Cell-Based Sensors for Quantifying Cell Health and Disease
Progression in Engineered Systems
by
Sarvesh Varma
B.A.Sc., Nanotechnology Engineering, University of Waterloo, 2010 S.M. Electrical Engineering and Computer Science
Massachusetts Institute of Technology, 2013
SUBMITTED TO THE DEPARTMENT OF ELECTRICAL ENGINEERING AND COMPUTER SCIENCE IN
PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY
AT THE
MASSACHUSETTS INSTITUTE OF TECHNOLOGY JUNE 2017
@ 2017 Massachusetts Institute of Technology. All rights reserved.
Signature of the author
Signature redacted
Sarvesh Varma Department of Electrical Engineering and Computer Science May 19, 2017
Certified by
Signature redacted
Professor of Electrical Engineering and
Accepted by Joel Voldman Computer Science Thesis Supervisor
Signature redacted
MASSASCUSETTS ~NTITUTE OF TECHNOLOGY V(Leslie
U/ A. Kolodziejski Professor of Electrical Engineering and Computer Science Chair of the Committee on Graduate StudentsCell-Based Sensors for Quantifying Cell Health and Disease
Progression in Engineered Systems
by
Sarvesh Varma
Submitted to the Department of Electrical Engineering and Computer Science on May 19, 2017, in partial fulfillment of the requirements for the degree of
Doctor of Philosophy in Electrical Engineering and Computer Science ABSTRACT
Healthy cells create healthy beings, while dysfunctional cells cause disease. Studying disease requires an understanding of how cells become dysfunctional from physiological states. Gaining this insight in vitro involves subjecting cells to relevant microenvironments and utilizing methodologies for assaying cells. Critically, to obtain accurate and unbiased insight, it is important to ensure that the cellular microenvironment remains representative, and that the assay methodology itself does not adversely perturb cell state. This thesis presents an approach where cells 'report' upon their healthy or stressed states, which could be assessed to either learn disease mechanisms, or quantified to design 'cell-friendly' methodologies. We engineered cell-based sensors that emit stress-regulated fluorescence, and applied them to characterize how distinct microenvironments regulate cell health. Here, we describe two endeavors that highlight the utility of this approach.
We first developed cell stress sensors for a diverse bioinstrumentation community to quantify the impact of engineered systems and methodologies upon cell health. Using NIH3T3 cells, we engineered sensors that report on stresses induced by DNA damage, heat shock, or fluid shear stresses. Each sensor provides sensitive and specific responses to induced pathways (relevant to several cell types), and can be used for a multiplexed stress-readout. The sensors do not require additional reagents and can be conveniently quantified with flow cytometry and real-time imaging. Successful distribution and adoption of the sensors by external users enabled quantitative characterization of flow sorting systems in the context of cell health, which was not explored before. Hence, the cell-sensor methodology designed as an 'open source' tool, could potentially serve as a novel standard for quantifying cell stress, and broadly for designing 'cell-friendly' methodologies.
We further utilized cell-based sensors to gain biological insight into stress-regulated diseases. We focused on atherosclerosis, a flow-regulated cardiovascular disease that remains a major cause of morbidity and mortality worldwide. We engineered a novel microfluidic platform to study atherogenesis in vitro that overcomes several limitations of existing models. This device emulates in vivo microenvironments by applying programmable spatiotemporal flow profiles observed from human patients directly upon cultured human cells. Utilizing an endothelial cell-based sensor (that reports on vascular health), device flows were validated with known biomarkers and endothelial signatures. Subsequently, these sensors were used to gain novel insight upon atherogenesis through the impact of hemodynamic flows upon endothelial function.
Overall, this thesis presents a facile and quantitative approach to investigate complex cell-stress emergent from diverse bioinstrumentation or within a disease microenvironment, which can be utilized to discover how environmental conditions regulate cell physiology, and human health.
To my gurus and guides from all walks of life,
my parents, and my sister
for their unwavering support, immeasurable love,
and unbelievable tolerance.
Acknowledgments
More than any part of this thesis, I've been looking forward to write this section- to express my gratitude to each and every person who helped this thesis to come into existence. While the thesis may be submitted under my name, none of this work would be possible without the heroes behind the scenes who've helped me get to where I am now.
First and foremost, I'd like to express my utmost gratitude to my thesis advisor, Prof. Joel Voldman. Whether he knows it or not, Joel is a remarkable wizard. He's always been a person of few-but-prudent (and frequently amusing) words. Over our meetings throughout graduate school, he has said things which made no sense to my infinitesimal brain immediately, but struck me (-minutes-years) later as words of great wisdom and deep insight. I'm very grateful to him for teaching me how to ask the right questions, and importantly, at the right time. Teaching by examples, he helped me learn attributes of an effective communicator (as a teacher, writer, or a speaker), as well as that of a careful listener and reader. I'm indebted to him for generously providing me with intellectual insight, personal and academic advice, along with the freedom to pursue my ideas, particularly in tough times. It's too soon to say if I'd ever do this all over again, but if it comes to it - I'd wish for Joel to be on my side.
Next, I'd like to acknowledge the incredible support of my committee members Prof. Tim Lu and Prof. Guillermo Garcia-Cardeha. As leaders in their respective fields, they both have served as excellent inspirations to me throughout the years. They both have always shared their precious time with me whenever I needed research or academic advice. Guillermo has been a critical collaborator without whom a significant portion of this thesis would not been fruitful. I am indebted for his kind support in this endeavor. Most importantly, I am grateful for him for believing in me, and sharing his infectious passion for his research. Likewise, I am grateful to Tim to providing me invaluable academic and career advice. From the very beginning, I've had a thing for synthetic biology and hence getting the opportunity to assist in teaching it with Tim has also been a privilege. I hope, and look forward to growing these relationships further, and to continue learning from their mentorship in the future.
They say a PhD is a marathon and not a sprint. To run either, you have to first arrive at the track and not to get lost. There have been several individuals who helped me navigate there
mentorship of teachers and professors from my past and present. From Canada, I'd like to
acknowledge the key roles of Profs. Ian Mitchell, Peter Norton and Rajni Patel in letting me
embark on a research oriented path. I'm indebted to Prof. Frangois Lagugn6-Labarthet whose
immense support and mentorship basically took my academic career to another level. Likewise,
I'm absolutely blessed to have learned under the guidance of Prof. Ron Martin, who holds more
inspiration-per-human-being than I've ever seen. I'm also grateful to Prof. Cart Hansen for
providing me the unique opportunity to learn state-of-the-art microfluidics in his lab, much of
which was directly useful in this thesis. In the US, I'm grateful to Prof. Todd Thorsen for
furthering my knowledge in microfluidics in his lab as well. His brilliance and passion as an
experimentalist remains a continuing inspiration for me. I'm equally thankful to Profs. Al
Grodzinsky, Roger Kamm, Roger Mark, Forbes Dewey Jr., and Prof. Rohit Karnik for kindly
providing me invaluable advice on a broad range of topics throughout the years.
This work has also benefited immensely from the assistance of my collaborators. It has been a
pleasure to work alongside Catherine and Anna on the cell sensor project. Likewise, it was a
pure stroke of luck to meet and collaborate with Andrew Box on the multiplexed sensors. Thanks
to his dedication and meticulous experimentation, we could successfully perform our case
studies with various FACS systems. I'd also like to thank Ben Slegtenhorst for his prompt help,
particularly in providing me endothelial cells whenever I needed some. I'm also thankful to my
UROP Yu-chi Kuo for helping me build the valve controller.
One of the reasons I could survive the turmoil that comes with research was the support of my
labmates and coworkers. I am thankful to all the current and past members of the Voldman lab
for putting up with me all these years. Particularly, I'd like to thank Michael Vahey for
exemplifying what a brilliant, hardworking and caring graduate student looks like at MIT. Thanks
to Salil Desai for not only initiating the cell sensor work, but also for his critical and practical
advice when I joined the group. Thanks to Yi-Chin Toh for teaching me important lessons in
perfusions, and for showing me how to take impeccable lab notes. I'm thankful to Laralynne
Przybyla for sympathizing with my perfusion and cell-culture woes, for being a biology guru,
and for providing great company in the lab. I'm thankful to other graduate students in the
lab-Nicha Apichitsopa, Aalap Dighe, Lisa Liu, Jaemyon Lee, Alex Jaffe, among others, for fostering
a warm and friendly lab environment. It's also been an absolute pleasure to work with, and
share the office space with wonderful postdocs in the lab. Specifically, I'd like to thank Marc
Castellarnau, Javier Prieto, Tao Sun, Thibault Honegger and Per Augustsson for elevating my
spirits, and generally for making grad school so memorable. It has been equally fun to share
the lab with visiting personnel such as Minoru, Morirtz, Rodrigo Balam, among others. In
addition, I'd like to extend my gratitude to my colleagues and friends from the
8thfloor labs,
who helped create a stimulating and friendly work environment. Special thanks goes out to
Lidan Wu, Han Wei Hou, Aniruddh Sarkar and Mark Scott for helping me out if I needed any
urgent reagents or equipment. Similarly, outside the Voldman Lab, I'm grateful to Raymond
Lam, Marco Cartas, Sumeet Kumar and Anupam Singhal for their creative tips'n'tricks in
troubleshooting research.
I would also like to acknowledge the support from the outstanding staff and members of various
departments and facilities I worked in throughout grad school. I thank the EECS dept.,
particularly their graduate office for being so welcoming and supporting at all times. I am also
thankful to the kind staff of RLE and MTL. Particularly, I'm indebted to Kurt Broderick for his
microfabrication prowess, and importantly for being a living example of wisdom and humility.
I'm equally thankful to Dennis Ward for his critical help in microfabrication processes and for
his overall cleanroom cheer. Additionally, I'd like to thank the MIT libraries for frequently
helping me dig up obscure articles to validate or discredit my own obscure work.
I want to specifically acknowledge certain special individuals who kept me sane and happy in
grad school. I'm super fortunate to have spent many of these years in the caring friendship of
Hao-Wei, Burak, Dan, Joe, Caspar, Jason, Betty, Tracy, Farhan, Vivek, and Mega, to name a
few. I will always cherish every moment I spent with them- in good and tough times, and remain
forever indebted for their loving companionship. I owe you all.
Last but not the least, I have no words to thank the masterminds that helped me get to where
I am. This thesis is just an example (that too an insignificant one) compared to all that they
have helped me get through in life. For this, and everything else I'm eternally grateful to my
parents and sister for their unconditional love, encouragement, and blessings. Like it or not,
this is on you. And on that bombshell it's time to end. Thanks for reading!
Table of Contents
Chapter 1 Caring for Cells in Engineered Microsystems and Microenvironments... 12
1.1 Introduction to the Thesis ... 13
1.2 Cell Health in Microsystems ... 13
1.3 Cell Stress Responses... 15
1.4 Potential Stressors within Microsystems ... 18
1.5 Methods for Assessing Cell Health in Microsystems... 23
1.6 Current Challenges in Assessing Cell Health within Microsystems ... 28
1.7 Motivating a Transcriptional Cell-Sensor Assay ... 31
1.8 Using Cell-Sensors to Monitor Stressors Regulating Human Disease ... 34
1.9 Overview of Thesis... 38
Chapter 2 Engineering cell-based sensors for stresses prevalent in microsystems... 40
2 .1 Intro d u ctio n ... 4 1 2.2 DNA Damage Sensor ... 42
2.3 Fluid Shear Stress Sensor ... 54
2.4 Heat Shock Sensor ... 68
2 .5 C o nclusio n s ... 78
2 .6 M eth o d s... 7 9 2.7 Acknowledge ments and Contributions ... 85
Chapter 3 Multiplexed Cell-based Sensors for Assessing the Impact of Engineered Systems and M etho ds o n Ce ll H ealth ... 86
3 .1 Intro d u ctio n ... 8 7 3.2 Assembling a Library of Colored Cell Line Controls ... 89
3.3 Generation of Cell-Sensors for Multiplexing ... 92
3.4 Multiplexed Sensor Responses to Chemical Stressors ... 94
3.5 Multiplexed Sensor Responses to Physical Stressors ... 98
3.6 Utilizing Sensors to Assess Cell Damage by Cell Sorters... 100
3.7 Discussion and Conclusions ... 106
3 .8 M etho d s... 10 9 3.9 Acknowledgements and Contributions ... 111
Chapter 4 Atherofluidic System for Modeling Human Atherogenesis and Pathophysiology In Vitro ... 1 1 2
4.1 Introduction ... 113
4.2 Current Lim itations in Studying Atherogenesis ... 114
4.3 Aim s and Approach... 117
4.4 Adaptation of Endothelial FSS Sensor in M icrofluidic Platform s... 117
4.5 Branched M icrofluidic Device ... 120
4.6 Atherofluidic System ... 131
4.7 Endothelial Responses to Spatiotem poral Flows ... 156
4.8 Conclusions ... 165
4.9 M ethods... 166
Chapter 5 Contributions ... 171
5.1 Cell-based Sensors as a Quantitative Assay for Cell Stress ... 172
5.2 Adoption of Sensor M ethodology by External Users ... 174
5.3 Branched M icrofluidic Platform for Studying Anastom osis ... 175
5.4 Atherofluidic System ... 176
Table of Figures
Figure 1-1 Cell responses to applied stress dosages. ... 17
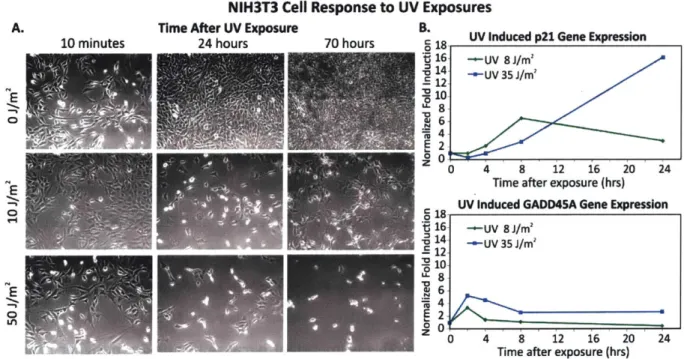
Figure 2-1 NIH3T3 cell response to UV treatm ents. ... 44
Figure 2-2 Quantification of DNA damage by comet assay and gene expression. ... 45
Figure 2-3 Plasmid map of the DNA damage sensing construct and inserted p21 promoter sequence...46
Figure 2-4 Illustration of DNA damage sensor design, construction and characterization...47
Figure 2-5 Sensitivity analysis of DNA damage sensor clones after MMS exposures with flow cytometry...47
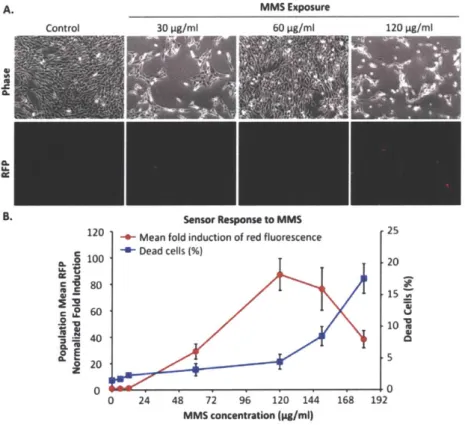
Figure 2-6 DNA damage sensor response to M MS exposures. ... 48
Figure 2-7 Cross-sensitivity analysis of DNA dam age sensors. ... 50
Figure 2-8 Specificity of DNA damage sensors to p53-p21 pathway activation ... 51
Figure 2-9 DNA Damage sensor response to UV-C exposures... 52
Figure 2-10 Dose response of DNA damage sensors to silver nanoparticles. ... 53
Figure 2-11 Schematic depicting the relevant mechanisms regulating and activating EGR1 upon flow. ... 57
Figure 2-12 Illustration of FSS sensor design, construction and characterization...58
Figure 2-13 Chem ical characterization of FSS sensors. ... 60
Figure 2-14 Microscopy and flow cytometry analysis of cells following FSS. ... 61
Figure 2-15 FSS characterization of cell sensors... 62
Figure 2-16 Characterization of inertial microfluidic margination device. ... 64
Figure 2-17 Illustration of heat shock sensor design, construction and characterization...69
Figure 2-18 Sensitivity analysis of heat shock sensor clones... 70
Figure 2-19 Heat shock sensor induction by arsenite and thermal shock...71
Figure 2-20 Sensitivity analysis of heat shock sensors. ... 72
Figure 2-21 Heat Shock sensor response quantified by microscopy. ... 73
Figure 2-22 Cross-sensitivity analysis of heat shock sensors... 74
Figure 2-23 Therm al Shock upon heat shock sensors. ... 76
Figure 2-24 Heat shock sensor response to therm al dosing. ... 77
Figure 2-25 Geometry and dimensions of the microfluidic device for FSS studies ... 81
Figure 3-1 Multiplexed cell stress sensors for quantifying the impact of engineered systems and methods on cell h e a lth ... 8 8 Figure 3-2 M u ltiplexing strategy...89
Figure 3-3 Images of the library of color cell lines used for the multiplexing template...90
Figure 3-4 Flow cytometry template for the library of color cell lines used for multiplexing. ... 92
Figure 3-5 DNA damage sensor response characterization following chemical induction...93
Figure 3-6 DNA damage sensor response characterization following UV exposures...93
Figure 3-7 FSS sensor response characterization following chemical induction. ... 94
Figure 3-8 Sensor multiplexing and analysis using flow cytometry... 95
Figure 3-9 Cross-sensitivity analysis via m icroscopy. ... 96
Figure 3-10 Cross-sensitivity analysis via flow cytom etry. ... 97
Figure 3-11 Real tim e m onitoring of single-cell sensors... 98
Figure 3-12 M ultiplexed sensor response to UV exposures. ... 99
Figure 3-13 NAC treatm ent on FSS sensors...100
Figure 3-14 Sensor Adoption and Characterization at Stowers Flow Core...104
Figure 3-15 . Characterization of sensor activation by FACS system s. ... 105
Figure 4-1 Atheroprotective and atheroprone spatiotemporal flow profiles in vivo. ... 113
Figure 4-2 M acroscale system s for applying flow profiles in vitro. ... 116
Figure 4-3 Endothelial KLF2-GFP FSS sensor design and response to distinct flow profiles...118
Figure 4-4 Endothelial FSS sensor culture and perfusion in microfluidic devices. ... 119
Figure 4-5 End-to-side distal anastomosis artery-graft configuration and flow profiles...121
Figure 4-6 Design and functionality of a m icrofluidic branch device. ... 121
Figure 4-7 Endothelial cell responses to flows in the branched microfluidic device...123
Figure 4-8 Low-Oscillatory flow generation on the branched device...126
Figure 4-9 G enerating spatial flow profiles. ... 130
Figure 4-10 A therofluidic System . ... 132
Figure 4-11 Tem poral W aveform Generator m odule design. ... 133
Figure 4-12 Cell culture m odule design and operation ... 135
Figure 4-13 Optim izing helical flow s w ith channel geom etries...142
Figure 4-14 Particle flow trajectories in SG and SH channel cross-section...143
Figure 4-15 Flow network analysis of the Low-Oscillatory Flow Generator...144
Figure 4-16 Temporal Waveform Generator module on the Atherofluidic device. ... 147
Figure 4-17 Low-Oscillatory flow generation on the Atherofluid ic device. ... 149
Figure 4-18 Simultaneous generation of atheroprotective and atheroprone spatio-temporal flow profiles...151
Figure 4-19 Cell culture m odule functionality. ... 155
Figure 4-20 KLF2-GFP sensor response to helical, chaotic or no flow. ... 157
Figure 4-21 KLF2-GFP sensor response spatiotem poral flows. ... 158
Figure 4-22 Second Generation KLF2-GFP sensor response spatial flows...161
Figure 4-23 First and Second Generation KLF2-GFP sensor responses to spatial flows. ... 162
Chapter
1
Caring for Cells in Engineered
Microsystems and
Microenvironments
1.1
Introduction to the Thesis
This thesis describes designing, and utilizing cell-based sensors to quantify activation of cell stress mechanisms induced by diverse physical stressors relevant to engineered platforms, and also to human diseases. This work was pursued with two related endeavors. The first relates to developing cell-based stress sensors for a broad bioinstrumentation engineering community. These sensors can assist device designers and users in obtaining specific biological insight about cell health, which would be challenging to measure or interpret otherwise. Such sensors can serve as a standard for this community to identify and establish 'cell-safe' methodologies, thereby extending the utility and adaption of such technologies. This work is described in Chapters 2-3 of this thesis.
The other endeavor of this thesis was to utilize cell health sensors for gaining insight into microenvironmental stress-regulated human disease progression. Specifically, this was performed by deploying endothelial cell-based sensors (that report on vascular health) within an engineered platform that recreates both physiological and pathological microenvironments
relevant to atherosclerosis. The synergistic application of such sensors within the relevant disease-mimic platform provided unique biological insight about human atherogenesis, which could not have been achieved with alternative techniques. This work is described in Chapter 4 of the thesis.
This chapter describes the motivation and background for the thesis, while the last chapter discusses its contributions and implications. Thesis overview and overall chapter outlines are described in Section 1.9.
1.2
Cell Health in Microsystems
A cell's health and biological function are closely regulated by its microenvironment. Adverse perturbations caused by external stimuli (e.g., injury, molecular signals) can steer cellular homeostasis towards a diseased state. To this end, countless platforms and technologies (including microfluidics) have been developed to gain insight into disease biology, as well as for
engineering diagnostics and therapeutics. In their application, these devices typically leverage a variety of physical forces and biochemical factors to study and manipulate a broad range of cell types1 2. Paradoxically, the device microenvironment itself may inevitably impose undesirable
changes upon cell health, thereby biasing or invalidating the device's utility. However, how does one decouple any device-imposed biological artifacts from the intended biological study and application? In other words, how does one ensure that the device microenvironment is designed
and maintained in a way that does not stress or harm cells? Critically, adoption of microfluidic technologies and their utility to the end-users will be limited if there is a lack of standards that ensure cell-safe design and operation. In this chapter, I address these concerns, review the
relationship between microenvironmental stimuli and cell stresses, and propose transcriptional cell-based sensors that report on stress pathway activation, as a potential solution for establishing standards for measuring cell health in microsystems. Furthermore, focusing on a stressor ubiquitous to microscale devices, and one that bears strong relevance to human cardiovascular diseases - fluid shear stress, I will propose the application of cell-based sensors to investigate flow-regulated disease progression within a microfluidic platform. I anticipate such a cell-sensor approach will help the community progress towards establishing cell-health standards, thereby enhancing the value and relevance of microscale technologies-and platforms. Furthermore, the application of cell-based stress sensors in disease-mimicking platforms could enable novel biological insight that would be challenging to obtain by alterative methodologies.
Prior to establishing any standards, relating to health, it is important to define what cell-health is, what it means for different cells, and how may be perturbed in microsystems. Within engineered platforms, one important consideration is to define which cells are intended to be used within a particular device. There are two broad categories of devices that study or manipulate cells. First, there are platforms that are designed for specific models, for instance devices to study stem cell fate choice3, hepatocyte toxicity"' , neuronal injury and regeneration', 7, cell mechanobiology8, etc. A key aspect in the validation of such in vitro models is to recapitulate specific phenotypes. Hence, device usage protocols are established to demonstrate such functions9"10 in order to convince the relevance of such systems to a broader audience (e.g. biologists, physicians, etc.).
The second category consists of devices that are not specific to a particular cell genotype or phenotype. Examples of such devices include cell sorting systems that utilize optical1
-13, electrical14-'6, magnetic17-19, acoustic20
, 21, or hydrodynamic inertial forces2 2, 23, etc. Other
examples include devices that are meant to prepare cells for subsequent assays24-2 7,
droplet-based platforms 28-31, or platforms meant for automated cell culture3 2-3 4 or perfusion culture35-38.
In such devices, assessment of the device microenvironment can arguably be harder because there may not necessarily be a 'standard' or well-defined cellular function that could be measured for validation, as for the cell-type specific devices. The impact of any particular device operating conditions may also not have been previously explored in the context of cell health, making it difficult to find relevant assays and prior work. In addition, considering the large diversity of physical forces and biochemical factors utilized in the application of both categories of platforms, it is often challenging to find conditions that minimize cell stress, and broadly do not alter its biological state. In this chapter, I will focus on this second class of devices, and will present considerations for the device designer and device user on how to assess the impact of microsystems upon cell health.
1-3 Cell Stress Responses
A dysfunctional or damaged cell is an undesirable consequence that needs to be prevented to maintain a healthy physiological state. However, what this healthy state refers to is dependent on a few important aspects. In this section, I will provide a framework for defining cell-health, that is applicable to many phenotypes and of relevance to the microsystem engineer.
Mammalian cells consist of parenchymal cells (e.g. cardiomyocytes, neurons, hepatocytes, etc.), that provide specific functions, which are integral to their healthy state. On the other hand, stromal cells serve to assist parenchymal cells by providing structural support, nutrient delivery, immune surveillance etc. While the in vivo purpose and functions may differ among cells, they are all susceptible to stress damage in similar ways. Specifically, stressors can inflict injury to at least four critical aspects: 1) cell membranes/organelles 2) ATP generation processes 3) protein synthesis and 4) genome integrity and replication processes- all of which can influence the other.
particular cell. Importantly, these states will differ between the natural in vivo microenvironment and typical in vitro culture conditions and depending on the cellular phenotype and origin, the relevant reference must be considered as the healthy state. Additionally, within in vitro culture, primary cells and transformed cell lines (including cancer cell lines) will maintain distinct homeostatic equilibria for each of the essential cellular processes. For cancer or diseased cells this equilibrium is an 'unhealthy' state, which may be of interest to recapitulate within disease-mimic models. With this context, a stressor is defined as a stimulus that steers the cell away from its preexisting equilibrium state. In addition, any perturbation which causes ATP depletion, loss of ionic homeostasis (Ca"*, Na', K', etc.), generation of reactive free radicals, mitochondrial damage, pH imbalance, defects in membrane permeability, genetic mutations, etc. is a stressor.
Any external stimulus can potentially harm otherwise healthy cells based on the stimulus' intrinsic characteristics, duration of exposure, etc. For example, brief exposure to high-energy radiation (e.g. gamma radiation) could damage cells, as could prolonged exposure to comparatively lower-energy radiation (e.g. UV radiation) at the same intensity. Here, we refer to this overall "amount of stress" as a 'stress dosage'. Typically, a cell would be able to adapt or repair itself following low stress dosage (Figure 1-1). Moderate dosages can stress a cell beyond its tolerance, where it may be forced to change irreversibly to a dysfunctional phenotype. Likewise, high stress dosages or inability to adapt to stress dosage can lead to cell death. Importantly, irreversible damage can activate cell death mechanisms before the effects manifest themselves into ultrastructural and histochemical changes (typically taking ~min-hrs), followed by changes visible by light microscopy, and then gross morphological changes (typically taking "hrs-days).
Healthy Cell
V adajp~tation
i
dysfunction * necrosisSrepair
edifferentiation
* apoptosisInability to Adapt Inability to Adapt 5
Reversible Irreversible Cell Death
Damage Damage
Stress-Induced States Microsystems Affecting Cells Phenotype A 0
0
Phenotype B E
Phenotype C Operating Conditions
Phenotype D * * I ? 0 ?4? ?
Stress Dosage Cell State
Figure 1-1 Cell responses to applied stress dosages. Illustration showing how phenotype-dependent cell health may be perturbed by varying doses of stress stimuli within microsystems. Cellular damage and its impact on cell fate depends on the magnitude of stress dosage. The resultant cell state further depends on the inherent stress tolerance of a given phenotype. In addition to the biological complexity, the broad diversity in engineered system design and operating conditions can further impact cell state in important and generally unknown ways.
Given the broad diversity of cell types, there is wide variation in cells' sensitivity to environmental
stress. A particular dose can either stress, irreversibly damage, or even kill a particular cell
depending on its genotype and phenotype (Figure 1-1). We are particularly interested in
sub-lethal stress, as such stress may not lead to obvious variations in phenotype, but can still affect
phenotype in important ways. Examples of observable sub-lethal and reversible changes include
cell swelling, fatty changes, while irreversible changes include lysosomal rupture, membrane
breakdown, apoptotic DNA fragmentation, etc. Within mammalian cells there are several stress
pathways: pro-survival mechanisms as well as apoptosis pathways that are conserved among
species and various tissue types. Hence, instead of laboriously 'screening' functionalities of
various cell types against device conditions, probing the state of such conserved pathways and
mechanisms provides an opportunity to discover conditions that may likely be stressful to a
variety of cell types.
1.4
Potential Stressors within Microsystems
Any aspect of cell culture, handling, manipulation or analysis affects phenotype to some degree. Several studies have revealed how different aspects of the cellular microenvironment regulate cell state39, such as the culture substrate chemical composition40
, 41 its mechanical properties42
-44, the culture medium composition45 and culture architecture (2D vs. 3D)46,47. In addition, cells may encounter additional extracellular cues within microsystems such as light exposure from microscopy, which may induce phototoxicity48, or exposures to a variety of fluid shear stresses
(FSS), which may affect their physiology49. Importantly, exposure to stressors within microsystems impinge upon conserved stress pathways that regulate cell state and function. For instance, reactive oxygen species (ROS) generated by phototoxicity can damage DNA and activate p53-regulated DNA repair and cell-cycle arrest mechanisms. More generally, excessive intracellular ROS as well as cellular heating can damage proteins which are then recognized and degraded by protein folding and trafficking chaperones (heat shock proteins). Mechanical injury, including that by FSS can activate multiple mechanisms such as ROS, Calcium signaling, mitogen-activated kinase pathway (MAPK), inflammatory NF-KB pathway and more. Calcium imbalance and oxidative stress can both stress the endoplasmic reticulum, which initiates the unfolded protein response pathway50. On the other hand, MAPK/ERK pathway regulates cell cycle and proliferation mechanism and its over-activation or deregulation can steer cells towards a cancerous stage51. Additionally, NF-KB pathway importantly regulates expression of inflammatory signals and cytokines and similar to the other mechanisms, it closely regulates the homeostasis between survival and apoptotic pathways5 2. While the complex inter-pathway crosstalk and its relationship to multifactorial stress dosages is not fully characterized or understood, there is a definitive risk of activating stress pathways with stressors within microsystems. Hence, when introducing cells to device microenvironments it becomes important to identify potential cell-stressing stimuli and use that information to design appropriate devices and operational states where adverse effects and technical bias are minimized 3
, s4. As the diversity of such stimuli is essentially infinite, I will first review major stressors that are commonly found in cell-based microsystems.
Shear stress. Since cells are cultured, sorted, manipulated, etc. in liquid environments, cell-based
operations often involve fluid flows with, or around, cells. Such flows consequently impart FSS upon cells. Out of the many types of mechanical stresses that can be imparted55, FSS is the ubiquitous mechanical stressor in microfluidic systems. Depending on the application, the intensity and duration of applied FSS can vary significantly across platforms, and furthermore the impact of such stresses upon cells is dependent on the cell phenotype49. For instance, flow-based
microfluidic sorters can impart short-but-intense FSS (100-1000s dynes/cm2 for ms-sec
durations) while cell culture devices can subject cells to 'chronic-but-gentle' FSS (0.001-1 dyne/cm2
for hrs-days duration)49'56,57. Several microfluidic devices also provide moderate FSS
for ~min durations. In many cases published values of FSS in devices are lacking58, however they can be inferred through analytical estimates using the fluid properties and flow rate, along with device dimensions.
FSS can have beneficial properties towards cells, such as maintenance of endothelial cell function 9. Nevertheless, in most microsystem applications it is viewed as a stressor0 63
Consequently, FSS has been lowered by decreasing fluid flow rates, designing high-aspect-ratio chambers, and by other geometric designs (e.g. microwells) that may shield cells from applied FSS49. However, the 'dosage' at which FSS becomes a stressor is not always obvious. Despite
lowered magnitudes, reported 'safe FSS' setpoints vary drastically among devices, even among those working with the same cell types. For instance, Villa-Diaz et al. cultured human embryonic stem cells (hESCs) in their microfluidic platform and reported that long-term exposure to the device FSS of 0.6 dynes/cm2 did not affect cell adhesion, and did not impact hESC differentiation64. In contrast, Titmarsh et al. reported an optimal hESC culture FSS of 0.005 dynes/cm2, beyond which cells would detach and show signs of differentiation in their platform 65. On the other hand, Yoshimitsu et al. reported that device FSS of 0.01 dyne/cm2 did not affect the self-renewal marker Oct3/4 in human induced pluripotent stem cells after 3 days of culture66. These examples provide evidence for a -100x variance in what was concluded as non-harmful FSS, making it challenging to identify absolute deleterious effects within a low FSS regime. This is important because many devices are designed to deliver FSS values to be below a certain setpoint
(hence 'safe' for cells), however such notions may not always be generally applicable across cells or platforms.
Dose-dependent FSS activates complex biological cascades and mechanisms, such as activation of mechanosensitive pathways, calcium signaling, etc. in mammalian cells (reviewed elsewhere67). Critical regulators of in mammalian cell stress also include reactive oxygen species, which are generated and regulated at a controlled rate in healthy cells. However, external stimuli (e.g. FSS, light, heat, etc.) can generate excess ROS leading to oxidative stress, where damage can be inflicted upon cellular membrane lipids, proteins, and nucleic acids. In this context, several studies support FSS as a cell stressor. For instance, studies have demonstrated FSS can cause intracellular ROS and oxidative stress, and can also lead to compromised viability2
Light. Light is continually used in a broad variety of operations within microsystems, particularly for monitoring and manipulating cells. Visual cell observation is often an integral part of microsystem use. Visible light has been used to image live cells, with and without fluorescent molecules present as proteins or staining dyes, at tissue-scale resolution to near-molecular resolution68 69. However most cells in vivo are not exposed to light, and microscopy-induced
photoxicity7071 can become a stressor in microsystems. Ultraviolet light, which is known to cause
cell stress via DNA damage and ROS7 2, has been utilized in microsystems for cell encapsulation via photopolymerization73-75. UVA and violet light (340-380nm) is typically used for imaging blue fluorophores (such as Hoechst 33342), blue light (460-500nm) is used for imaging green fluorophores (such as GFP, or Calcein-AM); and green light (528-553nm) is used to image red fluorophores (such as ethidium homodimer-1). However, such exposures can potentially be toxic to cells by direct effects by or indirect effects such as by induced ROS, which can enhance cell damage. Purschke et al. reported that 3-day time-lapse imaging of unstained rat embryonic fibroblast cells at 15 minute intervals could induce ~15-20% cumulative apoptosis in the culture76.
With Hoechst 33342 staining, cumulative apoptosis was relatively higher; however, such cell death could be reduced by lowering exposure energy dosage, reducing stain concentration, and reducing imaging frequencies.
Sublethal damage from phototoxicity is often measured by means of a comet assay, which allows for quantifying DNA single-strand and double-strand breaks77. DNA lesions in the form of
damaged bases such as 8-oxoguanine or formamidopyrimidines can be recognized by enzymes such as Fpg glycosylase that then cleave DNA at the lesion site, creating a strand break which could then be quantified by the comet assay78. Incorporating this method, Ge et al. reported Calcein-AM induced phototoxicity in TK6 lymphoblast cells when cells were imaged from 2-15 minutes in blue, green and red fluorescence channels48. Importantly, they reported that
prolonged exposure of unstained cells was most genotoxic (strand breaks and oxidative damage) for the blue, then green and then red channels. Also, they reported that Calcein-AM stained cells were significantly more damaged than cells expressing GFP and imaged with identical conditions, highlighting the relative phototoxicity of the stain compared to fluorescent proteins. Such wavelength-dependent DNA damage by UV and visible light imaging has also been reported in cells types such as AS52, U2OS, COS-7 and HeLa cells72
,9.
In other applications, optical forces have been applied in microsystems to sort cells with the use of lasers11-13, 80-82. Optical tweezers typically employ NIR-wavelength lasers, which could stress
cells by direct photon damage or by indirect photothermal heating. Kong et al. performed a comparative analysis of exposing HeLa cells to laser systems of UVA to NIR range and reported varying degrees of DNA crosslinking damage and single strand breaks in the tested wavelength range 3. In aqueous environments, a 1064nm NIR laser at 100 mW was reported to raise the temperature by 1-22C 4
, which can potentially initiate heat stress in cells maintained at physiologic temperature. Though the exact nature and severity of insult will depend on the laser wavelength, it is also regulated by optical power and exposure durations, both of which have been typically minimized in microsystems in hopes to minimize cell damage1 2
. While not all optical modalities and parameters will inherently be damaging, context-specific phototoxicity has been studied extensively and several precautionary measures have been identified to minimize detrimental effects, comprehensively reviewed elsewhere7 0 85' 86.
mentioned, laser light exposures can raise culture medium temperatures, typically on the order of 1 K/100 mW 4'
87. Electric fields that have been used to manipulate cells, such as via
dielectrophoresis, can lead to Joule heating in conductive liquids (~16 K for 4V for 50 pm spaced electrodes in culture media, scaling quadratically with voltage88). Depending on the ambient
temperature, such elevated temperatures can heat cells significantly greater than the in vivo temperature of 372C. Integrated electrodes in microsystems have been used to maintain physiologically heated cell culture89, however applying higher temperatures with such electrodes can induce substantial heat stress. This aspect was explored in microdevices to intentionally apply elevated temperatures to study cellular heat shock9091, to heat cells for mimicking cell injury92
and even to lyse cells93.
Cells may also experience thermal stimuli by other physical forces such as from radiation forces in acoustophoresis or surface acoustic waves microfluidic devices. Specifically, loses in the piezoelectric transducers used in these devices generate voltage-dependent heat which can couple into the device fluidic environment94 91. Such heating can cause drifts in the resonance frequencies and hinder device performance94, hence many devices have incorporated heat sinks
or active temperature controllers to maintain operational temperatures (typically pinned around room temperature96 97). While subjecting cells to ambient temperature may not activate heat
shock pathways, prolonged exposures can activate unique cold shock stress pathways in mammalian cells9 8'99. Overall, cell exposure to significant temperature changes above or below their physiologic range can occur in a variety of platforms, and, in return, pose risk of cell damage in microsystems.
Nutrient stress & oxygen. Long-term cell culture devices need to provide cells with sufficient nutrients and oxygen, while removing waste products to maintain cellular homeostasis. Nutrient deprivation may happen when lowering device culture FSS by restricting media flow rate, and consequently lowering the nutrient delivery and waste perfusion rates. Another route towards nutrient depletion in such culture devices is by the adsorption and losses of nutrients to the device materials, such as PDMS. Specifically, PDMS is known to adsorb proteins and absorb hydrophobic macromolecules, imparting nutritional imbalance upon cells100. Furthermore,
evaporation in PDMS microfluidic devices can lead to osmotic changes that can hinder cell growth and development63, 101. Detailed strategies for addressing such concerns in microsystems have been reviewed elsewhere49,102
While depriving cells of carbon sources or failing to remove waste products such as lactate is universally harmful, oxygen has a more varied effect on cell physiology. Metabolically active cells such as hepatocytes require high amounts of oxygen03'
104, while other cells types (e.g., stem
cells105, 106) have enhanced phenotypes at the low oxygen tensions found in most in vivo microenvironments. While prolonged changes in oxygen tension can manifest into functional adaptations, short-term exposures are less commonly found in microsystems but remain to be explored in the context of cell health.
1-5 Methods for Assessing Cell Health in Microsystems
There are several qualitative and quantitative assays that provide information about cell health. Among these, some assays provide a gross/global view of cell state (generic assays) and some provide more specific information. Depending on the context and convenience, one choses among some of these assays below:
Generic Assays: Cell health has been reported both by assaying cells directly, and by indirect assessment of the cellular microenvironment. While there exist many examples of the latter, (e.g. monitoring medium pH'07'108 or dissolved oxygen levels108 109), it is generally more common to
assay cells directly.
When beginning to investigate if a device is not detrimental to cells, the most obvious (hence most popular) measurement reported is that of cell viability" 22, 10-114, followed by assessment
of cell morphology and proliferation3 2 3 33, ,
13-16. Viability itself can be quantified by a variety of
assays"7, though it is most conveniently done so by using colorimetric or fluorescent probes which can imaged within microsystems. In many cases"6' 118, viability has been assessed by exclusively labeling live cells using cell-permeant Calcein-AM stain, which becomes fluorescent and cell membrane-impermeant by intracellular esterases in viable cells. On the other hand,
others1
9-1
22have measured fractions of cells with compromised membranes (labeled dead), with
Trypan blue stain.
While simple, the drawback of using these assays is that they do not label the "other" cells. A
better approach is to have two stains, so that both live and dead cells are positively labeled. These
'live/dead' stains1 3, 1 3, 123-125 typically use Calcein-AM (which makes live cells fluoresce green) and
ethidium homodimer-1 (which makes dead cells fluoresce red). This method is particularly useful
as it quantifies relative impact of proliferation and of any cytotoxicity.
Viability-only assays fail to identify early apoptotic (and likely stressed) cells, as these cells do not
have a compromised membrane and are undetectable by Trypan blue, propidium iodide (PI),
ethidium homodimer-1, and 7-aminoactinomycin D (7-AAD) stains. Phosphatidylserines, which
flip from the cytoplasmic to extracellular leaflet during early apoptosis, can be stained with
annexin V. Though not applied for device design or usage optimization, annexin V staining
combined with P1126 or with 7-AAD121 have been conducted within microfluidics to identify viable
(double negative), early apoptotic (annexin V positive, PI negative), or late apoptotic and dead
(double positive) cells. A variety of other applications and advancements of viability and
apoptosis assays within microfluidic devices has been reviewed elsewhere
2.
Viability can also be discerned by measuring cellular metabolism, such as by monitoring culture
glucose consumption rates
29, or by monitoring the activity of cytochrome p450 intracellular
enzymes (in the case of hepatocytes'
0,
130).The metabolic dye
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) is applicable to a broad variety of cells, where the
cell-soluble tetrazolium is metabolized to cell-incell-soluble blue formazan crystals that can be measured
by colorimetric methods"
7. The MITT assay has been also been utilized to report on cell
proliferation as a means of representing cell heath"s,131. Although the readout of the MTT assay
is sensitive to cell metabolism, it is also sensitive to the number of cells in the assay, the
incubation time, requires colorimetric measurement, does not report on how many dead cells
are in the system, and the short path length of microsystems makes colorimetric assay difficult.
Given these challenges, one would choose MTT over live/dead assay when the system is not
amenable to detailed microscopic imaging, or where metabolism itself is of primary interest.
While it is universally important for all cells to be viable, it is not necessary for them to continue proliferating. For instance, cardiac cells or neurons in vivo do not proliferate following mature differentiation. In contrast, endothelial cells and smooth muscle cells proliferate in certain pathological context or in response to injury. Primary cells may be coaxed to proliferate in vitro, however their proliferation capacity will depend on their lineage, maturation, as well as culture conditions. Hence it may not always be obvious which proliferative rates or trends may be good or bad for those cells. On the other hand, cell lines are more comfortable with in vitro culture, and their growth rates are more easily characterized. In many instances, cell proliferation has been measured qualitatively, and used to infer that cells did not incur any damage within different microsystems3 4
, 37, 132, 133. Less-common generic assays include measurements of cell
shape and elongation1 2 3, spreading134, migration1 35, or other aspects of cell morphology37, 64, 89, 131
Specific Assays: A viable cell does not necessarily imply that the cell is stress-free or unperturbed. With this rationale, many groups have investigated cell health in their platforms beyond the generic assays. One broad way to group these assays is based on whether they affect upstream, short-term, and relatively broad aspects of cell phenotype, or downstream, long-term, and more specific aspects.
Some groups have investigated short-term changes (seconds-min range) in response to the device conditions. Such assays typically focus on events that occur quickly and are thus associated with intracellular signaling molecules. For instance, Perroud et al. measured nuclear factor kappa-B (NF-KB, transcription factor that regulates inflammatory programs) translocation via engineered reporters, and extracellular signal-regulated kinase (ERK) phosphorylation (a mitogen activated protein kinase) via staining, within cells that were sorted through their microfluidic device13. EI-Ali et al. focused more broadly on the stress-inducible mitogen-activated protein kinase (MAPK) pathway136, through the phosphorylation of ERK, JNK and p38 kinases in cells passed through their device1 2. Importantly, NF-KB, ERK, and MAPK are involved in transducing
Calcium is an intracellular messenger important in regulating several essential cellular functions, and disturbances in its homeostasis can lead to many diseases37. Yin et al. measured calcium flux in CHO cells in response to a large range of FSS (~0.01-10 dynes/cm2) in their devices and noted
FSS-dose dependent induction of Ca"* signals, which matched signals from chemical agonists, even at moderate FSS'2 1
Cellular ROS and its direct effects can also be assessed. A common example is the probe 2'-7'-dichlorodihydrofluoresce diacetate (DCFDA), which is taken up by a variety of cells, where it fluoresces in response to intracellular ROS138. Wu et al. used DCFDA to measure cell stress
induced by electric fields in their device3s, Lo et al. used the same measurement against oxygen
gradients in their device139, and Chin et a/. used it to measure ROS induction in response to shear
stress140. An immediate effect of ROS is DNA damage, which can be characterized via a variety of assays. Ultrasound-induced DNA damage in acoustofluidics compared to that by Doxorubicin was investigated by measuring histone y-H2AX phosphorylation41, which is known to occur following
DNA double strand breaks14 2. Other examples of short-term cellular responses to stress include
changes in membrane receptor display, as well as expression of immediate-response stress genes. Adams et a/. investigated platelet activation in their acoustophoresis device in response to the applied acoustic fields96. Specifically, they reported that expression of CD62 did not immediately vary in platelets with or without device acoustic fields, compared to off-chip prothrombin-treated positive controls. Wang et al. developed a microfluidic platform which utilized optical forces in sorting cells". Here, they measured the viability of sorted HeLa cells, and measured the expression of heat shock sensitive HSPA6 gene and multifactorial cell stress sensitive Fos gene immediately after sorting to assess if the device activated stress pathways.
Short-term molecular assays have the advantage that they can be run quickly after exposure, rather than requiring extended culture. This feature is appealing for the device user or designer. They also have a higher level of specificity than generic assays, as they focus on individual molecules and pathways. The drawback is their assay complexity; since they may require genetically modified cell lines (in the case of NF-KB translocation) or immunofluorescence with
phosphor-specific antibodies (in the case of kinase assays).
For longer term changes (-hours), an assay will focus on gene expression or function. Hur et al. performed microarray analysis of 100s of genes in MCF7 cells sorted through their inertial microfluidic device, against unsorted controls to assess whether there were any global transcriptional changes as a result of sorting2s. Similarly, Sharei et al. developed a microfluidic
platform for intracellular delivery, and measured expression profiles POUF5 and ALP genes within mouse embryonic stem cells that passed through the device, to assess if certain conditions could initiate cell differentiation programs1 . On the other hand, instead of checking expression profiles at a single timepoint, some groups have utilized cell-based sensors that can provide an integrated response to environmental stressors. Specifically, Davidsson et al. utilized a luciferase-based sensor in HeLa cells (activated by MAPK through G-coupled protein receptors14 3) to
monitor long-term cell health their microfluidic platform4 4. Similarly, Au et al. utilized a
cell-based heat shock sensor (along with microarray analysis and comet assay) to investigate if their digital microfluidic platform biased cell health54. Regmi et al. used a caspase-activation FRET reporter to monitor apoptosis activation, simultaneously labelling live and necrotic cells in continually circulating tumor cells within a microfluidic flow system145. In this way, their approach allowed for classifying live, dying and dead cells in response to the flow conditions. Late-term changes in cell health (-days) have also been reported. Lopacinska et al. measured neuronal-like PC12 cell viability with Calcein staining, cell cycle with PI staining, metabolism with MTT, and gene expression changes with microarrays in response to common culture substrates used in microsystems such as polydimethylsiloxane (PDMS), polymethylmethacrylate (PMMA) and polystyrene'. As another example, Villa-Diaz et a. measured expression of OCT3/4 and NANOG genes in cultured hESCs to assess if their device FSS altered pluripotency after days of culture64.
Assays focusing on expression or function have the advantage of specificity; upregulation of a gene involved in the heat shock pathway is strong evidence that the cell underwent stress. Additionally, expression assays do not require many cells (a few thousand is routine, and single cell is possible), and the results can be quantitative (e.g., fold upregulation). The drawbacks are that 1) it takes ~hrs for expression to occur, which can decrease throughput, and 2) the exact time from stress to readout will affect the assay results, since the amount of mRNA or protein
present will depend on both synthesis and degradation. Additionally, most of these assays are endpoint, and so require cell recovery and isolation, though live-cell reporters do exist.
Though extensive device optimization or characterization using a variety of cell health assays is not commonly found in literature, the examples from various groups discussed above do demonstrate a few important points. First, the microsystems community does care about cell health in their platforms, and further, it does employ a variety of techniques and assays to investigate the phenomenon. Second, there are several examples of groups using similar assays (e.g. viability, proliferation), but there lacks a consensus when it comes to the applying assays specific (and likely more informative) to cell health. We believe that the latter is related to certain inherent challenges in adapting established cell health specific assays within the device design and workflow.
1.6 Current Challenges
in Assessing
Cell
Health
within
Microsystems
To systematically assess cell state in device microenvironment, one has to consider the vast variety of assays (generic and specific) used for assessing cell health. In this regard, it is common to adapt standard assays within engineered microsystems, or apply them to cells which have experienced the device microenvironment. While some assays, such as imaging-based assays, are easily translatable to microscale technologies, there exist challenges in finding assays that are specific to cell health, quantitative, and conveniently adaptable. Some of these challenges are elaborated below.
i.6.i Challenges and Tradeoffs
Challenge of low cell numbers: In microscale platforms where cells are retained in small volumes (for culture, analysis, manipulation, etc.), there are inherent limitations on the number of cells available for running any assay. 'Flow-through' devices such as sorters that do not work with rare cells, or instead work with cell lines are an exception to this limitation, since the cell availability is directly related to the starting amount of cells. In most other cases, with limited cell numbers
it becomes challenging to run a standard biological assay that was not designed for low cell numbers. For instance, trying to assess cell metabolism in microenvironments through alkaline phosphatase activity (e.g. of osteoblasts146, 147) or cytochrome p450 functionality (e.g. of
hepatocytes148) has required macroscale analysis machines, which typically require large cell
numbers (~ 100,000 cells). Additionally, with 10s-1000s of cells, it becomes challenging to run a protein assay such as a western blot or a genetic assay such as qRT-PCR as each of these methods may typically require 10-10OX more cells to get enough signal91,14 9
,150. Though similar limitations may also be present when quantifying cell viability off-chip with a hemocytometer or flow cytometer5 1, microscopy-friendly viability stains provide a easily adaptable and quantifiable assay to small cell numbers in microsystems. However, assays for other aspects of cell health are yet to be customized, and importantly, translated into microsystems working with small cell
populations.
Complexity of Specific Assays: Many specific assays are destructive, costly and complex, and hence technically prohibitive. Assays of intracellular protein levels or gene expression have been performed by our group152 and others3 2",2 3 while developing various platforms. However, within
the microfluidic community the usage of such methods to interrogate device bias is not routinely performed for certain reasons.
First, it is usually not obvious as to which cell health biomarkers to measure, and hence which assay to run. Second, many relevant assays (such as qRT-PCR, western blotting, flow cytometry) require processing of intra- or extracellular material, and may require steps such as washing or mixing biological materials, and further isolation, purification, etc. Any such additional steps need to be incorporated into the device workflow based on practicality and convenience. Obtaining starting materials (cells or cellular constituents) can be done by either taking the cells off-chip for processing, or bringing the assay reagents on-chip. Retrieving immobilized cells from microfluidic devices is often a non-trivial task. While attached cells can be harvested by flushing enzymatic dissociation buffers116,153, one has to be careful about potential cell losses in the fluidic network, or handling processes. Alternatively, cells can be retrieved with optical methods154
sophisticated technical expertise. On the other hand, while bringing assay reagents to cells is
possible through specific fluidic architectures
156or device designs
32,
157,