HAL Id: hal-03086011
https://hal.archives-ouvertes.fr/hal-03086011v2
Submitted on 13 Apr 2021
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Early Left-Planum Temporale Asymmetry in newborn
monkeys (Papio anubis): A longitudinal structural MRI
study at two stages of development
Yannick Becker, Julien Sein, Lionel Velly, Laura Giacomino, Luc Renaud,
Romain Lacoste, Jean-Luc Anton, Bruno Nazarian, Cammie Berne, Adrien
Meguerditchian
To cite this version:
Yannick Becker, Julien Sein, Lionel Velly, Laura Giacomino, Luc Renaud, et al..
Early
Left-Planum Temporale Asymmetry in newborn monkeys (Papio anubis): A longitudinal
struc-tural MRI study at two stages of development.
NeuroImage, Elsevier, 2021, 227, pp.117575.
ContentslistsavailableatScienceDirect
NeuroImage
journalhomepage:www.elsevier.com/locate/neuroimage
Early
Left-Planum
Temporale
Asymmetry
in
newborn
monkeys
(
Papio
anubis
):
A
longitudinal
structural
MRI
study
at
two
stages
of
development
Yannick
Becker
a,b,
Julien
Sein
b,
Lionel
Velly
b,
Laura
Giacomino
b,
Luc
Renaud
b,
Romain
Lacoste
c,
Jean-Luc
Anton
b,
Bruno
Nazarian
b,
Cammie
Berne
a,
Adrien
Meguerditchian
a,c,∗a Laboratoire de Psychologie Cognitive, UMR 7290, Université Aix-Marseille / CNRS, 13331 Marseille, France b Institut des Neurosciences de la Timone, UMR 7289, Université Aix-Marseille / CNRS, 13005 Marseille, France c Station de Primatologie, CNRS, UPS846, 13790 Rousset, France
a
r
t
i
c
l
e
i
n
f
o
Keywords: Hemispheric specialization Lateralization Language evolution Development MRI Baboona
b
s
t
r
a
c
t
The“language-ready” braintheorysuggeststhattheinfantbrainispre-wiredforlanguageacquisitionprior tolanguageexposure.Asapotentialbrainmarkerof suchalanguagereadiness,a leftwardstructuralbrain asymmetrywasfoundinhumaninfantsforthePlanumTemporale(PT),whichoverlapswithWernicke’sarea.In thepresentlongitudinalinvivoMRIstudyconductedin35newbornmonkeys(Papioanubis),wefoundasimilar leftwardPTsurfaceasymmetry.Follow-uprescanningsessionson29juvenilebaboonsat7-10monthsshowedthat suchanasymmetryincreasesacrossthetwoagesclasses.Theseoriginalfindingsinnon-linguisticprimateinfants stronglyquestiontheideathattheearlyPTasymmetryconstitutesahumaninfant-specificmarkerforlanguage development.SuchasharedearlyperisylvianorganizationprovidesadditionalsupportthatPTasymmetrymight berelatedtoalateralizedsysteminheritedfromourlastcommonancestorwithOld-Worldmonkeysatleast 25–35millionyearsago.
1. Introduction
Languageanditstypicalfunctionalandstructuralasymmetricbrain organizationwereinitiallyconsideredasuniquetoHomosapiens evolu-tion(Crow,2004),suggestingaspecific“language-ready” braindating backto350000ago.Therefore,brainlateralizationinseveralregions washypothesizedasoneofthekeyfeaturesofthelanguage-readybrain, asmosthumansshowagreatercorticalactivationinthelefthemisphere formostlanguagefunctions(Vigneau etal.,2006).Forinstance,the leftPlanumTemporale(PT)-aregionwhichoverlapswithWernicke’s area-wasfoundparticularlyactivatedinavarietyofauditorylanguage processingtaskslikephonologicalauditorydecoding(Shapleskeetal., 1999)andincludingthemainperceptioncomponentoftheaudio-motor loopforphonologicalprocessing(Vigneauetal.,2006).Inthe pioneer-ingworkofGeschwindandLevitsky(1968),aleftward PT asymme-trywasalsofoundattheanatomicallevel,suggestingitsrelationship withfunctionalbrainasymmetryforlanguagetasks(Josseetal.,2006;
Tzourio-Mazoyeretal.,2018).
Abbreviation:PT,PlanumTemporale.
∗Correspondingauthorat:LaboratoiredePsychologieCognitive,UMR7290,Université Aix-Marseille/CNRS,13331Marseille,France.
E-mailaddress:adrien.meguerditchian@univ-amu.fr(A.Meguerditchian).
Additionally, the“language-ready” braintheory suggeststhatthe infant brain is pre-wired for language acquisition (e.g. Dehaene-Lambertz etal.,2002).Indeed,studieshave reportedthatallhuman infantsseemtohaveaninnate,inheritedreadinessforlanguage acqui-sition,independentlyfromculture.Forinstance,newbornsareinitially abletodistinguisheveryphonemebeforeselectivelydiscriminatingonly phonemesrelatedtothelanguagetheyareexposedtoKuhletal.(2008). During their first year, infants will be also sensitive to vocal sounds,their nativeprosodyandvowels,infertheabstractstructure ofspeechandconnectwordstotheirreferents(Dehaene-Lambertzand Spelke,2015).
Theneuralstructureforsuchalanguagereadinessremainsunclear. Nevertheless,whiteandgreymatterorganizationininfantsreveals sim-ilararchitectureincomparisonwithadults(Duboisetal.,2010). Inter-estingly,similartoadults,three-month-oldinfants’BOLDresponsesto speechshowedamorepronouncedactivationofthePTintheleft hemi-sphere(Dehaene-Lambertzetal.,2002),rasingthequestionwhetheror not thePTmightbefunctionallylateralizedfrombirthon.
Addition-https://doi.org/10.1016/j.neuroimage.2020.117575
Received27July2020;Receivedinrevisedform8October2020;Accepted16November2020 Availableonline4December2020
1053-8119/© 2020TheAuthor(s).PublishedbyElsevierInc.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Y. Becker, J. Sein, L. Velly et al. NeuroImage 227 (2021) 117575 ally,structuralPTleftwardasymmetrywasalsoshowninpost-mortem
fe-tusesorinfantbrainsinearlydevelopment(WitelsonandPallie,1973;
Wada,1975;Chietal.,1977)andwithin-vivostructuralMRIimages (Duboisetal.,2010;Hilletal.,2010;Glaseletal.,2011).Sucha struc-turalPTleftasymmetrymaybestablishedduringthelasttrimesterof fetallife(Chietal.,1977)andarelaterincreasingduringdevelopment, suggestingitslinkswithlanguagedevelopment(Chietal.,1977).
However, several studies in nonhuman primates questioned the structural PT asymmetry as a human-specific marker for the brain specialization for language.Manual delineation of post-mortembrain (Gannon et al., 1998) and in-vivoMRI scans(Hopkins etal., 1998;
HopkinsandNir,2010;Marieetal.,2018),showedlargerleftPTsin bothapesandbaboons,suggestingthatthisneuroanatomicalfeatureis sharedalsowithOld-Worldmonkeys.
Whethersuchanearlyneuroanatomicalfeatureextendedtoother nonhumanprimateinfantsisunknownalthoughthiscomparative ques-tionremainscriticalfordetermining itssupposedhumanuniqueness troughevolutionanditsrelationtoapre-wiredbrainforlanguage ac-quisition.
Therefore,theaimofthepresentlongitudinalin-vivoMRIstudyin nonhumanprimatesistoinvestigate,thestructuralneuroanatomicalPT
asymmetriesin35babooninfants(Papioanubis)andits development acrossagethroughmanualdelineationoftheregion’ssurface.The ear-liestpost-natalageclassincludes33newbornsatthecritical neurodevel-opmentalperiodbelow3months(aswellastwo5-monthsoldoutliers) inwhichthesynaptogenesisismaximalandthemyelin,synapsesand cellbodiesarethusnotfullymature(Scottetal.,2016).Thefollow-up MRIlongitudinalscanningandPTdelineationincludes29ofthose35 baboonsattheolderjuvenileageclass(i.e.,from7to10months). 2. Methods
2.1. Subjects
Subjectsrangedfrom4to165daysofage(Mean:32.63;SD:6.13) andincluded21males and14 females.Outofthose35baboons,29 werelaterrescannedasecondtime,rangingfrom218to362daysof age(Mean=278.62;SD=30.11)(seetableinsupplementarymethods withsubjects’details).
AllmonkeysarehousedinsocialgroupsattheStationde Primatolo-gieCNRS(UPS846,Rousset,France)andhavefreeaccesstooutdoor areasconnectedtoindoorareas.Allsubjectsarebornincaptivityfrom 1(F1)or2generations(F2).Woodenandmetallicstructuresenrichthe enclosures.Feedingtimesareheldfourtimesadaywithseeds,monkey pelletsandfreshfruitsandvegetables.Waterisavailableadlibitum.
2.2. Animalhandling
Minimallyinvasivemedicationwasrealized,andnopremedication wasneeded.Mothers fromthenewbornsubjectswerecaptured with theirinfantthenightbeforethescanattheStationdePrimatologiefor check-upsandweretransportedtogetherthefollowingdayoftheMRI session.
Upon arrival at the MRI center, the mother of the focal subject wassedatedwithanintramuscularinjectionofketamine(3mg/kg)and medetomidine(30𝜇g/Kg)aswellastheirfocalinfantifabove5months old. Focal newborn below 5 months were not sedated anddirectly broughttothepreparationroom forthefollowingprocedures. Focal infantswerethenanesthetizedunder6-8%sevofluraneinductionwith amask.Acatheterwastheninsertedintothecaudalarteryforblood-gas sampling,andtrachealintubationwasperformedforsteadycontrolled ventilationusingananestheticventilator(Cato,Drager,Germany). End-tidalcarbondioxidewasmonitoredandusedtoadjustventilationrate (0.2to0.3Hz)andend-tidalvolume.TheanesthesiainsidetheMRI machinewasthenmaintainedusing 3%sevofluranevia acalibrated
vaporizerwithamixtureofair0.75L/minandO20.1L/min).
Periph-eraloxygensaturation,heartrateandbreathingrate,weremonitored throughoutexperiments.
All animal procedures were approved by the “C2EA -71 Ethi-cal Committee of neurosciences” (INTMarseille) under the number APAFIS#13553-201802151547729v4,andhasbeenconductedatthe StationdePrimatologieunderthenumberagreementC130877for con-ductingexperimentsonvertebrateanimals(Rousset-Sur-Arc,France). All methodswereperformedinaccordance withtherelevantFrench law, CNRSguidelinesandtheEuropean Unionregulations(Directive 2010/63/EU).
2.2.1. Imagingprotocol
FromSeptember2017toMarch2020,in-vivoimagingwasperformed usinga3TclinicalMRIscanner(MAGNETOMPrisma,Siemens, Erlan-gen,Germany)equippedwith80mT/mgradients(XR80/200gradient systemwithslewrate200T/m/s)anda2-channelB1transmitarray (TimTX TrueForm).Forthesessionsatt0(“newborn” ageclass)and att1(i.e.,from7to10monthsold),theanimalswerescannedinthe supineposition,withtwo11cmreceive-onlyloopcoils:oneunderthe headandanotheronearoundthefaceoftheanimal.Theholdingofthe twocoilsandtheanimalheadwasprovidedthroughtheuseofa pearl-tecbag(VacFixSystem)andsomestraps.Protectionfornoisereduction wasattachedaroundtheears.AttheendoftheMRIsession,whenfully awakedfromanesthesia, baboonswerecarefully putbackwiththeir motherandthentransportedbackattheStationdePrimatologiefor im-mediate(ordelayed)reintroductionintotheirsocialgroupsunderstaff monitoring.
2.2.2. Structuralacquisitionprotocol
T1w images were acquired using a 3D Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) (Mugler and Brooke-man, 1990) sequence(0.4 mmisotropic, FOV= 103×103×102.4 mm,matrix=256×256slicesperslab=256,sagittalorientation, read-outdirectionofinferior(I)tosuperior(S),phaseoversampling=10%, averages=3,TR=2500ms,TE=3.01ms,TI=900ms,flip-angle=8°, bandwidth=300Hz/pixel,nofatsuppression,pre-scannormalization). T2wimageswereacquiredusingaSamplingPerfectionwithApplication optimizedContrastusingdifferentangleEvolutions(SPACE)sequence (Mugleretal.,2000)(0.4mmisotropic,FOV=154×115.5×102.4 mm,matrix=384×288,sliceperslab=256,sagittalorientation, read-outdirectionItoS,phaseoversampling=0%,averages=2,TR=3200 ms,TE=393ms,bandwidth=566Hz/pixel,nofatsuppression,echo trainlength=790msandpre-scannormalization).Thetotalacquisition timeforstructuralscanswas65min(35minforT1wand30minfor T2w).
2.3. PreprocessingofanatomicalMRI
AnatomicalT2wimagesofthefirstscanningsessionandanatomical T1wimagesofthesecondscanningsessionwerenoisecorrectedwiththe spatiallyadaptivenonlocalmeansdenoisingfilter(Manjónetal.,2010) implementedinCat12toolbox(http://www.neuro.uni-jena.de/cat/) in-cludedinSPM12(http://www.fil.ion.ucl.ac.uk/),whichrunson MAT-LAB(R2014a).
Next,eachimagewasmanuallyorientedusingITK-Snap3.6 accord-inganteriorandposteriorcommissuresplaneandtheinterhemispheric fissureplane.
2.4. ManualdelineationofthePT
ManualdelineationofthePTinthepresentstudyfollowedthesame procedurethanthepreviousMRIstudyonthePTasymmetryofadult baboons(Marieetal.,2018).However,becauseoftheimmaturebrains ofthenewbornsubjects,T2wMRIsignalwasusedinsteadofT1wMRI signalformanualdelineationforthefirstlongitudinalscans.
Infact,theT2wMRIsignalissensitivetothefreewaterpresentin voxels.Theproliferationofmembranesduetosynaptogenesisandthe processofmyelinationwilldecreasetheproportionoffreewaterinthe graymattervoxelsandthusdarkentheimagesduringmaturationofthe firsthumanyearpost-natal.Therefore,ahighercontrastisgeneratedin comparisontoT1wimagesinearlyimmaturebrains,whichhelpsfor betterdelimitation(Dehaene-LambertzandSpelke,2015).
Followingtheproceduresusedinhumans(Larsenetal.,1989),great apes(Hopkinsetal., 1998;HopkinsandNir,2010; Cantalupoetal., 2003)andOld-Worldmonkeys(Marieetal.,2018;Lyn etal.,2011), thesurfaceofthePThomologwasmeasuredinthecoronalplane.
TheT2w imagesof every subject wereimported in ITK-Snap.In there,theregionofinterestwasmanuallytracedinthecoronalplanon theindividualnativespacewiththeITK-Snaptool“Paint-BrushMode” withfeatureroundbrushsize1,usingatouchpad-drivenpointer (Wa-comCintiq® 13HD).
Coronalplaneswereusedbecausetheydisplaythefulldepthofthe sylvianfissure,ofwhichthePlanumTemporaleisitsfloor.Asdescribed byMarieetal.(2018),delineationofthePTwasperformedasfollowed: TheposteriorborderofthePTwascharacterizedbythelastcaudalslice displayingtheSylvianfissure.Theanteriorborderwasdefinedbythe fullclosureof theInsulasulcusandgreymatter.This techniquewas chosenduetotheinconsistencyofthepresenceoftheHeschl’sgyrus (seeMarieetal.,2018;Lynetal.,2011 fordiscussions).
Foreachslice,thedelineationwastracedonthemostventral bound-arybetween thesulcusandthegreymatter.Inorder tobalancethe rater’spossiblehandednessbias,tracingforeachsubjectwasrandomly undertakeneitherfromthemostmedialtothemostlateralpixelofthe Sylvianfissureorfromthemostlateraltothemostmedialpixel.This stepwasrepeatedonthenextslice,movingposteriorlyuntiltheSylvian fissurefelloutofview.
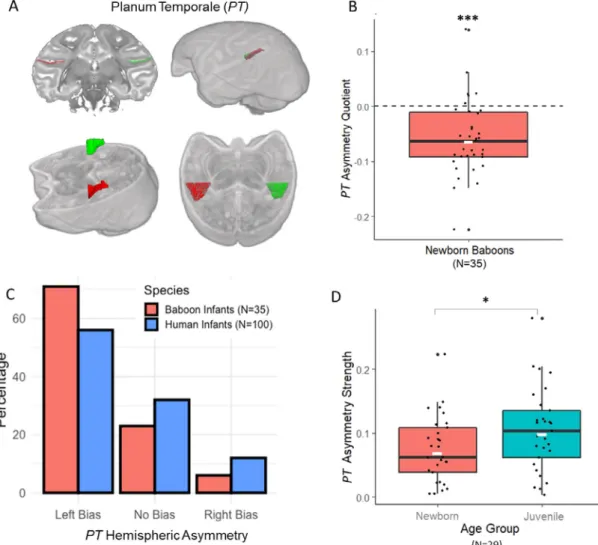
Next,asurfaceareawasgeneratedacrossallslicesforeach hemi-sphereindependentlyinagivensubject(seeFig.1.A).Foreachsubject, anAsymmetryQuotient(AQ)oftheleft(L)andtheright(R)surface areaswascomputedAQ=(R– L)/[(R+L)×0.5]withthesign in-dicatingthedirectionofasymmetry(negative:leftside,positive:right side)andthevalue,thestrengthofasymmetry.Further,asreportedby
HopkinsandNir(2010)forhumansandgreatapes,theAQwasalsoused toclassifythesubjectsasleft-hemisphericbiased(AQ≤–0.025),right biased(AQ≥0.025),ornonbiased(–0.025<AQ<0.025).Athreshold of0.025representsa2.5%differenceinsurfaceareabetweenleftand rightPT.
Asecondrater,blindtotheside,confirmedthemeasuresofthePT
inasubsampleof15individualsforbothhemispheres(interrater corre-lationcoefficientfor30PTtracingwasr(30)=0.94,p<0.0001).
2.5. Statistics
StatisticswereconductedwithR3.6.1(RCoreTeam(2017).R:A lan-guageandenvironmentforstatisticalcomputing.RFoundationfor Sta-tisticalComputing,Vienna,Austria.URLhttps://www.R-project.org/.)
3. Results
3.1. PTstructuralasymmetry
WefoundasignificantleftwardasymmetryofthePTsurfaceata group-levelin35newbornbaboons(t0)accordingtoaonesamplet-test inthe35subjects’AQscores(seeFig.1.B),MeanAQ=-0.058±0.067 SD;t(34)=-5.15,p<0.0001.CategorizationofindividualAQshowed alsoamajorityofleftwardPT-biasedindividuals(seeFig.1.C):25 ba-boonsexhibitedaleftwardhemisphericPTbias(71.4%)whereas2 ex-hibitedarightwardPTbias(5.7%)and8noPTbias(22.9%),a distribu-tionquasi-identicalthantheonefoundinhumaninfants(Wada,1975). Wefoundnodifferenceofdistributionbetweeninfantbaboonsand in-fanthumans accordingtochi-square(p=0.25forthreegroups“Left
bias,Rightbias,nobias” andp=0.20fortwogroups“Leftbias,Right bias”). Thenumber of leftward PT-biased baboonswas significantly greaterthanthenumberofrightwardPT-biasedsubjectsaccordingto chi-squaretest(𝜒2=19.59,p<0.0001).
3.2. Ageclasses’comparisonofPTlateralization’sstrength
Follow-upanalysisamongthe29rescannedbaboonswhenreaching 7to10monthsofage(t1)showedasignificantincreasedstrengthof thePTasymmetry(MeanAbsoluteAQscore,M.=0.105±0.065SD) incomparisontotheirearliestageclassaccordingtoapairedsample t-test(MeanAbsoluteAQscore,M.=0.073±0.049SD),t(28)=-2.39,
p=0.024(seeFig.1.D)aswellasasignificantcorrelationbetweenthe twoMRIsessions,r(29)=0.55,p<0.002.
3.3. Left,rightPTsurfaceareas
At t0, the mean PT surface areas were in the left hemisphere:
M.= 50.34mm2 ±9.27SD(inmales M. =51.07 mm2 ± 9.80SD;
inFemalesM.=49.24mm2±9.17SD);andintherighthemisphere: M.=47.44mm2±8.93SD(inmalesM.=48.53mm2±9.35SD;in
FemalesM.=45.79mm2±7.78SD).
At t1, the mean PT surface areas were in the left hemisphere:
M.=58.48mm2 ±8.0SD(inmalesM.=59.23mm2±8.07SD;in
Females M. =57.67 mm2 ±7.75SD); andin therighthemisphere: M.=55.73mm2±8.65SD(inmalesM.=57.07mm2±9.99SD;in
FemalesM.=54.28mm2±6.64SD). 3.4. Age,sex,brainsizeeffect
MultiplelinearregressionanalysesshowedthattherightPTsurface (p=0.001),theleftPTsurface(p=0.02)andage(p=0.033)predict
PTasymmetrystrengthbutnotthesubject’ssexandbrainvolume.At t0andt1,nosignificantdifferencesofmeanAQs,meanLeftPTsurface, andofmeanRightPTsurfacewerefoundbetweenmalesandfemales. 4. Discussion
Ourresultsshowedthatearlypost-natalnonhumanprimateinfants present a significant human-like neuroanatomical asymmetry of the Planum Temporalesurface(PT) infavor ofthelefthemisphere.This findingisclearlyconsistentwithearlyPTasymmetryfoundinhuman newbornsandinfants(Chietal.,1977;Duboisetal.,2010;Glaseletal., 2011;Hilletal.,2010;Wada,1975;WitelsonandPallie,1973)although measurementmethods,Left-Right-Ambiclassificationthresholdand sta-tisticalpowerintermsofsamplesizedifferaswellasageclass equiva-lencewhichoverallmakeinterspeciescomparisonchallenging. Never-theless,thedistributionisquasi-identicaltotheonesreportedinboth humaninfantsandhumanadults(GeschwindandLevitsky,1968)but alsoinadultchimpanzees(HopkinsandNir,2010)andadultbaboons (Marieetal.,2018).Ourfindings arealsosomewhatconsistentwith averaged-brainleftwardasymmetriesfoundininfantRhesusmacaques withinlargetemporalclusterswhichseemtooverlapwithPTaccording toanautomatedsource-basedmethod(Xiaetal.,2019).Sucha sim-ilarage-relatedphenomenonwasalsodescribed inhumaninfantsby
Wada(1975).Interestingly,wefoundthatthedirectionofindividualPT
asymmetryisconsistentacrossageclasseswhileitsstrengthisincreasing withage.Incontrast,nosexorbrainsizeeffectswerefoundon direc-tionorstrengthofPTasymmetry.Thisfindingisnotconsistentwiththe ideathatincreaseinPTasymmetryinHominidaeevolutionwasdueto increaseinbrainvolume(Pilcheretal.2001).Additionally,ifstrength ofPTasymmetryisaffectedbysexinhumanadults(Hirnsteinetal., 2019),itseemsnotthecaseinadultbaboons(Marieetal.,2018), in-fantbaboonsandhumaninfants(Duboisetal.2010).
Thisfindinginanon-linguisticspeciesclearlyquestionsthe histori-calideathatsuchamaturationaleffectofthePTasymmetry’sstrength
Y. Becker, J. Sein, L. Velly et al. NeuroImage 227 (2021) 117575
Fig.1. (A)RepresentationoftheasymmetricPlanumTemporale(PT)inthebaboonbrainonaT2wimageaccordingtoacoronalsection,3Dbrainrenderand obliquesectionorientedalongtheSylvianFissure(theleftPTisinredandtherightPTingreen).(B)MeanAsymmetryquotient(AQ)forthePlanumTemporale surfaceofthenewborninfantbaboons(N=35).NegativeMeanAQscoreindicatesleftwardhemisphericasymmetryatapopulation-level.Thelongblackline representsthemedian,thewhiteshortlinethemean.∗∗∗p<0.0001(C)Subjectsdistribution(inpercentage)asafunctionofthedirectionoftheirPTasymmetryin
babooninfants(N=35,inred)versusinhumaninfants(N=100,inblue,fromWada,1975).(D)VariationofstrengthofthePlanumTemporalesurface’sasymmetry (MeanabsoluteAQscore)amongthe29baboonsscannedlongitudinallyattwoearlystagesofdevelopment:Newborn(inred)versusJuveniles(i.e.,from7to10 monthsinblue).∗p<0.05.
is relatedtolanguagedevelopment in humaninfants (Wada, 1975). One couldask whethertheexistence oftheasymmetry shortlyafter birthhasaninnate,andthus,geneticcomponentashypothesizedfor humaninfants(Hilletal.,2010)or towhat extentitisrather influ-encedbypre-andpost-natalexperience.Inanycase,thecollective find-ings clearlyprovide additionalsupportfor thephylogenetic continu-itybetweenhumanandnonhumanprimatespeciesaboutsuchabrain asymmetricfeature.Suchacontinuityextendedattheearliest postna-talstageofdevelopmentacrossbothspeciesmayquestiontheearlyPT
asymmetryasahumannewborn-specificmarkerofthelanguage-ready brain.
Structurallateralizationofsuchalanguageareamaynotsolely ac-countforapre-wiredbrainforlanguageacquisitionasitwassupposed forhumanbabies(Dehaene-Lambertzetal.,2002).
However,itremainsunclear which factoris drivingsucha com-monearlyasymmetricfeatureofthebrainanatomyamonghumanand nonhumaninfants. One potential explanation is that earlyPT struc-turalasymmetrymighthavenothingtodowithdevelopmentof lan-guagelateralization,givensomestudiesinadultsreportednomatch be-tweenstructuralandfunctionalasymmetryofthisregion(Keller,2011;
Greve,2013).
Nevertheless,themostrecentstudyaddressingthisquestioninadults contradictssuchahypothesis(Tzourio-Mazoyeretal.,2018).Although the lackof matchwas confirmed between structural and functional asymmetryofthePTinalanguagetask,structuralPTasymmetrywas foundassociatedwithfunctionallateralizationofanadjacentauditory areaattheendoftheSylvianfissure,suggestingitslinkswithlanguage lateralization.
Therefore,anotherpotentialexplanationisthattheearlyPT struc-turalasymmetryinbothhumanandnonhumaninfantsmightpredict thedevelopmentofhomologcommunicativefunctions,whichstillform a foundationfor coreaspectsof thehumanlanguagesystem. Deter-mining such commondeveloping functionsbetween speciesremains highlyspeculative,giventhelackoflongitudinalstudiesininfantson theemergenceofbrain-behaviorrelationships.Nevertheless,basedon neuroimagingstudiesinnonhumanprimatefocusingonadults,itmight benotexcludedthatsharedpropertiesofcommunicativesystemsin hu-manandnonhumanprimatescouldberelatedtoPTstructural asym-metry. For instance,previous studiesin monkeys andapes have re-portedhuman-likefunctionallateralizationforprocessing conspecific calls.However,itsoverlapwithPTanatomicalregionremainsunclear aswellasthedirectionofthefunctionallateralization(i.e.,towardleft
versus right hemisphere) which are inconsistent across the liter-ature (e.g. Poremba et al.; 2004; Gil-da-Costa and Hauser 2006;
Petkovetal.2008;Jolyetal.,2012).Alternatively,someauthorshave proposedthatpropertiesofthecommunicativegesturalsystemin non-humanprimates couldconstitute anotherpotential functional candi-dateof PTspecialization.Infact,whereas productionof communica-tivemanualgestureshavebeenfoundhighlylateralizedinfavorofthe right-handinboth baboonsandchimpanzees(Meguerditchianetal., 2013),acontralateralrelation betweenPTstructuralasymmetryand handpreferencesforcommunicativegesturewasreportedinadult chim-panzees(HopkinsandNir,2010;Meguerditchianetal.,2012).These lat-terfindingshavethussuggestedthatsharedpropertybetweengesture signalinginapesandlanguagesystemin humansmightbeboth ulti-matelyrelatedtothisasymmetryfeatureofthetemporallobeanatomy (Meguerditchianetal.,2012).Whethersimilargesturalfunctional spe-cializationofthestructuralPTasymmetryexistsinbaboonshasbeennot investigatedyetalthoughbothchimpanzeesandbaboonshaveshown similarleftwardstructuralasymmetryofthePTaswellassimilar right-wardpatternsofgesturalcommunication’smanuallateralization.
Furtherstudiesinoursampleofinfantbaboonswouldhelpus de-terminatethepotentialrelationshipbetweentheseearlyPTstructural asymmetryanddevelopmentofmanuallateralizationofcommunicative gestures.
Inconclusion, the presentfinding in nonhuman infants provides additionalsupporttothehypothesisof acontinuity between nonhu-manandhumanprimatesconcerningleftwardstructuralPTasymmetry. Sharingsuchananatomicalfeatureofthebrainatthisearlier postna-talstageofdevelopmentreinforcedthustheideaofitscommonorigins fromourdistantevolutionaryancestor,datedback25–35millionyears ago,althoughitspotentiallinkwiththelanguage-readybrainremains anopenquestion.
Declaration of Competing Interest Authorsdeclarenocompetinginterests. Acknowledgments
General: WeareverygratefultothevetMarieDumasyfor supervis-ingthefirsthealthandanesthesiamonitoring,EmilieRaphaforgreat assistanceandanimalcare,FredericCharlin,aswellasthecarestaff of theStationdePrimatologie,suchasValérieMoulin,BrigitteRimbaud, RichardFrancioly,thevetsPascalineBoitelle,AlexiaCermolacce& Jan-nekeVerschoor,andthebehavioralmanagerPauMolina.Wethankalso KepKeeLohforEnglishcorrections.
Funding
TheprojecthasreceivedfundingfromtheEuropeanResearch Coun-cilundertheEuropeanUnion’sHorizon2020 researchandinnovation programgrantagreementNo716931 -GESTIMAGE-ERC-2016-STG (P.I.AdrienMeguerditchian),fromtheFrench“AgenceNationaledele Recherche” ANR-16-CONV-0002(ILCB)andtheExcellenceInitiativeof Aix-MarseilleUniversity(A∗MIDEX).ThisMRIacquisitionsweredone
attheCenterIRM-INT(UMR7289,AMU-CNRS),platformmemberof FranceLifeImagingnetwork(grantANR-11-INBS-0006).
Author contributions
Y.BandA.Mpreparedthepaperandtherevision. Y.B.performed the tracing andanalyses. C.B. performed the interrater tracing. J.S. parametrized theMRI sequences andoptimizedthe MRI acquisition setup.B.N.designedthebaboons’monitoringprograms.L.V.,L.R.,R.L. andL.G.designedthespecificproceduresofwelfare,anesthesia, mon-itoringandpreparationof baboonsin theMRImachine. J.L.A super-visedandcoordinatedtheMRIsession.A.M.designedandsupervised thestudyandMRIacquisitions.
Data and materials availability
Alldataisavailableinthesupplementarymaterials. Data availability statement
Ourdatawillbeavailableonlineuponpublicationandisattached tothissubmissioninthesupplementarymaterial.
Supplementary materials
Supplementarymaterialassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.neuroimage.2020.117575. References
Cantalupo, C., Pilcher, D.L., Hopkins, W.D., 2003. Are planum temporale and sylvian fis- sure asymmetries directly related?: A MRI study in great apes. Neuropsychologia 41, 1975–1981. doi: 10.1016/S0028-3932(02)00288-9 .
Chi, J.G., Dooling, E.C., Gilles, F.H., 1977. Gyral development of the human brain. Ann. Neurol. 1, 86–93. doi: 10.1002/ana.410010109 .
Crow, T.J. , 2004. The Speciation of Modern Homo Sapiens. OUP/British Academy . Dehaene-Lambertz, G., Dehaene, S., Hertz-Pannier, L., 2002. Functional neuroimaging of
speech perception in infants. Science 298, 2013–2015. doi: 10.1126/science.1077066 . Dehaene-Lambertz, G., Spelke, E.S., 2015. The infancy of the human brain. Neuron 88,
93–109. doi: 10.1016/j.neuron.2015.09.026 .
Dubois, J., Benders, M., Lazeyras, F., Borradori-Tolsa, C., Leuchter, R.H.-V., Mangin, J.F., Hüppi, P.S., 2010. Structural asymmetries of perisylvian regions in the preterm new- born. NeuroImage 52, 32–42. doi: 10.1016/j.neuroimage.2010.03.054 .
Gannon, P.J., Holloway, R.L., Broadfield, D.C., Braun, A.R., 1998. Asymmetry of chim- panzee planum temporale: humanlike pattern of Wernicke’s brain language area ho- molog. Science 279, 220–222. doi: 10.1126/science.279.5348.220 .
Geschwind, N., Levitsky, W., 1968. Human brain: left-right asymmetries in temporal speech region. Science 161, 186–187. doi: 10.1126/science.161.3837.186 . Gil-da-Costa, R., Hauser, M.D., 2006. Vervet monkeys and humans show brain asymme-
tries for processing conspecific vocalizations, but with opposite patterns of laterality. Proc. R. Soc. B: Biol. Sci. 273, 2313–2318. doi: 10.1098/rspb.2006.3580 .
Glasel, H., Leroy, F., Dubois, J., Hertz-Pannier, L., Mangin, J.F., Dehaene-Lambertz, G., 2011. A robust cerebral asymmetry in the infant brain: the rightward superior tem- poral sulcus. NeuroImage 58, 716–723. doi: 10.1016/j.neuroimage.2011.06.016 . Greve, D.N., Van der Haegen, L., Cai, Q., Stufflebeam, S., Sabuncu, M.R., Fischl, B., Brys-
baert, M., 2013. A surface-based analysis of language lateralization and cortical asym- metry. J. Cogn. Neurosci. 25, 1477–1492. doi: 10.1162/jocn_a_00405 .
Hill, J., Inder, T., Neil, J., Dierker, D., Harwell, J., Van Essen, D., 2010. Similar patterns of cortical expansion during human development and evolution. Proc. Natl. Acad. Sci. 107, 13135–13140. doi: 10.1073/pnas.1001229107 .
Hirnstein, M., Hugdahl, K., Hausmann, M., 2019. Cognitive sex differences and hemi- spheric asymmetry: a critical review of 40 years of research. Laterality 24, 204–252. doi: 10.1080/1357650X.2018.1497044 .
Hopkins, W.D. , Marino, L. , Rilling, J.K. , MacGregor, L.A. , 1998. Planum temporale asym- metries in great apes as revealed by magnetic resonance imaging (MRI). NeuroReport 9, 2913–2918 .
Hopkins, W.D., Nir, T.M., 2010. Planum temporale surface area and grey matter asymme- tries in chimpanzees (Pan troglodytes): the effect of handedness and comparison with findings in humans. Behav. Brain Res. 208, 436–443. doi: 10.1016/j.bbr.2009.12.012 . Joly, O., Ramus, F., Pressnitzer, D., Vanduffel, W., Orban, G.A., 2012. Interhemispheric differences in auditory processing revealed by fMRI in awake Rhesus monkeys. Cere- bral Cortex 22, 838–853. doi: 10.1093/cercor/bhr150 .
Josse, G., Hervé, P.-Y., Crivello, F., Mazoyer, B., Tzourio-Mazoyer, N., 2006. Hemi- spheric specialization for language: brain volume matters. Brain Res. 1068, 184–193. doi: 10.1016/j.brainres.2005.11.037 .
Keller, S.S., Roberts, N., García-Fiñana, M., Mohammadi, S., Ringelstein, E.B., Knecht, S., Deppe, M., 2011. Can the language-dominant hemisphere be predicted by brain anatomy? J. Cognit. Neurosci. 23, 2013–2029. doi: 10.1162/jocn.2010.21563 . Kuhl, P.K., Conboy, B.T., Coffey-Corina, S., Padden, D., Rivera-Gaxiola, M., Nelson, T.,
2008. Phonetic learning as a pathway to language: new data and native language magnet theory expanded (NLM-e). Philos. Trans. R. Soc. B: Biol. Sci. 363, 979–1000. doi: 10.1098/rstb.2007.2154 .
Larsen, J.P., Ødegaard, H., Grude, T.H., Høien, T., 1989. Magnetic resonance imaging - a method of studying the size and asymmetry of the planum temporale. Acta Neurolog- ica Scandinavica 80, 438–443. doi: 10.1111/j.1600-0404.1989.tb03906.x . Lyn, H., Pierre, P., Bennett, A.J., Fears, S., Woods, R., Hopkins, W.D., 2011. Planum tempo-
rale grey matter asymmetries in chimpanzees (Pan troglodytes), vervet (Chlorocebus aethiops sabaeus), rhesus (Macaca mulatta) and bonnet (Macaca radiata) monkeys. Neuropsychologia 49, 2004–2012. doi: 10.1016/j.neuropsychologia.2011.03.030 . Manjón, J.V., Coupé, P., Martí-Bonmatí, L., Collins, D.L., Robles, M., 2010. Adaptive non-
local means denoising of MR images with spatially varying noise levels. J. Magn. Resonance Imaging 31, 192–203. doi: 10.1002/jmri.22003 .
Marie, D., Roth, M., Lacoste, R., Nazarian, B., Bertello, A., Anton, J.-L., Hopkins, W.D., Margiotoudi, K., Love, S.A., Meguerditchian, A., 2018. Left brain asymmetry of the Planum Temporale in a nonhominid primate: redefining the origin of brain special- ization for language. Cereb Cortex 28, 1808–1815. doi: 10.1093/cercor/bhx096 .
Y. Becker, J. Sein, L. Velly et al. NeuroImage 227 (2021) 117575 Meguerditchian, A., Gardner, M.J., Schapiro, S.J., Hopkins, W.D., 2012. The sound of one-
hand clapping: handedness and perisylvian neural correlates of a communicative ges- ture in chimpanzees. Proc. Biol. Sci. 279, 1959–1966. doi: 10.1098/rspb.2011.2485 . Meguerditchian, A., Vauclair, J., Hopkins, W.D., 2013. On the origins of human handed-
ness and language: a comparative review of hand preferences for bimanual coordi- nated actions and gestural communication in nonhuman primates. Dev. Psychobiol. 55, 637–650. doi: 10.1002/dev.21150 .
Mugler, J.P., Bao, S., Mulkern, R.V., Guttmann, C.R.G., Robertson, R.L., Jolesz, F.A., Brookeman, J.R., 2000. Optimized single-slab three-dimensional spin-echo MR Imag- ing of the brain. Radiology 216, 891–899. doi: 10.1148/radiology.216.3.r00au46891 . Mugler, J.P., Brookeman, J.R., 1990. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn. Reson. Med. 15, 152–157. doi: 10.1002/mrm.1910150117 .
Petkov, C.I., Kayser, C., Steudel, T., Whittingstall, K., Augath, M., Logothetis, N.K., 2008. A voice region in the monkey brain. Nat. Neurosci. 11, 367–374. doi: 10.1038/nn2043 . Pilcher, D.L., Hammock, E.A.D., Hopkins, W.D., 2001. Cerebral volumetric asymmetries in non-human primates: a magnetic resonance imaging study. Laterality 6, 165–179. doi: 10.1080/713754406 .
Poremba, A., Malloy, M., Saunders, R.C., Carson, R.E., Herscovitch, P., Mishkin, M., 2004. Species-specific calls evoke asymmetric activity in the monkey’s temporal poles. Na- ture 427, 448–451. doi: 10.1038/nature02268 .
Scott, J.A., Grayson, D., Fletcher, E., Lee, A., Bauman, M.D., Schumann, C.M., Buono- core, M.H., Amaral, D.G., 2016. Longitudinal analysis of the developing rhesus mon-
key brain using magnetic resonance imaging: birth to adulthood. Brain Struct. Funct. 221, 2847–2871. doi: 10.1007/s00429-015-1076-x .
Shapleske, J., Rossell, S.L., Woodruff, P.W.R., David, A.S., 1999. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res. Rev. 29, 26–49. doi: 10.1016/S0165-0173(98)00047-2 .
Tzourio-Mazoyer, N., Crivello, F., Mazoyer, B., 2018. Is the planum temporale surface area a marker of hemispheric or regional language lateralization? Brain Struct. Funct. 223, 1217–1228. doi: 10.1007/s00429-017-1551-7 .
Vigneau, M., Beaucousin, V., Hervé, P.Y., Duffau, H., Crivello, F., Houdé, O., Ma- zoyer, B., Tzourio-Mazoyer, N., 2006. Meta-analyzing left hemisphere language ar- eas: Phonology, semantics, and sentence processing. NeuroImage 30, 1414–1432. doi: 10.1016/j.neuroimage.2005.11.002 .
Wada, J.A., 1975. Cerebral hemispheric asymmetry in humans: cortical speech zones in 100 adult and 100 infant brains. Arch. Neurol. 32, 239. doi: 10.1001/arch- neur.1975.00490460055007 .
Witelson, S.F., Pallie, W., 1973. Left hemisphere specialization for language in the newborn: neuroanatomical evidence of asymmetry. Brain 96, 641–646. doi: 10.1093/brain/96.3.641 .
Xia, J., Wang, F., Wu, Z., Wang, L., Zhang, C., Shen, D., Li, G., 2019. Mapping hemispheric asymmetries of the macaque cerebral cortex during early brain development. Hum. Brain Map. doi: 10.1002/hbm.24789 .