Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
9th ASCE Joint Specialty Conference on Probabilistic Mechanics and Structural Reliability [Proceedings], pp. 1-6, 2004-07-26
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=fb130681-50e7-4ae8-a59f-4a475748db9f https://publications-cnrc.canada.ca/fra/voir/objet/?id=fb130681-50e7-4ae8-a59f-4a475748db9f
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at Probabilistic study of chloride-induced corrosion of carbon steel in concrete structures
Probabilistic Study of Chloride-Induced Corrosion of Carbon Steel in Concrete Structures
Z. Lounis, M.ASCE, J. Zhang, and L. Daigle
NRCC-47071
A version of this document is published in / Une version de ce document se trouve dans :
9th ASCE Joint Specialty Conference on Probabilistic Mechanics and Structural Reliability, Albuquerque, New Mexico July 26-28, 2004, pp. 1-6
9th ASCE Joint Specialty Conference on Probabilistic Mechanics and Structural Reliability
P
ROBABILISTICS
TUDY OFC
HLORIDE-I
NDUCEDC
ORROSION OFC
ARBONS
TEEL INC
ONCRETES
TRUCTURESZ. Lounis, M.ASCE, J. Zhang, and L. Daigle National Research Council, Ottawa, ON, Canada
Zoubir.Lounis@nrc-cnrc.gc.ca, Jieying.Zhang@nrc-cnrc.gc.ca, Lyne.Daigle@nrc-cnrc.gc.ca.
Abstract
This paper presents an approach for the probabilistic modeling of the chloride-induced corrosion of carbon steel reinforcement in concrete structures that takes into account the uncertainties in the physical models of chloride penetration into concrete and corrosion of carbon steel, as well as the uncertainties in the governing parameters, including concrete diffusivity, concrete cover depth, surface chloride concentration and threshold chloride level for onset of corrosion. The parameters of the models are modeled as random variables and the distribution of the corrosion time and probability of corrosion are determined by using Monte Carlo simulation. The approach is applied to predict the level of corrosion in the top layer of reinforcing carbon steel of a highway bridge deck that was exposed to chlorides from deicing salts for forty years. The statistics of the governing parameters are generated from a detailed field survey using non-destructive evaluation methods. The predictions of the proposed model agree very well with the field data, which illustrates the capability of probabilistic models to characterize the corrosion response of carbon steel and the actual condition of reinforced concrete structures.
Introduction
The corrosion of carbon steel reinforcement in concrete structures leads to concrete fracture, loss of bond between steel and concrete, and reduction in strength and ductility. As a result, the safety, serviceability and durability of concrete structures are reduced, while their life cycle costs are increased. Normally, concrete protects carbon reinforcing steel from corrosion by forming a passive filmaround the steel due to the high alkalinity of the concrete pore solution. When chloride ions from deicing salts or seawater penetrate into the concrete and reach the steel surface, they disrupt the pasive film and initiate corrosion. The corrosion of carbon steel will start as soon as the chloride content reaches a threshold level, which defines the resistance of carbon steel to corrosion. Consequently, the onset of corrosion is governed by the surface chloride concentration, concrete diffusivity, concrete cover depth of the steel, corrosion threshold level, as well as moisture level in terms of the pore solution, and the availability of oxygen.
In this paper, the prediction of the time to onset of corrosion is based on a semi-analytical model that assumes a diffusion model for chloride transport with the use of the apparent diffusivity and surface chloride concentration that take into account some of the uncertainty associated with the transport model. Given the heterogeneity of concrete and time- and space-dependence of its properties, and variability of the chemistry of the pore solution and microstructure, a probabilistic modeling of the chloride contamination of concrete and corrosion of carbon steel is developed. The proposed model incorporates the uncertainty associated with the analytical models of chloride transport and corrosion initiation, as well as the uncertainty associated with the governing parameters and predicts the distributions of chloride concentration and corrosion time using Monte Carlo simulation. The prediction capability of the proposed probabilistic model is illustrated in a case study of a corrosion-damaged concrete bridge deck reinforced with carbon steel and that was exposed to chlorides from deicing salts for forty years.
Corrosion Mechanism of Carbon Steel in Concrete Corrosion Process in Reinforced Concrete
Concrete is a porous material with microcracks that provide a relatively easy access to aggressive agents, such as ogygen, water and chlorides, which can penetrate from the ambient environment as shown in Figure 1. When chlorides (from de-icing salts, sea water spay, or cast into concrete by use of admixtures, water with Cl−, or contaminated aggregates) are introduced onto the steel surface, the corrosion protection is damaged. Steel corrosion is an electrochemical process in which the steel is dissolved (Uhlig, 1963). Figure 1 sketches the general corrosion process of steel in concrete, in which the steel loses its ions at the anode by the oxidation reaction (Fe→Fe++ + 2e−). The electrons are released and transported to the cathode for the reduction of oxygen (O2 + 2H2O + 4e− → 4OH−). The flow of ions through the concrete pore solution and electrons forms an electrical current for the reactions to proceed. As a result, the eletrical resistivity of concrete, availability of oxgen, and chemistry of the pore solution are the determining factors for the corrosion kinetics of the carbon steel in a concrete medium ( Tuutti, 1982). The concrete pore solution is naturally alkaline with a pH level between 12.5 and 13.5 (Hewlett, 1998). Embedded in such an environment, the steel is protected from corrosion by passivity, which is a condition of corrosion resistance of an active metal caused by the formation of a thin surface film of corrosion products (Uhlig, 1963). The composition of the passive film is uncertain, it is either ferrous (Fe2+) or ferric (Fe3+) oxide.
P e n e t r a tio n o f C l−, , O2, a n d H2O th r o u g h m ic r o c r a c k s a n d p o r e s F e⇒ F e2 + + 2 e− 2 O H−⇐1 /2 O2+ H2O + 2 e − e le c tr o n s A n o d e C a th o d e
Figure 1. Steel reinforcement corrosion process in concrete
The corrosion rate of a passivated steel in concrete can be less than 1 µm/year (Andrade, 1995). The hydroxyl ions (OH−) act as a corrosion inhibitor in concrete that is opposing the corrosive action of chloride ions, which destroy the protective passive film. Although, the interaction mechanism between the chlorides and passive film is not well understood, the chlorides initiate corrosion by disrupting the passive film, reducing the pH level (Jones, 1992), or acting as a catalyst of the oxidation (Uhlig, 1963). It is generally accepted that the concentration ratio of Cl−/OH− in the pore solution at a threshold value determines the onset of corrosion. Hausmann [1967] suggested 0.6 as the threshold value. Chloride content less than 0.2% to 0.6% by weight of cement is recommended in most specifications. Given the inherent complexity and heterogeneity of concrete as a corrosion medium, there exists large uncertainty in the chloride threshold value.
Chloride Transport Model
A reliable prediction model of chloride penetration into reinforced concrete structures is critical for predicting the time to onset of corrosion of carbon steel. The governing transport mechanisms of chlorides into structures are the ionic diffusion in saturated concrete and water absorption in partially saturated concrete. Chloride diffusion is a transfer of mass by random motion of free chloride ions in the pore solution resulting in a net flow from regions of higher concentration to regions of lower concentration (Kropp et al., 1995). The rate of chloride ingress is proportional to the concentration gradient and the diffusion coefficient of the concrete (Fick’s first law of diffusion).Although chloride ingress into concrete is complex, Fick’s law of diffusion may be applied to quantify the chloride ingress. Since in the field, chloride ingress occurs under transient conditions, Fick’s second law of diffusion can be used to predict the time variation of chloride concentration for one-dimensional flow, as follows:
] x C D [ x t C ∂ ∂ ∂ ∂ = ∂ ∂ (1)
Under the assumption of a constant diffusion coefficient, and boundary condition C=Cs and initial condition C=0 for x>0, t=0, Crank’s solution of Eq. (1) yields:
)] Dt 2 x ( erf 1 [ s C ) t , x ( C = − (2)
where: C(x,t) = chloride concentration at depth x after time t; Cs = chloride concentration at the surface; D = diffusion coefficient; and t = time of exposure.
Despite its simplicity and extensive use, this model has some shortcomings: (i) the diffusion coefficient is not a constant but rather depends on time, temperature, and depth because of the heterogeneous nature and aging of concrete; and (ii) the exposed surface is subjected to a continually changing chloride exposure. In general, the values of the surface chloride concentration and “apparent” diffusion coefficient can be estimated from Eq. (2) by determining the best-fit curve through field data obtained from chloride profiles at different depths and exposure times. The time to onset of corrosion (ti) depends on the diffusion coefficient of concrete, surface chloride concentration, depth of concrete cover, and the value of the threshold chloride level. Using Eq. (2), and assuming Cth as the threshold chloride concentration, and dc as the concrete cover depth, the time to onset of corrosion (ti) is determined as follows:
2 s th 1 2 c i
)]
C
C
1
(
erf
[
D
4
d
t
−
=
− (3)Probabilistic Characterization and Prediction of Corrosion of Carbon Steel Identification of Model and Parameter Uncertainties
As discussed in the previous section, there is a considerable level of uncertainty associated with the mechanism of corrosion of reinforcing steel embedded in concrete, as well as with the mechanism of chloride penetration into concrete, which may be grouped into aleatoric uncertainty and epistemic uncertainty. The aleatoric uncertainty arises from the physical or inherent uncertainty identified with the random nature of the basic parameters that govern the chloride penetration and corrosion mechanisms. This uncertainty is associated with: (i) variability of the concrete cover depth, which is a function of the quality of construction and quality control; (ii) uncertainty of the chloride concentration at the surface, which is considerable in the early stages of exposure; and (iii) uncertainty of the chloride diffusion coefficient, which depends on time, temperature, and depth. The apparent chloride diffusion coefficient is determined by fitting the solution of Fick’s second law of diffusion to measured chloride profiles expressed in terms of total chloride concentrations (including both free and bound chlorides).
The epistemic uncertainty arises from the uncertainty in the models for chloride transport and corrosion initiation. The model uncertainty results from the use of a simplified physical model of the actual phenomenon, such as: (i) assumption of chloride transport mechanism governed by diffusion; (ii) use of simplified models of the diffusion coefficient and driving chloride concentration; and (iii) use of simplified chloride threshold level to define the corrosion resistance of carbon steel. The epistemic uncertainty also arises from statistical uncertainty due to the choice of a given probability density function or estimating statistical parameters from a limited sample size, as well as from ignoring the variable correlation or parameter-dependence of some of the variables. In light of the above, it is clear that a deterministic prediction model can be quite inadequate in predicting the actual structural response. Therefore, the chloride contaimination of concrete and the corrosion of reinforcing steel should be determined using probabilistic methods. The uncertainties associated with the surface chloride concentration, chloride diffusion coefficient, concrete cover depth, and threshold chloride level are considered by modeling them as random variables with probability density functions fCs(c), fD(D), fdc(dc), and fCth(Cth), respectively. These distributions are fitted to the data obtained from the field measurement of chloride profiles, measurements of corrosion activity, and observed damage on bridge structures.
Corrosion Prediction using Monte Carlo Simulation
Monte Carlo methods are the most widely used techniques for uncertainty analysis, with a wide range of applications. These methods involve sampling at “random” from the distribution of inputs to simulate artificially a large number of experiments until a statistically significant distribution of the structure response is generated (Melchers, 1987). The direct sampling Monte Carlo method is most widely used, although not as
efficient as those based on importance sampling. The probability of an event g(x)≤0 under consideration, typically termed “failure” (e.g. probability that the chloride content at the steel level exceeds a threshold level), may be expressed as (Melchers, 1987):
(4)
x
x
x
)
0
]
f
x(
)
d
(
g
[
I
....
P
f=
∫ ∫ ∫
≤
where I[ ]is an “indicator function” that equals 1 if [ ] is “true” and 0 if [ ] is “false” (Melchers,1987). Eq. (4) represents the expected value of I[ ].
Case Study
The predictive capability of the Monte Carlo method is illustrated on an aging corrosion-damaged concrete bridge deck that was exposed to chlorides from deicing salts for forty years. An extensive investigation of the deck that included detailed visual inspection, non-destructive and partial destructive evaluation of the deck was undertaken. The field data showed a considerable level of variability in all parameters that govern the corrosion of reinforcing steel and are summarized in Table 1 (Lounis and Mirza, 2001).
Table 1. Summary of data from field assessment
Variable Distribution Mean Value Coefficient
of Variation dc (cm) Normal 3.66 45% Cs (kg/m3) Lognormal 4.56 40% Da (cm 2 /year) Lognormal 0.51 30% Cth (kg/m3) Lognormal 1.35 10%
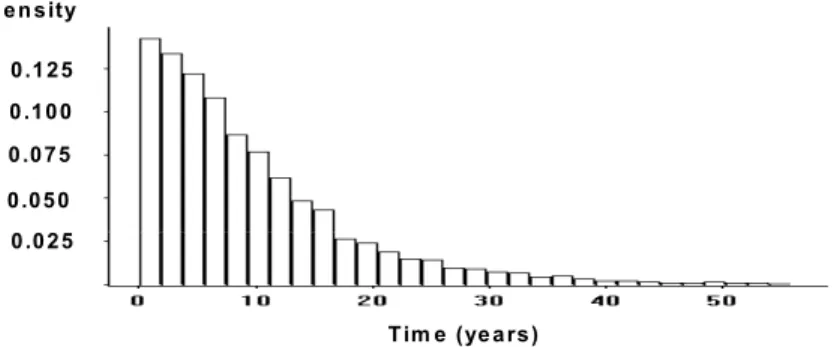
The random variable vector is x =[Cs, Cth, D, dc]T. Using the direct Monte Carlo simulation method, the distribution of the time to onset of corrosion is generated and is shown in Fig.2. It had a skewed distribution with a mean of 10.23 years and a coefficient of variation of 100%. T im e (ye a rs ) 0 .1 0 0 0 .0 7 5 0 .0 5 0 d e n s ity 0 .0 2 5 0 .1 2 5
Figure 2. Histogram of corrosion initiation time of carbon steel in Dickson Bridge deck
The cumulative distribution of the time to corrosion is also generated and is shown in Fig.3. It shows that the probability of corrosion is 92% after 40 years. From the detailed
field investigation of the bridge deck after 40 years (Lounis and Mirza, 2001), it was found that 85% of the top steel was corroded or had started to corrode.
0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 0 5 10 15 20 25 30 35 40 45 50 Time (years) Corrosion probability Probability of corrosion after 40 years = 0.92
Figure 3. Time-Variation of probability of corrosion of carbon steel in Dickson Bridge deck
Conclusions
This paper illustrated the application of a probabilistic approach for the modeling and prediction of carbon steel reinforcement corrosion in bridge structures that are subjected to the application of deicing salts during winter. The proposed probabilistic model provided a very good prediction of the extent of corrosion of the reinforcing steel in the top mat of a deteriorated reinforced concrete bridge deck as the difference between the model prediction and actual condition was about 7%.
References
Andrade, C. (1995), “Qualification of Durability of Reinforcing Steel,” Concrete Technology, Proceedings
International RILEM Workshop, pp. 157-176, E & FN SPON.
Hausmann, D. A. (1967), “Steel Corrosion in Concrete: How Does it Occur?” Material Protection, 6, pp.19-21.
Hewlett, W. (1998), Lea's Chemistry of Cement and Concrete, 4th edition, Arnold. Jones, D. A. (1992), Principles and Prevention of Corrosion, 2nd Ed., Prentice Hall.
Kropp, J., et al.(1995), “Transport Mechanisms and Definitions”, In Performance Criteria for Concrete
Durability, J. Kropp & H.K. Hisldorf (eds.), E&FN SPON, pp. 4-14.
Lounis, Z, and Mirza, M.S. (2001), “Reliability-based Service Life Prediction of Deteriorating Concrete Structures”, In Concrete under Severe Conditions, Banthia, N (ed.), pp. 965-972.
Melchers, R.E. (1987), Structural Reliability- Analysis and Prediction, Ellis Horwood Ltd., Chichester. Tuutti, K. (1982), Corrosion of Steel in Concrete, Swedish Cement and Concrete Research Institute. Uhlig, H.H. (1963), Corrosion and Corrosion Control, John Wiley & Sons Inc.