Amphiphilic gold nanoparticles: mechanisms for
interaction with membranes and applications in drug and
vaccine delivery
Prabhani U. Atukorale
Bachelor of Engineering, Biomedical Engineering
Vanderbilt University, Nashville, TN, 2005
Master of Science in Engineering, Biomedical Engineering
Johns Hopkins University, Baltimore, MD, 2007
Submitted to the Department of Biological Engineering
in Partial Fulfillment of the Requirements for the Degree of
Doctor of Philosophy
at the
,MASSACHUSETTS
INSTTTEOF TECHNOLOGY
Massachusetts Institute of Technology
JUN 18 2014
May 2014
t
a~i
LIBRARIES
C 2014 Massachusetts Institute of Technology
All rights reserved
Signature of Author:
-Signature
redacted-Department of Biological Engineering May 23, 2014
Certified by:
Signature redacted
Darrell J. Irvine
Professor of Biological Engineering and Materials Science Thesis SupervisorSignature redacted
Accepted by:
Forest M. White Associate Professor of Biological Engineering Chairman, Graduate Program Committee for Biological Engineering
Members of Thesis Committee
Darrell J. Irvine
Professor of Biological Engineering and Materials Science & Engineering
Thesis Supervisor
Francesco Stellacci
Professor of Materials Science & Engineering
Ecole Polytechnique Feddrale de Lausanne, Switzerland
Jacquin C. Niles
Associate Professor of Biological Engineering
Thesis Committee Chair
Amphiphilic gold nanoparticles: mechanisms for interaction with
membranes and applications in drug and vaccine delivery
Prabhani U. Atukorale
Submitted to the Department of Biological Engineering
On May 9, 2014 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biological Engineering at the
Massachusetts Institute of Technology
Abstract
Materials that can interact with and transit membranes without toxic bilayer disruption or poration are of great interest in the drug delivery field. These materials can presumably bypass endocytosis to directly enter the cell cytosol through the plasma membrane, which is often the desired site of action for therapeutics, thereby avoiding potential and likely cargo degradation if trapped in endosomes. Alternatively, if these materials are endocytosed, they are often able to escape the endosome by interacting with and transiting the endosomal membrane, and moving into the cytosol. Here, we present a unique membrane-interacting, amphiphilic gold nanoparticle (amph-AuNP) system, which we show can interact with, embed within, and even penetrate through multiple adjacent lipid bilayers without evidence of membrane disruption or poration.
By virtue of these key properties, we also present these amph-AuNPs as effective carriers for
therapeutic molecules. Using a one-step reaction, we synthesized small -2-4 nm core size amph-AuNPs with an amphiphilic ligand shell comprised of one or two alkanethiols. Each amph-AuNP is coated by a mixture of long-chain mercaptoundecanesulfonate (MUS) terminated by a water-soluble sulfonate group, and in some cases, short-chain hydrophobic octanethiol (OT). First, we describe our efforts to adapt and develop a method to synthesize giant multilamellar model membranes to study amph-NP-membrane interaction in a well-defined setting. We show that giant membranes can be synthesized and fine-tuned by varying lipid composition and buffer salt concentration, can be fluorescently labeled with lipid tracers, and can be analyzed robustly with confocal microscopy and flow cytometry. Second, we describe our systematic analysis of amph-AuNP and membrane characteristics that influence mechanisms of NP association with bilayers. We study effects of general membrane properties such as electrostatics and phase that govern NP-membrane interactions, and found that NP penetration of bilayers was blocked under conditions where strong electrostatic repulsion or gel-phase lipids were employed. We further studied effects of AuNP core diameter, surface charge, and surface hydrophobicity on NP-membrane interactions at the nanoscale. We found that MUS particles with an optimal gold core size -2-3nm in diameter and MUS:OT particles of a broader size range were capable of inducing hemifusion between liposomal membranes, while MUS:OT 2:1 particles of intermediate hydrophobicity were capable of spontaneously aggregating within the bilayer of vesicles to form Janus egg-like morphologies. Third, we built on these NP membrane-embedding properties to explore and characterize NP-embedding in erythrocyte membranes, with particular attention to the glycocalyx and membrane fluidity, for the future application of constructing therapeutic erythrocyte 'pharmacytes' in situ. Finally, we describe work in engineering amph-AuNPs to carry short antigenic peptide cargoes for in vivo vaccine applications, where immunization experiments have shown much promise for antigen-ferrying amph-AuNPs in eliciting robust and long-lasting CD8+ T cell responses.
Thesis supervisor: Darrell J. Irvine
Table of Contents
Acknowledgments ...
5
L ist of F igures ...
7
L ist of T ables ...
9
Chapter 1: Introduction ...
10
Membrane-interacting materials ... 10Inorganic nanoparticles that interact with membranes ... 11
Advantages of gold nanoparticles for biological applications ... 12
Soluble, amphiphilic gold nanoparticles that interact with membranes ... 13
Scope and aims of this thesis ... 14
Chapter 2: Synthesis and characterization of giant model membranes ...
16
Introduction ...
. . 16
Methods and Materials ...
20
Results and Discussion ...
21
C onclusions ...
. . 24
Chapter 3: Characterization of amphiphilic gold nanoparticle design and bilayer
properties that govern interactions with membranes ...
25
Introduction ...
. . 25
Methods and Materials ...
27
Results and Discussion ...
30
C onclusions ...
. . 43
Chapter 4: Characterization of amphiphilic gold nanoparticle interaction with
erythrocytes ...
45
Introduction ...
. . 45
Methods and Materials ...
46
Results and Discussion ...
49
C onclusions ...
. .
58
Chapter
5:
Conclusions and Future Directions ...
59
Amphiphilic gold nanoparticles for peptide vaccine applications in vivo ...
59
Acknowledgements
My years of graduate school at MIT have been some of the best, most challenging, and most
rewarding of my life. With the remarkable clarity that comes only with hindsight, I take time to thank deeply the people who have made this growth and development possible. My efforts would be nothing without the support I have had.
To my advisor, Prof. Darrell Irvine: you have my first acknowledgment. Thank you for your passion and creativity. Thank you for encouraging me and giving me the freedom and independence to grow, and for always instilling perspective and confidence when the path appears bleak. I count myself lucky for being a part of your lab over the years that I have, to have the unique experience of seeing your lab burgeon as it has.
To members of my thesis committee: Prof. Francesco Stellacci, for fostering our collaboration across the Atlantic. You have my thanks for your astute guidance, diligence, and encouragement every step of the way. To my committee Chair, Prof. Jacquin Niles: thank you for the time and effort you have invested, for your critical feedback that has always been constructive.
To my collaborators: Prof. Alfredo Alexander-Katz, the start of our collaboration with you was a pivotal juncture of my project and I will remember you with thanks and appreciation not only for our discussions but also for working at the bench with me. To Reid Van Lehn: many many thanks for our numerous discussions, brainstorms, and analysis of data over the last years, you have always been helpful. To Randy Carney, Ahmet Bekdemir, and Paulo Silva at EPFL: thank you for teaching me how to synthesize particles and for being the ever-helpful, expert synthesizers as we needed. To Mark Ames-Droz: thank you for your dedication in learning in the lab besides me when we first started the project.
To Irvine lab members past and present: Greg Szeto, Haipeng Liu, and Chris Jewell, thank you for your mentorship and for assistance with experiments - you have always been helpful and positive. To Kelly Moynihan: your energy is infectious and you have my thanks for the time and patience you put in to help me with in vivo experiments. To Sabrina Yang: thank you for helping me with TEM and for your discussions and feedback. To Chyan Ying Ke and Yiran Zheng: thank you for your smiles and cheer at the bench for the past years. To Bonnie Huang, Melissa Hanson, Talar Tokatlian, Sudha Kumari, Kavya Rakhra, Marianne Bandeira Melo, and Pete DeMuth: thank you for your help, energy, and fun in the lab. I will dearly miss you all. To my UROP Priyanka Saha: thank you for your dedication, hard work, and positive attitude that made all the difference.
To Jeff Wagner and Jim Abshire: thank you for providing erythrocytes as I have needed them. To Cary Opel and Robbie Williams: thank you for your help with in vivo work.
To members at core facilities: Dong Soo Yun (Koch Institute Nanotech Materials Core), thank you for working with me over many diligent months at the cryoTEM, the results of which have been of much importance in my thesis. To Glenn Paradis and Mike Jennings (Koch Flow Cytometry Core): thank you for your always helpful assistance. To Nicki Watson (Whitehead): thank you for much effort you put into preparing cell EM samples for my work.
To my husband Sumeda, you are my muse, my encourager, and one of my truest, deepest, and best friends. Your love inspires me to live with passion, joy, and incredible energy and I thank you for it.
To my sister Vajini, you have been my rock for as long as I can remember. Throughout all our lives, your love and friendship has grounded me, nourished me especially when I most needed it, and kept me balanced.
To my parents, for the un-ending courage you have given us, for the love you have shown us without condition, for your genuine kindness and never-ending positivity, for the tremendous resilience and open-mindedness you have without bounds, I thank you now and till the end of time. No accolade can ever match what you have given us and every success I have or will ever have I owe in near-entirety to you. You are my heroes.
List of Figures
Figure 1.1. Membrane-embedding schematic of antimicrobial peptide protegrin ... 9
Figure 1.2. Oleic acid-coated iron oxide NPs associated stably between liposome bilayer leaflets after surfactant dialysis ... 9
Figure 1.3. AuNPs coated with dodecanethiol prepared either by extrusion or surfactant d ialy sis ... 1 1 Figure 1.4. Schematics of long-chain MUS and short-chain OT used for amph-AuNP sy nth esis ... 1 1 Figure 1.5. Depictions of three formulations of amph-AuNPs ... 11
Figure 1.6. Evidence of amph-AuNP entry into cells via non-endocytic pathways ... 12
Figure 2.1. Examples of GUV studies of lipid domain formation ... 14
Figure 2.2. Examples of cell-attached and cell-free GPMVs ... 15
Figure 2.3. Confocal images of 'model cell' GUVs with artificial cytoskeletons ... 16
Figure 2.4. GUV studies to elucidate HIV- 1 TAT transit across membranes, interaction with the cytoskeleton, and eventual translocation ... 17
Figure 2.5. Brightfield confocal micrograph of all-DOPC GMVs prepared in water ... 20
Figure 2.6. Examples of GMVs synthesized by varying lipid composition, buffer salt concentration, and fluorescent tracers, and analysis by both confocal microscopy and flow cytom etry ... 2 1 Figure 2.7. GMV fluorescence quantification by flow cytometry after treating with various A uN P s ... . . 22
Figure 3.1. Scheme of one-step reaction from a modified Brust-Schiffrin synthesis for am ph-A uN P s ... 23
Figure 3.2. Schematic depiction of our proposed mechanism of amph-AuNP insertion and embedding within bilayers based on the ability for surface ligands to 'snorkel' between the aqueous/lipid interface ... 23
Figure 3.3. Atomistic molecular dynamics simulations for membrane-embedded MUS:OT 1:1 and all-MUS amph-AuNPs ... 24
Figure 3.4. Atomistic molecular dynamics simulations for free energy of membrane-embedding for all-MUS, MUS:OT 2:1, and MUS:OT 1:1 AuNPs of various core sizes and surface ligand m orphologies ... 24
Figure 3.5. Black lipid experiments in support of simulation results ... 25
Figure 3.6. Confocal micrographs of all-DOPC GMVs cotreated with calcein and BODIPY-labeled all-MUS amph-AuNPs ... 28
Figure 3.7. Line scans of confocal micrographs of all-DOPC GMVs treated with BODIPY-labeled MUS:OT 2:1 NPs and quenching analysis in various solvents and with liposomes ... 29
Figure 3.8. Computational quantitation of potential mean force of BODIPY-grafted onto an AuNP when in solution versus when embedded within a bilayer ... 31
Figure 3.9. Flow cytometry quantification of all-DOPC GMVs treated with BODIPY-labeled all-MUS, MUS:OT 2:1, and MUS:brOT 2:1 ... 32
Figure 3.10. Confocal analysis of membrane electrostatics with DOPC/DOPG GMVs ... 33
Figure 3.11. Confocal and FACS analysis of membrane phase with DPPC GMVs ... 35
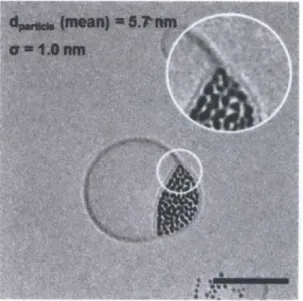
Figure 3.12. Cryo TEM analysis for batches #1-4 ... 37
Figure 3.13. Cryo TEM analysis for batches #5-7 ... 40
Figure 3.14. Summary of size distribution analysis for batches #1-7 ... 41
Figure 4.1. Confocal and FACS analysis of uptake of MUS:OT 2:1 amph-AuNPs into erythrocytes ... 48
Figure 4.2. Erythrocytes treated with unlabeled MUS:OT 1:1 AuNPs at 37*C ... 49
Figure 4.3. BODIPY-MUS:OT 2:1 interactions with erythrocytes after treatment with m em brane-altering com pounds ... 52
Figure 4.4. GMV studies for model erythrocyte membranes ... 55
Figure 5.1. Antigens and haptens for which AuNPs have been used as carriers in vivo ... 58
Figure 5.2. Peptide construct and loading onto amph-AuNPs ... 59
Figure 5.3. Tetramer results for pilot immunization with SIINFEKL ... 61
Figure 5.4. Tetramer results for follow-up immunization post boost #1 ... 63
List of Tables
Table 5.1. Groups of mice used in pilot immunization study ... 62
Table 5.2. Groups of mice used in follow-up immunization study ... 63
Chapter
1
Introduction
Membrane-interacting nanomaterials
Materials that can interact with and transit membranes without toxic bilayer disruption or poration are of great interest in the drug delivery field. These materials can presumably bypass endocytosis to directly enter the cell cytosol through the plasma membrane, which is often the desired site of action for therapeutics, thereby avoiding potential and likely cargo degradation if trapped in endosomes. Alternatively, if these materials are endocytosed, they are often able to escape the endosome by interacting with and transiting the endosomal membrane, and moving into the cytosol. Two very well-studied, biologically-derived classes of membrane-interacting materials are cell penetrating peptides (CPPs) and anti-microbial peptides, and the mechanisms by which they associate with membranes vary.
Naturally-occurring CPPs can be transcription factors secreted and internalized by cells, derivatives of HIV-1 TAT (transcription-activating factor) involved in viral replication, or membrane translocating sequences that shuttle pre-proteins to specific organelles.1-3 CPPs
often have short -1 1-amino acid arginine-rich sequences, called protein transduction domains (PTDs) that are thought to be involved with membrane interaction and entry into the cell.4
Studies have shown that CPPs stimulate high levels of endocytosis to mediate cargo entry-, while other studies have shown evidence for passive transport into the cell0'1". More recent findings have helped to elucidate one key mechanism, however, where cationic, arginine-rich PTDs are thought to first interact with the proteoglycans of the cell, slightly permeabilize membranes by creating saddle-splay curvature through multidentate hydrogen bonding of lipid headgroups, attach to and polymerize the underlying cytoskeleton, and stimulate endocytosis, particularly of large cargos.4'
Anti-microbial peptides, by contrast, are produced by the innate immune system of multicellular organisms as a defense mechanism against bacterial invasion. '14 Individual peptides are often linear, small (~3 nm), and amphiphilic, with cationic endgroups and a hydrophobic center, and by virtue of these properties, are able to insert into bacterial cell membranes, group together to create pores, and thus induce microbial cell death (Fig.
A
+ -4 + 4.0
+I
OA
B4Figure 1.1. Membrane-embedding schematic of antimicrobial peptide protegrin. A) Peptides are
small, amphiphilic, and linear, with a hydrophobic center and charged, cationic endgroups, and B) can insert and embed in bacterial membranes and group together via disulfide interactions to form pores and subsequent cell death. Adapted from [15].
The striking properties of natural CPPs and anti-microbial peptides have inspired the search for synthetic nanomaterials that can interact with biological membranes and cells. Synthetic cationic polymers and dendrimers have also been shown to interact with membranes, but do so by electrostatic attraction with lipid headgroups, and are known to create toxic pores in membranes and in some cases induce cell lysis. 19-22
Inorganic nanoparticles that interact
with membranes
Certain examples of inorganic nanoparticles (NPs) have been engineered to interact with membranes. For example, iron oxide NPs
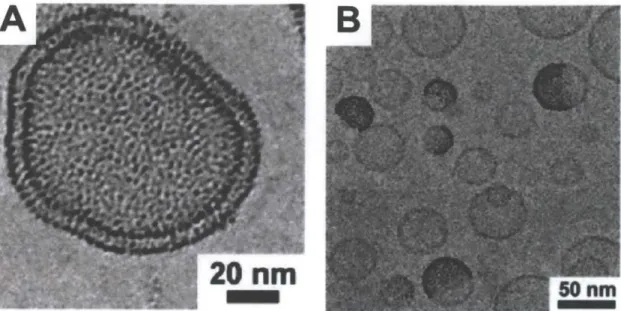
-5 nm in core diameter coated with a hydrophobic layer of oleic acid and associated with liposomes by surfactant dialysis stably associated between bilayer leaflets (Fig. 1.2). CdSe quantum dots with a hydrophobic surface have been coated with amphiphilic ligands such as phospholipids or detergents and, within a
4-5 nm core size range, have been shown to stably embed within synthetic membranes.4
Mesoporous silica NPs
-100
nm in size haveFigure 1.2. Oleic acid-coated iron oxide NPs associated stably between liposome bilayer leaflets after surfactant dialysis. Adapted from [23].
been shown to adsorb to erythrocyte membranes via interaction of NP surface silanol groups with cell membrane phospholipids.
Advantages of gold nanoparticles for biological applications
In the search for novel membrane-interacting nanomaterials, gold nanoparticles (AuNPs)
26-31
have particular advantages of interest for applications in the delivery of therapeutics. Their behavior and function in vitro and in vivo depend on their structural properties, and synthesis methods are amenable for easy fine-tuning to achieve desired structures. They have optical and electronic responses that depend very specifically on size and shape, can be synthesized with a high surface area-to-volume ratio, and have been shown to enter a wide variety of cell types. They can also be functionalized with various functional groups such as thiols, amines, and phosphines, which can be conjugated to therapeutic cargo molecules.26 They are also relatively inert in a biological setting. AuNPs have also been extensively tested and characterized in in vivo cancer applications as cytokine12 and siRNA3 carriers and as
sensitizers to subsequent radiation therapy.3 4 3 5 Further, Phase I clinical trials have shown low toxicity.3 2 In addition to cancer, Au-based drugs are clinically used for the treatment of rheumatoid arthritis and for in vivo applications in HIV/AIDS, asthma, and malaria.36
AuNPs have also been designed to interact with membranes and several specific examples warrant special discussion here. First, AuNPs less than 2 nm in core diameter were synthesized with a hydrophobic dodecanethiol surface shell and associated with DOPC liposomes either by extrusion or detergent dialysis.37 Extrusion led to very dense loading of AuNPs into vesicles by NP embedding within membranes, where there was matching of the hydrophobic NP surface with the hydrophobic interior of the bilayer (Fig. 1.3A). In contrast, detergent dialysis led to the formation of Janus liposomes, where AuNPs were able to 'unzip' a portion of the vesicle bilayer and were found to be stably associated between bilayer leaflets (Fig. 1.3B). A follow-up study also reported that small -1.8 nm core AuNPs coated with a dodecanethiol or hexadecanethiol shell associated avidly with liposomes when AuNPs and lipids were first dried and annealed with choloroform prior to hydration.38 In contrast, there was no AuNP-membrane loading with larger, -4.1 nm core NPs. Finally, computational analysis of 2.2 nm core AuNPs coated with a mixture of hydrophobic octanethiol ligands and amphiphilic dodecanethiol ligands that are either terminated with a carboxylate group (anionic) or an ammonium group (cationic) showed that anionic and hydrophobic AuNPs were able to interact comparatively less with cell membranes due to their surface charge, while cationic AuNPs interacted avidly with membranes but to the extent of causing membrane poration and toxicity.39 We highlight here that these systems of
membrane-interacting AuNPs studied to date largely fall into the classes of insoluble and hydrophobic or toxic and cationic, and there are no examples yet of water-soluble, non-perforating, membrane-interacting AuNPs, which is the subject of the work presented in this thesis.
Figure 1.3. AuNPs coated with dodecanethiol prepared either by A) extrusion for a dense loading of liposomes, or B) detergent dialysis for the formation of Janus vesicles. Adapted from [37].
Soluble, amphiphilic gold nanoparticles that interact with membranes
Here, we have studied a uniquemembrane-interacting AuNP system, which we show can interact with, embed within, and even penetrate through multiple adjacent lipid bilayers without evidence of membrane disruption or poration. By virtue of these key properties, we also present these NPs as effective carriers for therapeutic molecules. Using a one-step reaction modified from the Brust-Schiffrin method, we synthesized small -2-4 nm core size amphiphilic AuNPs (amph-NPs) with an amphiphilic ligand shell comprised of one or two alkanethiols (Fig.
1.4).41 Each AuNP is coated by a mixture of
long-chain
mercaptoundecanesulfonate
(MUS) terminated by a water-soluble sulfonate group, and in some cases, short-chain hydrophobic octanethiol (OT). We
can synthesize amph-AuNPs with 1:1 MU
varying hydrophobicity by varying
their surface ligand composition: Figure 1 e.g., all-MUS, MUS:OT 2:1, and AuNPs
(
MUS:OT 1:1 (Fig. 1.5). Atomistic solution.0-Na*
O=S=O
HS
Sodium 11 -mercaptoundecane
sulfonate: MUS
HSO'
Octanethiol: OT
Figure 1.4. Schematics of long-chain MUS and short-chain OT used for amph-AuNP synthesis. Adapted from [41].
3:OT
2:1 MUS:OT
all-MUS
.5. Depictions of three formulations of
amph-1:1 MUS:OT, 2:1 MUS:OT, and all-MUS) in
endocytosis
inhibited
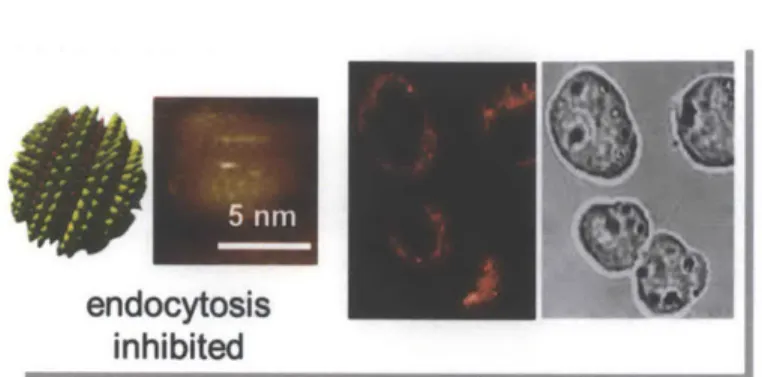
Figure 1.6. Evidence of amph-AuNP entry into cells via non-endocytic pathways. MUS:OT-2:1 AuNPs as visualized with scanning tunneling microscopy, STM (left panel), and labeled with BODIPY for visualization with a confocal microscope when incubated with DC2.4 cells at 40C (right panel). Adapted from [47].
entire cytosol but strikingly did not enter the nucleus observed that amph-AuNPs ferry short DNA oligos endocytosis is pharmacologically inhibited.4 8
molecular dynamics simulations of
these amph-AuNPs carried out by our collaborators in the Alexander-Katz laboratory (MIT Materials Science) emphasize the degrees of freedom available to the surface ligands, implying that they can
morph and adapt their
conformations based on their environment.42-46 Further, from prior studies with this system, it was observed that, under conditions where endocytosis was inhibited in dendritic cells, amph-AuNPs
(MUS:OT 2:1) were still seen to
enter the cells and permeated the (Fig. 1.6). In other work, we also into B16F10 melanoma cells when
Scope and aims of this thesis
Given our discussion of membrane-interacting nanoparticles and the promise they hold for biological delivery applications of therapeutic cargoes in Chapter 1, the core premise of this thesis is to study in experimental mechanistic detail the methods by which amph-AuNPs interact with lipid membranes. We will also describe work in which we build on these findings to develop applications for these membrane-interacting amph-AuNPs.
Chapter 2 details our efforts in adapting a synthesis method for giant model membrane vesicles. We describe the need for model membranes to study AuNP-membrane interactions in a well-defined setting and develop a protocol of synthesis. We also characterize our results from this method, discuss methods of analysis, and provide a broad summary overview of the results of our various syntheses.
Chapter 3 describes our systematic analysis of amph-AuNP and membrane characteristics that influence mechanisms of NP association with bilayers. We study effects of general membrane properties such as electrostatics and phase that govern NP-membrane interactions. We also study effects of AuNP core diameter, surface charge, and surface hydrophobicity on NP-membrane interactions. We use analysis of membranes both at the micro- and nanoscale levels in these studies. Novel NP-lipid hybrid structures we have observed in these studies may be the basis for inspiration of novel materials.
Chapter 4 discusses our efforts to build on the membrane-embedding properties of amph-AuNPs to achieve NP embedding in erythrocyte membranes for the future application of constructing therapeutic erythrocyte 'pharmacytes' in vivo and in situ. Here, we characterize
in detail erythrocyte membrane properties that most influence amph-AuNP embedding, with particular attention to the glycocalyx and the property of membrane fluidity.
These chapters complete the requirements of this thesis and Chapter 5 details our conclusions. Further, Chapter 5 also includes future directions and applications we have developed for this system of amph-AuNPs and also includes a summary of our work in engineering amph-AuNPs to carry short antigenic peptide cargoes for in vivo vaccine applications. We detail our methods of peptide construct design and conjugation onto AuNPs, review our pilot co-culture and in vivo immunization results, and finally describe our follow-up immunization studies where amph-AuNPs carrying model peptide antigens have shown exceptional promise in eliciting a robust CD8+ T cell response. We end this chapter with a discussion of our hypothesis for mechanisms of NP action and a review of the future experiments we will carry out to test them.
Chapter 2
Synthesis and characterization of giant model
membranes
Introduction
Advantages of giant model membranes and applications for which they have
been developed
Over past decades, membrane biophysicists have sought to study the properties of cell membranes, also with the purpose of developing nanomaterials to transit them.49 Giant
unilamellar vesicles (GUVs, Fig. 2.1) have been developed for this purpose and, by virtue of their ease of synthesis, analysis, and largely spherical structures 20-50 pm in diameter, they offer significant advantages over planar, supported lipid bilayer systems, the latter of which
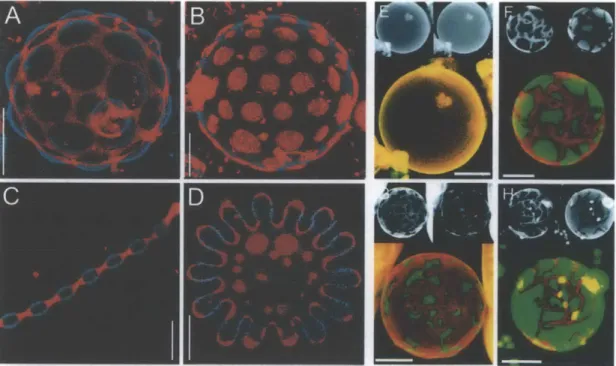
Figure 2.1. Examples of GUV studies of lipid domain formation. Images from two-photon
microscopy showing liquid-order (perylene, blue) and liquid-disorder (rhodamine-DPPE, red) phase coexistence in GUVs made of a mixture of SM/DOPC/cholesterol. A) 56%/24%/2% at
250C, B) 61.5%/13.5%/25% at 250C, C) 63%/7%/30% at 440C, and D) 58.5%/10.2%/31.3% at
50*C, scale bars 5 pm. Images from confocal microscopy, also showing phase co-existence in
GUVs made of DLPC/DPPC and in one case, cholesterol, at 250
C (DiI-C20, red and BODIPY-PC, green). E) All fluid phase, 100% DLBODIPY-PC, F) 40%/60%, G) 20%/80%, H) 50%/50% and 5%
have been widely-used to study the membrane pore-forming activities of cationic nanoparticles. Many GUV studies have been focused on understanding the biophysics of membrane domain formation, using compositions of lipids with low and high melting temperature (Tm).5 4-6 Examples include liquid-order and liquid-disorder phase separation in
GUVs made of sphingomyelin (SM, high Tm), dioleoyl-phosphatidylcholine (DOPC, low Tm), and cholesterol at various temperatures (Fig. 2.1A-D)." Phase separation and domain formation in GUVs can be easily visualized by confocal and two-photon microscopy, which also enables analysis of the myriad lipid structures that can form with various compositions of phase domains. This is illustrated by data from the literature shown in Figures 2.1E-H, showing confocal micrographs of GUVs made of dilauroyl-phosphatidylcholine (DLPC, low Tm), dipalmitoyl-phosphatidylcholine (DPPC, high Tm), and in some cases, cholesterol, which show phase separation at 25*C.56 In particular, GUV studies of the formation of membrane domains have widely been used to probe the existence of lipid rafts, which are thought to be liquid-ordered regions enriched in sphingomyelin and cholesterol, and these studies have many applications in understanding cell signaling, endocytosis, and also passive transit of molecules through bilayers.57,58 To employ GUV synthesis technology but still preserve the
complexity of whole cell membranes, studies have also described the synthesis of giant plasma membrane vesicles (GPMVs) that are either cell-attached or cell-free (Fig. 2.2).9 This work has been insightful in understanding phase separation of lipids and proteins in representative cell membranes. While GUVs have been visualized by two-photon microscopy and confocal microscopy, fluorescence correlation spectroscopy (FCS) has been used to quantify bilayer lipid fluctuations particularly in phase-separation studies.
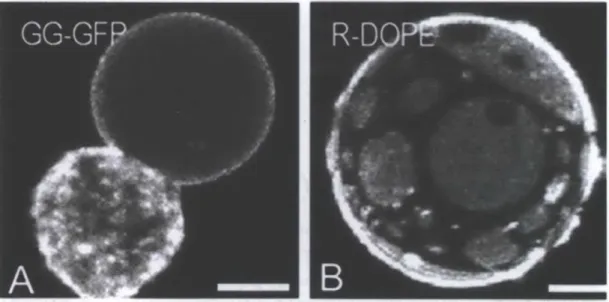
Figure 2.2. Examples of cell-attached and cell-free GPMVs. Confocal micrographs of A) a rat basophilic leukemia (RBL) cell with an attached GPMV (GG-GFP = geranyl-geranyl-EGFP), and B) a cell-free GMPV from an NIH 3T3 fibroblast with fluorescent membranes with evident phase
Model membranes to study membrane interaction and penetration of
nanomaterials
Model membranes have also been used to study mechanisms of membrane interaction of nanomaterials that are designed to bypass endocytosis and directly transit into the cytosol or to escape endosomes. This application is of special relevance to us. The widely-studied cell-penetrating peptide HIV-1-TAT is a particular example. Studies with neutral and anionic GUVs with both liquid-ordered and liquid-disordered domains and single-molecule tracking analysis showed that the cationic TAT CPP appears to float (not aggregrate) on bilayers and preferentially inserts more deeply into the headgroup region of anionic lipids.60 Follow-up
studies have also shown that cationic TAT can penetrate highly anionic GUVs (made with 40% DOPS, phosphatidyl-serine), especially when they have regions of negative curvature. Further, these studies have shown that this membrane translocation can induce nanometer-sized pores in the membrane.6 1 Experiments have also been done as part of the first steps to
create artificial cells, where GUVs have been synthesized with spectrin and ankyrin proteins
Figure 2.3. Confocal images of 'model cell' GUVs with artificial cytoskeletons. Vesicles are made with lipids from porcine brain extracts (labeled with DiD C18, red), with added spectrin/ankyrin proteins (labeled with phalloidin-Alexa 488, green) that anchor actin filaments to the interior face of GUV membranes to induce the formation of dense actin bundles. Adapted from
[62].
that anchor actin filaments to the GUV interior membrane and create actin bundles, which allows the study of cytoskeletal rearrangements (Fig. 2.3).62 Recent studies with actin-filled GUVs have provided key elucidation to the mechanism of membrane interaction and translocation of TAT (Fig. 2.4).2 Upon incubation with TAT peptide, filamentous actin (F-actin)-filled GUVs displayed membrane instabilities and promoted growth of F-actin bundles, which, in some cases morphed into filopodium-like protrusions reminiscent of membrane ruffling and macropinocytosis. Schematic results shown in Figure 2.4E indicates that cationic TAT may induce pores creating saddle-splay curvature, attach to and polymerize the underlying F-actin cytoskeleton, and stimulate endocytosis, particularly of large cargoes.1 2
GUVs have also been used for studying synthetic nanomaterial interactions with membranes, for example in the case of 20 nm polystyrene nanoparticles with cationic surfaces that were found to create transient pores in the artificial membranes.63
Methods
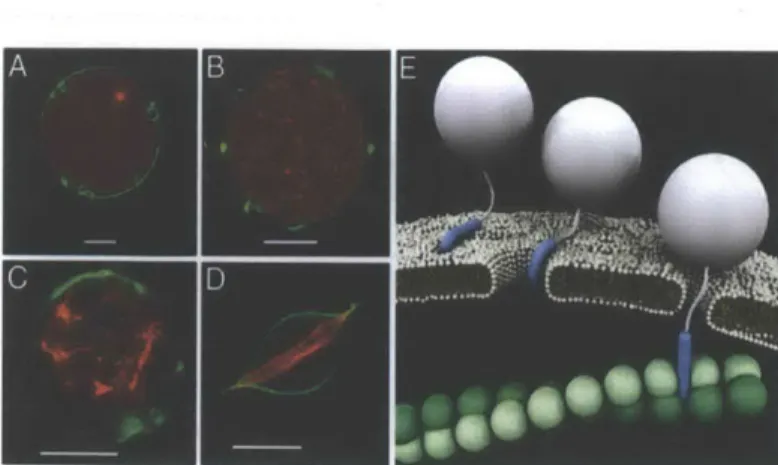
Figure 2.4. GUV studies to elucidate HIV-1 TAT transit across membranes, interaction with the cytoskeleton, and eventual translocation. Confocal micrographs of GUVs made with 40%/40%/20% PE/PC/PS (DiO, green) that A)
encapsulate globular actin (G-actin) that is subsequently polymerized into F-actin, and form B)-D) dimple-like stabilities in membrane and growth of F-actin bundles induced after incubation with TAT. E) Schematic depiction of TAT interaction with membrane and simulation of endocytosis. Scale bars 10 pm. Adapted from [12].
of
model
membrane synthesis
The most commonly used techniques for model membrane synthesis are electroformation (more traditionally) and gentle hydration. We note here that lipid vesicles can be formed to be unilamellar or multilamellar
- or, more often, a mixture of both - depending on the synthesis technique used. Multilamellar structures often result from
dense coatings of lipid films and when there is electrostatic attraction between lipid headgroups. Further, vesicle harvests often have to be handled gently and even slight agitation is prone to induce the fusion of unilamellar vesicles into multilamellar structures. Electroformation typically results in unilamellar vesicles (GUVs).64 Gentle hydration,
however, can result in both GUVs and GMVs (giant multilamellar vesicles).64
Electroformation
The electroformation method, while widely used for the preparation of unilamellar vesicles especially, is sensitive to lipid composition, buffer concentration, and apparatus setup.64-7 6
I
this technique, lipid films are formed on indium tin oxide (ITO) electrodes separated by a Teflon spacer and sealed in an aqueous chamber before the application of a potential at a specific voltage and frequency. GUV harvests are then cooled for an extensive 18-36 hr period prior to imaging. The established electroformation methods work most optimally for neutral lipids and low ionic strength buffers and are sensitive to electrode material and applied voltage. Common artifacts of this method are lipid hydrolysis and oxidation.
Gentle hydration
In the gentle hydration method, lipid films are incubated at a temperature well above the Tm of lipids in the composition (usually 45-55*C) and hydrated gently for 1-2 days, without
agitation, allowing the natural formation of giant vesicles.64' -.
Temperature of incubation is the most important parameter for high vesicle yield in this synthesis technique. In contrast to electroformation, this method has also been used for the formation of vesicles of negatively-charged lipids. Gentle hydration has also been used to successfully form vesicles
in buffers with physiological levels of salt (~150 mM). Best yields, however, result from low or zero ionic strength conditions.
Given our previous studies and our proposed mechanism of membrane interaction and embedding for our amph-AuNP system4 2
,4 7
,48
, we opted to adapt and modify a synthesis method of gentle hydration to develop a simple, tunable synthesis method for giant model membranes that allows us to study this phenomenon with robust analysis. Our method largely results in the formation of multilamellar structures, although there is a notable population of unilamellar vesicles. GMVs are an apt model of study for our particular amph-AuNP system since they not only enable us to investigate NP-membrane embedding in outer bilayers but also to study embedding and penetration of NPs through adjacent lipid bilayers.
Methods and Materials
Materials
All lipids and cholesterol were purchased from Avanti Polar Lipids. Stocks were kept in
chloroform and stored at -20'C in aliquots.
GMV synthesis by gentle hydration
Lipid stocks in chloroform were added to glass scintillation vials, where total lipid was 1 pimole per vial. When fluorescent lipid tracers were necessary to label GMVs, dyes were added at 0.1-0.5 mol%. Chloroform was allowed to evaporate overnight at 22'C, in order to form lipid films on glass. Uncapped vials were then incubated in a water bath at 70'C for > 6 hr, and this step allowed these films to hydrate without immersion in solution. Sucrose buffer
(50 mM in water or PBS) was then added at 2 mL per vial, and vials were capped and further
incubated at 70'C overnight, allowing GMVs to form. GMVs were gently harvested with minimal agitation or mixing the following day, after first cooling to 22'C. When a larger proportion of unilamellar vesicles were desired, lipid films were first prepared on well-scratched glass surfaces.
Confocal microscopy
Confocal samples were prepared in 8-well LabTek chambers (Nunc) and, prior to adding GMVs, we first prepared solutions of 50 mM glucose in water or PBS with amph-AuNPs. GMV harvests in sucrose were mixed 1-2x with a pipette and added at a 1:4 ratio in a sample well. Notably, samples were only rocked back and forth after preparation and there was no additional mixing of GMVs with AuNPs in the well. Samples were incubated 1-3 hr at 22'C, unless otherwise noted. Samples were then imaged with a LSM 510 confocal microscope (Carl Zeiss). Z-stacks were collected using an optical slice thickness of 1 pm, and images were analyzed with Zeiss LSM software.
Flow cytometry
Flow cytometry samples were prepared in 96-well round-bottom plates (BD) or polystyrene flow cytometry tubes (BD Falcon). We first prepared solutions of 50 mM sucrose in water or PBS with amph-AuNPs in sample wells. GMV harvests in sucrose were mixed 1-2x with a pipette and added at a 1:1.5 ratio per sample well. Samples were rocked gently back and forth after preparation - but there was no additional mixing of GMVs with AuNPs in the wells. Samples were incubated at 1-3 hr and 22*C, unless otherwise noted. Samples were then analyzed with a LSR Fortessa HTS flow cytometer (BD) and data was analyzed using FlowJo software.
Results and Discussion
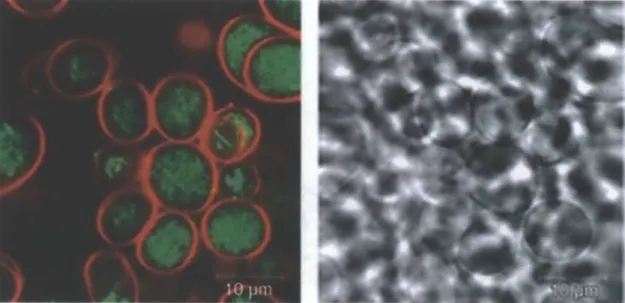
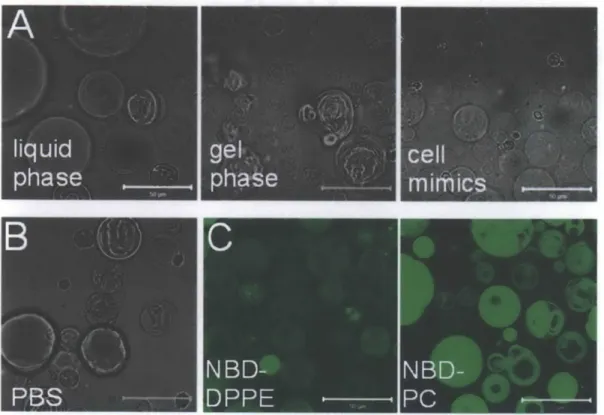
Our synthesis technique provided high yields of GMVs, an example of which are shown in Figure 2.5 for an all-DOPC composition in water. This method yields both unilamellar and multilamellar structures, the latter of which are important in order to study penetration of amph-AuNPs through adjacent bilayers. In many cases, there are vesicles within vesicles. Depending on the lipid and buffer composition, GMVs are typically -20-50 pm in size.
Figure 2.6 is a summary of the results of various GMV synthesis conditions. GMVs can be made to have different phases (liquid phase with DOPC, gel phase with DPPC, and a mixture of the two) and with multiple types of lipids and cholesterol (example shown here is for a composition mimicking the outer leaflet of erythrocytes) (Fig. 2.6A). This method also allows them to form in physiological buffers and salt such as 150 mM NaCl4 2, PBS (Fig. 2.6B
and unpublished data included in this thesis), and even RPMI medium complete with 10% fetal bovine serum (FBS) (data not shown). When forming GMVs by our technique of gentle
Figure 2.5. Brightfield confocal micrograph of all-DOPC GMVs prepared in water.
hydration in physiological buffers, we note that the all-DOPC formulation has very low yield and inclusion of some fraction of negatively-charged lipid (for example, DOPC/20% DOPG)
was necessary for a good yield of GMVs. This observation has also been seen in the
Figure 2.6. Examples of GMVs synthesized by varying lipid composition, buffer salt concentration, and fluorescent tracers, and analysis by both confocal microscopy and flow cytometry. GMVs prepared A) in liquid phase with an all-DOPC composition, in gel-phase with an all-DPPC composition, or mimics of erythrocyte outer leaflets with DOPC/SM/DOPE/cholesterol/GMl, B) in phosphate-buffered saline (PBS), and C) with 0.1-0.5 mol% NBD-DPPE and NBD-PC as fluorescent lipid tracers. Scale bars 50 pm.
literature, where 10-20% of DOPG, DOPS, phosphatidic acid (PA), or cardiolipin have been shown to be necessary for formation of GUVs that are otherwise composed of DOPC and
DOPE (phosphatidylethanolamine).8 We also note here that studies using the traditional
method of electroformation have shown that vesicle formation in the presence of salt has been challenging, and this challenge has been circumvented by modifying the standard electroformation protocol' 67
'76 and using PEGylated lipids68. GMVs can also be synthesized with fluorescent membrane tracers, with images shown in Figure 2.6C for NBD-DPPE and
NBD-PC at 0.1-0.5 mol%. We have also used fluorescently-labeled cholesterol, dyes of the
Di family, and labeled DOPE (data not shown).
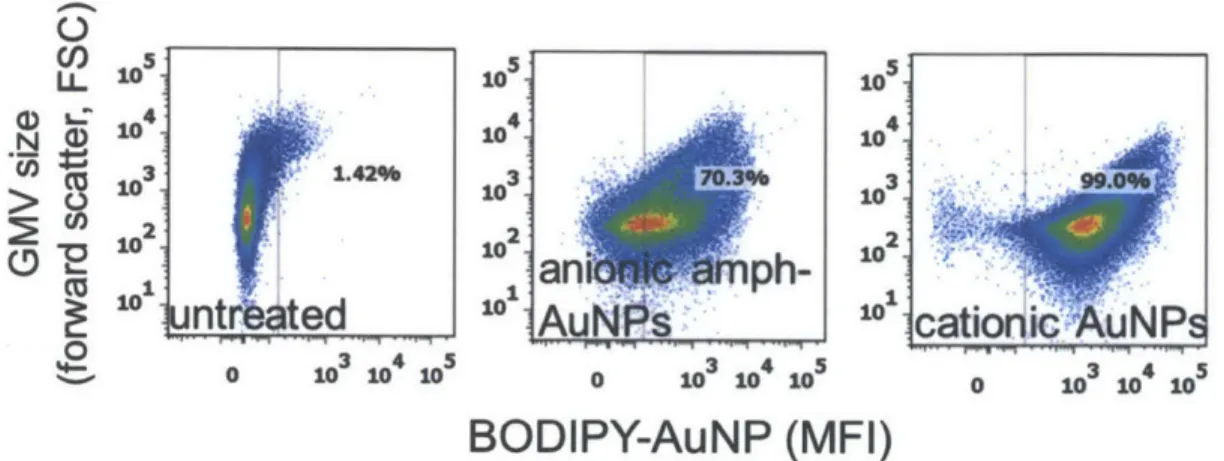
Further, in addition to visualization by confocal microscopy, GMVs can also be analyzed robustly in large populations by flow cytometry, which allows ready quantification of fluorescence. Figure 2.7 has an example of fluorescence of untreated all-DOPC GMVs, compared to those treated with anionic amph-AuNPs and cationic AuNPs). We have observed that the exception to using flow cytometry for fluorescence quantification of GMVs is when they are prepared in buffers containing salt, where flow cytometry trends do not agree with those seen with confocal imaging. This observation might be due to alteration in buffer salt concentration and hence NP-membrane interactions due to sample mixing with
flow cytometer sheath fluid, which is deionized water, as it flows through the analyzer chamber. When our syntheses conditions have high ionic strength buffers, therefore, we analyze fluorescence intensities of confocal images when quantification is necessary.
Cl) Jo.S.S LL 10
5
105 N 10 10 10 3 1.42% 3 7) 3 . 1 0 10a
10 -10ntrated
1A
N
cationic
NP
3O 3 3. 4 5 0 103 4 05 0 103 104 05 0 103 104 105BODIPY-AuNP (MFI)
Figure 2.7. GMV fluorescence quantification by flow cytometry after treating with various AuNPs.
Conclusions
We have developed a simple, tunable synthesis method for giant multilamellar model membranes by gentle hydration. Using this method, we can synthesize GMVs with varying lipid composition, buffer salt concentration, and fluorescent lipid tracers, and visualize membranes by confocal microscopy and analyze fluorescence of large populations of vesicles using flow cytometry. This technique will enable us to study amph-AuNP interactions with membranes, and also to quantify how changes in membrane properties influence these interactions. This method is employed in the model membrane experiments of Chapters 3 and 4 of this thesis.
Chapter 3
Characterization of amphiphilic gold nanoparticle design
and bilayer properties that govern interactions with
membranes
Introduction
In this chapter, we present our analysis of our unique membrane-interacting amph-AuNP system, which we show can interact with, embed within, and even penetrate through multiple lipid bilayers without membrane disruption or poration. Using a one-step reaction and a
modified Brust-Schiffrin scheme, MUS and OT
alkanethiol ligands are dissolved in methanol and HAuC14 is dissolved in ethanol.40'1 The two components
are mixed together and the Au is reduced by the gradual addition of sodium borohydride. This step nucleates the AuNPs and they grow with an amphiphilic surface layer
(Fig. 3.1).
I
Solvent-accessible surface (SASA) Buried surface Screened 0 Coulombic 0 repulsion 6 0 Ao=s=o
HS Sodium 11-mercaptoundecene sufonate: MUS A -AuLigand Spring model for "snorkeling- bilayer deformation
Figure 3.2. Schematic depiction of our proposed mechanism of amph-AuNP insertion and embedding within bilayers based on the ability for surface ligands to 'snorkel' between the aqueous/lipid interface. Adapted from [42].
Octanethol: OT HAuC14 NaBH4 EtOH NaH o=s=o AJ s
's
Figure 3.1. Scheme of one-step reaction from a modified Brust-Schiffrin synthesis for amph-AuNPs. MUS and OT
alkanethiol ligands are added
to HAuCl4. Sodium
borohydride is then added to reduce the Au and to nucleate NPs coated with an amphiphilic ligand layer. Adapted from [41].
In very recent work, we showed using computational modeling and experimental
studies with model
membranes that, within a specific size range, these AuNPs actually appear to prefer a membrane-embedded state, where free energy is minimized. In this state, the
hydrophilic sulfonate headgroups in the AuNP ligand shell 'snorkel' to the aqueous/lipid interface much like transmembrane proteins.4 2
Figure 3.2 is a schematic depiction of this mechanism, and highlights that amph-AuNPs are not merely electrostatically attracted to the membrane surface to interact with the periphery of the membrane, but rather, within a certain core diameter range, surface ligands are able to splay and rearrange in response to the
A
Qfo
MUS OT
Figure 3.3. Atomistic molecular dynamics simulations for membrane-embedded A) MUS:OT
1:1 amph-AuNPs, and B) all-MUS amph-AuNPs. Adapted from [42] and unpublished work
from Reid Van Lehn, Alexander-Katz group.
Striped (S) Random (R) Mixed (M) 50 0- -50- -100- -150- -200- -250--U-aI-MUS 2:1 MUS:O ps T -A- R Vjr M 1:1 MUS:OT s -4.R -. M
. 1
2
3
4 5
6
0 8
9 10.0
0.0
1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 10.0Nanoparticle gold core diameter (nm)
Figure 3.4. Atomic molecular dynamics simulations for free energy of membrane-embedding for all-MUS, MUS:OT 2:1, and MUS:OT 1:1 AuNPs of various core sizes and surface ligand morphologies. Adapted from [42].
environment so that the hydrophobic alkyl portions of the ligands are found, very stably, next to the hydrophobic interior of the membrane bilayer. Figure 3.3 shows a schematic depiction of NP-membrane embedding for
MUS:OT 1:1 (Fig. 3.3A) and
all-MUS amph-AuNPs (Fig. 3.3B). The results of our simulations for all-MUS,
MUS:OT 2:1, and MUS:OT 1:1
amph-AuNPs suggested first, that all three formulations embed within membranes, but the core diameter for optimal embedding where free energy is lowest changes with NP type (Fig. 3.4).42 All-MUS NPs
embed optimally at a core diameter of 2 nm, MUS:OT 2:1 NPs embed optimally at a core
0
- --- - -- - -- -- --- - .
-diameter of 3.8 nm, and MUS:OT 1:1 NPs embed optimally at a core -diameter of 4.5 nm. Second, these simulations also indicated that embedding did not depend on surface morphology of NP, where we tested effects of ligand arrangements from striped to random to mixed. Rather, our key conclusions from this initial study were that optimal NP membrane-embedding depended on NP core diameter and surface hydrophobicity. Black lipid experiments with size-fractionated MUS:OT 2:1 and MUS:OT 1:1 NPs further supported these computational results (Fig. 3.5). Notably, when amph-AuNPs were added to giant model membrane vesicles, there was significant NP-membrane interaction, embedding, and even penetration through adjacent bilayers, also in the presence of physiological levels of salt (data shown in this chapter). Further of note, membrane embedding by these particles was observed without any experimental evidence of membrane poration or disruption.
U Sim: embedding E Expt: embedding 0 Expt no embedding Figure 3.5. Black lipid
experiments in support of
2:1 simulation results
MUS:OT IM i-n - experimentally showing
higher levels of membrane
1:1 embedding correlating to NPs
MUS:OT FU:H 1* with higher surface
hydrophobicity (MUS:OT 1:1 0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 10.0 versus MUS:OT 2:1).
Nanoparticle gold core diameter (nm) Adapted from [42].
In this chapter, we build on these initial studies and provide thorough experimental support with giant model membranes and liposomes for our proposed mechanism of membrane interaction. We first investigate the mechanism by which fluorescently labeled amph-AuNPs can serve as membrane 'sensors'. Then, we use model membranes to investigate the effects of general membrane properties (electrostatics and phase) on AuNP-membrane interactions. Finally, we use cryogenic transmission electron microscopy (cryoTEM) to understand how
NP design characteristics (core diameter, surface charge, and surface hydrophobicity) affect
AuNP-membrane interactions. This work will not only provide key experimental support and elucidation of our proposed mechanism of AuNP-membrane interaction, but will also inform
NP design criteria and therapeutic cargoes of choice to mediate optimum delivery in a
biological setting. In addition, this work can also provide inspiration for the design of new hybrid materials composed of hydrophobic metallic NPs and lipids.
Methods and Materials
Materials
HAuC 4 salt, OT ligand, and calcein were purchased from Sigma. BODIPY 630/650-X
succinimidyl ester was purchased from Invitrogen. MUS ligand was prepared in-house with a method described previously.47
Amph-AuNPs were also prepared in-house as described previously.41 All lipids and cholesterol were purchased from Avanti Polar Lipids.
Amph-AuNP synthesis, characterization, and labeling
We used a modified one-phase Brust-Schiffrin synthesis to prepare amph-AuNPs.40
Briefly, a solution of HAuCl4 was prepared in -200 mL ethanol and an equimolar mixture of thiol
ligands was prepared in methanol. These two solutions were mixed together and allowed to stir for 15 min. A saturated solution of NaBH4 in ethanol was then added slowly and
drop-wise and, following this step, the reaction was allowed to stir for an additional 3 hr. The flask was placed at 4'C overnight after which the precipitated particles were isolated by filtration through qualitative filter paper. The filtered particles were washed sequentially in ethanol and methanol and dried with further washes in acetone. Before characterization or analysis, insoluble NPs were first removed by dissolving ~10 mg dry NPs in 1 mL water, centrifuging at 14,000 x g for 2 min, and isolating the soluble NPs that remain in the supernatant. Size distributions of NP batches were determined using transmission electron microscopy (TEM). ImageJ software was used to quantify the sizes of NPs from high magnification TEM images, which were subsequently used to calculate the mass extinction coefficient.
A BODIPY-Cl -SH dye construct was prepared in-house by adding an alkylthiol linker to
BODIPY 630/650-X, succinimidyl ester. Amph-AuNPs were labeled by place-exchange of BODIPY-C 1-SH with surface ligands by incubating 30 pimoles of dye construct per mg of NPs over 2-4 days at 22'C with constant shaking or stirring. Labeled AuNPs were purified and separated from excess dye by precipitation and washing in acetone at least 5x. Washed amph-AuNPs were dried overnight and solubilized in water for use.
GMV synthesis
GMVs were synthesized as described in Chapter 2. For testing of membrane electrostatics, we used GMVs of all-DOPC in water, 80% DOPC/20% DOPG in water, and 80%
DOPC/20% DOPG in PBS. For testing of membrane phase, we used GMVs of all-DOPC, 50% DOPC/50% DPPC, and all-DPPC. For testing of effects of membrane cholesterol, we
used all-DOPC GMVs and GMVs of 75% DOPC/25% cholesterol.
Liposome synthesis
To prepare unilamellar liposomes -80 nm in diameter, prepared lipid films at 1 pimole lipid per glass scintillation vial, reconstituted in 200 pL buffer (water or PBS), and vortexed for 30 sec every 10 min for 1 hr. Hydrated lipid solutions were then transferred to 1.5 mL Eppendorf tubes and sonicated with a probe sonicator for 5 min on ice with alternating cycles of 6W and 3W for 30 sec intervals. Liposomes were cooled to 22*C before use.
Computational simulations were carried out by our collaborator Reid Van Lehn (Alexander-Katz laboratory, MIT Materials). The potential of mean force (PMF) for moving the BODIPY fluorophore away from the NP surface was estimated using atomistic molecular dynamics simulations. The NP was modeled using a recent parameterization of mixed-monolayer-protected NPs based on the GROMOS 54a7 force field.44'8 -83 BODIPY was parameterized using a 'building block' approach using a combination of existing molecules in the GROMOS library and quantum chemical calculations.84'85 The PMF was calculated using the weighted histogram analysis method8
6,8 7 from umbrella sampling simulations of a NP either in solution or embedded in the bilayer. All simulations were performed with Gromacs version 4.6.1.
Confocal microscopy
For GMVs, confocal samples were prepared as detailed in Chapter 2. BODIPY-MUS:OT 2:1 amph-AuNPs were used at 0.07 or 0.28 mg/mL. Samples were imaged and analyzed as described above.
Flow cytometry
For GMVs, flow cytometry samples were prepared as described in Chapter 2.
BODIPY-MUS:OT 2:1 NPs were added at 0.07 or 0.28 mg/mL. Samples were analyzed as detailed
above.
Cryogenic transmission electron microscopy
Cryo TEM samples were prepared in 100 [tL volumes. First, amph-AuNPs were prepared in 70 pL buffer (water or PBS) and 30 pL of liposomes were added (150 nmole lipid) and
mixed with a pipette. AuNPs were added for a final concentration of 0.28 mg/mL. In sample preparation for cryo-electron microscopy, 3 uL of AuNP-liposome sample was dropped on a
lacey copper grid coated with a
continuous carbon film and blotted to remove excess sample without damaging the carbon layer by a Gatan Cryo Plunge III. The grid was mounted on a Gatan 626 single tilt cryo-holder equipped in the TEM column. The specimen and holder tip were cooled down
by liquid nitrogen, which is maintained during transfer into
the microscope and subsequent imaging. Imaging on an JEOL 2100 FEG microscope was done using minimum dose method necessary to avoid sample damage under the electron beam. The microscope was operated at 200 kV and with a magnification in the ranges of
10,000-60,000 for assessing particle size and distribution. All images were recorded on a
Results and Discussion
Nanoparticle-bound dye as a sensor of membrane embedding
To study the mechanisms that govern membrane interaction of amph-AuNPs, we prepared giant multilamellar vesicles (GMVs) with synthetic lipids, as detailed in Chapter 2 of this thesis.4 2
Cell-sized GMVs allowed us to study the effects of individual membrane characteristics on AuNP-bilayer interactions using confocal microscopy and flow cytometry. We recently reported that when DOPC GMVs were co-incubated with the membrane-impermeable tracer calcein and BODIPY-labeled MUS:OT amph-NPs, calcein remained excluded from the vesicle interiors, but nanoparticles were observed to strongly absorb to the GMVs and penetrate throughout multilamellar vesicles.4 2
Similar membrane penetration was also observed for -2.5 nm gold core MUS amph-NPs (Fig. 3.6). However, we also noted an unexpected aspect of this result: Although no BODIPY signal was detected except associated with membranes of the vesicles, the supernatants of these samples, which could be sampled without drawing up GMVs that settled to the bottom of the imaging well, clearly showed a
purple absorbance indicating the presence of free NPs remaining in solution. To rule out a quenching effect of calcein on the dye-labeled NPs in solution, we imaged BODIPY-labeled
MUS:OT 2:1 amph-AuNPs incubated with DOPC GMVs in the absence of calcein, and saw a similar lack of background BODPY signal in the medium surrounding the GMVs (Fig. 3.7A),
despite the clear presence of free NPs remaining in the supernatant. Linescans of these images showed areas of very high fluorescence (2000-4000 times above background) where AuNPs colocalized with lipid, but no signal above background in the surrounding solution. The fluorescence signal associated with the membranes was also not autofluorescnece of the lipids, as no signal above background was detected by confocal imaging of GMVs alone (data not shown). Intensity 4000- 3000- 2000-
100
0-0 20 40 60 80 Distance (pm)E
I
100 120 140(b)
15000-1L 2 10000.550001
0
CO
ni + + +U0
free
BODIPY
HS-C1 1-BODIPYf
free
BODIPY
HS-C1 1-BODIPY§
1
2
concentration (ALM) water DMF 3(c)
N
water 4 00 0 2 mM liposomes - 3000, E 3 mM liposomesS20001
L300-a
C 100:...20011W
0
BODIPY-
HS-C11-MUS:OT BODIPY
2:1
only
Figure 3.7. A) Line scans of confocal micrographs of all-DOPC GMVs treated with BODIPY-labeled MUS:OT 2:1 NPs at 0.07 mg/mL. B) Solvent quenching analysis of free BODIPY and
C1 1-BODIPY in water and DMF. C) Quenching analysis of BODIPY-MUS:OT 2:1 and HS-C II-BODIPY in water and liposomes.
Prior work has shown that some molecules exhibit environment-dependent fluorescence."-9' To test whether fluorescence of the neutral BODIPY dye is dependent on its solvent environment, we prepared solutions of free BODIPY 630/650-X dye and of the alkanethiol form of the dye used for particle conjugation (HS-C1 1-BODIPY) in either water or the less
(a
polar solvent DMF. Strikingly, despite clear solubility at micromolar concentrations, both forms of the BODIPY dye showed very weak fluorescence in pure water, relative to the organic solvent (Fig. 3.7B). We also attempted this analysis with non-polar solvents such as toluene and hexane, but were limited by poor solubility of both the free dye and the alkanethiol conjugate in these solvents. Given these results, we next determined the impact of interaction with lipids on the fluorescence of the free or nanoparticle-bound dye.
BODIPY-labeled MUS:OT 2:1 amph-AuNPs or free HS-C1 1-BODIPY were added to DOPC
liposomes (80 ± 20 nm in diameter) and fluorescence spectra were measured for a range of dye/NP concentrations (Fig. 3.7C). BODIPY-labeled amph-AuNPs in water without liposomes had a fluorescence close to background and free HS-C 11 -BODIPY fluorescence showed very weak fluorescence at the same equivalent dye concentration (4 pM). However, in the presence of liposomes, fluorescence levels increased substantially, with a linear increase in signal with BODIPY concentration for both the free and nanoparticle-bound dye. Given that the background fluorescence levels of BODIPY-AuNPs without liposomes is the same as of water alone, we also noted that fluorescence of AuNPs with lipsosomes was low in general compared to the construct alone because there is likely very low labeling of AuNPs
- and not likely due to Au core-mediated quenching of the dye. If the latter were true,
background levels of samples with AuNPs but without liposomes would also be lower. We also note here that the difference in raw intensity values of fluorescence signal between Figures 3.7B and 3.7C is due to slightly difference excitation/emission wavelengths. We used 642/665 nm and an optimal gain setting to collect the data in Figure 3.7B, but since liposome samples had a high background signal at this setting, we used 636/665 nm and a manual gain to collect the data in Figure 3.7C.