomenon that not only causes reduction in gas capture capacity but also boosts the corrosion of the equipment and adds to the toxicity of the environment. Most alkanolamines, especially MEA, degrades quite fast in the presence of oxygen (Figure 1.2) [13]. Degradation phenomenon results in loss of almost 2.2 kg of MEA per tonne of CO2 captured. Thus disposal and make-up of the degraded solvent considerably heave the
costs [14]. CH2CH2OH :N H H Fe3+ CH2CH2OH +.N H H C CH2OH :N H H H Fe3+ CH CH2OH :NH CH CH2OH :N H H OO MEA CH CH2OH :N H H OO H H2O H2O CH O H 2
+
NH3 CH O CH2 O H NH3+
Imine :NH CH CH2OH Imine Peroxide Peroxide Radical O2 -H+ Hydroxyacetaldehyde Formaldehyde Imine Radical Aminium Radical MEA H2O -H2O25 No single mechanism can be used to generalize the degradation phenomenon of alkanolamines under different operating conditions. This is quite obvious from the analysis of degradation products at different temperatures. At 100 °C, 1,2-ethanediol, 1,3,5-triazine, N-butylformamide, 1,4,7,10,13,16-hexaoxacyclooctadecane and 1,2,3,6-tetrahydro-1-nitropyridine are the degradation products while at 120 °C, methylpyrazine, 7-oxabicyclo[2.2.1]hept-5-en-2-one, 1-propanamine, ethylamine, 1,3,5-triazine, and 3,3-(1,2-ethanediyl)bis(syndone) are obtained in case of MEA-H2O-O2-CO2 system [13].
Degradation of amines results in the production of heat stable salts that are impossible to regenerate under the prevailing conditions of solvent regeneration in gas capture unit. Increase in temperature, O2 partial pressure, MEA initial concentration, and CO2 loading
significantly raise the rate of oxidative degradation (Table 1.1) [15].
Table 1. 1. Initial rates of oxidative degradation of MEA under different operating conditions.*
Initial rate [kmol/(hm3)]
Temperature (°C)
Initial MEA concentration (kmol/m3) O2 concentration (mol/m3) 0.044 160 2 3.994 0.065 160 3 3.994 0.082 160 4 3.994 0.208 160 11 3.994 0.104 170 3 4.293 0.117 170 4 4.293 0.380 170 8 4.293 0.431 170 10 4.293 0.007 120 4 3.154 0.056 140 4 3.500 0.070 170 3 3.305 *adapted from [15]
CO2 is also detrimental to alkanolamines as higher gas loading is found to increase the
degradation process. Studies have shown resemblance among MEA, DEA, and MDEA degradation in the presence of CO2; amines, oxazolidinones and imidazolidinones being the
main products. Thermal degradation phenomenon is negligible compared to CO2/O2
6
Figure 1. 3.Amine degradation: ■ thermal; ■ CO2 induced (adapted from [16]).
Certain other impurities like SO2, in the flue gas also have adverse effect on amine
degradation (Figure 1.4). Corrosion inhibitors used in amine systems also found to trigger solvent degradation, see Figure 1.5 [17].
0 10 20 30 40 50 60 70 80 90 100 0.34 951 1.36 89 2.33 01 3.34 95 4.33 98 5.35 92 6.34 95 7.33 98 8.33 01 9.34 95 10.34 11.33
■Thermal degradation: 4 mol.kg-1 amine, 140 °C, 15 days
■ CO2 induced degradation: 4 mol.kg-1 amine, 140 °C, 15 days, 2MPa CO2
(%) M DE A DMAE AMP M EA MAE DEA HE EDA DMP TM EDA N,N -d iM EDA N,N,N ’-t riM EDA N,N’ -d iM EDA
7
Figure 1. 4.Effect of SO2 on MEA degradation [17].
Figure 1. 5. Effect of corrosion inhibitor (NaVO3) on MEA degradation [17]. 0 0.001 0.002 0.003 0.004 0.005 0.006 0.007 0 50 100 150 Time (hr) R at e of de gra da ti on ( m ol /L. hr) 6% O2 6% O2 + 6 ppm SO2 6% O2 + 11 ppm SO2 0 1 2 3 4 5 6 0 50 100 150 200 250 Time (hr) M E A c on ce nt ra ti on ( m ol /L) with NaVO3 without NaVO3
8
Moreover, regeneration of alkanolamine to enable smooth recycling is not cost effective. Among alkanolamines, MEA is the most efficient CO2 capture agent with high CO2
-captured product (carbamate) stability which correspondingly accounts for high energy requirements during regeneration, a major drawback leading to hiking costs of the process [18].
1.2.3. Corrosion of equipment
All amine treating plants face corrosion problems. Bottom of absorbers and regenerators, pumps and valves are more vulnerable to corrosion due to high gas loading and elevated temperatures. Corrosion is also a major concern in the safety of plants, causing weakening of the equipment that may lead to explosion of pressure vessels. High CO2 loading,
increased concentration of amine/O2, elevated temperatures and higher solution velocities
all cause corrosion rate to accelerate (Figures 1.6). Corrosion is the result of anodic (iron dissolution) and cathodic (reduction of oxidizers present in the solution) electrochemical reactions [19]. Dissociation of water/protonated amine, hydrolysis of CO2 and amine
regeneration reactions provide oxidizing species enabling corrosion process to continue [20]. Most significant anodic/cathodic reactions leading to corrosion are:
2 2 3 3 2 2 2 2 2 2 2 2 2 2 Fe Fe e HCO e CO H H O e OH H (1.4)
9
Figure 1. 6. Effect of various parameters on the corrosion rate of carbon steel C1020 in aqueous MEA (basal conditions: MEA conc. 5 kmol/m3; gas loading 0.4 mol CO2/mol
MEA; 80 °C temperature) [19].
Other impurities like SO2 also gear up wear and tear of the equipment [21]. Presence of
SO2 speeds up the corrosion phenomenon through the formation of hydrogen ions as shown
below: 2 2 3 2 3 3 2 1 2 2 2 2 2 4 SO H O H HSO HSO H SO SO O H O H SO (1.5)
Or SO2 may react with O2 and iron causing direct corrosion of steel:
2 2 4 4 2 2 2 3 2 2 4 2 4 2 4 2 4 6 2 4 4 4 2 4 4 Fe SO O FeSO FeSO O H O Fe O H O H SO H SO Fe O FeSO H O (1.6) 1.2.4. Corrosion inhibition
By employing suitable inhibitors, corrosion phenomenon may be effectively suppressed up to 80%. A number of corrosion inhibitors, based on arsenic, antimony, vanadium, copper
10
(like NaVO3, CuCO3) are being used in order to control and prevent corrosion that not only
adds to the capital cost but most of these are toxic and hazardous to life as well.
Degradation also plays its role in this regard as this phenomenon not only depletes the active CO2 capturing species but the resulting products also enhance the corrosion rate by
lowering the inhibition ability. Presence of certain salts like NaCl greatly lowers the inhibitor efficiency which might be due to the attack of Clˉ ions on the passive film [22]. Presence of heat stable salts (acetate, formate, oxalate, etc) increase the corrosion rate, probably by introducing additional oxidizing agents [23].
A number of organic (including thiourea, salicylic acid) and inorganic (vanadium, antimony, copper, cobalt, tin and sulfur compounds) inhibitors have been exploited. Sodium metavanadate (NaVO3) is the most trusted in amine based CO2 capture plants that
can reduce corrosion rate to less than 1 mpy (0.0254 mm/year). In spite of their successful use, the probable consequences of inhibitors‟ toxicity (more toxic than absorption solvents, Table 1.2) on human health and environment are of great worry. The more strict regulations in case of toxic/hazardous substances in very near future may limit the use of such compounds due to high disposal costs [24].
Table 1. 2. Toxicity of absorption solvents and conventional inhibitors.* Chemical LD50-orala (mg/kg) Mouse Rat Absorption solvents Monoethanolamine (MEA) 700 1720 Diethanolamine (DEA) 3300 710 Conventional inhibitors Vanadium pentaoxide 23 10 Sodium metavanadate 74.6 98 Ammonium metavanadate 25 58.1 *adapted from [24]; a LD
50 (lethal dose) is the dose large enough to kill 50% of a sample of animals under test.
1.3. Physical Solvents
To cope with the problems of higher regeneration energy requirements, degradation and corrosion posed by chemical solvents (aqueous alkanolamines), physical solvents (Table 1.3) have been employed where there is higher CO2 concentration found in the feed gas
11 (such as pre-combustion capture). But these face their own downsides i.e. prerequisite of higher CO2 concentrations, elevated pressures, and refrigeration/cooling of the solvent/feed
gas. Moreover, most physical solvents are also liable for dissolution of heavier hydrocarbons in reasonable quantities [11].
Table 1. 3. Physical solvent processes.*
Process name Solvent Licensor
Fluor solvent Propylene carbonate (PC) Fluor Corporation
SELEXOL Dimethyl ether of polyethylene glycol
(DMPEG) Dow Chemical Company
Purisol N-Methyl-2-pyrrolidone (NMP) Lurgi
Rectisol Methanol Lurgi
Sulfinol Sulfolane and MDEA/DIPA
(Mixed physical/chemical solvent) Jacobs *reproduced from [11]
1.4. Ionic Liquid Solvents
An exciting new class of solvents known as ionic liquids (ILs), entirely composed of ions, are being synthesized and investigated for diverse applications such as organic/inorganic reactions, catalysis, metal extraction, gas separations, etc. The prime advantage of using ionic liquids is that these have no detectable vapor pressure and hence don‟t contribute to atmospheric pollution. Also, owing to the availability of numerous constituent ion pairs, thousands of binary ionic liquids are potentially possible and by choice application specific solvent can be synthesized [25,26].
In the following pages, from the perspective of carbon dioxide capture, the literature has been reviewed to have a thorough knowhow about the use of these novel species.
1.5. Ionic liquids for CO
2capture - Development and progress
*Abstract/Résumé
Innovative off-the-shelf CO2 capture approaches are burgeoning in the literature, among
which, ionic liquids seem to have been omitted in the recent Intergovernmental Panel on Climate Change (IPCC) survey. Ionic liquids (ILs), because of their tunable properties,
12
wide liquid range, reasonable thermal stability, and negligible vapor pressure, are emerging as promising candidates rivaling with conventional amine scrubbing. Due to substantial solubility, room-temperature ionic liquids (RTILs) are quite useful for CO2 separation from
flue gases. Their absorption capacity can be greatly enhanced by functionalization with an amine moiety but with concurrent increase in viscosity making process handling difficult. However this downside can be overcome by making use of supported ionic-liquid membranes (SILMs), especially where high pressures and temperatures are involved. Moreover, due to negligible loss of ionic liquids during recycling, these technologies will also decrease the CO2 capture cost to a reasonable extent when employed on industrial
scale. There is also need to look deeply into the noxious behavior of these unique species. Nevertheless, the flexibility in synthetic structure of ionic liquids may make them opportunistic in CO2 capture scenarios.
Des approches de capture du CO2 innovantes sont en plein essor comme le révèle la
littérature actuelle, parmi lesquelles, les liquides ioniques semblent avoir été omis dans la récente revue du GIEC (Intergovernmental Panel on Climate Change, IPCC). Les liquides ioniques (ILs), en raison de leurs propriétés ajustables, large gamme de liquide, stabilité thermique et pression de vapeur négligeable, apparaissent comme des candidats prometteurs rivalisant avec les amines dans les contacteurs gaz-liquide classiques. En raison de la solubilité importante de gaz, les liquides ioniques à température ambiante (RTILs) sont très utiles pour la séparation du CO2 des gaz de combustion. Leur capacité
d'absorption peut être grandement améliorée par fonctionnalisation avec un groupement amine, mais avec une augmentation concomitante de la viscosité rendant le contrôle du procédé difficile. Toutefois cet inconvénient peut être surmonté par la mise en oeuvre de membranes à base de liquides ioniques, en particulier lorsque les pressions et températures élevées sont impliquées. En outre, en raison de la perte négligeable de liquides ioniques lors du recyclage, ces technologies permettront aussi de réduire le coût du captage du CO2
dans une mesure raisonnable lorsqu'elles sont utilisées à l'échelle industrielle. Il est également nécessaire d‟examiner attentivement le caractère toxique de ces espèces. Néanmoins, la souplesse de la structure de synthèse des liquides ioniques peut les rendre abordables dans les scénarios de capture du CO2.
13
1.5.1. Introduction
Recent concept of using ionic liquids (Figure 1.7) for CO2 capture is gaining interest due to
their unique characteristics, i.e., wide liquid range, thermal stability, negligible vapor pressure, tunable physicochemical character and high CO2 solubility. An important
drawback much discussed in the case of ILs is their high viscosity. However, by choosing an appropriate combination of cation and anion, the viscosities can be adjusted over an acceptable range of <50 cP to >10,000 cP. For CO2 capture at high temperatures and high
pressures, such as in integrated gasification combined cycle (IGCC) pre-combustion capture, IL viscosity is less of a concern for its sharp decrease at elevated temperatures, though thermodynamics of CO2 absorption untowardly dictates poor abatement
performances. Therefore, paths pursued in recent research works include the use of ionic liquids for carbon dioxide capture involving room-temperature ionic liquids (RTILs), task-specific ionic liquids (TSILs) or supported ionic-liquid membranes (SILMs) [27-30].
Figure 1. 7.Some cations and anions constituting ionic liquids (ILs).
1.5.2. CO2 capture by room-temperature ionic liquids (RTILs)
Considerable research work is being done showing high carbon dioxide solubility in certain RTILs, especially in those having imidazolium-based cations. Depending on their thermal stability and CO2 selectivity (in general over nitrogen and smaller hydrocarbons), ILs are
stronger candidates (for CO2 capture) compared to certain conventional solvents such as
methanol, ethanol, and acetone [31]. RTILs portray a typical behavior of a physical solvent;
14
that is, increase in CO2 partial pressure results in linear increase in the gas solubility
whereas temperature exerts opposing effect on CO2 absorption in the RTIL [32]. The
solubility of carbon dioxide, ethylene, ethane, methane, argon, oxygen, carbon monoxide, hydrogen and nitrogen in 1-n-butyl-3-methylimidazolium hexafluorophosphate, [bmim][PF6] in the temperature range between 10 and 50 °C and pressures up to 13 bar
proves the superiority of IL over various organic solvents like heptane, cyclohexane, benzene, ethanol and acetone (see Figure 1.8 and Table 1.4). Dissolution enthalpy and entropy values suggest stronger interaction of CO2 with the IL, [bmim][PF6]. The relatively
higher solubility of CO2 may be attributed to its quadrupole moment and dispersion forces.
Owing to their negligible volatility and thermal stability under the explicit conditions, ILs are unlikely to contaminate the gas stream. Raeissi and Peters verified the thermal stability of 1-n-butyl-3-methylimidazolium bis[trifluoromethylsulfonyl]imide, [bmim][Tf2N], by
conducting the gas capture experiments in the temperature range of 40–177 °C and pressures up to 140 bar. Even after keeping at 177 °C for more than 10 h, the ionic-liquid conferred reproducible results for CO2 solubility [33,34]. Mass transfer of the gas is of
much importance especially where gas is to undergo a chemical interaction. Hence, during fabrication of an appropriate ionic liquid, drawbacks posed by high viscosity must be addressed [34].
Figure 1. 8. Solubilities of CO2, C2H4, C2H6, CH4, Ar and O2 in [bmim][PF6] at 25 °C
(adapted with permission from [34]). 0 0.05 0.1 0.15 0.2 0.25 0 2 4 6 8 10 12 14 Pressure (bar) M o le F ra c ti o n CO2 C2H4 C2H6 CH4 Ar O2
15
Table 1. 4.Henry‟s constants (bar, at 25 °C) for gases in different organic solvents.*
[bmim][PF6] heptane cyclohexane benzene ethanol acetone
CO2 53.4 84.3 133.3 104.1 159.2 54.7 C2H4 173 44.2a - 82.2 166.0 92.9 C2H6 355 31.7 43.0 68.1 148.2 105.2 CH4 1690 293.4 309.4 487.8 791.6 552.2 O2 8000 467.8 811.9 1241.0 1734.7 1208.7 Ar 8000 407.4 684.6 1149.5 1626.1 1117.5 CO nondetect 587.7 1022.5 1516.8 2092.2 1312.7 N2 nondetect 748.3 1331.5 2271.4 2820.1 1878.1 H2 nondetect 1477.3 2446.3 3927.3 4902.0 3382.0 *adapted with permission from [34]; a for ethylene in hexane
The experimental and simulation studies have shown that CO2 is significantly soluble in
alkylimidazolium-based ILs. The origin of this high solubility could be related more to the anion moiety that enhances interactions by favoring peculiar distributions of CO2 molecules
around the cation [35]. Alkyl-side chain length of the imidazolium cation of the ILs also affects CO2 solubility to a certain extent (Table 1.5). Fluorine substituted side chains
greatly augment the uptake of CO2 compared to the corresponding non-substituted side
chains but at the expense of an increase in viscosity [36-39].
Table 1. 5.Henry‟s constants for CO2 in different ionic liquids.*
Ionic Liquid HCO2 (bar)
C3mimTf2N 37 ± 7
C3mimTf2N with constant-density gas 39 ± 1
C3mimPF6 52 ± 5
C4mimTf2N 37 ± 3
C4mimTf2N with 2.7 wt% polyethylenimine 38 ± 3
C6mimTf2N 35 ± 5
C8mimTf2N 30 ± 1
C8mimTf2N with 20% relative humidity 30 ± 2
C8mimTf2N with 40% relative humidity 27 ± 4
C8F13mimTf2N 4.5 ± 1
C8mimTf2N (58 mol%)/C8F13mimTf2N (42 mol%) 15 ± 1
1,4-dibutyl-3-phenylimidazolium bis(trifluoromethylsulfonyl)imide 63 ± 7 1-butyl-3-phenylimidazolium bis(trifluoromethylsulfonyl)imide 180 ± 17 *adapted with permission from [39]
The nature of anion seems to have a stronger influence on gas solubility than that of the cation. Ionic liquids possessing [Tf2N] anion show higher CO2 solubility among
imidazolium-based RTILs (Table 1.6). A number of factors like free volume, size of the counter ions, and strength of cation-anion interactions within the ionic liquid structure seem
16
to govern CO2 solubility in RTILs. Higher gas solubility with increase in alkyl-side chain
may be the result of increased free volume available for CO2 with corresponding decrease
in cation–anion interactions [40,41]. The thermal stability and negligible volatility of RTILs make them quite viable for prolonged use. Hou and Baltus found that even after regenerating the ionic liquid six times, by N2 purging followed by vacuum application at 70
°C, there was practically no change in gas capture capacities [40].
Table 1. 6.Henry‟s law constants of CO2 in ionic liquids.*
Ionic Liquid HCO2 (bar)
10 ˚C 20 ˚C 25 ˚C 30 ˚C 40 ˚C 50 ˚C [bmim][Tf2N] 28 ± 2 30.7 ± 0.3 34.3 ± 0.8 42 ± 2 45 ± 3 51 ± 2 [pmmim][Tf2N] 29.6 ± 0.6 34 ± 3 38.5 ± 0.9 40.4 ± 0.6 46 ± 3 53 ± 2 [bmpy][Tf2N] 26 ± 1 31.2 ± 0.1 33 ± 1 35 ± 2 41 ± 4 46 ± 1 [perfluoro-hmim][Tf2N] 25.5 ± 0.2 29.2 ± 0.4 31 ± 2 32 ± 2 36 ± 4 42 ± 2 [bmim][BF4] 41.9 ± 0.2 52 ± 2 56 ± 2 63 ± 2 73 ± 1 84 ± 4 *adapted from [40]
The equilibrium pressure not only depends on temperature but also on CO2 concentration.
At 60 bar, CO2 solubility in 1-ethyl-3-methylimidazolium
bis[trifluoromethylsulfonyl]imide, [emim][Tf2N], is found to be 60 mol%. When compared
with 1-ethyl-3-methylimidazolium hexafluorophosphate, [emim][PF6], the gas is found
more soluble in IL with [Tf2N]− anion. The difference is further pronounced at higher CO2
mole fraction (Figure 1.9). Such data is confirming the effect of anion on CO2 interaction
with IL [42]. Fluoroalkyl group enhances CO2 solubility, thus making [emim][Tf2N] more
17
Figure 1. 9.CO2 solubility in [emim][Tf2N] and [emim][PF6] (adapted from [42]).
Room-temperature ionic liquids can be effectively used for hydrogen purification with high selectivity for CO2/H2 separation. Selectivity of the ionic liquid, [bmim][PF6], for CO2/H2
mixtures constituting 45–50 wt% H2 is in the range of 30-300. Selectivity drops at higher
temperature but enhances with pressure increase [43-45]. Hence this setup may be employed in CO2 capture from pre-combustion power plants. A pressure-swing
adsorption/desorption method can be employed for H2 purification by RTILs. CO2 showed
good solubility in 1-ethyl-3-methylimidazolium 2-(2-methoxyethoxy)ethylsulfate, [emim][MDEGSO4] at 30 °C in the pressure range of 8.54–67 bar, and expectably
increasing with pressure rise (Table 1.7). Pyrrolidinium and ammonium based RTILs like 1-n-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide ([bmpy][Tf2N]) and
trimethyl(butyl)ammonium bis(trifluoromethyl)sulfonyl)imide ([N(4)111][Tf2N]) have also
been investigated for H2 purification showing CO2 absorption capacity comparable to
imidazolium-based RTILs in the temperature range of 20-140 °C [46,47]. Regarding H2S/CO2 selectivity, H2S was found almost three times more soluble than CO2 in
1-(2-hydroxyethyl)-3-methylimidazolium tetrafluoroborate ([hemim][BF4]). However, owing to
the greater concentration of CO2 in the flue gases, higher partial pressure of CO2 diminishes
0 100 200 300 400 500 600 700 800 900 0 0.1 0.2 0.3 0.4 0.5 0.6 P ( ba r) x (CO2) [emim][PF6] [emim][Tf2N]
18
this advantage. This observance illustrates that RTILs can be efficiently tailored to remove H2S and CO2 concurrently [48,49].
Table 1. 7.CO2 solubility data in [emim][MDEGSO4].*
P/bar mCO2 a/(molCO2 . kgIL-1) P/bar mCO2 a/(molCO2 . kgIL-1)
30 ˚C 40 ˚C 8.540 0.3850 8.650 0.3301 14.72 0.6654 14.97 0.5713 28.67 1.3239 28.88 1.1162 42.30 2.0404 42.81 1.7053 55.21 2.7357 56.62 2.2899 62.30 3.0936 63.50 2.5606 50 ˚C 60 ˚C 8.420 0.2743 8.470 0.2380 15.12 0.4911 15.21 0.4257 29.38 0.9587 29.61 0.8235 43.59 1.4509 43.95 1.2359 57.32 1.9205 57.70 1.6254 65.20 2.1710 66.36 1.8551 70 ˚C 8.560 0.2110 15.22 0.3737 29.87 0.7171 44.27 1.0655 58.68 1.4097 67.10 1.6008
*adapted from [44]; a with buoyancy correction
The viscosity of common RTILs is quite high, [bmim][BF4] (79.5 cP) is found to be 40
times more viscous compared to 30% MEA (monoethanolamine) solution at the same temperature (33 °C) [50]. To cope with the viscosity constraints, RTILs may be mixed with some common organic solvents or water. Addition of water (IL aqueous solutions) helped overcome viscosity problems as shown in Table 1.8 [51]. However, inclusion of such liquids will come with their drawbacks as well. Besides, the advantage comes at the expense of a decrease in gas capture capability. This is evident from the behavior of an ionic liquid [Choline][Pro] (Figure 1.10) examined in pure form as well as after mixing with polyethylene glycol (PEG 200) at temperatures 35–80 °C and ambient pressure [52]. Gas solubility decreased with increasing amount of PEG 200, under constant temperature and pressure conditions. This is explicable because of the low CO2 solubility in PEG 200.
19 amount of PEG 200 has been found favorable. This may be due to the decrease in viscosity and/or solvent role of PEG 200.
Figure 1. 10. Proposed mechanism for chemical absorption of CO2 by the TSIL (adapted
from [52]).
Table 1. 8.Viscosity values for different compositions of tri-iso-butyl(methyl)phosphonium tosylate/water mixtures.*
Mass fraction IL ± 0.0001/(w/w) η±σa (cP) 0.0000 0.89 0.1250 1.65±0.08 0.2500 2.6±0.1 0.3750 4.0±0.2 0.5000 6.9±0.3 0.6250 11.6±0.5 0.7500 23.0±0.7 0.8720 68.0±2.0 1.0000 1320±13
*adapted from [51]; a standard deviations
Another more workable option, in case of alkanolamine systems, may be the replacement of aqueous medium with some stable and non-volatile room-temperature ionic liquid in order to combine the advantages of both, i.e., negligible vapor pressure, higher thermal stability and lower heat capacity of ionic liquids, and fast capture kinetics and low viscosity of certain alkanolamines [53]. Switching the CO2 capture product (carbamate in this case)
into a foreign phase would pull the equilibrium-limited CO2 absorption towards higher CO2
conversion values, unlike in conventional aqueous amine solutions with soluble carbamate salt (Figure 1.11). Thus, it can be inferred that to take advantage of useful properties of ILs,
20
amine-IL solutions need to be investigated more deeply as potential replacement solvents for aqueous amine scrubbing systems.
Figure 1. 11. [hmim][Tf2N]-MEA solution: (a) fresh sample; (b) on CO2 exposure;
showing precipitated MEA-carbamate (reprinted with permission from [53]).
Regarding natural gas purification, certain hygroscopic imidazolium-based ionic liquids like [bmim][PF6], [C8mim][BF4] and [C8mim][PF6] have the ability to dehydrate the gas
stream as well [54-56]. Also, the presence of water along with acetate ion in some ionic liquids akin to [hmim][acetate] and [bmim][acetate] may facilitate the capture phenomenon through weak bonding with CO2 [57]. Diminished corrosion of the equipment, almost
one-third the heat capacity of (especially imidazolium-based) RTILs, compared to the aqueous systems, may help rationalize the large scale application of these unique species for CO2
capture [20,53,58–60].
In short, room-temperature ionic liquids especially imidazolium-based RTILs may be employed in natural gas/hydrogen purification or in CO2 capture from fossil fuel based
power plants. Regarding regeneration, room-temperature ionic liquid based materials may be easily recovered either by pressure sweep process coupled with vacuum treatment, by applying heat or by bubbling nitrogen through the absorbent [50,52]. However,
task-21 specific ionic liquids or RTILs mixed with amine bearing species require temperature sweep regeneration involving vacuum heating [39].
1.5.3. CO2 capture by task-specific ionic liquids (TSILs)
As discussed above, CO2 is sufficiently soluble in room-temperature ionic liquids (RTILs).
However, the CO2 capture ability can be significantly enhanced by introducing basic
character in the ILs. Functionalization of ionic liquids with a suitable moiety (like amine) may be opted in this regard [61,62]. CO2 absorption ability of TSILs can reach up to
threefold that of the corresponding RTILs. The enhanced effect of pressure in case of TSILs was observed by the fact that there was a steady increase in gas loading with rise in pressure, providing evidence both for chemical as well as physical sorption. The effect is not so apparent in case of aqueous amine solutions which possess stoichiometric limitations [50]. Reversible sequestration of CO2 has been achieved by attaching primary amine
moiety to an imidazolium cation, without any decrease in the ionic-liquid stability. For five consecutive cycles of gas absorption/desorption, the regenerated TSIL ([pabim][BF4]) did not show any loss of efficiency. [pabim][BF4] exhibits better CO2 capture competence
compared to [hmim][PF6], owing to chemical capture phenomenon in the former. The TSIL
when exposed to CO2 for 3 h at room temperature and pressure, the mass gain was 7.4%
which corresponds to 0.5 molar uptake of CO2 (maximum theoretical value for CO2 capture
as amine carbamate). The proposed mechanism of interaction between CO2 and
[pabim][BF4] is shown in Figure 1.12. The inclusion of water in the ionic liquid was found
to increase the CO2 holding capacity which might be due to the formation of additional
bicarbonate species [63,64].
22
In spite of the tunable approach towards TSILs, these functionalized species exhibit much higher viscosities as compared to the corresponding RTILs or other commercially available CO2 scrubbing solutions, posing too serious complications to be applicable on an industrial
scale. CO2 capture by TSILs causes a sharp increase in viscosity, resulting into a gel-like
material [65]. This drawback may be avoided by utilizing mixtures of TSILs and RTILs or TSILs may be adsorbed onto porous membranes.
Comparison of CO2 capture by ionic liquids with that by conventional aqueous amine
solutions (30 wt% MEA/MDEA) illustrates that the absorption activities of ionic liquids resembles that of common physical solvents (Figure 1.13). Nonetheless, CO2 absorption
ability increases significantly on functionalization of ionic liquid with primary amine moiety. Task-specific ionic liquids, [Amim][BF4] and [Am-im][DCA], perform like
chemical solvents at low pressures (≤1 bar). However, at higher pressures, they pursue the performance of room-temperature ionic liquid, [bmim][BF4]. On the other hand, aqueous
amine solutions accomplish the maximum capacity at about 2 bar and any further increase in pressure does not seem feasible. Whereas functionalized ionic liquids (TSILs) carry on steady CO2 absorption with ascending pressure even beyond the stoichiometric limit
[50,66]. This behavior shows that TSILs possess both chemical as well as physical tools for gas capture.
23
Figure 1. 13. Molar CO2 loads in solvent volume (for MEA/MDEA, consider aqueous
solution volume): data for ionic liquids at 30 °C [50]; data for MEA and MDEA at 40 °C [66].
1.5.4. CO2 capture by supported ionic-liquid membranes (SILMs)
A number of studies have been performed to explore the prospects of supported ionic-liquid membranes involving RTILs or TSILs or both in CO2 capture applications. To take
advantage of thermal/chemical stability and essentially no volatility, and to deal with the limitations due to viscosity and also to increase the contact area between gas and ionic liquid, supported ionic liquids may prove a better choice in CO2 separation from flue gases.
RTIL, [bmim][Tf2N], supported on porous alumina membrane revealed very encouraging
results in favor of CO2 separation ability [67]. The SILM with [bmim][Tf2N] shows higher
CO2/N2 selectivity of 127 than that with [C8F13mim][Tf2N] (72). Furthermore, the
fluorinated ionic liquid is much more viscous than [bmim][Tf2N] that tends to cause a
decrease in CO2 diffusivity. A proposed process diagram regarding the application of SILM
in a coal-fired power plant is shown in Figure 1.14. SILMs may compete economically with commercial amine scrubbing provided permeance and selectivity are optimized. Ionic liquids like [bmim][PF6] adsorbed to a porous (ceramic or zeolite) material may be
24
employed for CO2 separation by introducing pressurized gas on one side and collecting the
CO2-depleted gas downstream of the porous medium [68].
Figure 1. 14. Proposed setup for CO2 separation by SILM in a coal-fired power plant
(adapted from [67]).
In another study [69,70], [bmim][BF4] was adsorbed onto polyvinylidene fluoride (PVDF)
polymeric membrane. The mass ratio of IL/membrane in SILMs was kept 0.5–2.0. With the increase of IL content, the permeability coefficient was seen to increase abruptly. Rise in temperature resulted in a corresponding increase in membrane free volumes caused by increased mobility of polymeric chains. This development stimulated simultaneous increase in permeability. However, the selectivity for CO2 decreased when compared with CH4. This
is because CH4 show more diffusion selective property than solubility selective property
and so its solubility is more affected by membrane structure. The rise in pressure demonstrates a positive effect on selectivity. Through optimization of operating conditions, 25-45 CO2/CH4 selectivity was achieved. The solubility behavior of CO2, H2, CO and CH4
in two ionic liquids, [bmim][Tf2N] and [emim][Tf2N] makes their usage interesting as
separation membranes [71]. The solubility of CO2 in the two ionic liquids reaches up to 60
mol% compared to that of H2 that remains up to 7 mol% at 90 bar. The pressure increase
![Figure 1. 1. Reaction scheme including reactions between amine, CO 2 /carbonate and protons (reproduced from [10])](https://thumb-eu.123doks.com/thumbv2/123doknet/7449556.221244/25.918.150.661.296.679/figure-reaction-scheme-including-reactions-carbonate-protons-reproduced.webp)

![Figure 1. 14. Proposed setup for CO 2 separation by SILM in a coal-fired power plant (adapted from [67])](https://thumb-eu.123doks.com/thumbv2/123doknet/7449556.221244/46.918.112.708.183.551/figure-proposed-setup-separation-silm-fired-power-adapted.webp)

![Figure 2. 3. CO 2 absorption isotherms for DEA/[hmim][Tf 2 N] surfactant stabilized emulsions obtained at 25°C](https://thumb-eu.123doks.com/thumbv2/123doknet/7449556.221244/74.918.125.613.118.470/figure-absorption-isotherms-dea-surfactant-stabilized-emulsions-obtained.webp)
![Figure 2. 6. 13 C NMR spectrum of crystalline carbamate (retaining traces of [hmim][Tf 2 N]) taken in DMSO-d6 solvent](https://thumb-eu.123doks.com/thumbv2/123doknet/7449556.221244/78.918.110.791.410.793/figure-spectrum-crystalline-carbamate-retaining-traces-taken-solvent.webp)


![Figure 3. 5. Linear polarization curves of carbon steel 1020 at 25 °C: a) in aqueous alkanolamines; b) in alkanolamine+[bmim][BF 4 ] mixtures](https://thumb-eu.123doks.com/thumbv2/123doknet/7449556.221244/96.918.120.602.133.891/figure-linear-polarization-curves-aqueous-alkanolamines-alkanolamine-mixtures.webp)