Publisher’s version / Version de l'éditeur:
Technical Translation (National Research Council of Canada), 1959
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/20386548
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at Compacted Snow Road. 1. Properties of Snow
Eriksson, R.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=d74586b3-070e-4440-8d4d-eecee291e190 https://publications-cnrc.canada.ca/fra/voir/objet/?id=d74586b3-070e-4440-8d4d-eecee291e190
NRC TT-849
NATIONAL RESEARCH COUNCIL OF CANADA
TECHNICAL TRANSLATION 849
THE COMPACTED SNOW ROAD
I.
PROPERTIES OF SNOW
BY R. ERIKSSON
o FROM
SVENSKA SKOGSVARDSFORENINGENS TIDSKRIFT, (2): 157 - 188, 1954
TRANSLATED BY H. A. G. NATHAN
THIS IS THE FIFTY· FIFTH OF THE SERIES OF TRANSLATIONS PREPARED FOR THE DIVISION OF BUILDING RESEARCH
OTTAWA
NATIONAL RESEARCH COUNCIL OF CANADA Technical Translation 849
I. Properties of snow
Snens egenskaper)
T1tle: The compacted snow road.
(Den snepackade カセァ・ョN I.
Author: R. Eriksson
Reference: Svenska Skogsv&rdsfOreningens Tidskrift, (2): 157-188,
1954. (SDA*Meddelande no. 52)
Translator: H.A.G. Nathan, Translations Seotion, N.R.C. Library
•
SDA=
Forestry Society and Royal Domain Administration, Researchprefacセ
Although snow roads are of economio interest in Canada, particularly to its pulp and paper industry, there is as yet very little published information on their construction and performance which is generally available in English to engineers.
As one contribution toward solving this problem the National Research Council, through its Associate Committee on Soil and Snow Mechanics, is cooperating with Professor Seheult of the University of New Brunsvlick, on a project to evaluate current practice in the construction and use of snow roads in Canada and other countries of the world. One of the first steps has been to translate into English the more pertinent publications available on the subject.
Sweden, through experiment and field practice has gained much valuable experience on the construction of snow roads. This translation, pUblished with the per-mission of the author, is the third of a series of
reports to be translated which describe this Swedish ex-perience. The Division of Building Research records its appreciation of the translation by Mr. H.A.G. Nathan.
Ottawa,
November 1959
R.F. Legget, Director
THE COMPACTED SNOW ROAD
I. PROPERTIES OF SNOH
Foreword
Since the winter of 1938-39, when the first systematic experi-ments to replace the plowing of roadways by a method of compacting
the snow with crawler tractors were carried out in Sweden, this type of road, the compacted ウョoャセ road, has been increasingly used for the transport of timber by crawler tractor or horse in regions with heavy snowfalls, i.e., the interior and northern sections of Sweden. Extensive measurements to determine the strength of roads and tests by means of various instruments and methods were carried out by the SDA as early as 1950 in association with the staff of the Lycksele experimental station.
Recently I reported on the experience gained in the actual use of the snow roans in 1940, 1946, 1950 and 1953. It is now consider-ed appropriate to submit the results collectively, beginning with a report by Mr. Rune Eriksson on the physical phenomena associated with the various ways of treating a snow cover and on certain experiments in connection with measuring the strength of compacted snow roads. These measurements were carried out by the SDA. The team consisted of Messrs. E. Ribendahl, E. Malmberg, R. Nilsson and R. セウエ「・イァN
some of whom are still with the SDA.
In the next number of this periodical a report will be given on the practical experiments carried out to date.
A.C:son Leijonhufvud Introduction
Using snow to construct rOads is rather like setting a thief to catch a thief. Snow is most frequently considered as an obstacle to traffic which must be ploughed off the road as fast as possible
(Fig. 1). If the ウセッキ is compacted instead, it may under favourable conditions carry heavy vehicles and, therefore, a snow road may be a
-4-favourable solution to many transport problems from the economic point of view. This is clearly shown by the compacted snow roads which were tested with good results for a number of years in the northern parts of Sweden (A.C:son Leijonhufvud, 1950).
For a rational execution and utilization of this new road tech-nique more knowledge of the properties of snow as a bUilding material is required.
The investigation described here was intended as a contribution towards this end.
During a large part of the year snow covers the ground in
Sweden. Snow interferes with the economy in many ways and thus af-fects the cost of traffic, transportation, outdoor work, etc.
Furthermore, its removal is expensive. Therefore, the knowledge of the properties of snow is also important for problems other than those directly connected with the compacted snow roads. Snow is a complex and unstable material, as anyone who studies closely the many forms of falling snow or anyone concerned with the problem of wax-ing skis will certainly agree •. In order to discover the relation-ships between the various properties of snow, the fundamental physic-al properties of ice, water and water vapour must be known.
Ice, Water and Water Vapour
Ice, water and water vapour consist of the elements of hydrogen and oxygen in the proportion of 2 - 16 by weight. Two positive hy-drogen ions and one negative oxygen ion combine to form a vapour mOlecule. An idea of the size of this mOlecule is provided by the distance between the centre of the hydrogen ion and that of the
oxygen ion. This distance is 0.0000000095 cm. (Fig. 3) (Pauling,
L.,
1952) •Ice
In ice the molecules are fixed in certain positions by the forces of attraction acting between them. The molecules are regularly
oriented. Each molecule is in contact with only four neighbour molecules (Fig. 4) Hp。オャゥョァセ
L.,
1952). Hence they are so widely
-5-spaced that ice has an abnormally low density (specific gravity). The orientation of the molecules in space determines the
crystalline structure of ice. Therefore the ice crystal acquires a basically hexagonal form in approximately the same way that bricks laid side by side produce the rectangular elements of a building. In other words, the ice crystallizes in the hexagonal system. All crystals forming snow, white frost, hail and sea ice have this in common. These crystals tbus have the same physical properties with respect to elasticity, deformation, melting point, vapour pressure, etc. The properties of ice crystals described below apply also to the other crystals (Table I).
At very low temperatures the molecules ill the ice crystals re-main qUietly in their positions. When the temperature increases the molecules become more active. Each molecule bounces to and fro in the small region separating it from its neighbours and pushes the
I
neighbour molecules more and more vigorously.
Two pieces of ice which are pressed against one another will co-here after some time (cf. below). The completeness with which the pieces of ice are joined depends on the strength and duration of the compression as well as on the relative orientation of the systems of crystallization of the two pieces. If the orientations of the sys-tems of crystallization are exactly the same for the two pieces of ice, they freeze together completely. Then the joint between them differs in no way from the pieces of ice themselves. Using the
"brick" analogy again, when two straight brick wal.Ls are parallel and stand in a straight line, they can be joined together as one wall without difficulty. However, if the walls are inclined slightly
with respect to one another, the bricking up would result in a trans-ition zene in which the bricks could not be placed in their regular order. It may be assumed that the pieces of ice behave in a similar way. If the systems of crystallization are differently oriented in the two pieces of ice, the Joint forms a zone of transition between them. Such a joint 1s weaker than the pieces of ice themselves
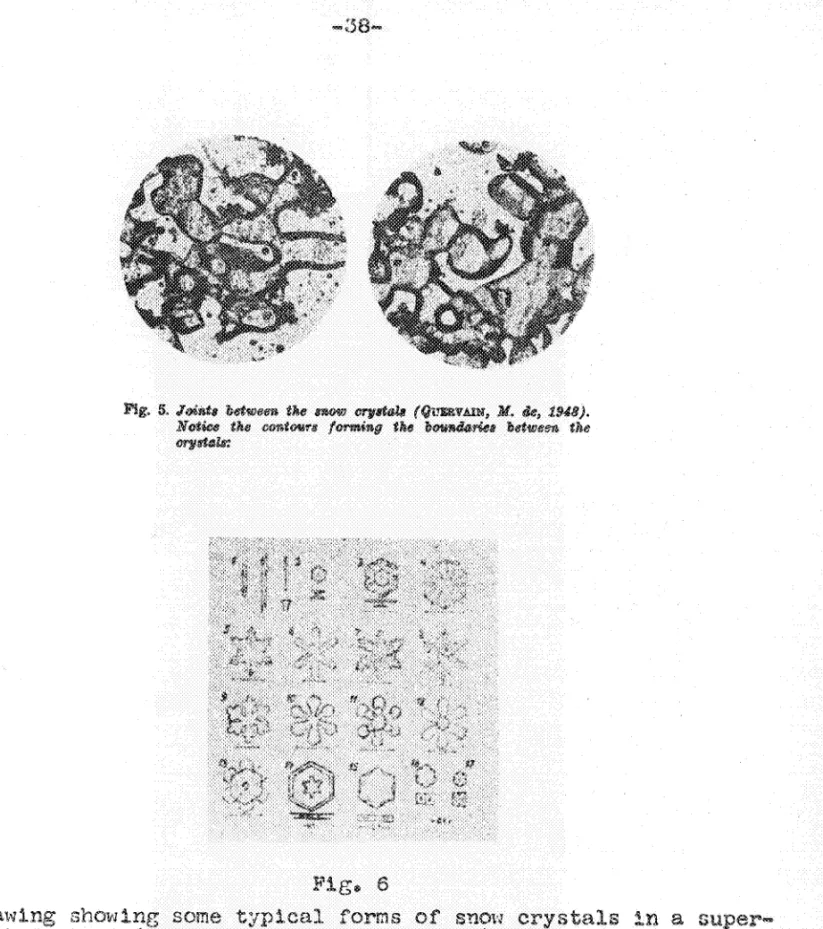
-6-It may be expecten thRt the joints which form between the crystals in a layer of snow with their systems of crystallization oriented at random are more unstable tllen the crystals themselves (Fig. 5) (Quervain, M. de, 1950). "ilien the ice melts the dissolu-tion begins in these joints and it is here that the crystals fall apart. Because of the special structure of the material forming the
joint it may be assumed, moreover, that the disintegration is due to impurities in the material (Bucher, E., 1948).
Water
In water the molecular motion 1s so vigorous that the molecules cannot be kept in fixed positions. The molecules are freely mobile and the higher the temperature the more vigorously they move. How-ever, the forces of attraction are sufficient to hold the molecules together within a constant volume. Like ice, water thus has a
definite volume. As distinguished from ice, which is characterized by a regular molecular orientation, water generally has an arbitrary or unstable structure. To some extent the forces of attraction af-fect the fonm of bulk water. This is clearly evident in the case of small quantities of water, e.g. drops and capillaries. The
fOllowing generally applies: the forces of attraction attempt to contract the water surface to a minimum. This is called surface tension (Table I). A small drop thus has a spherical form and hence the smallest possible surface for its volume.
Water vapour
Like water, water vapour consists of freely mobile molecules, but unlike water, they are so widely separated that the effect of the forces of attraction is almost negligible. In contrast to liquid water, water vapour thus has an indefinite volume.
The vapour molecules do not float about qUietly. They travel at an average speed of several hundred metres per second and the
higher the temperature the faster they will move (Dorsey, E.N., 1940).
However, the movement of vapour through a layer of air (i.e., the diffusion), does not tal:e place at this high speed. Like ourselves, the vapour mOlecules have "traffic problems". OccaSionally they
-7-atmosphere with which water vapour is mixed in nature. セセ・ウ・ col-lisions greatly retard the movement of vapour through still air. For example, the vapour diffuses at a fairly slow rate through the air in the pores of a snow cover.
When a solid is in contact with vapour its surface is incessant-ly bombarded by molecules. tィ・セ・ cause a slight impact on the sur-face each time. The total effect of the vapour molecules hammering at the surface is known as the partial pressure of the water vapour or vapour pressure. The latter increases with the number of vapour molecules per unit volume and with the temperature.
Ice - Water
From the molecular point of view the transition from ice to water may be described in the following way. When an ice crystal is warmed
the mOlecules are set in motion, and this motion becomes increasingly vigorous as the temperature increases. At the melting point the
motion becomes so vigorous that the mOlecules slip past one another and change their relative positions (Pauling, L., 1952).
As the melting progresses the loose structure of the ice
partially collapses. The molecules approach one another more closely and the water acquires a greater 1ensity than that of the ice. How-ever, many links are preserved and aggregates of molecules with the expanded structure are still found in the water at freezing point. When the temperature increases further some of these aggregates are destroyed, accompanied by a further increase in the density. Only at a temperature of more than +4°C. is this effect exceeded by the normal expansion, which is due to the fact that the increased motion of the molecules requires more space (Pauling, L., 1952).
If the loose structure of the ice is exposed to a sUfficiently
high pressure, it collapses. The ice melts under pressure. The
lower the temperature the higher will be the pressure required for the collapse of the structure. In other words, pressure decreases the melting point of ice (Table II). However, at temperatures below -22°C. water no longer forms under high pressure, instead an ice of a structure different from that of ordinary ice forms
pao
-8-If the two pieces of ice are compressed at moderate temperature, the ice melts in the area of contact. The actual area of contact is of course only a fraction of the apparent area of contact. There-fore, the specific pressure may become very high. If the pressure then eases, the water in the area of contact freezes again and the pieces of ice cohere. This process is known as regelation.
When ice is melting the resulting water spreads as a coat over the ice and collects in cavities and inward recesses, e.g. at points of contact between pieces of ice, accorning to laws determined by the forces of attraction between the molecules. If this water freez-es again, the shape of the ice forming becomfreez-es approximately the
same as that of the water cover before freezing. In this way the pieces of ice become rounded and the cohesion between two adjacent pieces of ice is reinforced.
セセィ・ョ water freezes, the grOWing ice crystal does not readily
accept impurities and. these tend to collect at the freezing planea When the more concentrated solution at the freezing plane finally freezes, a "joint" with a lower melting pOint than that of the indi-vidual crystals is formed. Therefore on uniform warming of the ice this layer melts first. An example of this is the "rotten" ice sometimes encountered in spring (Dorsey, E.N., 1940).
When snow is treated with salt a layer of salt solutions forms around the snow particles. This layer has a considerably lower melt-ing point than that of ice in general. Such snow therefore becomes soft and does not immediately freeze together even at temperatures considerably Lower- than ooC. Slushy snow mixed キセ th mud, which is frequently encountered on city streets with heavy traffic, is another example of the dissolving effect of foreign substances on snow.
Ice - セj。エ・イ Vapour
'l'he exchange be twe en ice and water- vapour is of fundamental im-portance for tre origin and transformation of snow.
As mentioned above, the molecules in the ice vibrate to and fro with respect to one another. In some cases the vibration may be-come so Vigorous that a molecule in the crystal surface overbe-comes the attraction from adjacent molecules, is ejected from the crystal
-
-9-and forms a free vapour molecule. This will become more likely as the vibrations become more vigorous and the temperatures increase. The rate of evaporation from the ice surface thus increases as the temperature increases.
A few of the free vapour molecules above the ice surface will revert to the ice surface during their motion and adhere. The rate at which the molecules condense in this way is proportional to the number of vapour molecules above the ice surface.
The vapourization from an ice surface will increase the number of vapour molecules above it until the rate of condensation equals that of the vapourlzation. The vapour pressure at this position of equilibrium is known as the vapour pressure of ice at the prevailing temperature.
If the temperature of ice decreases, vapourization takes place at a rate slower than condensation, and the number of vapour mole-cules will decrease until a new state of equilibrium is attained. The vapour pressure of ice thus decreases as the temperature de-creases (Table III).
When the vapour pressure in the ambient air is higher than the vapour pressure of ice, the crystal grows. The molecules are then arranged in approximately the same way as bricks whenl the bricklayer lays brick to brick and one layer of brick after the other. In this way plane crystal faces are formed (Burton et al., 1951).
A brick which lies at an edge or at a projecting abutment be-tween two courses so that only two of its surfaces are joined to other bricks has a weaker hold and is easier to remove than a brick, which, for example, has five surfaces joined to other bricks. The conditions are approximately the same for the molecules in an ice crystal as for brick. When the vapour pressure in the ambient air is lower than that of the crystal, the edges and angles evaporate more rapidly than other parts of the crystal. Therefore, the crys-tals become rounded.
If two surfaces with different vapour pressures, due to, for example, different temperatures at the surfaces, are close to one another, the molecules will move from the surface with the higher
..
-10-vapour pressure (the QXイァセイ number of free vapour molecules) to the surface wi tr the Lower- vapour pressure.
The transition from the solid state to the gaseous and vice versa, as described here, also takes place in other sUbstances, e.g. camphor and naphthalene, and is known by the common term of
sublimation.
Water - Water Vapour
Like ice, water is in equilibrium with vapour at any temperature when the molecules of the vapour have a certain concentration. The pressure which corresponds to this concentration above a plane water surface is called the water's vapour pressure at the temperature in question (Table IV).
Formation of Snow Crystals
When masses of moist air rise to heights of reduced atmospheric pressure they cool due to expansion. As a result, the relative
humidity of the atmosphere increases. If, at the same time, the temperature drops below the freezing point while the relative humid-ity of the atmosphere exceeds the saturation point relative to ice, the climatic medium has been created in which snow crystals can grow (Fig. 6). However, the formation of snow crystals normally requires that the humid air is II fertilizedII with sui table nuclei, from \'lhich
the crystallization may her;in. It is assumed that these crystal nuclei consist of extremely small water drops, dust particles, etc., with special physical and chemical properties, but their exact
nature is not fully known. Since this is a ーイッ「ャセュ chiefly of
meteorological interest, it 1s omitted here. It must suffice merely to pOint out that snow crystals may begin to grow under special con-ditions at high humidity and low temperature.
In the presence of these conditions the snow crystals grow due to the fact that vapour molecules flow in from the supersaturated air and orient themselves according to the fixed crystal Rtructure. The vapour thus changes directly to a solid form. Hence the snow crystals are formed by sublimation.
-11-Precipitation results when the crystals have grown to such a size that they can no longer kee p floating in the atmosphere without falling to the ground. The growth of the crystal surfaces varies
、・ー・ョセゥョァ on the degree of supersaturation and on the temperature.
The basal planes of the crystals have some properties which differ from those of the lateral faces.
Since the snow crystals fall through humid air the supply of vapour is more extensive to some parts of the crystals than to other parts. These and other causes contribute to the fact that the growth of the crystals varies widely with the external conditions.
'#hen the snow crystals gTow in clouds of small super-cooled water drops, which are qUite common in the atmosphere, .the vapour moves from the water nrops to the snow crystals, because of the lower
vapour pressure of the latter (cf. Table III and IV). The snow crystal!:) thus grow at the expense of the water drops.
This difference between the vapour pressure of ice and that of water varies With the temperature and attains its maximum at approxi-mately -15°C. Therefore, it may be expected that the snow crystals in a cloud will grow fastest at about this temperature. This was also clearly shown by the investigations of v.rall and Nakaya , They pointed out that the relationships between the crystal shape and the temperature constitute a good basis for the classification of the highly diversified crystals of new snow. Therefore a summary of the above investigation is given here (cf. Fig. 6 and 7).
Snow crystals begin to fall at -2°C., but this is rather the exception. As the temperature drops the formation of snow becomes more common. Between _2° and -7°C. only needle-shaped crystals up to 2 mm. in length are encountered. Between _8° and -10°C. disc-shaped crystals form; their diameter scarcely· exceeds 1.5 mm.
Between -12° and -18°C. the rate of growth is at its maximum and may reach approximately 5 mm. per hour. In this temperature range most
of the finely-branched forms of snow crystals grow. These
star-shaped crystals may attain a size of approximately 5 mm. Between -180 and -24°C. the finely-branched shapes rapidly deteriorate,
-12-At temperatures below -25°C. the crystals become still smaller and assume a prismatic form.
'I'he thickness of all these crystals is small and will scarcely exceed 0.1 mm. compared with other snow crystals. This minimum crystal length seems to be more characteristic of the properties of new-snow crystals than their maximum length. Therefore, in the ex-periments carried out here, the minimum measure (the diameter of the sphere which may 'he assumed to be inscribed in the snow crystal) was used to denote the particle size.
Heavy snowfalls originate from clouds of great vertical thick-ness in which neither the temperature nor the humidity of the
atmos-phere are uniform. Crystals form at any height and during their
relatively slow fall (0.3 - 1 m. per second) they are exposed to
changing influences. Therefore, they do not become uniform. As a
matter of fact, heavy snowfalls usually produce a collection of dif-ferently shaped crystals.
Hail which forms when snow crystals pass through series of melt-ing and freezmelt-ing processes can' accompany a snowfa Ll ,
Snow crystals can pass through clouds in which they becqme covered with small water drops which then freeze, forming silvery white fluffy pellets. The latter may be more or less spherical and are known as soft hail (Fig. 8).
Hoar-frost is deposited on the snow cover by sublimation. Eime is formed from fog and mist due to the fact that small water drops freeze to small ice grains on contact with cold objects. On mountain tops exposed to humid winds the contribution of rime to precipitation is considerable.
In wooded regions the contribution of hail, hoar-frost and rime to the snow cover is usually small. During the winter preclpitation in solid form consists chiefly of snow crystals with their greatly diversified fragile shapes.
p:a
Nevv SnO"J
In calm weather the snow crystals fall gently to the ground in-dividually or in accumulated flakes. They support themselves and the ones falling on top of them at a few very small surfaces. There-fore, the newly formed snow cover is a sort of very brittle open
network of thin snow crystals. The more branches that the snow crystals have, the more air the snow cover will contain and the lighter and more porous it \'lill be. The newly formed cover, there-fore, has a very low specific gravity (Table VI).
Under the effect of the wind the brittle projections of the snow crystals are broken (Fig. 9). The fine crystalline parts may become packed. The snow cover \"lhich forms in a snovr storm, i.e., so-called wind-packed snow, tterefore is denser and harder.
The newly formed snow cover may contain a large number of dif-ferent crystalline forms, more or less broken up and more or less hard-packed. However , all new snow has a common significant prop-erty, namely that each new-snow crystal has a laree surface in pro-portion to its volume.
The Transformation of Snow
As soon as the snow crystals land on the ground, and indeed even while they are falling, they begin to age. The local climate in the snow cover differs from that in the humid atmosphere, where the crystals originate. In the snow cover the supply of material from the humid atmosphere has ceased and a slow transformation of the snow crystal begins (Bucher, E., 1948).
The transformation of the crystal takes place by sublimation, mel ting t'o l.Lowed by freezing, and by the motions of the vapour and
the water of melting in the snow.
Sublimation takes place at temperatures below the melting point. The lower the temperature the slower the rate of transformation by this means \'1ill be, but even at the Lowest temperatures the snow 1s thus transformed grAdually. The corners, edges and finely branched parts of the crystals evaporate relatively fast in dry air, as a result of which the crystals become rounded (Fig. 10). The needles,
-14-discs and stars in the new snow are thus transformed into a more compact gr-a
m ,
This is primarily the way fine-grained snow forms(Fig. 11).
l:!ith the sublimation of vapour there is a tendency towards the formation of distinct crystal faces. However, it may be assumed that in the upper layer of the snow cover the thaWing and freezing which punctuate the cold periods, and variations in the moisture content result in more rounded forms. Deeper layers are better insulated against variations in temperature and moisture. In these layers temperature and moisture may remain fairly constant for a long time, at least in the northern parts of Sweden. In such a local climate the processes of sublimation go on more or less uninterrupted by melting and freezing. This may be the reason for the formation of snow particles with distinct crystal shapes. It may also be assumed that the movement of the moisture upwards from the warmer ground is important for the formation of such crystals (Quervain, M. de, 1950).
This type of snow is frequently encountered in Lapland in mid and late winter and is usually covered by an insulating crust. This was observed and described by an Englishman studying snow as early as about 1800. He used the term "Lapland snow" (Seligma.n, G., 1936).
It is characterized by snow particles which are fairly large and have distinctly shRrp edges. These snow particles lack cohesion and slide
away when stepped upon. Such snow is often called depth hoar
(Fig. 12).
Melting and evaporation affect the edges, corners and finely branched parts of the crystals more rapidly than the compact parts. The water of melting is absorbed about the latter parts and between the larger snow particles. Due to surface tension the surfaces are rounded off and the snow particles are drawn into one another. The cohesion in the snow increases. The more rine-grained the snow the more noticeable this process will be.
If melting is f'oL'Lowed by freezing, a more coarse-grained snow results \'li th the same rounded form that melting and surface tension produce and with stronger joints between the grain than before. In such a case a hard crust results.
p
-15-It may be reasonably assumed that the snow melts to some extent even at temperatures below ooC. It was recently pointed out that セエ
temperatures which are not too low the aging Gnow crystals are en-veloped by a very thin flUid membrane in which foreign substances that had reduced the melting point are dissolved (Wall, E., 1947).
In early winter and mid-Winter heat moves upwards from the ground through the snow cover. Experiments have shown that a large proportion of this heat trqnsport is associated with evaporation from the lower layers of snow and subsequent condensation in the upper layers (Yosida, Z. and Kuroiwa, D., 1950). By this means material is moved upwards from below. This is assumed to be of great import-ance for the transformation of uhe snow cover. The porosity of the
snow controls the rate of this transformation. In dense layers, e.g. wind-packed snow, the movement of the vapour is thus hindered and therefore the transformation becomes relatively slow (Quervain, M. de, 1950). It may thus be expected that compacting retards the transformation of material.
The combined effect of the mentioned processes determines the transformation of snow. Broadly speaking, it consists in the
break-ing elm'm of the finely br-anched new-snow crystals into more compact
grains, the larger ones growing at the expense of the smaller onesj
as the young cuckoo in his foster セッュ・ thrives at the expense of
his smaller "brothers and sisters". Therefore, the fine-grained snow becomes increasingly coarser with time and changes to depth hoar or more rounded particles which have frozen together.
A good insight into the structure of a snow layer is provided by microphotographs of thin sections, which may be obtained by a special method. A number of such photographs are shown in Fig. 13. It can be seen how the snow structure in a layer has changed with time. The structure of a layer of depth hoar is shown in Fig. 14 in a photograph taken in a similar way.
The extent and amount of a snowfall varies from place to place. The grain size, shape of grain and cohesion in the snow cover will depend on the external conditions to which each layer is exposed. As a result, the snow cover consists of layers of different
thick-pz
-16-nesses and properties. Therefore, the composition of the snow cover will vary from location to location, from day to day and from year to year.
Deformation and Strength of Snow
An illustrative description of the deformation of glacial ice, which must be considered an extreme form of packed snow, is given
in an account of a journey by Mark Twain.
He was on the Gorner Grat in Switzerland with the Gorner glacier below. Knowing that the glacier moved, because Baedeker said so, he made a bold decision. He would travel to Zermatt with the glacier. A few passages from his book are given below.
"I had the expedition march down to the glacier along a mule track and took up a favourable position in the centre, since the centre moves fastest according to Baedeker. However, for the sake of economy I placed the heavy luggage close to the edge in order to have it forwarded as freight.
"I waited and lilai ted and i!!lai ted, but the glac ier did not move. It grew dusk, darkness fell, but we did not move.
ItI asked the expedition to pitch camp, mounted a guard and gave orders to call me as soon as Zermatt came in sight.
"I woke up at 10.30 the next morning and looked around. We had not moved one inch.
"I thought to myself: 'Now the old wreck has run aground again". I opened Baedeker to find out whether he said anything about such an annoying delay. I found a few words which threw some light on the situation. 'The Gerner Glacier', said Baedeker, 'moves at a rate of one inch per day'. I had never felt so indignant or
been so frustrated. I made a hasty calculation: an inch per day,
I
Or 30 feet per year; the distance to Zermatt is nearly four miles. The time イ・セオゥイ・ェ for the trip with the glacier would be slightly mOre than 500 years".
On the whole, the report is instructive. It conveys the know-ledge that glaciers do creep and also throws some light on the slow rate at which they creep.
-17-Other forms of snow also creep but more smoothly, since the vis-cosity decreases with decreasinG specific gravity, decreasing grain size and increasing temperature (Bucher, E., 1948). Therefore, at relatively high temperatures and prolonged periods of loading, snow may be considered as a viscous compressible fluid. At low
tempera-tures and under temporary stresses, such as those arising from traffic loads, snOH removal, etc., the flow in the snow will be unimportant, and unrter- these condit i ons snow behaves almost like a solid, compressible body. Porous snow differs from fluids and most of the solid materials by its great compressibility. A fluid or a metal which is subjected to strong (hydrostatic) pressure on all sides decreases its volume very little. For example, when sand and clay are subjected to pressure from all directions they are only slightly compacted and recoil strongly when the pressure is removed. Porous snow, on the other hand, is readily compressed to a fraction of its initial volume and remains compressed after the pressure has been removed. For example, 10 centimetres of new snow may he com-pressed to one centimetre. It is difficult to find any analogy for this. A 10-metr'e layer of carefully piled fragile crystal glass
(by giving free rein to the imagination almost anything becomes con-ceivable) might possibly be assumed to correspond to new snow. The crushed prOduct, which would be the result if a house were put on top of this layer corresponds to packed snow.
Porous new snow has practically no bearing capacity. Vehicles sink so deeply down into it that it is difficult to move them. How-ever, naturally Or artificially packed snow may have considerable bearing capacity and it is the bearing capacity of this type of snow, which has a specific gravity of approximately 0.3 and more, that is of prime interest here.
With a view to preventing and decreasing the damaging effects of avalanches, extensive studies have been carried out to determine the strength properties of snow. The results thus obtained help to illustrate the bearing properties and enables comparisons to be made between snow and ッエィ・セ road foundations, such as sand and clay.
-18-!"Aol{ec1 snow in whi ch the cohesion between the snow particles is
either lacking or very weak thus has bearing properties which most closely resemble those of sand.
The depth hoar mentionecl above, treated coarse-grained snow and even cold fine-grained snow immediately after treatment are typical examples. Like coarse sand, this kind of snow セャゥ、・ウ out of the way when stepped upon or when small wheels or skids pass over it.
According to the theory of materials with internal friction, under wl'lch this coarse sand is classified, the stresses are propa-gated only by normal forces and frictional forces between the
particles. The internal friction in such material determines the lateral sliding of the material under vertical load and tlluG its bearing strength. Therefore, macadam, which has a coefficient of internal friction of approximately 1, has a higher bearing capacity than sand with a coefficient of internal friction of approximately 0.7.
For depth hoar a coefficient cf internal friction of approxi-mately 0.7 was obtained from measurements at -4°C. Higher values were obtained for other types of snow. However, the internal fric-tion cannot be as accurately determined in snow as in sand because of processes of ウィゥヲエゥョセ and melting, etc., and the formation of
joints in the snow (Bader, G. et 0.1., 1939).
The load 1tihich a long plate resting on material with internal friction can support is proportional to the square of the plate's width and is characteristic of this material. Therefore, it may be expected that if the width of one of the runners of a sleigh, which rests on cold snow (which resembles sand) is doubled, the load may be more than doubled without the runner sinking deeper into the snowe However, the compressibility and flow properties of snow complicate
the situation to some extent.
セヲオ・ョ the snow particles in a compacted snow are frozen together
the bearing capacity is increasede The snow thus acquires greater cohesion and, along with this, pressure may result in pure shear
stresses and tensile stresses as well. This kind of snow and
p
-19-and the relation between the maximum load -19-and the size of the load area, for example, differs here from that for sand.
The cohesion between the snow particles can he measured, e.g. by tensile tests. Such tests have shown that a tensile strength
of more than 1 kGffi./sq.cm. is not unusual in packed snow and that the tensile strength increases with increasing density, decreasing grain size and decreasing temperature of the snow (Bucher, E., 1948).
The constant change in the properties of snow and in the
usually ゥョィッュッセ・ョ・ッオウ structure make the bearing properties very var-iable and complex. Therefore, the value of indirect, relative
measurements of the bearing capacity of packed snow is often de-batable. Such measurements should be supplemented by direct
full-scale measurements.
Snow Classification
Snow classification is usually based on the measurements of: (a) the snow temperature,
(b) the shape of the snow particles, (c) the size of the snow particles,
(d) the cohesion 「・エセ・・ョ the snow particles,
(e) the relative proportions of ice, air and water.
In order to classify a snow cover accurately these factors are determined for each snow layer.
The snow temperature (a) may be measured vlith an ordinary ac-curate thermometer. In the present investigation the temperature was usually measured 10 centimetres above the snow surface and
10 centimetres below it. For more accurate determinations the
temperature must be measured at many more levels.
With respect to the particle shape (b) a distinction has been made here only between new snow and granular snow. It was not
con-sidered necessary to differentiate between different types of new-snow crystals, as was done in Canada (National Research Council of Canada, 1949; Klein, G.J., 1946; Pearce, D.E. and Gold, L.W., 1949; Schaefer et al., 1951).
セLNセMMMMMMMMMMMMMMMMMM
_ _1IIIIIIi-20-':rhe particle size was measured v.Jith a microscope provided v:ith a scale. The diameter of the ereatest sphere assumed to be in-scribed in the snow particle was chosen as the crt cer-i on of the particle size. By measuring in this way, a low value is obtained for the particle size of the thin shapes of nev: snow and a greater value as the snow is aging. The mean value for the particle size has been established from at least 10 measurements of snow particles
selected at random.
Neither melting snow nor water was included in the determina-tion. If desired, the water content may be determined by calori-metric methods. The proportion of snow and air (d) in a snow layer was determined by weighing a given volume of undisturbed snow.
In order to measure the cohesion between the snow particles a proctor needle was used. It is normally employed in simple field tests for the determination of bearing properties of different solI types (Fig. QUIセ
The proctor needle is very simple in design. In principle, it is a spring balance whi.ch gives the maximum load over a small sur-face. It consists of a housing in which a spring is suspended. Plates of varying ウゥセ・ウ may he fastened to a shaft at the lower part of the housing. In the upper part there is a rod which rests on a spring with its lower end while it is provided with a handle at its upper end. This rod is graduated in such a way that the force with which the plate is pressed against the soil can be read by
means of a slide ring. The proctor needle is graduated from 5 to 40 kgm. and the plate areas vary from 0.25 to 0.32 sq. em. This arrangement was found to he very suitable for measuring the bearing properties of packed snow.
Snow-Compacting Tests
As early as the winter of 1950 the SDA experimental station at Lycksele was carrying out tests on compacted snow roads. It must SUffice here to describe only the introductory tests, which in-cluded measurements of the cohesion and bearing capacity ッィエ。セョ・、
-21-general idea of hovr the bearing capac i ty of snow increased with time after treatrr.ent. TI1e effects of temperature and of the dif-ferent methods of treatment were also to be det er-mi ned ,
The tests included comparisons between the following methods of treatr.1ent:
1. Compaction bv crawler tractor
A crawler tractor was driven twice back and forth over a test strip of previously undisturbed snow with tracks edge to edge. Im-mediately after compaction the top snow layer was smoothed with a board in order to obtain an even measuring surface.
2. Compacting with a crawler tractor follovJed by dragging
Compaction viaS carried out as described above and followed, after some time, by dragging vlith a special drag (Leijonhufvud, A.C:son, 1950).
3. Stirring of snow
The snow was stirred vigorously with a stick, after which the surface was smoothed.
4. Compaction by tam12.ing
The snow was compacted by vigorous tamping with a wooden pole.
5. Screening
Screening was carried out by passing snow from the different
layers of a snow cover through a screen of 2 mm. mesh size. A
portion (approximately 305'0 of the granular snow in the bottom layer could not be passed through the screen and was not included in the test. During the screening the snovr was allovJed to fall from a
height of approximately 1 metre. The surface was 'not smoothed after screening, so that compression of the snow was avoided. However, the snow became more compact than before screening owi ng to the destruction of the open structure.
In these comparison tests the snow was classified in the manner mentioned above. The bearing capacity of the surface was measured with the proctor needle, 1, 2, 5 and 24 hours after the treatment. The manner in which the bearing capacity (as measured with the proctor needle) increased after the treatment is shown in Fig. l6a-d.
-22-The results obtained from the tests scatter and no clear relation-ship be twe en the method of treatment, the specific gravity and the
「・。イゥョセ t.J capacitv could be established.セ However, it is evident that
compaction with the crawler tractor followed by dragging in every case produced proctor values for the bearine capacity that were hiVler than those obtained by the other methods.
It appears that ゥョ」イ・。ウゥョセ temperatures have a decreasing ef-fect on the bearing strength of a compacted snow cover. This is evident from comparisons of Fig. 16a-d. TIle effect of the tempera-ture is also illustrated by Table VII, which 1s hased on a prolonged study of a compacted snow road. The manner in whLch the bearing capacity decreased with the rising spring temperatures is clearly evident from this table.
The relationship hetween the load area and the bearing strength per unit area is evident from Fig. 17, which shows the results ob-tained from meesur-emerrt s with proctor needles having various plate sizes and with pressure hoxes placed on plates of various sizes and loaded with a tractor t r-ac k shoe. It is qui te clear that the bearing strength does not increase in direct proportion to the size of the load area, which in the diagram would have corresponded to a hori-zontal 11ne. As the size of the load area increases the pressure per uni,t area which the snow can bear w1 thout gi vi.ng way decreases.
The bearing capacities measured with the proctor needle at dif-ferent depths in a compact snow road almost two months old are shown in Table VIII. Although the air temperature was high when the
measurements were taken, so that the upper layers セ・イ・ not as hard packed, the bearing capacity for the proctor plate decreased with in-creasing depth below the surface.
Discussion of the Tests Increase in bearing capacity after compaction
The sinking of vehicles and horses into the snow may be due to the fact that some of the snOH is compacted while some is pushed
aside. In the first case the snow foundation decreases in volume and in the second it changes its form. Therefore, it may be said that the capacity of the snow to prevent a load from sinking, i.e., the
-23-bearing capaclt y , depends on the r-e si stance of the 8nO\'1 to changes
in volume and to deformations.
By treating a cold, loose snow cover, e.g. by applying load by tamping or shaking, the loose structure is crushed and the snow is packed. Because of increasen oensity, the resistance of the snow to changes ln volume increases simultaneously with the development of resistance to deformation due to friction between the snow
particles.
The zones of contact which form between the snow particles as the result of compaction are probably very small. This indicates that even thouGh the force which compresses the snow particles may appear small, the pressure per unit area may nevertheless be high. At temperatures close to DoC. reeelation may thus glve rise to
forma-tion of joints between the snow particles.
When melting snow is packed down, the membranes formed by melt-ing water between the snow particles contribute to the cohesion of the snow by surface tension, just as moisture imparts strength to fine-grained solIs.
The contribution of regelation and surface tension to the co-hesion 1s instantaneous and is of little or no importance at lower temperatures. セャゥエィ respect to the sharp increase in the bearing capacity of compact snow observed during the day following the
treatment we have to look to another phenomenon for an explanation. Since the bearing capacity in compacted snow increases with time at very low temperatures, it is reasonable to assume that this increase is related to the sublimation of vapour セゥエィゥョ the zones of contact between the compressed snow particles. This is shown by the fact that the hearing capacity increases at a relatively slow rate. It should take a certain time for the vapour to diffuse into the cracks between the snow particles which have been bridged by
crystallization. In this way the strengthening of the joints between the snow particles セ。ケ go on for a long time after compaction.
The experiments in which the snow was stirred and screened show that the cohesion of the snow increased without any actual compac-tion. It may be assumed that in this case the hardening of the snow
-24-!
,
I
f
1s due partly to the crushing of the snow particles during treatment. However, the hardening probably was brought about by the mixing of snow of different temperatures during the treatment (Shalshov, 1948; Taylor, 1953). In the usually vrarmer- bottom layer of a snow cover the vapour preRsure is higher than in the colder top layers. Thus, after mixing, a movement of molecules from the layers of the warmer
snow to those of the colder snow takes place, and also to the zones of contact between the cold and warm snow particles. Therefore, it may be assumed that the mixing of cold and warm snow contr1butes to the formation of joints in the snow.
The joints wht.ch are formed as a result of regela tion, sub-limation or melting and freezing provide the compacted snow with cohesion. Without the latter, snow would behave like coarse sand. Wide surfaces, such as the tracks of tractors, should find sufficient
support, but narrow and small surfaces, such as sledge runners, horses' hooves and the load area of motor vehicle wheels will sink down considerably since the snow is pressed sideways. Therefore, a cohesive upper snow laye!" is almost as important for the compacted snow road as a surface course is for an ordinary road on a founda-tion of coarse sand and coarse gravel.
The thermal conductivity of snow is increased by compaction (Table IX). Th1s facilitates the freezing of the foundation of the road. This is important for the bearing capacity of bogs, etc. The stablli ty of compacted SnO\lT roads
Uninterrupted sublimation seems to have a tendency to change the snovi structure in such a way that uistinct, even crystal surfaces form. Consequently, the joints between the particles weaken with time or dissolve completely. The depth hoar mentioned above, which is frequently encountered underneath dense insulat1ng· layers of snow, is an example of this. Therefore, this type of snow must be expected in the bottom layer of a compacted snow road and was
actually observed at the bottom of old compacted snow roads in the Lycksele district. However, in the upper layers, melting and sub-sequent freezing, the addition of new snow and intense compression prevent the joints from weakening.
-25-Therefore, it may be expected that in late winter the compucted snow road will consist of a coherent frozen snow slab resting on
less compact snow. The road may thus behave as if it were hollow und break-throughs of the outer layer may prove troublesome. However, this can be avoided by continuous compaction of the road and settle-ments.
During prolonged periods of thawing the jOints usually melt faster than the snow particles themselves. If subsequently the particles are coarse, then the snow has no cohesion, owing to the fact that the water layer does not have sufficient strength to hold the large snow particles together. On the other hand, fine-erained snow may be compact even though it is melting.
Dissolution of the road by heat is facilitated by impurities in the snow. Foreign substances, ttJhich are diaso Lved in the snow, reduce the melting point in the layer about the snow particles and may thus make the snow slushy. Dark substances, moreover, absorb more solar heat than the white snow and thus contribute to melting.
The relatively dense surface of the compacted snow road pre-vents evaporation. This should contribute to the stability of the road. For example, the compacted snow under ski tracks across fields sometimes remains long after the other snow has melted away.
Effect of the size of the load surface on the bearing strength of
セ
In the experiments the maximum force required for pressing a fairly small plate through a compacted snow layer was measured. It is of course very important to clarify to vrhat; extent this measure-ment is a criterion of the ability of a compacted snow road to
carry different types of vehicles. We now encounter the problem of how the bearing capacity depends on size and form of the load
sur-face. This is a complex problem, since the material in the compact-ed snow road varies from the road surface down to the ground, from fall to spring, from year to year and with the changes in weather and temperature.
A general relationship, be twe en the bearing capacity and size and form of the load surface on different snow foundations cannot be
NセiiBBBBGBMMMMMMMMMMMMMMMMM⦅BBGB
セL
-26-I
I
establi shed on the strength of the e xper-Irne nt.a.L data alone. For the special CRse under investigation here, the measurements showed that the bearing capacity of a plate did not increase in proportion to the increase in its size. Doubling of the plate size thus implies that the load could he increased only to a value which was less than double. Since the イッ。セ investigated eVidently was typical, it may be expected for the normal compacted snow road that the maximum pressure per unit area, at which breaking through takes place, de-creases with increased load surface. However, it is possible that the behaViour may be different in certain cases. As mentioned above, the experimental data do not suffice to establish a general relationship between the bearing capaCity and size and form of the load surface on different snow foundations.
In the case of a small pressure plate the stresses in the snow are cnnfined to the region about the plate. Therefore, the value for the bearing strength which may be obtained with the two small pressure plates of the proctor needle will depend primarily on the cohesion of セセ snow in the upper ,layers.
The effect of the snow type on compaction
In fine-grained snow good cohesion is readily obtained by com-paction vrht Le poor results are usually obtained in coarse-grained snow. A possible explanation for this is the fact that there are many more points of contact in fine-grained snow than in coarse, granular snow. Therefore, the cohesion in fine-grained snow is
greater than in coarse-grained, a condition that also became evident in the tensile tests mentioned above.
A prereqUisite for obtaining sufficient bearing strength by com-paction alone thus s<';ems 1';0 be the presence of a certain amount of fine-grained ウセッキ in the snow cover (SIPRE, Report 2 and 3).
Just as gravel roads may be made more compact by mixing the gravel with a binder (e.g. clay), it may be assumed that greater cohesion in coarse-grained snow is obtained by mixing it with fine-grained snow and then compacting the mixture.
r..···,····,··
••. ts:
-27-!
I
セヲ・」エ of the temperature on the bearing capacity
The higher the snow temperature the more easily the snow will be compresseda Thaw in association with compaction should provide additional strength for the road, provided that cold weather follows
(Taylor, 1953).
However, if crawler tractors are used, compaction is rendered considerably more difficult at temperatures around ooC. The snow sticks to the tracks because of melting due to pressure and subRe-quent freezing (regelation), and accumulations of hard snow are found which may even break the tractor tracks. In view of this, compacting by crawler tractor should be carried out at temperatures
of at least a few 、・セ・・ウ below ooC. (Leijonhufvud, A.C:son, 1950).
If the snow cover is mixed in conjunction with compacting, the great difference in エ・セー・イ。エオイ・ of the snow layers should have a favour-able effect on the ィ・セイゥョァ capacity of the snow.
The bearing capacity of the snow some time after the compaction depends on the temperature. This is clearly evident from the ex-periments. This may be explained ,by the fact that the ice, and par-ticularly the joints between the snow particles, harden and grow as the temperature decreases. Therefore, an ever-increasing force is required to break the joints between the particles as the tempera-ture decreases. Consequently, the bearing capacity increases with decreasing temperature.
The dependence on temperature greatly limits the use of the compacted snow road with respect to time and locality. The mean temperature during the time the road is used must. thus be somewhat below ooC. Only brief increases in temperature up to or above the melting point are admissible. No criteria for this can be laid down as yet, but must first be established by practical tests.
The effect of the treatment on the bearing capacity
Specific gravity measurements and determinations of the bearing capacity with the proctor needle as well as SUbjective estimates showed that the strongest compaction of snow was obtained by crawler tractor, provided, of course, that the compaction with the tractor 1s followed by dragging.
-28-After compaction with the tractor the snow surface is lumpy; there is no even, firm イッ。セ surface. Dragging produces a more homo-geneous surface and presumably a more compact top layer. Since the measurements with the proctor needle primarily provide a criterion of the aohesion in the surface, the striking increase in bearing capacity (as measured with the proctor needle) as a result of
dragging can thus be explained to some extent. Therefore, it might be assumed that the values obtained by measurement overemphasize
the importance of dragging.
The effect of repeated compaction with the tractor on the same day is not great enough to justify the increased amount of work. After the first compaction the bearing strength of the snow probably
increases so much that on the next compaction some hours later the visible results of this treatment are sliGht. On the other hand, repeated compaction may be expected to have a considerable effect as the snow cover gr-ows by precipi tat ton (Taylor, 1953).
i
\Jihen the crawler tractor is used for compaction the compression is greatest in the surface layer and decreases セjゥエィ increasing
depth, since the load pressure is c1istributed over an ever greater area as the pressure penetrates more deeply into the snow. In order to obtain improved compaction in deep snow and possibly also a mix-ture of the snow layers, the same principle may be applied as in the stabilization of the ground for an airfield, etc. So-called
"sheepsfoot" rollers are オウ・セ in certain cases here. These rollers have lugs which penetrate ieep down into the foundation when the roller moves. The compactness obtained by the snQw when it is
trampled on by a herd of reindeer indicates that good results may be expected from these rollers.
The greater the pressure to which the snow is exposed from ail sides the more closely packed the snow will become. With increasing pressure the compactness increases at first rapidly and then more and mOre slowly. However, this pressure cannot be increased merely by reducing the load area, e.g. the track width of the tractor. The load area must be fairly large in relation to the depth of the snow so that the snow is not forced outwards. Therefore, in order to
-29-obtain great pressure from all sides the vehicle compacting the snow must be heavy. Otherwise, Great pressure may be attained dynamically by impact effect.
Even in this case the load area must he large and a fairly
ャ。イセ vibrating plate or something similar may perhaps be a suitable device for this.
If the compacted snow road is constructed merely by compressing the snow to a higher specific weight, it is left to the snow alone to form the jOints between the snow particles. However, this forma-tion of joints can be promoted, partly by treatment with vlater,
steam or heat and partly by intense mixing of the snow in conjunction with the compaction. In order to obtain satisfactory results by
means of the w.ethods mentioned, large quantities of heat are re-'1uired, involving an unwieldy apparatus (Taylor, 1953). The latter method, in which differences in temperature in the snow cover are utilized, is preferable since it permits the use of simple tools.
It appears possible to make the 」ッュセエ」エ snow roads strong enough to support not only tractors, horses and sledges but even trucks (SIPRE 2 and 3). If the construction and maintenance costs can be kept close to the present low level, the economic importance of these roads should increase considerably. However, continuous practical tests are required in order to develop sUitable methods and to determine the economical use of this road construction technique in different climatic regions.
Summary
Winter roads constructed by packing dovm the snow or by beating it have been used for several years in northern Sweden for trans-porting forest products. The use of these roads constitutes an economical solution of many transport problems.
In order to make more efficient and widespread use of this road construction technique it seems desirable to obtain more data on the properties of snow as a bUilding material. The present investi-gation Has car-r-Led out from, this point of vie\'l.
1"
If
I
I
-30-',11th the aid of data from the literature an attempt was made to clarify the physical background of the bearing capacity of snow. A brief de sc r i p t.Lou is given of the fundamental physical properties of ice, water and vapour and of the deformation, transformation and strength of snow.
The experiments comprised:
1. Treatment of snow with various simple methods. The
rela-tive bearing capacity of snow was measured with a proctor needle at definite intervals after treatment.
2. Establishment of an experimental series with various test loads on compacted snow.
3. Continuous measurements of a compacted snow road with a proctor needle while the road was in use.
It was found that the relative bearing capacity increased with the time after the treatment. This applied to all the methods of treatment employed. It was also found that the bearing capacity increases as the temperature decreases.
Among the methods of treatment employed, that of compacting the snow with a crawler tractor followed by dragging produced the high-est value for the relative bearing capaclty, whereas low values were
obtained by compaction with a crawler tractor alone. The specific
gravity of snow packed down by various metbods ranged from 0.;:> to
0.4.
An increase in the size of the load surface reduced the pressure per unit area that the snow could carry without failure.
Some working hypotheses concerning the bearing capacity were established on the basis of data from the literature and the results obtained from the experiments. These hypotheses are summarized
below.
It is assumed that the sinking of a vehicle or a horse into a compacted snow road is due to the fact that some of the snow under the load surface becomes compressed and some is pushed aside. Thus, not only does the snow change in volume but also in form. Sinking may be prevented by the ヲッャャッセゥョァ methods:
1. By packing the snow thoroughly when constructing the road. This increases the resistance of the s110VJ to changes in volume, while
simultaneously the friction betv.een the snow particles makes the snow resistant to changes in form.
2. By promoting the formation of jOints between the snow par-ticles, thus making the snow more cohesive.
It is assumed that the formation of joints between the particles is the result of regelation and of sublimation. The processes of mel ting and sur-s enuerrt freezing, which take place when the
tempera-ture occasionally rises above ooC., also promote the formation of joints. Better results are obtained when the cold snow is mixed intensively with the snow from the bottom layers and when it is crushed. The joints between the particles make it possible for the snow to resist tensile and shearing stresses. The ultimate stress limits seem to increase with decreasing temperature, ィゥセャ・イ specifIc density, smaller particle size and with increasing time after the
treatment.
Melting or prolonged processes of sublimation seem to impair or dissolve the jOints between the snow particles.
The bearing capacity of a·compacted snow road may be improved and its life extended by strengthening the joints between the snow particles. This may be done by crushing the particles more thorough-ly, by mixing fine-grained and coarse-grained snow, by mixing snow of different layers (having differant temperatures) and by compress-ing snow more thoroughly from all sides, even in the lower layers. Compacting the snow at temperatures higher than those at which
compaction is carried out at present, heating, spraying of water or steam, etc., may also prove useful in this respect.
-32-L1terature
BADKH,G.rn. f l.: DcrSchnee und seine Metamorphose. Geotechn. Ser. Hydr., Lief 3, Bern 1939.
BEKKER,1\1. G.: Snow studies in Germany. National Research Council of Canada. Technical memorandum no 20., Ottawa, May HJ51. (InnehiUler bl. a. 200
lit-teraturhanvisningar. )
BENTLEY.W. A. och HUMPHREYS,"V. J.: Snow crystals. New York 1931.
BUCHER, E.: Beitrag zu den theoretischen Grundlagen des Lawinenverbaus, Davos 19,1,8.(Innchiiller,hI. a. 236 littereturanvlsningar.)
BURTON, CABRF..RA och FRANTZ: A theory of growtlh of real crystals. Phil. Trans. A 243, 1951.
BYERS, R. H.:General meteorology. New York 19,1,4.
Canadian paperspresentedat the Oslo meetings of the international union of geodesy and geophysics Aug. 1948. N aiional Research Council of Canada, Ottawa,
Dec. 1949.
doiuゥセ[yL E. N.: Properties of ordinary watersubstance, 1940.
HOPPLER, F.: Die Plastizitiit des Eises. Kolloid Z. 97 (1941).
Journal of Glaciology (Tidskrift med artiklar och resumeerom sno ).
KIJElN, G. J.: Mcbhod of measuring the significant characteristics of a snOW-COHI'. National Research Council of Canada, Report no 1\11\1-192, Ottawa, Nov,
19·1,6.
LEI.JONHUFVUD, A. C :SON: Den snopackade basvagcn, SDA Aktuell Information av intern natur , nr 30, 1950.
LIJNDQHI:;T, G.: Dc svenska.fjiillcnsnatur,
N AK.\YA m. fl.: .J.of Fac of Sc. '1"hc Hokkaido Imp. Univ, ser. 2, Sapporo, .Japall
nr 1 (1936), I (1937),2 (1938) oeh 3 (1939).
NAK,\YA, U.: An electron microscope study of snow crystal nuclei. Journal of Glacio-logy, April 1953.
PAULING, L.: Allman kemi, Uppsala 1952.
PEAH(E, D. E. och GOIJD, L. "V.: The canadian snow sun'ey 19'17-1950. National Research Council of Canada, Ottawa, Dec. 19·1,9.
Pt:USSON,B. O. E.: Hestiindighct.och barighet hos ett istacke , Sv, viigforeningens
tid-skrift nr 10, 1918.
PUTKISTO, K.: Snopackad vag ·for hasttransport. Mctsateho mcdrl. nr 89.
QUERVAIX, 1\1. de: Das Korngcfilge von Schnee. Schwciacrische Mineralogischc und Petcrographische Mitteilungen 28 (19,1,8),518.
Die Metamorphose des Sehncekristalls. Vcrhandlungen dcr Schweizerisehcn N aturforscheudcn Gescllschaft, Daves 1950.
SCHAEFER, V. J., KLEIN, G. J., QUF.JWAIN, H. de : Dr8lft of an international snow classification presented at the congress of the UGGI, Bruxclles 1951.
SHAKHOV, A. A.: FizicheS'kie profess)' v snegovom pokrovo (Fysrkaliska processer j
ett snotacke ). Ixvcst iia Akademii N auk SSSR, Seriia geograficheskaia i geo-fiziches'kaia.. 1948, 12: 239-248.
SIPRE Report 2. First SIPRE Snow compaction conference, Dec. 1950.
SIPRE Report 3. Socond "'IPRE Snow compaction conference, May 1951.
SIPRE Report ,1,. Review of the properties of snow and ice, July 1951.
SF.I.lGMAl", G.: Snow structure and ski fields, London 1936.
St rasvc , 1!1l3. 10 P/.1,), :H-35, Chief engineer for traffic and wintcr maintenunce, Todt organization: Specification for the construction of winter rOlH1s (vnow roads) .
TAYLOR. A.: Snow compaction. Jan. HJ;313, SIPRE Report 13.
"'ALL. E.: Chcr die Entstehung der Sclmcckristallc I."'·iss. Arb. des dcutschen
me-tcorologischen Dienstcs Imf ranaosischen Besetzungsgebier. 3 Jalng., 1 Band
QYᄋセWN
YOSIDA, Z.och KUROIWA, D.: Sublimation in thc interior of snow layer. Low tempe-rature science. Sapporo 1950.
YOSID.\,Z.oeh KOZHL\, K.: Change in shape of snow crystals L Institute of Low Temperature Science, Hokkaido Univeristy.
-33-Table I
l¥'(odulus of elasticity for ice E
=
90900 (1 - 0.00558t) kgm./sq.cm. Strength of sea ice in linear compression: values between 5 and125 ksm./sq.cm. were measured. The values most commonly ob-tained are near 25 kgm./sq.cm.
Specific heat of ice 0.5 cal./gm.oC., l.e., amount of heat required for heating 1 gram of ice one degree Centigrade.
Heat of fusion 80 cal./gm., i.e., amount of heat required to melt 1 gram of ice.
Specific heat of 1Jrat'?r 1 cal./BID. °C., i.e., amount of heat required for heating 1 gram of water one degree Centigrade.
Heat of vapour-t aat ron (wat.e r - vapour) 540 cal./gm., i.e., amount of heat required for vapourization of 1 gram of water.
Surface tension water-atmosphere at OoC.
=
76 dynes/em. = 0.77 mgm./mm.Table II
Melting point of ice under hydrostatic pressure
Melting point °C. 0.0 -5.0 -10.0 -15.0 -20.0 -25.0
Pressure in atm. 1 590 1090 1540 1910 2045
1 atm.
=
1.0333 kgm./sq.cm.fable III
Vapour pressure of ice
Temp. in °C. -50° -40° -30° -20° -15° -10° -5° _0.0°
Vapour pres- 0.029 0.096 0.286 0.776 1.241 1.950 3.013 4.579
Hg
.
The water vapour above a water surface increases as the temperature increases and is affected by the surface in such a way that it increases with the convexity of the curvature. The vapour pressure is thus greater above a small drop of water than above a plane or a
concave surface (Table V).
Temp. -50 0 -40 0 -30 0 -20 0 -15 0 -10 0 -50 0 0 +5 0 +10 0 +150 +200
°c.
Vapour pres- 0.048 0.142 0.483 0.942 1.436 2.149 3.163 4.579 6.543 9.209 12.788 17.535 sure mm. Hg Table IV I U セ I 1 0.108 100 0.0011 1000 000001The specific gravity of snow
The vapour pressure above small drops of water Vapour pressure of water
Table V
New porous snow ••••••••••••••••••• 0.005 - 0.05
Slightly wind-packed snow... 0.06 • 0.1
Firmly wind-packed snow •••.••••••• 0.1 - 0.3
Old coarse-grained snow ••••••••••• 0.3 - 0.6
Density of ice (spec. grav.) 0.92 Table
V.I
Radius of curvature セ Hセ
=
0.001 mm.) ••••• Increase in vapour pressure in%...•
Table VII
Proctor-needle measurements of a 」ッュー。」セウョッキ road subjected to traffic
Values obtained with the proctor needle
Date of Temp. Number Plate Measured pressure in Specific pressure in
measure- °C. of no. kgm./sq.cm. kgm./sq.cm.
ment
measure-ments Sleigh Road Sleigh Road
runner centre runner centre
track track 27/2-51 -13° 21 0.25 39.0:t2.6 156.0
"
-13° 32 0.25 19.2:t8.3 76.8 20/3 _5° 10 0.25 40.0:t0 160.0•
-5° 26 0.25 27.8:t5.4 111.2 2/4 -5° 16 0.25 42.0:t0 168.0"
_5° 15 0.25 23.3:t6.0 93.2 12/4 +6.5 18 0.25 39. 7:t0. 94 158.8 -n +6.5 28 0.25 10.5!3.4 42.0 It +6.5 14 0.50 16.0:tl.4 32.0 I CN en I
-36-Table VIII
セセ・ same road measured wlth the proctor needle at dlfferent depths below the surface
Date of Temp. Number Plate Depth Values obtalned wlth
measure- °C. of no. below the proctor needle
ment measure- road Measured Spec1flc
ments level pressure pressure
ln kgm. 1n kgm./ sq. em. 12/4 +6.5° 10 0.25 8 cm. 40.3 :to.64 161.2 II It It It 20 em. 32.0:t4.0 128.0 It
"
"
It 30 em. 19.7!2.6 78.8 It"
II It 40 em. 10.4!1.1 41.6 Table IX Thermal conductlvlty (k)k for lee
=
20-25 ml11lwatt/cm.oC. at temp. between 0 and -30°C. k for snow=
0.29 HQKQPPᄋセRI • (m1111watt/cm.oC.) Hセ=
spec.grav.).}f
•
Spec1f1c grav1ty kgm./11tre k
s now m1ll1watt/cm.oC.
Accord1ng to J. Devaux
0.125 0.25 0.50
Fig. 3..& Gャj。セ m.olem.lk (P.UILXNG, L., 1951).
Fig. 1.81WtlJ as13"obstacle.
Flgl1l 4:
The crystal structure of ice (Pauling, LQ, QYURIセ The molecules shown on the left have been drawn to scale