© The Author 2015. Published by Oxford University Press on behalf of the Maryland Psychiatric Research Center. All rights reserved. For permissions, please email: journals.permissions@oup.com
Nonverbal Social Communication and Gesture Control in Schizophrenia
Sebastian Walther*,1, Katharina Stegmayer1, Jeanne Sulzbacher1, Tim Vanbellingen2,3, René Müri2,4,
Werner Strik1, and Stephan Bohlhalter2,3
1University Hospital of Psychiatry, Bern, Switzerland; 2Department of Clinical Research, University Hospital, Inselspital, Bern,
Switzerland; 3Neurology and Neurorehabilitation Center, Kantonsspital Luzern, Luzern, Switzerland; 4Department of Neurology,
University Hospital, Inselspital, Bern, Switzerland
*To whom correspondence should be addressed; University Hospital of Psychiatry, Bolligenstrasse 111, 3000 Bern 60, Switzerland;
tel: +41-31-930-9483, fax: +41-31-930-9958, e-mail: walther@puk.unibe.ch
Schizophrenia patients are severely impaired in nonver-bal communication, including social perception and ges-ture production. However, the impact of nonverbal social perception on gestural behavior remains unknown, as is the contribution of negative symptoms, working memory, and abnormal motor behavior. Thus, the study tested whether poor nonverbal social perception was related to impaired gesture performance, gestural knowledge, or motor abnormalities. Forty-six patients with schizophre-nia (80%), schizophreniform (15%), or schizoaffective disorder (5%) and 44 healthy controls matched for age, gender, and education were included. Participants com-pleted 4 tasks on nonverbal communication including nonverbal social perception, gesture performance, gesture recognition, and tool use. In addition, they underwent comprehensive clinical and motor assessments. Patients presented impaired nonverbal communication in all tasks compared with controls. Furthermore, in contrast to controls, performance in patients was highly correlated between tasks, not explained by supramodal cognitive deficits such as working memory. Schizophrenia patients with impaired gesture performance also demonstrated poor nonverbal social perception, gestural knowledge, and tool use. Importantly, motor/frontal abnormalities nega-tively mediated the strong association between nonverbal social perception and gesture performance. The factors negative symptoms and antipsychotic dosage were unre-lated to the nonverbal tasks. The study confirmed a gener-alized nonverbal communication deficit in schizophrenia. Specifically, the findings suggested that nonverbal social perception in schizophrenia has a relevant impact on ges-tural impairment beyond the negative influence of motor/ frontal abnormalities.
Key words: social cognition/negative symptoms/
pantomime/imitation
Introduction
Social impairments are a central feature of schizophre-nia, and social cognition has been suggested as determi-nant for functional outcome.1,2 Social cognition includes processes of social interaction, ie, perception, interpreta-tion, and responding to social relevant stimuli.3 Various domains of nonverbal communication are impaired in schizophrenia, such as facial emotion recognition4 and imitation,5 recognition of emotional prosody,6 use of co-speech gestures,7,8 and imitation of hand gestures.9,10
Successful nonverbal communication relies on both correct perception and expression of information. How nonverbal perception and expression influence each other in schizophrenia is poorly understood, as are the asso-ciations with clinical phenomena. Gestures are heteroge-neous, complex expressive behaviors that may accompany speech including movements of fingers, hands, and arms. They may substitute or aid language comprehension and provide clues on cognitive action representation.11–13 Here, we refer to hand and finger gestures related to transitive and intransitive symbolic information. Transitive ges-tures are tool related and require simulating the specific action in absence of the object (eg, signaling the use of a comb or a hammer). Intransitive gestures convey highly overlearned, emblematic information (eg, signaling stop or waving good bye). Both transitive and intransitive ges-tures are important components of everyday nonverbal communication. Schizophrenia patients use spontaneous hand gestures less frequently than healthy subjects.7,8 In hand imitation tasks, patients perform less accurate than controls.5,9,14 First studies report clear-cut gestural deficits in up to 67% of schizophrenia patients.14 These gestural deficits encompass spatial and temporal errors as well as body part as object errors (eg, using the index finger when asked to demonstrate how to use a tooth brush).10,14,15 Gesture deficits in schizophrenia have been linked to
negative symptoms, motor abnormalities (ie, parkinson-ism and catatonia), frontal lobe dysfunction, and work-ing memory impairments.5,8–10,14
Nonverbal social perception relates to the decoding of social relevant emotional information from various nonverbal cues such as facial affect, prosody, and body gestures.16 Deficits of nonverbal social perception have been demonstrated in schizophrenia by using multimodal tasks,16 including poor recognition of hand gestures. In fact, schizophrenia patients tend to misinterpret hand gestures.17 Poor nonverbal social perception was reported to correlate with conceptual disorganization, but not with negative or positive symptoms.16
Correct gesture use is thought to rely on action rep-resentation and knowledge of the symbolic content of gestures.11 Thus, to understand gestural deficits in schizo-phrenia, we need to test the association between nonverbal social perception, gestural knowledge, and gesture pro-duction. As mentioned above, clinical phenomena such as working memory deficits, negative symptoms, and motor abnormalities hamper gesture production in schizophre-nia. Their contribution to gestural knowledge and nonver-bal social perception requires elucidation. Finally, it has to be clarified whether impairments in the performance of transitive gestures resemble deficits in symbolic representa-tion of acrepresenta-tion rather than true impairments of acrepresenta-tion. In other words, do patients suffer from impaired simulation of tool use or from actual defective tool use? For instance, apraxic stroke patients also perform poorly when panto-miming tool use but benefit from the physical properties of the tool during demonstration and actual use.18
The present study aimed to investigate whether ges-ture deficits in schizophrenia were related to nonver-bal social perception, gesture knowledge, or actual tool use. Furthermore, we investigated the impact of clinical symptoms such as negative symptoms, working memory impairment, and motor abnormalities (parkinsonism, neurological soft signs [NSS], dyskinesia, and catatonia) on domains of nonverbal communication. We hypoth-esized that schizophrenia patients were impaired in all tasks on nonverbal communication (nonverbal social perception, gesture performance, gesture knowledge, and tool use). Furthermore, we expected poor nonverbal social perception and presence of motor abnormalities to impair gesture performance in schizophrenia.
Methods
Subjects
In total, 46 patients and 44 healthy control subjects matched for age, gender, and education were included in this study. Subjects were recruited from the inpatient and outpatient departments of the University Hospital of Psychiatry, Bern, Switzerland. Healthy controls were recruited among staff and via advertisement. All subjects were right handed as determined by the Edinburgh handedness inventory.19
Exclusion criteria included substance abuse or dependence other than nicotine; past or current medical or neurologi-cal condition impairing movements, such as dystonia, idio-pathic parkinsonism, or stroke; and history of head trauma with concurrent loss of consciousness. Exclusion criterion for patients was a history of electroconvulsive treatment. Exclusion criteria for controls were a history of any psy-chiatric disorder as well as any first-degree relatives with schizophrenia or schizoaffective disorder.
All participants were interviewed with the Mini International Neuropsychiatric Interview.20 Patients were further interviewed with the Comprehensive Assessment of Symptoms and History.21 Diagnoses were given according to Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria (n = 37 schizophrenia, n = 2 schizoaf-fective disorder, and n = 7 schizophreniform disorder). All but 4 patients received antipsychotic pharmacotherapy. Clinical and demographic data are given in table 1. All par-ticipants provided written informed consent. The protocol was approved by the local ethics committee.
Procedures
Nonverbal Communication Tests. Participants
under-went behavioral tests on 4 tasks on nonverbal com-munication related to hand gestures (for detailed
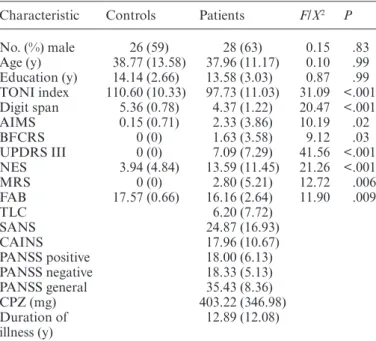
Table 1. Demographic and Clinical Characteristics, Mean (SD)
Characteristic Controls Patients F/X2 P
No. (%) male 26 (59) 28 (63) 0.15 .83 Age (y) 38.77 (13.58) 37.96 (11.17) 0.10 .99 Education (y) 14.14 (2.66) 13.58 (3.03) 0.87 .99 TONI index 110.60 (10.33) 97.73 (11.03) 31.09 <.001 Digit span 5.36 (0.78) 4.37 (1.22) 20.47 <.001 AIMS 0.15 (0.71) 2.33 (3.86) 10.19 .02 BFCRS 0 (0) 1.63 (3.58) 9.12 .03 UPDRS III 0 (0) 7.09 (7.29) 41.56 <.001 NES 3.94 (4.84) 13.59 (11.45) 21.26 <.001 MRS 0 (0) 2.80 (5.21) 12.72 .006 FAB 17.57 (0.66) 16.16 (2.64) 11.90 .009 TLC 6.20 (7.72) SANS 24.87 (16.93) CAINS 17.96 (10.67) PANSS positive 18.00 (6.13) PANSS negative 18.33 (5.13) PANSS general 35.43 (8.36) CPZ (mg) 403.22 (346.98) Duration of illness (y) 12.89 (12.08)
Note: AIMS, Abnormal Involuntary Movement Scale; BFCRS, Bush Francis Catatonia Rating Scale; UPDRS III, motor part of the Unified Parkinson’s Disease Rating Scale; NES, Neurological Evaluation Scale; MRS, Modified Rogers Scale; FAB, Frontal Assessment Battery; TLC, Thought Language and Communication Scale; SANS, Scale for the Assessment of the Negative Syndrome; CAINS, Clinical Assessment Interview for Negative Symptoms; PANSS, Positive and Negative Syndrome Scale; P values adjusted for multiple comparisons.
description, see supplementary material). In all tasks, higher scores indicate superior performance. The test of upper limb apraxia (TULIA)22 is a comprehensive assessment of gesture production in 2 domains: fol-lowing demonstration by the examiner (imitation) or on verbal command (pantomime). Performance of 48 items was videotaped. Evaluation rated content and temporal-spatial errors (for details, see supplementary
material). Total scores range 0–240. All ratings were
performed by a single rater blind to diagnoses and clini-cal presentation (S.W.), who had been trained by the test developers (T.V. and S.B.). Intraclass correlations exceeded .83.
The Tool-Use test18 additionally examines the demon-stration and actual use of tools. Specifically, 3 conditions using a scoop and a hammer are evaluated: pantomime (without the tool), demonstration (with the tool), and actual use (with a recipient object). Each tool is tested in 3 trials per condition. Performance was videotaped and later evaluated considering grip formation, move-ment execution, movemove-ment direction, and spatial errors. Total scores range 0–72. Tool use was evaluated by 2 rat-ers blind to diagnoses and clinical presentation, who had been extensively trained. Interrater reliability met intra-class correlations of .86.
The modified postural knowledge task (PKT)23,24 is a gesture recognition task. Participants were presented with cartoons of persons carrying out 10 intransitive and 10 transitive actions, while the distal parts of the execut-ing limbs are not shown. Below each cartoon, 3 images of limb positions are given including the correct one. Participants have to choose the correct match. In addi-tion, 10 images of 3 hands holding objects were presented, again with 2 versions of incorrect grip and position and 1 correct. Total scores range 0–30.
We applied the Mini Profile of Nonverbal Sensitivity (Mini-PONS)25 to test social perception. The Mini-PONS includes 64 scenes from the original Mini-PONS,26 in which short videos of 2 s each present a white woman with altering facial expression, voice intonation, and/ or bodily gestures. Participants had to choose from 2 options the one that best describes the observed situa-tion immediately after watching the video, eg, saying a prayer or talking to a lost child. The total scores range 0–64.
Clinical Assessments. Furthermore, we investigated
motor abnormalities in the participants using clinical rating scales to assess abnormal involuntary movements, parkinsonism, NSS, and catatonic behavior. We applied the Abnormal Involuntary Movement Scale (AIMS),27 the motor part of the Unified Parkinson’s Disease Rating Scale (UPDRS III),28 the Neurological Evaluation Scale (NES),29 the Bush Francis Catatonia Rating Scale (BFCRS),30 and the Modified Rogers Scale.31 In addi-tion, we applied the Frontal Assessment Battery (FAB).32
Verbal working memory was assessed with the digit span backward from the Wechsler Memory Scale.33
In all motor rating scales, higher scores indicate the presence of motor abnormalities. In contrast, in the FAB and digit span, higher scores indicate superior perfor-mance. In all subjects, nonverbal intelligence was mea-sured with the Test of Nonverbal Intelligence (TONI).34
In the patients, we further assessed the Positive and Negative Syndrome Scale (PANSS)35 and the Thought Language and Communication scale36 to monitor formal thought disorder and 2 scales for negative syndrome sever-ity: the Scales for the Assessment of Negative Syndrome (SANS)37 and the Clinical Assessment Interview for Negative Symptoms (CAINS).38 The clinical assess-ments have been performed by a single expert psychiatrist (K.S.), who had been trained by the senior investigator to achieve interrater reliabilities of κ > .80.
The total assessment had approximately 5 hours dura-tion for patients and approximately 4 hours for controls. In many subjects, tests were performed on 2 consecutive days.
Statistical Analyses
Demographic and clinical data were compared between groups using ANOVAs or chi-square tests where appro-priate. In patients, the clinical rating scales were subject to principal component analysis (PCA) extracting com-ponents with eigenvalues > 1 and subsequent varimax rotation (for details, see supplementary material). PCA yielded 4 factors explaining a sum of 83.5% of the vari-ance: (1) negative symptoms (30.1%, including PANSS negative, PANSS general, CAINS, and SANS), (2) motor/ frontal abnormalities (25.7%, including UPDRS motor part, NES, BFCRS, FAB, and digit span backward), (3) positive symptoms/working memory (14.0%, including PANSS positive, PANSS general, and digit span back-ward), and (4) dyskinesia/catatonia (13.7%, including AIMS and BFCRS). Factor scores were extracted for further analyses.
First, we compared total scores of the tasks on non-verbal communication (TULIA, PKT, PONS, and Tool-Use) between groups using ANCOVAs controlling for TONI index score and digit span, as groups differed in these variables (table 1). Second, we explored whether performance in one of the nonverbal communication tasks was related to the performance of the other tasks for both groups separately. In controls, we were interested in whether associations were found in the absence of major motor or cognitive impairments. As both medication and age were shown to influence gesture performance and rec-ognition,10,14,39 we calculated the correlations between the 4 tasks using within-group partial correlations correcting for age and chlorpromazine equivalents (CPZ) in patients and correcting for age in controls. Third, we investi-gated within-group associations between the 4 nonverbal
communication tasks and the 4 clinical factors, age, and CPZ in stepwise regression analyses. Finally, we explored the association between nonverbal social perception and gesture performance using (1) partial correlations cor-recting for age, CPZ, and the factor motor/frontal abnor-malities, (2) a series of regression models to test whether motor/frontal abnormalities were mediating or moder-ating this association, and (3) a hierarchical regression analysis, in which motor/frontal abnormalities were the first step and nonverbal social perception was the second step to determine the effect on gesture performance. All analyses were conducted in SPSS22 (IBM). Tests were corrected for multiple comparisons (Pcorr = P × n), with n being the number of tests.
Results
Between-Group Differences in Nonverbal Communication
Controls had superior performance in the nonverbal intel-ligence test and the digit span backward and less motor abnormalities and frontal lobe dysfunction compared with schizophrenia patients (table 1). Patients had inferior performance in all tasks of nonverbal communication
(table 2) when controlling for nonverbal intelligence and
working memory. Applying the cutoff scores,14,18 45.7% of schizophrenia patients had gesture performance defi-cits (47.8% pantomime and 32.6% imitation) and 37.8% were impaired in the Tool Use task (37.8% pantomime, 11.1% demonstration, and 11.1% use).
Within-Group Correlations
Controls. In controls, none of the nonverbal
commu-nication tasks correlated significantly with any other. Partial correlations corrected for age indicated that gesture performance, gestural knowledge, and nonver-bal social perception were related to higher IQ (
supple-mentary table S2). In contrast, gestural knowledge and
nonverbal social perception were correlated with longer duration of education, and gesture performance was correlated with better working memory performance.
Finally, tool use performance was unrelated to any of the descriptive variables.
Patients. In patients, the performance between each
of the nonverbal communication tasks was strongly cor-related (table 3), except the correlation between nonver-bal social perception (PONS) and tool use at trend level. Particularly, gesture performance was closely linked to gestural knowledge, tool use, and nonverbal social per-ception. The correlations between communication tasks remained significant even when correcting for verbal working memory impairments, age, and CPZ (
supple-mentary table S3). Furthermore, nonverbal
communica-tion task performances demonstrated significant partial correlations with clinical variables when correcting for age and CPZ (supplementary tables S4 and S5).
Next, the contributions of age, CPZ, and the 4 clini-cal factors to the 4 nonverbal communication tasks were tested using stepwise linear regression models (table 4). All 4 tasks were associated with motor/frontal abnormal-ities. Beyond these associations, gesture performance was related to age, positive symptoms, and the dyskinesia/ catatonia factor. Furthermore, nonverbal social percep-tion was explained by positive symptoms/working mem-ory and tool use by dyskinesia/catatonia. The negative symptom factor and CPZ were excluded in each model.
Finally, we explored whether intact nonverbal social perception was critical for correct performance of hand gestures and whether motor/frontal abnormalities may hamper this association. Partial correlations suggested that nonverbal social perception was associated with better performance of hand gestures (r = 0.34, P = .04) when cor-recting for age, CPZ, and the factor motor/frontal abnor-malities. Figure 1 depicts the mediator analysis. In patients, superior nonverbal social perception is associated with cor-rect performance of hand gestures (top panel). Although adding motor/frontal abnormalities to the model decreased the effect of nonverbal social perception on gesture perfor-mance, the association still remained significant, while the explained variance was increased (lower panel). In other words, motor/frontal abnormalities only partially medi-ated the effect of nonverbal social perception on gesture performance. In addition, there was no interaction effect of
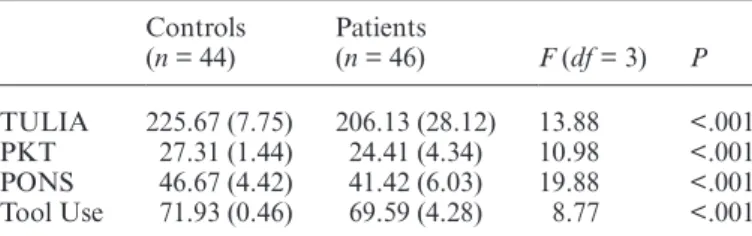
Table 2. Group Comparisons of Task Performance, Mean (SD) With Covariates TONI and Digit Span Backward
Controls (n = 44) Patients (n = 46) F (df = 3) P TULIA 225.67 (7.75) 206.13 (28.12) 13.88 <.001 PKT 27.31 (1.44) 24.41 (4.34) 10.98 <.001 PONS 46.67 (4.42) 41.42 (6.03) 19.88 <.001 Tool Use 71.93 (0.46) 69.59 (4.28) 8.77 <.001
Note: TONI, Test of Nonverbal Intelligence; TULIA, test of upper limb apraxia; PKT, postural knowledge task; PONS, Profile of Nonverbal Sensitivity; P values adjusted for multiple comparisons.
Table 3. Partial Correlations Between Tasks in Patients (n = 46), Corrected for Age and CPZ
PONS PKT Tool Use
r P r P r P
TULIA .57 <.001 .76 <.001 .75 <.001
PONS .52 .003 .38 .07
PKT .51 .005
Note: Abbreviations are explained in the first footnote to table 2.
nonverbal social perception and motor/frontal abnormali-ties on gesture performance (data not shown). Hierarchical regression analysis indicated that nonverbal social percep-tion had an effect on gesture performance beyond motor/ frontal abnormalities (model 1: motor/frontal abnormali-ties R2 = .48, F
(change)[1, 44] = 40.39, P(change) < .001, motor/
frontal abnormalities β = −.69, P < .001; model 2: non-verbal social perception ΔR2 = .10, F
(change)[2, 43] = 9.75,
P(change) = .003, motor/frontal abnormalities β = −.50, P < .001, nonverbal social perception β = .37, P = .003). Discussion
The present study on nonverbal communication deficits in schizophrenia yielded 4 main findings. First, we con-firmed impairments in nonverbal social perception and gesture production in schizophrenia.1,10,14,16,40 In addition, patients had deficits in gestural knowledge and actual tool use. Second, as hypothesized, in schizophrenia, deficits in
gesture performance were associated with impairments in nonverbal social perception, gesture knowledge, and tool use. In contrast, performance in these tasks was not corre-lated in controls. Third, motor/frontal abnormalities were the common factors associated with poor performance in all 4 nonverbal tasks in patients. Still, beyond motor abnormalities, the tasks were associated with distinct clini-cal factors. Fourth, poor nonverbal social perception was associated with impaired gesture performance and this association was only partially mediated by motor/frontal abnormalities. In contrast, a smaller ecological study failed to find an association between nonverbal social perception and spontaneous gesture use in schizophrenia.8
Motor Abnormalities Impaired Nonverbal Communication
Motor abnormalities are an intrinsic dimension of schizophrenia present even before the onset of the full blown disorder and often deteriorated by antipsychotic treatment.41–44 The motor phenomena include catato-nia, parkinsonism, abnormal involuntary movements, and NSS.42 We hypothesized that motor abnormalities would contribute to nonverbal social communication, particularly to expressive gestures or body posture and action imitation. Indeed, the factor motor/frontal abnor-malities (including parkinsonism, NSS, catatonia, frontal dysfunction, and working memory) correlated inversely with all tasks. In addition, the dyskinesia/catatonia fac-tor also correlated inversely with gesture production and tool use. Thus, the presence of motor/frontal abnor-malities impairs perception and expression of nonverbal communication in schizophrenia. Impaired gesture per-formance has been linked to motor abnormalities and frontal lobe dysfunction before.10 Consequently, patients with motor abnormalities are prone to impairments in nonverbal communication. This is not only relevant for chronic schizophrenia but also for subjects at risk for psychosis and first episode patients with schizophrenia. Both groups may experience motor abnormalities45,46
Table 4. Factors Impacting Nonverbal Communication Tasks
Model Rcorr 2 df F P Predictor β T P TULIA .60 4, 41 17.80 <.001 Motor/frontal −.56 −5.39 <.001 Age −.27 −2.60 .01 Positive/WM −.22 −2.33 .03 Dyskinesia/catatonia −.22 −2.28 .03 PONS .46 2, 43 20.06 <.001 Motor/frontal −.53 −4.81 <.001 Positive/WM −.45 −4.08 <.001 PKT .23 1, 44 14.37 <.001 Motor/frontal −.50 −3.79 <.001
Tool use .54 2, 43 27.41 <.001 Motor/frontal −.69 −6.78 <.001
Dyskinesia/catatonia −.26 −2.53 .02
Note: Abbreviations are explained in the first footnote to table 2. WM, working memory. Collinearity statistics: tolerance > 0.82, variance
inflation factor < 1.22.
Fig. 1. Association of social perception, motor/frontal abnormalities, and gesture performance. Upper panel: direct association between nonverbal social perception (PONS) and gesture performance (TULIA). Lower panel: inclusion of the mediator motor/frontal abnormalities (motor/frontal factor). Numbers indicate beta-weights. Note that the association between nonverbal social perception and gesture performance is weaker in the lower panel, suggesting a partial mediator effect of motor/ frontal abnormalities.
and deficits in social cognition.47 Therefore, it is conceiv-able that some of the social cognitive impairments are related to motor abnormalities. Indeed, young patients with schizotypal disorder use gestures less frequently than controls.48 Furthermore, subjects at ultrahigh risk of psychosis demonstrate content errors of spontaneous co-speech gestures.49
Link of Social Perception and Gesture Control: Mirror Neurons and Theory of Mind
Part of the strong relationship of social perception and gesture control was independent of motor/frontal abnor-malities as demonstrated by the mediation analysis. Furthermore, hierarchical regression confirmed that the impact of nonverbal social perception on gesture perfor-mance was greater than the negative impact of motor/ frontal abnormalities although regression models are not suited to finally prove causal relationships. In case a patient had sufficient nonverbal social perception abilities, the presence of significant motor/frontal abnormalities would, therefore, impair but not perish gesture performance.
A possible link between social perception and gesture control may be viewed in the light of embodied cogni-tion, in which gestures were suggested to mediate between action and its mental representation.11 Particularly, the mirror mechanism indicates that motor acts of others are understood by the same mechanism underlying the execution of these motor acts. Furthermore, the mirror mechanism of motor behavior is critical for the interpre-tation of goals and intentions of others.50 Another model claims that action semantics, ie, knowledge on specific actions and their meaning, is critical to understand action of others.51 Thus, the knowledge of the abstract meaning of gestures along with the action representation must, therefore, be critical for correct gesture performance.
The brain areas involved in gesture production, gesture observation, action imitation, and action observation are broadly the same: inferior frontal gyrus (IFG), insula and inferior parietal lobule (IPL).24,52–55 Imitation and observa-tion of hand gestures activate the right IPL and the medial prefrontal cortex, thus engaging the mirror neuron sys-tem and the mentalizing syssys-tem.55 In schizophrenia, aber-rant neural activation was detected during the processing of metaphoric gestures in the left IFG and left superior temporal sulcus (STS).56,57 Likewise, patients had abnor-mal activation during imitation and observation of fin-ger movements within the IPL and STS.58 Therefore, the cerebral motor system including cortical premotor areas may contribute to social cognitive deficits in schizophrenia during the perception, interpretation, and production of actions such as hand gestures and body postures.
Current psychological treatment programs to enhance social cognition efficiently improve emotion recognition but fail to impact more complex measures of nonverbal social perception.59 A specific add-on training of hand
gestures may increase and generalize the effects of cur-rent social skills training approaches. Furthermore, our data suggest considering some aspects of motor behavior when assessing social cognition. In fact, hand gestures and head and body movements are used for nonverbal expression in social interactions.
Negative Symptoms and Working Memory in Nonverbal Communication
Negative symptoms could have impact on social interac-tion, thus impair social cognition.3,60 The results of our study were conflicting: the negative factor of the PCA failed to correlate with any nonverbal communication task. However, in line with previous reports, impaired ges-ture performance, tool use, and gestural knowledge corre-lated with increased ratings in CAINS, SANS, and PANSS negative.5,9,10,14 Still, nonverbal social perception (PONS) lacked correlation with negative symptoms, as in other studies.1,16 The discrepancy between tests might be due to the composition of the negative factor in the PCA. In sum, our results argue against a general impact of negative symptoms on nonverbal communication in schizophrenia. Instead, negative symptoms may impact expression but not perception of nonverbal social interaction.
Schizophrenia has been associated with a general-ized supramodal impairment in working memory.61 In fact, imitation of hand gestures was linked to working memory in schizophrenia before.9 However, our results argue against a specific effect on gesture production and in favor of a generalized effect on nonverbal communica-tion. Indeed, the test of verbal working memory corre-lated with each of the 4 tasks (supplementary table S4). Furthermore, working memory was part of the factor motor/frontal abnormalities that correlated with all non-verbal tasks. The correlations between the tasks remain significant in schizophrenia even when controlling for working memory (supplementary table S3).
In controls, we found no correlation between the 4 nonverbal communication tasks. This lack of association would argue for distinct processes and abilities between gesture performance, gesture knowledge, tool use, and social perception. The scores of the gesture knowledge (PKT) and the Tool Use task clearly demonstrate a ceiling effect because these tests were developed for use in elderly subjects with apraxia.18,23,39,62 However, tasks of nonver-bal social perception (PONS) and gesture performance (TULIA) were designed to avoid ceiling effects and have sufficient variance in our sample. Still, nonverbal social perception and gesture performance were unrelated in healthy subjects in contrast to patients with schizophrenia.
Patients and controls were well matched for age, gender, and educational level. However, they still differed in nonver-bal IQ and working memory performance. Therefore, we had to include these parameters as covariates in the group comparisons. We investigated a heterogeneous group of
patients concerning age, duration of illness, and symptom severity. Therefore, gesture performance was better in this sample than in our previous study.14 In order to account for effects of treatment and age, CPZ and age were control vari-ables in partial correlation analyses. Of course, controlling for antipsychotic medication dose will not exclude medica-tion effects. Furthermore, although we excluded patients with current comorbid disorders based on diagnostic inter-views, we cannot completely exclude comorbidity effects of subsyndromal disturbances or undeclared past disorders. Conclusion
In conclusion, schizophrenia patients presented general-ized impairments in 4 tasks of nonverbal communication. In addition, patients with motor/frontal abnormalities had more difficulties in the tasks. Finally, irrespective of the neg-ative influence of motor/frontal impairments, there was a strong association between nonverbal social perception and gesture performance pointing to a mirror mechanism of gesture behavior. Future studies should focus on the under-lying brain alterations and establish whether specific inter-ventions on motor abnormalities may aid social cognition. Supplementary Material
Supplementary material is available at
http://schizophre-niabulletin.oxfordjournals.org.
Funding
This study received funding from the Bangerter-Rhyner Foundation (to S.W.) and the Swiss National Science Foundation (SNF grant 152619/1 to S.W. and S.B.). Acknowledgments
The authors thank Ms Nora Schaub who rated the Tool Use videos. The authors have declared that there are no conflicts of interest in relation to the subject of this study. References
1. Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: mod-eling the role of ability and motivation. Arch Gen Psychiatry. 2012;69:1216–1224.
2. Schmidt SJ, Mueller DR, Roder V. Social cognition as a medi-ator variable between neurocognition and functional out-come in schizophrenia: empirical review and new results by structural equation modeling. Schizophr Bull. 2011;37(suppl 2):S41–S54.
3. Green MF, Penn DL, Bentall R, et al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008;34:1211–1220.
4. Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019.
5. Park S, Matthews N, Gibson C. Imitation, simulation, and schizophrenia. Schizophr Bull. 2008;34:698–707.
6. Bach DR, Buxtorf K, Grandjean D, Strik WK. The influ-ence of emotion clarity on emotional prosody identification in paranoid schizophrenia. Psychol Med. 2009;39:927–938. 7. Troisi A, Spalletta G, Pasini A. Non-verbal behaviour deficits
in schizophrenia: an ethological study of drug-free patients. Acta Psychiatr Scand. 1998;97:109–115.
8. Lavelle M, Healey PG, McCabe R. Is nonverbal communica-tion disrupted in interaccommunica-tions involving patients with schizo-phrenia? Schizophr Bull. 2013;39:1150–1158.
9. Matthews N, Gold BJ, Sekuler R, Park S. Gesture imitation in schizophrenia. Schizophr Bull. 2013;39:94–101.
10. Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S. Impaired pantomime in schizophrenia: association with fron-tal lobe function. Cortex. 2013;49:520–527.
11. Cartmill EA, Beilock S, Goldin-Meadow S. A word in the hand: action, gesture and mental representation in humans and non-human primates. Philos Trans R Soc Lond B Biol Sci. 2012;367:129–143.
12. Kelly SD, Ozyürek A, Maris E. Two sides of the same coin: speech and gesture mutually interact to enhance comprehen-sion. Psychol Sci. 2010;21:260–267.
13. Obermeier C, Dolk T, Gunter TC. The benefit of gestures during communication: evidence from hearing and hearing-impaired individuals. Cortex. 2012;48:857–870.
14. Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S. Impaired gesture performance in schizophrenia: particular vulnerability of meaningless pantomimes. Neuropsychologia. 2013;51:2674–2678.
15. Martin P, Tewesmeier M, Albers M, Schmid G, Scharfetter C. Investigation of gestural and pantomime performance in chronic schizophrenic inpatients. Eur Arch Psychiatry Clin Neurosci. 1994;244:59–64.
16. Toomey R, Schuldberg D, Corrigan P, Green MF. Nonverbal social perception and symptomatology in schizophrenia. Schizophr Res. 2002;53:83–91.
17. Bucci S, Startup M, Wynn P, Baker A, Lewin TJ. Referential delusions of communication and interpretations of gestures. Psychiatry Res. 2008;158:27–34.
18. Randerath J, Goldenberg G, Spijkers W, Li Y, Hermsdörfer J. From pantomime to actual use: how affordances can facili-tate actual tool-use. Neuropsychologia. 2011;49:2410–2416. 19. Oldfield RC. The assessment and analysis of handedness: the
Edinburgh inventory. Neuropsychologia. 1971;9:97–113. 20. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The
Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psy-chiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33; quiz 34–57.
21. Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instru-ment for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623.
22. Vanbellingen T, Kersten B, Van Hemelrijk B, et al. Comprehensive assessment of gesture production: a new test of upper limb apraxia (TULIA). Eur J Neurol. 2010;17:59–66. 23. Mozaz M, Rothi LJ, Anderson JM, Crucian GP, Heilman
KM. Postural knowledge of transitive pantomimes and intransitive gestures. J Int Neuropsychol Soc. 2002;8:958–962. 24. Bohlhalter S, Vanbellingen T, Bertschi M, et al. Interference
with gesture production by theta burst stimulation over left inferior frontal cortex. Clin Neurophysiol. 2011;122:1197–1202.
25. Banziger T, Scherer KR, Hall JA, Rosenthal R. Introducing the MiniPONS: a short multichannel version of the Profile of Nonverbal Sensitivity (PONS). J Nonverbal Behav. 2011;35:189–204.
26. Rosenthal R, Hall JA, DiMatteo MR, Rogers PL, Archer D. Sensitivity to Nonverbal Communication: The PONS Test. Baltimore, MD: John Hopkins University Press; 1979. 27. Guy W. ECDEU Assessment Manual for Psychopharmacology.
Rockville, MD: US Department of Health, Education and Welfare; 1976.
28. Fahn S, Elton RL, Members UP. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent Developments in Parkinson’s Disease. Vol 2. Florham Park, NJ: Macmillan Healthcare Information; 1987. 29. Buchanan RW, Heinrichs DW. The Neurological Evaluation
Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res. 1989;27:335–350.
30. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93:129–136.
31. Lund CE, Mortimer AM, Rogers D, McKenna PJ. Motor, volitional and behavioural disorders in schizophrenia. 1: assessment using the Modified Rogers Scale. Br J Psychiatry. 1991;158:323–327, 333–336.
32. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626.
33. Wechsler D. Wechsler Memory Scale (WMS-III). San Antonio, TX: Psychological Corporation; 1997.
34. Brown L, Sherbenou RJ, Johnsen SK. Test of Nonverbal Intelligence. Austin, TX: PRO-ED; 1982.
35. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276.
36. Andreasen NC. Scale for the assessment of thought, language, and communication (TLC). Schizophr Bull. 1986;12:473–482. 37. Andreasen NC. The Scale for the Assessment of Negative
Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;49–58.
38. Forbes C, Blanchard JJ, Bennett M, Horan WP, Kring A, Gur R. Initial development and preliminary validation of a new negative symptom measure: the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res. 2010;124:36–42.
39. Mozaz MJ, Crucian GP, Heilman KM. Age-related changes in arm-hand postural knowledge. Cogn Neuropsychol. 2009;26:675–684.
40. Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006;163:448–454.
41. Whitty PF, Owoeye O, Waddington JL. Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull. 2009;35:415–424.
42. Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66:77–92.
43. Morrens M, Hulstijn W, Sabbe B. Psychomotor slowing in schizophrenia. Schizophr Bull. 2007;33:1038–1053.
44. Walther S, Ramseyer F, Horn H, Strik W, Tschacher W. Less structured movement patterns predict severity of positive
syndrome, excitement, and disorganization. Schizophr Bull. 2014;40:585–591.
45. Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psy-chotic symptoms in adolescents at high risk of schizophrenia. Arch Gen Psychiatry. 2008;65:165–171.
46. Peralta V, Campos MS, De Jalón EG, Cuesta MJ. Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Mov Disord. 2010;25:1068–1076.
47. Green MF, Bearden CE, Cannon TD, et al. Social cognition in schizophrenia, Part 1: performance across phase of illness. Schizophr Bull. 2012;38:854–864.
48. Mittal VA, Tessner KD, McMillan AL, Delawalla Z, Trotman HD, Walker EF. Gesture behavior in unmedicated schizo-typal adolescents. J Abnorm Psychol. 2006;115:351–358. 49. Millman ZB, Goss J, Schiffman J, Mejias J, Gupta T, Mittal
VA. Mismatch and lexical retrieval gestures are associated with visual information processing, verbal production, and symptomatology in youth at high risk for psychosis. Schizophr Res. 2014;158:64–68.
50. Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci. 2010;11:264–274.
51. Buxbaum LJ, Kalénine S. Action knowledge, visuomotor activation, and embodiment in the two action systems. Ann N Y Acad Sci. 2010;1191:201–218.
52. Bohlhalter S, Hattori N, Wheaton L, et al. Gesture subtype-dependent left lateralization of praxis planning: an event-related fMRI study. Cereb Cortex. 2009;19:1256–1262. 53. Fridman EA, Immisch I, Hanakawa T, et al. The role of
the dorsal stream for gesture production. Neuroimage. 2006;29:417–428.
54. Goldenberg G. Apraxia and the parietal lobes.
Neuropsychologia. 2009;47:1449–1459.
55. Mainieri AG, Heim S, Straube B, Binkofski F, Kircher T. Differential role of the Mentalizing and the Mirror Neuron system in the imitation of communicative gestures. Neuroimage. 2013;81:294–305.
56. Straube B, Green A, Sass K, Kirner-Veselinovic A, Kircher T. Neural integration of speech and gesture in schizophrenia: evidence for differential processing of metaphoric gestures. Hum Brain Mapp. 2013;34:1696–1712.
57. Straube B, Green A, Sass K, Kircher T. Superior temporal sulcus disconnectivity during processing of metaphoric ges-tures in schizophrenia. Schizophr Bull. 2014;40:936–944. 58. Thakkar KN, Peterman JS, Park S. Altered brain activation
during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am J Psychiatry. 2014;171:539–548.
59. Horan WP, Kern RS, Tripp C, et al. Efficacy and specific-ity of social cognitive skills training for outpatients with psy-chotic disorders. J Psychiatr Res. 2011;45:1113–1122. 60. Ventura J, Wood RC, Hellemann GS. Symptom domains and
neurocognitive functioning can help differentiate social cog-nitive processes in schizophrenia: a meta-analysis. Schizophr Bull. 2013;39:102–111.
61. Lee J, Park S. Working memory impairments in schizophre-nia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. 62. Randerath J, Li Y, Goldenberg G, Hermsdörfer J. Grasping
tools: effects of task and apraxia. Neuropsychologia. 2009;47:497–505.