HAL Id: hal-02417812
https://hal.archives-ouvertes.fr/hal-02417812
Submitted on 18 Dec 2019HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Corrosion of structural materials by liquid metals used
fusion, fission and spallation
J.-L. Courouau, D. Feron
To cite this version:
J.-L. Courouau, D. Feron. Corrosion of structural materials by liquid metals used fusion, fission and spallation. 1st IAEA Workshop on Challenges for coolants in fast spectrum system, Jul 2017, Vienne, Austria. �hal-02417812�
Corrosion of structural materials by liquid metals used in
fu-sion, fission and spallation
D. Féron and J.-L. Courouau
Den-Service de la Corrosion et du Comportement des Matériaux dans leur Environnement (SCCME), CEA, Université Paris - Saclay, France
Liquid metals (lithium, sodium, lead and its alloys Pb-Li or Pb-Bi) are used as coolants for fusion, fission or spallation reactors due to their thermal and nuclear properties. However, these liquid metals are corrosive when they come into contact with solid metallic materials. Preserv-ing structural alloys (no- and low alloyed steels, stainless steels, nickel based alloys…) in con-tact with these liquid metals requires the knowledge of the corrosion phenomena that may oc-cur: mainly liquid metal embrittlement and general corrosion with mass transfers within the heat transfer circuit.
Liquid metal embrittlement is a particular case of stress corrosion cracking in liquid metals which results in a decrease of the toughness or the ductility of the structural materials in asso-ciation with a brittle fracture surface. Wetting, temperature, stress and strain rates, solid and liquid metal compositions are key factors.
General corrosion mechanisms in liquid metals are governed by thermodynamics and may be divided into two main phenomena:
Reaction with impurities, oxygen mainly (and other soluble interstitial elements such as carbon, nitrogen, hydrogen and boron …).
Dissolution of alloying elements which is function of their solubility in the liquid metals. Liquid metal chemistry is then a key parameter for general corrosion of structural materials,. As the solubility is generally decreasing with temperature, mass transfer plays an important role in non-isothermal systems with dissolution in the hottest section and precipitation in the coldest parts of the circuits.
1 Introduction
Coolants required for use in advanced nuclear energy systems such as Fast Neutron Reactors (FNRs), Fusion reactors and Accelerator Driven system (ADS) need specific properties: high heat transfer coefficients are required since the power density in these systems is significantly higher than the previous generation of nuclear reactors. The operating temperatures are also higher in order to increase the efficiency of these systems, so their coolants should have high liquidus range. Liquid metals with high thermal conductivity, high boiling point and adequately high specific heat meet these requirement, even at low pressures. The coolant of FNRs should possess low neutron absorption cross section and be a poor moderator for achieving high neu-tron economy and for sustaining the hard neuneu-tron spectrum. Liquid sodium has been the coolant of choice for FNRs while liquid lithium or Pb-17Li alloy is considered as tritium breeder cool-ant in fusion systems. Liquid lead and Lead-Bismuth Eutectic (LBE) alloy are the candidate coolants for neutron breeder of ADS as well as for FNRs. The main thermo-physical properties of these liquid metal coolants are compared with those of water in Table 1.
In these advanced nuclear energy systems, controlling corrosion due to liquid metals stands as a major challenge. Corrosion of structural materials by liquid sodium, lithium, lead or its alloys may act according to various mechanisms [1, 2, 3, 4]: (i) intergranular penetration of the liquid
metal into the solid and embrittlement of the solid by the liquid, (ii) formation of intermetallic compounds, (iii) reaction with dissolved impurities (oxygen, carbon, nitrogen,…), (iv) tion of the solid into the liquid metal, … Moreover, in presence of a thermal gradient, dissolu-tion-deposition phenomena may occur between hot and cold areas. This may generate materials degradation in hot areas, deposition or even plugging in cold areas,…
For this overview of main corrosion phenomena of structural materials (mainly steels and stain-less steels) focus will be put on liquid metal embrittlement (§2), oxidation (§3) and dissolution (§4).
Table 1 – Thermo-physical properties of liquid metal coolants compared to water [1, 2, 4] Property Sodium Lithium Pb-Li Coolant Pb LBE H2O
Melting Point (K) 371 453.5 507 600.5 398 273 Boiling Point (K) 1156 1620 - 2018 1901 373 Density Kg/m3 at 773K 845 487 9486 10520 10150 0.99 at 323K Thermal conductivity at 773K (W/(K.m) 68.8 53.5 17 17.1 14.2 0.67 at 323K Heat capacity kJ/(kg.K) at 773K 1.269 4.212 0.187 0.150 0.146 1.339 at 323K
Vapour pressure (Pa) at 773K
2 at 573K 0.08 0.002 0.002 0.002 4
101325 Pa at 373K
2 Liquid metal embrittlement
Liquid metal embrittlement (LME) is the reduction in the elongation to failure that can be pro-duced when normally ductile solid metals are stresses while in contact with liquid metal [5]. In other terms, a ductile solid metal or alloy experiences a loss, which might be drastic, in the tensile elongation when exposed to liquid metal. This phenomenon stands as a major issue for metallic alloys exposed to liquid metals. It is specific to a pair solid/liquid metals. Main LME factors include:
- Wetting which is a prerequisite to LME. Pre-exposure at high temperature is often necessary to get a good wetting of the liquid metal on the solid alloy. For instance, af-ter a pre-exposure at 550°C during 1000 h in liquid lithium, austenitic and ferritic steels exhibit some susceptibility at 200-250°C in liquid lithium [6].
- Temperature: generally LME is more important around the melting point and de-creases when temperature inde-creases. For instance with the T91 steel (a 9% Cr marten-sitic steel) in a stagnant liquid Pb or LBE, the tensile tests revealed an embrittlement of the material, more pronounced at low temperature, that disappears as the tempera-ture is raised above 450°C [7, 8]. This behavior is explained by the reduction of the surface energy of the bare metal induced by the adsorption of the liquid metal. When the steel is submitted to low cycle fatigue tests in presence of the liquid Pb-Bi eutectic at 300°C, its lifetime is significantly reduced compared to tests performed in air. - Irradiation: on susceptible materials, LME is enhanced by irradiation. For instance,
LBE (lead–bismuth eutectic) embrittlement effects on ferritic/martensitic (FM) steels have been studied by conducting slow-strain-rate tensile (SSRT) testing on T91 and F82H ferritic steels either in argon or in liquid LBE after irradiation to doses up to about 20 dpa. Tests in argon revealed significant irradiation-induced hardening and
embrittlement effects (loss of ductility) as compared to the un-irradiated ones. Tests in LBE showed additional embrittlement effects induced by LBE, which increased with irradiation-induced hardening. As a consequence, the fracture strain of irradiated spec-imens was reduced to a very low level of about 2–3% [9].
- Solid metal microstructure and liquid metal impurities are also important parameters for LME susceptibility [1, 2, 3].
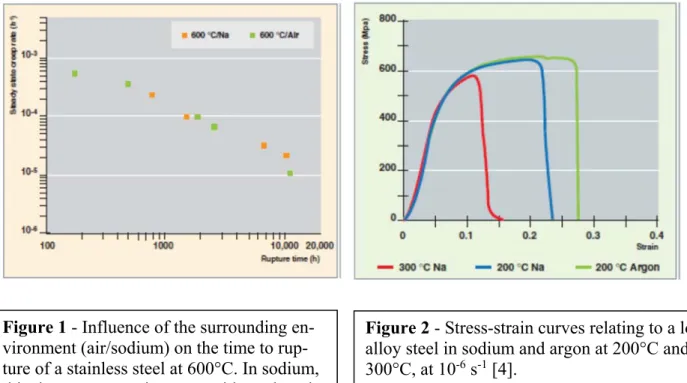
It is generally considered that exposure of ferritic and austenitic steels to pure liquid sodium (with a low impurity content), under constant-load test conditions, does not produce a major effect on the rupture strength of these steels [2, 10, 11]. Moreover, regarding stainless steels containing nitrogen (added to steel to improve its mechanical properties at high temperature, and, so, its resistance to failure), such as those of 316L(N) grade, they display comparable times to failure under creep in pure sodium and air, as shown on Figure 1. However, some creep-stressed specimens of AISI 304 austenitic steel when exposed to pure liquid sodium at 550°C presented reduced time to rupture and strain to rupture, associated with the last period of the creep rupture life, the range of tertiary creep [12]. The intergranular cracks were observed in the deformed parts of the specimen and are thought to be related to decarburization of the spec-imen. Accordingly, the same grade of steel presented a slight reduction of the ductile area in association with intergranular brittle fracture in area of high plastic deformation where marten-sitic transformation ( to ’) occurred in relation with very fine microstructure [13].
With impurity concentrations typical of sodium-cooled reactors, a certain susceptibility of un-alloyed or low-alloy steels (up to 9% chromium) can be observed during the very severe slow strain rate tensile tests, at temperatures between 200 °C and 400 °C, as displayed on Figure 2 for a low-alloy steel [9]. As evidenced by the observations carried out, microstructure and non-metallic impurities present in the alloy have a great impact on these phenomena of em-brittlement by liquid sodium. At higher temperatures, a sharp change of behavior can be seen, so that embrittlement by liquid sodium no longer seems to ever take place [10, 11].
Figure 1 - Influence of the surrounding
en-vironment (air/sodium) on the time to rup-ture of a stainless steel at 600°C. In sodium, this time to rupture is comparable to that ob-tained in air at the same temperature [4].
Figure 2 - Stress-strain curves relating to a
low-alloy steel in sodium and argon at 200°C and 300°C, at 10-6 s-1 [4].
Accidents (e.g. water or air leaks) may increase impurity concentration in liquid sodium and may lead to cracking, even if it is not a LME phenomenon: sodium hydroxide (NaOH) causes stress corrosion cracking (SCC) in both ferritic and austenitic steels in liquid sodium contami-nated by NaOH, if its concentration, temperature and stress are sufficiently high [14]. In addi-tion, stress corrosion cracking phenomena may occur owing to the presence of aqueous so-dium hydroxide when the component, covered by a residual soso-dium film, is contaminated by moisture, with local generation of aqueous sodium hydroxide. At temperatures higher than 80 °C for ferritic steels, and higher than 110 °C for austenitic steels, transgranular cracking may take place very rapidly. Such temperatures may occur during facility preheating after repair, and then during sodium filling. It is therefore required to avoid any residual presence of aque-ous sodium hydroxide on components, especially in gaps and clearances of structures and cir-cuits, which is obtained through relevant washing and drying procedures [4, 14].
In summary, regarding the susceptibility liquid metal embrittlement of structural materials (i.e. the diminution of strain or of the maximum stress before rupture):
Ferritic & martensitic steels, including ODS materials, are susceptible to LME in lead and its alloys (PbLi and PbBi) and some (low) susceptibility is observed in sodium and lithium at low temperatures [15].
Austenitic stainless steels exhibit some susceptibility at low temperatures in liquid lithium and a low or negligible susceptibility in liquid lead and its alloys.
No susceptibility has been observed in liquid sodium on austenitic steels. However, recent studies on both austenitic and ferritic/martensitic steels have shown that by in-creasing the oxygen activity in liquid sodium, a degradation of mechanical properties is observed, which was dependent also on other parameters (exposure time and tem-perature, wetting condition) [16, 17].
Other types of cracking may be observed, mainly in liquid sodium, due to the formation of sodium hydroxide during pollution periods (like during shutdowns).
3 Oxidation
The corrosion process will depend on the thermodynamic data which indicate the products to be formed. According to thermodynamics, reactions between elements from the solid alloy (iron, nickel, chromium…) and dissolved species (oxygen, carbon, nitrogen…) in the liquid metal can be predicted depending of the activity or the chemical potential of the dissolved spe-cies [1, 2, 3, 4, 18]. For oxygen which is the more common impurity, the formation of oxides solid compounds is given by predominance thermodynamic diagram called Ellingham diagram. This diagram represents the equilibrium oxygen partial pressure of metal oxidation reactions, as shown in figure 3. Following this diagram, it is not possible to form iron, nickel neither chromium oxides in liquid lithium, and in liquid lead-lithium. But in lead and in lead-bismuth eutectic (the BiO/Bi reaction is not represented in figure 3 but it is above the PbO/Pb equilib-rium), metallic oxides (Fe, Cr, Ni) can be formed if the oxygen content is high enough in liquid sodium, sodium oxide Na2O is more stable than NiO, magnetite Fe3O4 and even chromium
oxide below 500°C. Only ternary oxides like NaCrO2 are more stable than sodium oxide and
Figure 3 – Elligham diagram showing metal oxide stability as function of oxygen potential and
temperature [1, 2, 4, 18].
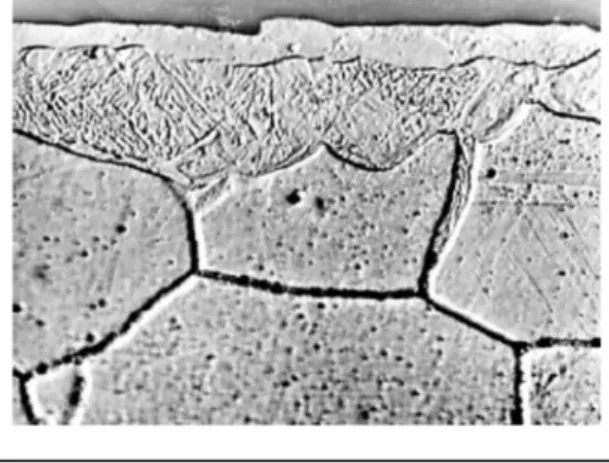
In lead and lead-bismuth alloy, one way of protecting materials against corrosion lies in the in situ formation of a protective oxide layer on the surface of materials (austenitic or martensitic steels). Such a layer can be formed on the solid’s surface by finely controlling the dissolved oxygen content, while avoiding lead oxide precipitation within the liquid metal. Yet, only through a strict control and accurate measurements of the oxygen content in the whole facility can this method prove to be successful. Ensuring the good behavior and resistance of these oxide layers first requires to understand their formation mechanism, then modelling the oxida-tion kinetics in order to predict long-term steel behavior under given condioxida-tions. Martensitic steels Fe-9Cr were particularly investigated in as much as they are considered as the candidate material for the spallation target window in hybrid reactors. In all the cases, a duplex oxide layer is seen to form on contact with steel (figure 4): it consists of an internal layer of Fe-Cr spinel on which a magnetite outer layer is superposed on contact with the liquid alloy. The nature of these two layers is identical whatever the test temperatures may be (between 470°C and 600°C).
So, controlling the dissolved oxygen content in liquid lead alloys is of key importance for cre-ating the thermodynamic conditions required for the formation of a protective oxide layer on the structural steel surface, consisting of iron and chromium oxides. The formation of this layer is much alike the in-situ building of a barrier against material transfer, which signifi-cantly reduces diffusion of alloying elements (especially, iron) towards the liquid. Yet, a strict control of the oxygen content in the whole facility is the condition required for this method to be fruitful. Moreover, dissolved oxygen activity has to be controlled within relatively narrow operating limits (figure 5), so as to reach an oxygen content not only higher than the threshold value for the formation of iron and chromium oxides – i.e. the condition required for protect-ing structures –, but also lower than lead oxide (PbO) solubility, that results in precipitation of solid crystals likely to be deposited on heat exchanger walls or to plug narrowed passage sec-tions partially or even fully [19].
Figure 4 - Backscattered-electron image of a cross-section of a steel (T91) immersed for 3600
hours into oxygen-saturated Pb-Bi alloy at 470 °C [4].
Figure 5 - Allowable range of oxygen concentration (Co in ppm) for the lead-bismuth
eutec-tic of primary coolant (420 °C-540 °C). The allowable area is represented by the green sec-tion, circumscribed by the vertical (cold and hot collector temperature) and horizontal lines that stand for the threshold concentrations in equilibrium with lead oxide formation at the coldest wall temperature (200 °C- 10-2 ppm), and with iron oxide dissolution at the hottest
wall temperature of the system (650 °C-5.10-4 ppm) [3, 4].
In liquid sodium, uniform corrosion rates increase with the oxygen activity, which indicates that the ternary oxide layer, if formed, has no protective effects. Uniform corrosion rates up to 500 µm per year has been observed at 650°C with 25 to 50 ppm of oxygen on austenitic stain-less steel (316L type). The corrosion rate of austenitic steels in liquid sodium between 450°C and 700°C is estimated to be proportional to oxygen concentration in the range 5 to 100 ppm. The exact mechanism at play in this oxygen enhanced dissolution is still to fully elucidate,
though the intermediate formation of Na-Cr-O and Na-Fe-O complexes were hypothesized since the 70ies to justify the straight increase of the iron solubility with the oxygen content [20, 21]. In any case, the policy to maintain the oxygen content to the lowest achievable level, typically lower than roughly 10 wppm, make the corrosion rates almost negligible on the long term in liquid sodium systems below 550°C.
The behavior of structural materials under oxidizing conditions (with high dissolved oxygen) can be summarized as follow:
- In lead and lead bismuth alloy, the formation of oxides may have protective properties and high chromium steels improve the corrosion resistance, but a strict range of oxy-gen concentrations needs to be followed in order to avoid dissolution of the oxides and precipitation of lead oxide. Mitigation includes protective coatings (typically Al2O3) or
steels with some aluminum content to form a self-healing protective layer.
- In liquid sodium, the corrosion rates increase with dissolved oxygen, though the full understanding of the effect of oxygen is still to be completed; the efficient oxygen control in reactor systems makes uniform corrosion rates low and not critical.
- No oxidation of iron, chromium, nickel… occurs in liquid lithium neither in lead-lith-ium due to the low oxygen activity in these liquid metals.
4 Dissolution
In liquid metals, the dissolution of a solid metal can occurs when (i) the oxides forms by the liquid metal (or alloy) are more stable than those formed from the solid metal (it is what hap-pens in liquid lithium or lead-lithium), or (ii) if the oxygen concentration is lower than the re-quired concentration needed to form oxides from the solid metals, like it may happens in so-dium, lead or lead-bismuth. The solubility of the main alloying elements becomes a key pa-rameter for the behavior of solid metals in liquid metals. As shown in table 4, nickel is the most soluble element whatever is the liquid metal. So nickel base alloys will not be suitable for these environments.
Table 2 – Solubility of the main alloying elements at 500°C (selected data coming from
refer-ences 2, 3, 9 & 11 / large scattering between available data)
Liquid metal Fe (ppm) Cr (ppm) Ni (ppm) Lithium 4 4 200 Sodium 0.2 0.005 1 Lead 0.8 0.1 5,000 Lead -Bismuth 5 50 50,000 Lead-lithium 1 10 3,000
General corrosion mechanisms of austenitic steels in liquid sodium were investigated in the seventies-eighties, and described in the literature devoted to this topic. They consist in a dis-solution of the surface elements in steel (Fe, Cr, Ni, Mn…), in contact with sodium, followed by their transfer and deposition, or diffusion, on the reactor structures. This deposition phe-nomenon represents the main vector of contamination of reactor structures by activated corro-sion products. The dissolution phase can be divided into successive time steps including
(i) The dissolution of austenite which occurs mainly at temperatures higher than 550°C, and consists in a selective dissolution of nickel present in the austenitic phase.
(ii) The formation of a ferritic layer linked to the preferential dissolution of the nickel present in steel from the outer austenitic layer which causes steel to be ferritized.
This ferritic layer is also dissolved, but at a slower rate, in liquid sodium (iron and chromium are also soluble in sodium to a lesser extent than nickel).
(iii) A steady state regime is reached when the thickness of the ferrite layer has reached its threshold value; the rates of alloying element dissolution and diffusion towards sodium correspond with a “stoichiometric corrosion“ of austenite, i.e. the relative quantities of metallic elements dissolved correspond with those of the initial alloy, as shown in figure 6.
In pure sodium (i.e. oxygen concentration lower than e.g. 5 ppm), corrosion corresponds with a dissolution of metallic elements in liquid sodium, as expressed here above. If temperatures are not too high (e.g. up to 550 °C), this phenomenon is relatively slow: corrosion is therefore limited.
Corrosion studies have evidenced that in the case of pure lithium and Pb-17Li alloy, corrosion occurs as a dissolution phenomenon; regarding pure lead and Pb-Bi alloy, the corrosion mech-anism varies according to the dissolved oxygen content in liquid metal: for oxygen concentra-tions lower than a critical minimum content needed to form oxides with solid metal alloying elements, corrosion takes place as a dissolution phenomenon, just as in lithium, Pb-17Li alloy or liquid sodium with low oxygen content.
Regarding 316L stainless steel, it undergoes a selective dissolution in these liquid metal envi-ronments as in liquid sodium: owing to the very high solubility of nickel in lead and lead al-loys, it is preferentially dissolved, thereby inducing the formation on the steel surface of a fer-ritic porous layer consisting of a network of channels filled with liquid alloy (figure 7). Anal-yses have evidenced a very high depletion in nickel, chromium and manganese, as well as an iron enrichment [4].
In Pb-17Li alloy, pure lead, as well as in Pb-Bi alloy (when the dissolved oxygen content is low), use of uncoated steel-316L is limited to moderate temperatures (below 350 °C approx.), at which it does not undergo significant dissolution. So is it for ferritic / martensitic steels, and strictly in flow velocity ranges which do not induce high corrosion rates. If temperatures are higher (above 350 °C approx.), steel dissolution rates become unacceptable, and materials protection has to be considered in order to ensure their corrosion resistance. This protection
Figure 6 - General corrosion of an austenitic
stainless steel in liquid sodium with low oxygen content (< 5 wppm, presumably 650°C, 8 m.s-1,
8000 h, and grain of 25-50 µm). The picture shows the emergence of a ferritic film at the surface (Ferrite / Ferrite and austenite / Sound austenite) [4].
Figure 7 – General corrosion of an
austenitic steel after immersion for 3000 hours at 500 °C into liquid Pb-Bi with a low oxygen content (7 10-8 wt.
can be achieved either by outer coatings, or through liquid alloy chemistry (oxygen and for-mation of an oxide protective layer) for lead or lead-bismuth.
The behavior of structural materials under reducing conditions (with low dissolved oxygen) can be summarized as follow:
- Preferential dissolution of nickel occurs under reducing conditions and leads to avoid nickel base alloys in most cases.
- In lead and its alloys (Pb-Li and Pb-Bi), the dissolution rates are more often too high above 350°C-400°C for ferritic and martensitic and austenitic steels. Mitigation measures include coatings.
- In liquid sodium, acceptable corrosion rates are observed up to 650°C in low oxygen content on ferritic and austenitic steels but more investigations are needed for long term behavior and modelling.
- In liquid lithium, other alloys like vanadium and niobium alloys are under considera-tion.
5 Conclusions and outlook
Among the corrosion issues which have not been detailed here above, two have to be men-tionned: (i) carburation/decarburation, nitruration/denitruration… which occurred at high tem-perature – typically above 550°C, and (ii) transfer processes which include dissolution in hot parts of the circuit and precipitation in the colder parts and which have major influence in non-isothermal systems leading to the issue of contamination by radioactive species (54Mn, 60Co).
These corrosion issues have to be included together with LME, oxidation and dissolution phe-nomena when the choice of structural materials has to be done.
In conclusive remarks, it could be outlined that
- In liquid sodium, corrosion phenomena, including liquid metal embrittlement, are con-sidered under control in expected environmental conditions (low oxygen, maximum temperature of 550°C/600°C…). Mechanisms and modelling are still needed for long term issues.
- In liquid lead and lead-bismuth, corrosion issues need mitigation strategies which are under development.
- More investigation are needed in liquid lithium either regarding mitigation or other al-loys like vanadium or niobium alal-loys.
Acknowledgements
The authors would like to thank very much CEA-Den for the authorization of reproducing part of the text and the above figures from the e-den monograph on corrosion and alteration of nu-clear materials (reference 4).
References
1 Yvon, P.: Structural materials for Generation IV nuclear reactors, Woodhead publishing, Cambridge, 2017 (ISBN 978-0-08-100906-2)
2 Féron, D.: Nuclear corrosion science and engineering, Woodhead publishing, Cambridge, 2012 (ISBN 978-1-84569-765-5)
3 Handbook on lead-bismuth eutectic alloy and lead properties, materials compatibility, thermal-hydraulics and technologies, EAN-NEA, OECD publications, Paris, 2007 (IBSN 978-92-64-99002-9)
4 Féron, D.; Richet, C.: Corrosion and alteration of nuclear materials, CEA-Den, Editions Le Moniteur, Paris, 2010 (ISBN 978-2-281-11369-3)
5 Nicholas, M.G.; Old, C.F.: Review liquid metal embrittlement. Journal of materials sci-ence 14 (1979) 1 – 18
6 Borgsted, H.U.; Grundmann, M.: The fracture of austenitic and martensitic steel in liquid lithium. Nuclear engineering & design, 3 (1986) 273-286
7 Legris, A.; Vogt, J.-B.; Verleene, A.; Serre, I.: Wetting and mechanical properties, a case study: Liquid metal embrittlement of a martensitic steel by liquid lead and other liquid metals. Journal of materials science 40 (2005) 2459 – 2463
8 Auger, T., Serre, I., Lorang, G., Hamouche, Z., Gorse, D., Vogt, J.-B., 2008. Role of oxidation on LME of T91 steel studied by small punch test. Journal of Nuclear Materials, 376 (2008) 336–340.
9 Long, B.; Dai, Y.; Baluc, N.: Investigation of liquid LBE embrittlement effects on irradi-ated ferritic/martensitic steels by slow-strain-rate tensile tests. Journal of Nuclear Mate-rials 431 (2012) 85–90
10 Hilditch, J.P.; Hurley, J.R.; Skeldon, P.; Tice D.R.: “The liquid metal embrittlement of iron and ferritic steels in sodium”, Corrosion science, Vol. 37, N° 3, (1995) 445-454 11 Skeldon, P.; Hilditch, J.P.; Hurley, J.R.; Tice D.R: The liquid metal embritllement of 9Cr
steel in sodium environments and the role of non-metallic impurities”, Corrosion science, Vol. 36, N° 4 (1994) pp. 593- 610.
12 Borgstedt, H.U., Mathews, C.K., Applied chemistry of the alkali metals. Plenum Press, New-York. 1987 (ISBN 13: 9780306423260)
13 Barkia, B., Auger, T., Courouau, J.L., Bourgon, J., 2017. Multiscale investigation of crack path and microstructural changes during liquid metal embrittlement of 304L austenitic steel in liquid sodium. Corrosion Science 127 (2017) 213–221.
14 Borstedt, H.U.; Champeix, L.: Corrosion in fast breeder reactors”, EFC publication N°1, The Institute of Metals, London, 1989, ISBN 0-901462-73-X.
15 Kimura, A.; Kasada, R.; Kohyama, A.; Konishi, S.; Enoeda; M.; Akiba, M.; Jitsukawa, S.; Ukai, S.; Terai, T.; Sagara, A.: Ferritic steel-blanket systems integration R&D—Com-patibility assessment, Fusion Engineering and Design 81 (2006) 909–916
16 Hémery, S.; Auger, T.; Courouau, J.L.; Balbaud-Célérier, F.: Liquid metal embrittlement of an austenitic stainless steel in liquid sodium. Corrosion Science 83 (2014) 1–5
17 Barkia, B.; Auger, T.; Courouau, J.-L.; Bourgon, J., 2017. Wetting by liquid sodium and fracture path analysis of sodium induced embrittlement of 304L stainless steel. Journal of Materials Research 1–9. https://doi.org/10.1557/jmr.2017.435
18 Balbaud-Celerier, F.; Martinelli, L.: Phénomènes de corrosion dans les métaux liquides. Techniques de l’Ingénieur, Paris, COR640 V1, mars 2017
19 Courouau, J.L.; Robin, R.: Chemistry control analysis of lead alloys systems to be used as nuclear coolant or spallation target. Journal of nuclear materials, Vol. 335 (2004), pp. 264-269.
20 Weeks, J.R.; Isaacs, H.S.: A general model for the corrosion of steels in high velocity sodium, in: Jansson, S.A. (Ed.), Presented at the Chemical aspects of corrosion and mass transfer in liquid sodium, The metallurgical Society of the American Institute of Mining, Metallurgical, and Petroleum Engineerst, New-York, 1973, pp. 207–222
21 Kolster, B.H.:Mechanism of Fe and Cr transport by liquid sodium in non-isothermal loop systems. Journal of Nuclear Materials 55 (1975) 155
![Table 1 – Thermo-physical properties of liquid metal coolants compared to water [1, 2, 4]](https://thumb-eu.123doks.com/thumbv2/123doknet/12723187.356800/3.892.115.822.335.615/table-thermo-physical-properties-liquid-metal-coolants-compared.webp)
![Figure 3 – Elligham diagram showing metal oxide stability as function of oxygen potential and temperature [1, 2, 4, 18]](https://thumb-eu.123doks.com/thumbv2/123doknet/12723187.356800/6.892.248.628.103.503/figure-elligham-diagram-showing-stability-function-potential-temperature.webp)
![Figure 4 - Backscattered-electron image of a cross-section of a steel (T91) immersed for 3600 hours into oxygen-saturated Pb-Bi alloy at 470 °C [4]](https://thumb-eu.123doks.com/thumbv2/123doknet/12723187.356800/7.892.220.682.105.467/figure-backscattered-electron-image-section-immersed-oxygen-saturated.webp)