Proc. Natl. Acad. Sci. USA Vol.93,
pp.
2714-2718,April
1996 Medical SciencesIncreased
mRNA
levels
for
components
of
the
lysosomal,
Ca2+-activated,
and
ATP-ubiquitin-dependent
proteolytic
pathways
in
skeletal
muscle
from head trauma
patients
(protein
breakdown/cathepsin/calpain/proteasome)
ODILE
MANSOOR*t,
BERNARD
BEAUFREREt:,
YVES
BOIRIEt,
CECILE
RALLIERE§,
DANIEL
TAILLANDIER§,
EVELINE
AUROUSSEAU§,
PIERRE
SCHOEFFLER*,
MAURICE
ARNAL§,
ANDDIDIER
ATTAIX§
*Service deReanimation,CentreHospitalo-Universitaire,63003Clermont-Ferrand, France;Centre de RechercheenNutrition Humaine:tLaboratoirede NutritionHumaine,Universit6d'Auvergne,BP321,63009 Clermont-Ferrand Cedex1, France;and§Unit6d'Etude duMetabolismeAzot6,Institut National de la RechercheAgronomique,63122Ceyrat,France
Communicated
by
VernonR.Young,
Massachusetts Instituteof Technology, Cambridge,
MA,December1, 1995ABSTRACT The cellular mechanisms
responsible
for en-hanced muscleprotein
breakdown inhospitalized patients,
whichfrequently
results in leanbody
wasting,
are unknown.To determine whether the
lysosomal,
Ca2+-activated,
andubiquitin-proteasome
proteolytic
pathways
areactivated,
we measured mRNA levels forcomponents
oftheseprocesses
in musclebiopsies
from severe head traumapatients.
Thesepatients
exhibitednegative nitrogen
balance and increased rates ofwhole-body protein
breakdown(assessed
by
['3C]leucine
infusion)
and ofmyofibrillar
protein
breakdown(assessed
by 3-methylhistidine urinary
excretion).
Increased muscle mRNA levels forcathepsin
D,
m-calpain,
and criticalcomponents
of theubiquitin proteolytic
pathway
(i.e.,
ubi-quitin,
the 14-kDaubiquitin-conjugating enzyme
E2,
andproteasome
subunits)
paralleled
thesemetabolicadaptations.
The data
clearly support
a role formultiple proteolytic
processes
inincreased muscleproteolysis.
Theubiquitin
pro-teolytic
pathway
could beactivatedby
alteredglucocorticoid
production
and/or
increasedcirculating
levels of interleukin1p
and interleukin 6 observed in head traumapatients
and account for the breakdown ofmyofibrillar proteins,
as wasrecently reported
in animal studies.Many
intensive carepatients experience
arapid
loss ofbody
proteins.
Tracerstudies haveshown,
inburned(1)
orseptic
(2)
patients,
increasedwhole-body
protein
breakdown. Elevatedmuscle
proteolysis
islargely
responsible
for thisincrease,
asshown
by
enhancedurinary 3-methylhistidine
excretion,
an indexofmyofibrillar
protein
breakdown(3).
However,
noneof the methodsto measureproteolysis
invivo,
namely,
wholebody
tracer
studies,
3-methylhistidine
excretion,
or tracer balanceacrosstheforearm
(a
methodallowing
directmeasurementof muscleproteolysis) (4),
gives any
information on thecellular mechanismsresponsible
forincreased muscleproteolysis.
Therearethree well characterized intracellular
proteolytic
systems
in mammalian skeletal muscle. Thelysosomal
path-way,
whichmostly
degrades
soluble and extracellularproteins,
is notinvolvedinthedegradation
ofmyofibrillar proteins
(5-7)
andcontributes littletooverall
protein
breakdown inmusclesincubated under
optimal
conditions(7-9).
TheCa2+-dependent proteinases (calpains)
degrade cytoskeletal
but notmyofibrillar proteins
(5-7)
and aremostly
involved in limitedproteolysis
of somespecific
target
proteins
(10).
Thethird,
and mostrecently
identifiedsystem,
is theATP-ubiquitin-dependent pathway
inwhichproteins
tobedegraded
arefirstcovalently
linked tomultiple ubiquitin
chains inaseveral-step
process
requiring
ATP,
theubiquitin-activating enzyme
El,
The
publication
costsofthis articleweredefrayed
inpartby
pagecharge
payment.This articlemusttherefore be
hereby
marked"advertisement"inaccordance with18U.S.C. §1734
solely
toindicate this fact.and oneof the
ubiquitin-conjugating
enzymes
(E2).
One of theubiquitin protein
ligases
(E3)
is sometimesrequired
forsub-strate
recognition
andubiquitylation
(11). Ubiquitylation
tar-gets
theproteins
forhydrolysis by
amultienzymatic complex
(the
26Sproteasome),
thefunctioning
ofwhich alsorequires
ATP(11).
Thissystem
classically catalyzes
theselective break-down of abnormalandshort-livedregulatory proteins
(11)
but is alsolikely
tobe theprimary
system
fordegradation
of thebulk of
myofibrillar proteins
according
to recent data inrodents
(6, 7).
However,
while increased activities oflysosomal
enzymes
haveoccasionally
been shown in the muscle ofcachectic
(12)
orinjured
(13) patients,
the roleof the twoothersystems,
andparticularly
of theATP-ubiquitin proteolytic
pathway,
hasneverbeendirectly
studiedin humanmuscle.Therefore,
to assesswhichproteolytic
systems
areactivatedin muscle of intensive care
patients,
we studiedseverely
head-injured
patients
becausethey
areclinically
known toexperience rapid
muscle loss(14).
Wehavedemonstrated that both wholebody
and skeletal musclemyofibrillar
protein
breakdownwereincreasedinthesepatients.
Thesemetabolicadaptations
correlated with enhancedexpression
of criticalcomponents
of thelysosomal,
Ca2+-dependent,
andATP-ubiquitin-dependent
proteolytic pathways
in musclebiopsies.
MATERIALS
ANDMETHODS
Subjects.
Theprotocol
wasapproved by
the Ethical Com-mittee ofClermont-Ferrandand informed writtenconsentwasobtained from the volunteers and from the
patients'
families. Sixhead-injured
patients
and fivecontrolsubjects
were stud-ied. The controlsubjects
werehealthy
young
volunteers[age
= 27 ± 3
years
(mean
±SEM);
body
mass index = 23 ± 2kg/m2;
four menand onewoman],
matched forage,
weight,
and
height
withthepatients
(age
= 28 ± 4years;
body
massindex=20+ 1
kg/m2;
four men and twowomen).
Thepatients
had exclusive and severe head
traumas,
as indicatedby
aGlasgow
comascale score between3and 8(normal
=15)
atadmission.
They
hadnoprior
diseaseandreceivedastandard-ized treatment
combining
artificial ventilation that wasad-justed
to maintain normoxia andhypocapnia,
sedationwithphenoperidin
andflunitrazepam, preventive
treatmentofep-ilepsy
withphenytoin
and of stress ulcer withsucralfate,
andartificialnutrition. Patients
requiring inotropic drugs,
steroids,
or barbiturates or who
developed
asepsis
(elevated
bloodwhite cell count
and/or
feverand/or
bacteremia)
during
thecourse of the
experiment
were excluded from thestudy.
Artificial nutritioninitially
consisted of intravenousglucose
Abbreviations: KIC,
a-ketoisocaproate;
IL-11, interleukin 1,3; IL-6,interleukin6; TNF,tumornecrosisfactor; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase.
STo
whomreprint requests
should be addressed. 2714(1.5
g
per
kg per
day)
followedby
enteralnutrition initiated betweendays
2and 4. Fromday
5 and untilcompletion
of thestudy,
all thepatients
receivedacontinuousnasogastric
feed-ing
at afixed rate of 1500ml/day (Nutrison
E+
Nutricia,
TheNetherlands) together
with 5%(wt/vol)
intravenousglucose
(250
ml/day)
providing
39 ± 2kcalper
kg
per
day
and 1.4+
0.09
g
ofprotein per kg per day
(i.e.,
1.6 kcal and 0.06g
ofprotein
per
kg
per
h).
Study
Protocol.Patientswerestudiedonday
8 after admis-sion.They
receivedaprimed
(6.9
g/mol/kg)
continuous(0.17
+
0.02Ltmol
per
kg
per
min)
infusion ofL-[1-'3C]leucine
for 10 hthrough
acentralvenouscatheter.L-[1-13C]Leucine
(99%
atom
percent
excess)
wasobtained from TracerTechnologies
(Sommerville,
MA)
and tested forapyrogenicity
andsterility
prior
to use.Bloodsamples
formeasurementofplasma
leucine anda-ketoisocaproate
(KIC)
13C
enrichments were takenthrough
anintra-arterialline,
prior
toandat30-min intervalsduring
the last 2 h ofinfusion. At the end of thetracerinfusion,
a30-to60-mg
musclebiopsy
wastaken froma vastuslateralismuscle,
after incision of the skin andaponevrosis using
aWecester-Blake
clamp.
The tissuewasimmediately
frozen inliquid nitrogen
andkept
at -80°C untilanalysis.
Enteral nutrition and other routine treatments were continuedthroughout
thestudy.
In addition to routine intensive caremeasurements,
blood and urinesamples
were also taken to measureplasma
interleukin1/3
(IL-13),
interleukin 6(IL-6),
andtumornecrosis factor(TNF) (days
4 and8),
24-hurinary
3-methylhistidine
and cortisol(days
4 and8),
andurinary
nitrogen
excretion(days
5-7).
Control
subjects
were studied in a similar manner.They
wereinstructed tofollow ameat-freediet,
providing
40 kcalper
kg
per
day
and 1.5g
ofprotein
per
kg
per
day,
for 3days
prior
to thestudy. They
received an identical 10-h tracerinfusion
except
that thetracerinfusionrate waslower(0.10
±0.01
tLmol per
kg
per
min)
and that arteriolized blood wasobtained from a hand vein
placed
in a heated box. Enteral nutritionwasadministeredassmall mealsgiven
every
20min,
to mimiccontinuous
feeding,
andprovided
1.4kcal and 0.06g
ofprotein
per
kg
per
h(equivalent
to 34kcal and 1.4g
ofprotein
per
kg
per
day).
Musclebiopsies
wereperformed
under local anesthesiawith 2%Xylocain
(Astra,
Sweden).
Twenty-four-hour
urinary
3-methylhistidine
excretion was measuredonthe
study day. Nitrogen
balance was notperformed.
Analytical
Methods. Plasma leucine and KIC 13Cenrich-ments were determined
by
gas
chromatography-mass
spec-trometry
as described(15) by monitoring
the ions ofmass/
charge
ratios303/302
and302/301
of thetert-butyldimethyl-silyl
derivatives of leucine andKIC,
respectively. Urinary
nitrogen
wasmeasuredby
pyrochemiluminescence,
3-methyl-histidinewasmeasuredby
liquid chromatography,
cortisolwasmeasured
by
standardRIA,
andcytokines
weremeasuredby
immunoradiometry
withTNF,
IL-1,3,
and IL-6 humanmono-clonal antibodies
(IRMA, Medgenix,
Fleurus,
Belgium).
NorthernBlot
Analysis.
Total RNAwasextracted from the musclebiopsies
asdescribedby
Chomczynski
and Sacchi(16).
Eight micrograms
of total RNA waselectrophoresed
in 1%agarose
gels containing formaldehyde.
RNA waselectro-phoretically
transferred to anylon
membrane(GeneScreen,
NEN)
andcovalently
bound to the membraneby
UV-crosslinking.
Resultsareonly
given
for five of sixpatients
and fivecontrols,
because when allsamples
werespotted
on thesame
membrane,
the sixthpatient
was notyet
included in thestudy.
The membranes werehybridized
with cDNAprobes
encoding
chickenpolyubiquitin (17),
rat 14-kDaubiquitin-conjugating
enzyme
E2(18),
HC2 and HC8 human protea-somesubunits(19),
and humanm-calpain
(20)
andcathepsin
D (21). The
hybridizations
wereperformed
at 65°C with[32P]cDNA
fragments
labeledby
randompriming
asdescribed(9).
Afterwashings
atthe sametemperature,
the filterswereautoradiographed
for 3-48 h at -80°C withintensifying
screens on
Hyperfilm-MP
films(Amersham).
Afterstripping
ofthe differentprobes,
thefilterswerereprobed
withacDNAfragment
encoding
thehousekeeping
gene
glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) (22)
to confirm thatchanges
seen were not due tononspecific changes
in all mRNAsor to unevenloading.
Densitometricsignals
forubiq-uitin,
the 14-kDaE2,
and HC2proteasome
subunit werequantified
by using
digital image
processing
andanalysis
(NIH
IMAGE version
1.43);
values were normalizedby
using
thecorresponding
GAPDH values to correct for variations in RNAloading.
CalculationsandStatistical
Analysis.
Nitrogen
balancewascalculated as the difference between
nitrogen
intake andurinary nitrogen
excretion(there
were nofeces fromdays
5to7).
No correctionwas made for miscellaneous losses.Steady
statesfor leucine and KIC
13C
enrichments andconcentrationswereobtainedoverthe last 2 hofinfusion
(five
timepoints),
asassessed
by
acoefficient of variation <5%andaslope
notsignificantly
different from zero in eachexperiment.
Total leucineflux,
an index ofwhole-body
protein
turnover, wascalculatedasthe ratio of
isotope
infusionrate(corrected
forisotopic
purity)
dividedby
theplasma
[13C]KIC enrichment,
which isrepresentative
of the enrichment of the intracellular leucinepool
(23).
This value includes the tracer infusion.Endogenous
leucine rate ofappearance,
an index ofwholebody
proteolysis,
wasequal
to total leucine flux minus thetracerinfusion and minus the enteral leucine intake. Asimilar calculationwas also done with
[13C]leucine
enrichment.Allthe resultsare
expressed
asthe mean+
SEM.Statisti-cally significant
differencesbetween thepatients
and controlgroups
weredeterminedby
anunpaired
Student'sttestfor allvalues,
except
mRNAlevels,
for whichaMann-Whitney
Utestwasused.
RESULTS
All
patients
wereinnegative nitrogen
balance(-6.9
± 1.6g
ofN
per
day;
range,
-3.3to-12.7g
ofNper
day).
Whole-body
protein
turnover(i.e.,
leucineflux)
andprotein
breakdown(i.e.,
endogenous
leucineproduction)
werehigher
(both,
P<0.01)
inpatients
(3.52
+
0.10and 2.51 ± 0.09,umol
per
kg
per
min)
than incontrols(2.36
± 0.15 and 1.38 ± 0.15jumol
per
kg
per
min),
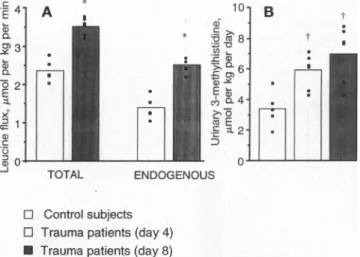
asshowninFig.
1. Similar resultswereobtainedwhen
[13C]leucine
enrichmentsweresubstituted for[13C]KIC
enrichments
(data
notshown),
theKIC/leucine
enrichment4 Q) 3) a a 2-E x 1-, C 0
0_0
10-TOTAL ENDOGENOUS E: 8 '.)-_ e 6 * f ·_ - 2--I'
0) B t Io0l
* E Controlsubjects
l Trauma
patients
(day
4)
* Trauma
patients
(day 8)
FIG. 1.
Whole-body
protein
kinetics inhead-injured
patients
atdays
4and 8 and incontrolsubjects.
Totalleucinefluxandendogenous
leucineflux,measuredby
isotope
dilution,areindexes ofwhole-body
protein
turnoverandbreakdown,respectively. (A)
Total leucine flux andendogenous
leucine flux.(B) Urinary 3-methylhistidine
(myofi-brillarprotein
breakdown).
*,P<0.001; t,P< 0.01,vs.controls.2716 Medical Sciences: Mansoor et al.
ratios
being
0.68 ± 0.03and 0.73 ±0.05 in thepatients
and in thecontrols,
respectively
(P
>0.05).
Urinary 3-methylhistidine
excretionwaselevated in
patients
atdays
4(5.94
± 0.51,umolper
kg
per
day)
and 8(6.98
±0.83,umolper
kg per
day) (both,
P<
0.01)
compared
with controls(3.40
±0.58t,mol
per
kg per
day)
(Fig. 1). Urinary
cortisol excretion was alsomarkedly
elevated atdays
4and 8(272
± 56 and 551 ± 120nmol/day,
respectively) (normal
values for theassay
= 30-100nmol/
day).
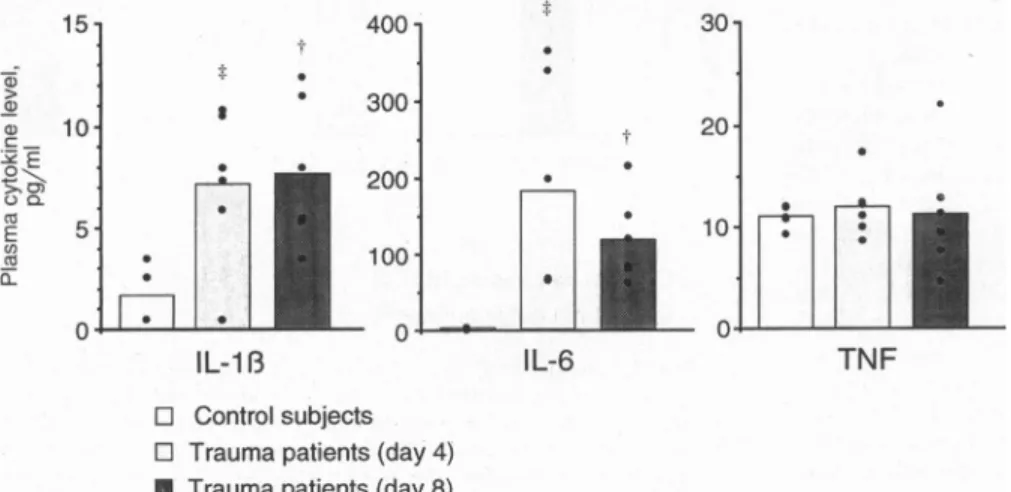
As shown inFig.
2,
plasma
TNF concentration wasunchanged,
whileplasma
IL-1j3,
andparticularly
IL-6,
were elevated inpatients
atday
4or 8(P
<0.02).
Northern blot
analysis
andhybridizations
showed that mRNA levels forcathepsin
D andm-calpain
rose in themuscles from head trauma
patients
compared
tohealthy
volunteers,
suggesting
an activation of bothlysosomal
andCa2+-dependent
proteinases
(Fig.
3).
Thesechanges
occurredwithout
any
significant
variation in GAPDHexpression
(1778
± 276 and 1786±369
arbitrary
densitometric units in controls andpatients,
respectively),
as alsoreported
in other musclewasting
conditions(8,9,30).
Recentobservationsindicatethatthe
ATP-ubiquitin-dependent proteolytic pathway plays
amajor
role in musclewasting
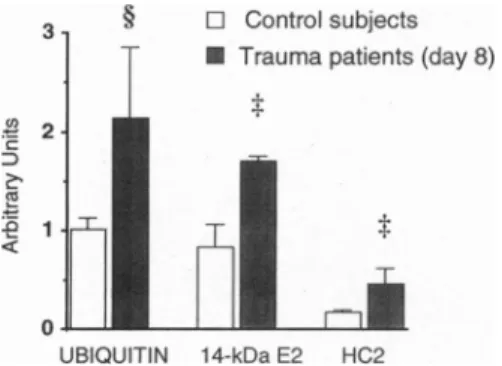
inanimal models(24). Fig.
3also shows that mRNA levels forbothtranscripts
ofubiquitin
were elevated in the muscles from headtraumapatients
(+
110%,
P<
0.05;
Fig.
4).
Sinceubiquitin
has various roles innonpro-teolytic
functions(25-27),
the RNA blots were alsoprobed
with the cDNA of the 14-kDa E2 that functions inE3-dependent ubiquitin conjugation
andprotein
breakdown(18)
andwith cDNAsencoding
the HC2 and HC8 subunits of the 20Sproteasome,
which is theproteolytic
core of the 26Sproteasome
thatdegrades
ubiquitin conjugates
(11).
Wefoundan increased
expression
of these othercomponents
(14-kDa
E2,
+105%,
P<0.02; HC2,
+171%,
P<0.02; HC8,
+113%,
P<
0.05)
oftheATP-ubiquitin-dependent proteolytic pathway
(Figs.
3 and4;
data not shown for the HC8proteasome
subunit).
DISCUSSION
Head-injured patients
exhibitednegative
nitrogen
balances,
enhanced rates of whole
body protein
breakdown,
and asustainedrise in
urinary
3-methylhistidine
excretion,
anindex of musclemyofibrillar protein
breakdown(3).
An increasedexpression
for criticalcomponents
of thelysosomal,
Ca2+-dependent,
andATP-ubiquitin-dependent
proteolytic
pro-cesses in muscle
biopsies
paralleled
theseadaptations,
thus,
strongly suggesting
that musclewasting
resulted from the simultaneous activation of these threeproteolytic pathways.
However,
it islikely
that a reduced rate ofprotein
synthesis
alsocontributedtomusclewasting
inourpatients,
asreported
(28, 29).
15 400 30O>~
,|
* 300 a, 10- 20 E |||
5
aI
200-n
;
03/)~~
10·1
* 100 QL--*
isIL-1
3IL-6
TNF
] Controlsubjects
[ Trauma
patients (day 4)
* Trauma
patients
(day
8)
tle informationis availableonthenatureof
proteo-ns
responsible
formusclewasting
in humans. Tooure,
thelysosomal pathway
is theonly degradative
at has beenreported
toplay
a role in cachexia in Lundholm et al.(12) reported
an involvement ofs in muscle
wasting
in cancerpatients.
IncreasedB and D activities were also
reported
in needleopsies
frompatients
with severe accidental traumalatter observation is
supported by
the increasednof
cathepsin
Dreported
herein.By
contrast,
theherthe
Ca2+-dependent
ortheubiquitin-proteasome
c
process
has neverbeen studied in humanmuscle,
ly
becausemeasuring
theiractivity
requires
large
mples.
Inthepresent
study,
the increasedexpression
ain andproteasome
subunitssuggests
aninvolvement twononlysosomal proteolytic
pathways
inincreasednuscle
protein
breakdown.However,
our observa-not indicate whether thechanges
in mRNA levels creasedtranscription
or alterations in mRNApro-Id
transport
or indegradation
rates. Theprecise
ce of
changes
in mRNA levels forcomponents
ofc
systems
ispresently
unknown,
although they
gen-relate with
changes
in muscleprotein
breakdownby
alternativemethods(e.g.,
invitrotechniques)
oreolytic
activities(for
review,
seeref.24).
dinate stimulation ofthe
ATP-ubiquitin-dependent
cpathway
witheithertheCa2+-dependent
(9)
ortheprocess
(8)
orboth(7)
prevails
in differenttypes
ofasting.
These observationssuggest
that these path-y serve to eliminate different classes of cellular(7).
However,
many observations in animal studiesate that neither the
lysosomal
nor the Ca2+-tproteinases
areresponsible
for the loss ofmyofi-oteins.
(i)
Inhibitors oflysosomal
acidification(e.g.,
line and
chloroquine)
or function(e.g.,
E64 and that inhibit thecysteine proteinases cathepsins
B,
H,
id the
Ca2+-dependent
proteinases)
do not affect listidine releaseby
incubated muscles(5-7). (ii)
Theand the
Ca2+-dependent
proteolytic pathways
doibute
significantly
toincreasedprotein
breakdown in ofsituations characterizedby
enhancedproteolysis
tarvation(8),
acidosis(30),
orcancer(9). (iii)
Evi-activation of eitherlysosomal
orCa2+-dependent
esis
lacking
inmany
instances of musclewasting
(8,
By
contrast,
myofibrillar protein
breakdownrequires
7),
and enhanceddegradation
ofcontractileproteins
ted with increased
expression
ofubiquitin
(6).
Ele-,NAlevels forubiquitin (8,
9, 30,
32,
33),
the 14-kDa24),
and/or
proteasome
subunits(9,
24, 30,
32)
arerved inseveral muscle
wasting
conditions in rodents. nulation ofubiquitin-protein conjugates
was alsoFIG.2. Plasma levels ofcytokinesin
con-trol
subjects
and inhead-injured patients
atdays
4and 8(radioimmunoassay).
t,P<0.01;t,P< 0.02,vs.controls.
Proc.
Natl. Acad. Sci. USA 93
(1996)
Proc. Natl. Acad. Sci. USA 93
(1996)
2717 C HT 2.2 kb : 3.5kb m-calpain C HT ._* 1.3kb GAPDH C HT C HT '_. 2.6kb 1.8kb 1.2kb1l.2kb
...~~~~~~~~~~~~~~~~...
ubiquitin 14-kDa E2 C HTe_
1.35kb HC2FIG.3.
Representative
Northern blots forcathepsin
D,m-calpain,
GAPDH,ubiquitin,
14-kDaE2,and theproteasomeHC2 subunit invastuslateralis muscle
biopsies
fromasingle
headtraumapatient
and controlsubject.
RNAwasisolated andelectrophoresed
through
adenaturing
1%agarose
gel,
transferredtonylon
membranes,andconsecutively hybridized
withthedifferent32P-labeledcDNAs.C,controlvolunteer;
HT,headtrauma
patient.
The size of thetranscript(s)
isgiven
in kb.Thetwo 14-kDa E2transcripts
arise from differentpolyadenylylation
sites(18).
The 1.8-kbtranscript,
whichcorresponds
tothemajor
mRNAspecies
intheratmuscle,isbarely
detectable inhumans.However,the 1.2-kbtranscript
was
mainly
affectedby
headtrauma, asobserved inthe muscles fromfasted(18)
andtumor-bearing
(9)
animals.reported
inthe musclesfromstarved,
denervated,
andtumor-bearing
rats(33,
34).
Furthermore,
proteasome
preparations
may
degrade
contractileproteins
(35,
36),
andthe accumula-tion ofubiquitin-protein
conjugates
occurredprimarily
inthemyofibrillar
fraction(34). (iv)
Itwasrecently
shown that theubiquitin proteolytic pathway,
which isprimarily
believed tocatalyze
the elimination of short-lived and abnormalproteins
(11),
is also involved in thedegradation
of the bulk oflong-lived
cellularproteins
(37).
Thus,
although
our datapreclude
the identification of substrates ofagiven proteolytic
system,
theclearoverexpression
ofmultiple
components
of theubiquitin
proteasome
proteolytic pathway
in musclebiopsies
fromheadtrauma
patients strongly
suggests
that thispathway
is involved in the breakdown of contractileproteins
in humans. The increasedwholebody
andmuscleproteolysis
observed in the headtraumapatients
could be duetoseveral factors.(i)
Ourpatients
had beenimmobilized for 8days
anddisuse isanimportant
factorleading
tomuscleatrophy.
An in vivohumanstudy
demonstrated thatatrophy
of an immobilized musclesolely
results from decreasedprotein synthesis
(38).
By
con-trast,muscle
wasting
seenafter section of the sciaticnerve(7)
oraftersimulatedweightlessness
(39)
inratsresultsprimarily
from increasedprotein
breakdown. It isnoteworthy
that the enhanced breakdown ofproteins
inthese conditions resulted fromthe coordinate activation oflysosomal,
Ca2+-dependent,
andATP-ubiquitin-dependent proteolytic
systems
(7, 39),
as shown here.(ii)
Thehigh circulating
levels of cortisol in ourpatients
mayalso have contributedtoincreasedprotein
break-down. Recent studies have shown thatglucocorticoids
are necessary for increasedexpression
ofubiquitin
in fastedanimals
(8)
orofproteasome
subunitsinacidoticrats(40),
anddexamethasone administration also resulted in increased mRNAlevels for the 14-kDa E2 inratskeletal muscle
(41). (iii)
3 §
C
Controlsubjects
* Trauma
patients
(day 8)
2 D J.
Tt
0 UBIQUITIN 14-kDaE2 HC2FIG. 4. Effectsofheadtraumaonabundance ofmRNAs
encoding
criticalcomponentsofthe
ubiquitin-proteasome proteolytic pathway
fromvastuslateralismuscles. RNAwasisolated andelectrophoresed
through
adenaturing
1%agarosegel,
transferredtonylon
membranes,and
hybridized
with 32P-labeledcDNAsencoding
ubiquitin,
the 14-kDaE2,ortheproteasomeHC2 subunitandGAPDH.Densitometricsignals
werenormalizedby
using
thecorresponding
GAPDH valuesto correctforvariations in RNAloading.
Valuesarethemean + SEM(n
=5).
§,P<0.05; $,P< 0.02,vs.controls.Several
cytokines, namely
TNF,
IL-1/3
(42),
orIL-6(43)
have beenreported
to increaseprotein
breakdown,
in skeletal muscle. Inparticular,
IL-13 isapossible signal
for enhancedATP-ubiquitin-dependent
proteolysis
inmuscle(24),
aswellas IL-6 inmyotubes
(44).
Thus,
thehigh circulating
levels of bothIL-1/
and IL-6 inheadtraumapatients
maycontributetothe activation of theubiquitin proteolytic pathway
in skeletal muscle.(iv)
Nutrition of thepatients
and controls was notexactly
identical;
the controls receivedslightly
(but
notsignif-icantly)
lessenergy(-5
kcal perkg
perday)
andwerefed for ashorterperiod
(10 h)
forpractical
reasons.However,
these minordifferencespresumably
donotaccountfor the dramaticmodificationsobserved.
Although
thepatients
didnotreceivesteroids and
P-agonists,
which are known for their catabolic and anabolicproperties, respectively,
we also cannotexclude that otherdrugs might
have affectedprotein
metabolism.Thus,
therespective
roles ofrest,glucocorticoids,
cytokines,
andother factorsasmediators of thecatabolic muscle response intrauma
require
furtherinvestigation.
In
conclusion,
weshowed thatthe mRNA levels for severalproteinases
or cofactors involved inprotein
breakdown arecoordinately
increased in the muscles fromhead-injured
pa-tients who exhibit enhancedwhole-body
andmyofibrillar
mus-cleprotein
breakdown. These datasuggest
that these mRNA levels in human musclebiopsies,
may be used as sensitive indicators of increasedprotein
breakdown sincecurrently
availabletechniques
formeasuring
in vivo rates ofprotein
breakdown do notprovide
any information aboutspecific
proteinases
that areresponsible
for musclewasting.
To ourknowledge,
ourobservations are also the first tosuggest
aninvolvement of the
ATP-ubiquitin-dependent
proteolytic
pathway
in musclewasting
inhospitalized
humansand,
there-fore,
support
furtherstudy
ofthe roleof thisspecific
proteo-lytic
pathway
inmuscleatrophy
duetoinjury.
We thank Dr.
Keiji
Tanaka(Institute
forEnzyme
research,To-kushima,
Japan)
forproviding
us with the cDNAs of the humanproteasomesubunits,Dr.Simon S.
Wing (McGill University,
Mont-real,
Canada)
for therat14-kDa E2cDNA,Pr. LucCynober
(Facult6
dePharmacie,Clermont-Ferrand)
for the3-methylhistidine
assays, Pr. GenevieveGaillard(Centre
JeanPerrin,ClermontFerrand)
forthecytokine
assays, and the staff members of theLaboratory
ofHuman Nutrition and of the Intensive Care Unit. This workwassupported by
research grants to D.A. and B.B. from the French Ministere de
l'Enseignement Sup6rieur
etde la Rechercheandthe InstitutNational de la RechercheAgronomique.
1. Wolfe,R.R.,
Goodenough,
R.D., Burke,J. F. &Wolfe,M. H.(1983)
Ann.Surg.
197, 163-171.2. Shaw,J. H.F.,Wildbore, M.&Wolfe, R. R.
(1987)
Ann.Surg.
205,228-294.
3.
Young,
V. R. & Munro,H. N.(1978)
Fed. Proc. Fed. Am. Soc.Exp.
Biol.37,2291-2300.4. Barrett, E. J. & Gelfand,R. A.
(1989)
DiabetesMetab. Rev. 5,133-148.
5. Lowell, B.B., Ruderman, N. B. & Goodman, M.N.
(1986)
Biochem. J. 234,237-240.C HT
cathepsinD
2718 Medical Sciences: Mansooretal.
6. Tiao, G.,
Fagan,
J.M., Samuels, N., James, J.H.,Hudson, K., Lieberman, M., Fischer,J. E.&Hasselgren,
P.0.(1994)
J.Clin. Invest. 94, 2255-2264.7. Furuno, K.,
Goodman,
M. N.&Goldberg,
A.L.(1990)
J. Biol. Chem. 265,8550-8557.8.
Wing,
S. S. &Goldberg,
A. L.(1993)
Am. J.Physiol.
264, E668-E676.9.
Temparis,
S.,Asensi, M., Taillandier, D., Aurousseau,E.,Lar-baud, D., Obled, A., B6chet, D., Ferrara, M., Estrela, J. M. &
Attaix,D.
(1994)
Cancer Res. 54,5568-5573. 10. Johnson, P.(1990)
Int. J.Biochem. 22,811-822. 11. Ciechanover,A.(1994)
Cell79, 13-21.12. Lundholm, K.,
Bylund,
A.C., Holm, J. & Schersten, T.(1976)
Eur. J. Cancer12,465-473.13. Guarnieri, G.,
Toigo,
G., Situlin, R., Del Bianco, M. A. &Crapesi,
L.(1988)
in Proteases II: PotentialRole inHealth andDisease,eds.Horl,W. H. &Heidland,A.
(Plenum,
NewYork),
pp. 243-256.14. Clifton, G.L., Robertson, C.S. & Grossman, R.G.
(1984)
J.Neurosurg.
60,687-696.15.
Collin-Vidal,
C.,Cayol,
M.,Obled,
C.,Ziegler,
F., Bommelaer,G.&
Beaufrere,
B.(1994)
Am.J.Physiol.
267,E907-E914. 16.Chomczynski,
P. &Sacchi,N.(1987)
Anal. Biochem. 162,156-159.
17.
Agell,
N.,Bond,U. &Schlesinger,
M. J.(1988)
Proc.Natl.Acad. Sci. USA85,
3693-3697.18.
Wing,
S. S. &Banville,D.(1994)
Am.J.Physiol.
267,E39-E48. 19. Tamura,T., Lee,D.H.,Osaka, F.,Fujiwara,
T.,Shin, S.,Chung,
C.H.,Tanaka,K. &Ichihara,A.
(1991)
Biochim.Biophys.
Acta1089,95-102.
20.
Imajoh,
S.,Aoki, K., Ohno, S., Emori, Y., Kawasaki, M.,Sugi-hara,H.&Suzuki, K.
(1988) Biochemistry
27,8122-8128. 21. Faust,P.L., Kornfeld,S. &Chirgwin,
J. M.(1985)
Proc. Natl.Acad. Sci. USA 82,4910-4914.
22. Fort, P.,
Marty,
L.,Piechaczyk,
M., ElSabrouty,
S., Dani, C.,Jeanteur, P. & Blanchard, J. M.
(1985)
Nucleic AcidsRes. 13,1431-1442.
23. Schwenk,W.F.,Beaufrere,B.&
Haymond,
M. W.(1985)Am.
J.Physiol.
249,E646-E650.24. Attaix, D., Taillandier, D.,
Temparis,
S., Larbaud, D.,Aurous-seau,E.,
Combaret,
L.&Voisin,
L.(1994) Reprod.
Nutr.Dev.34,583-597.
25. Jentsch, S., McGrath, J. P. &
Varshavsky,
A.(1987)
Nature(London)
329, 131-134.26. Glotzer, M.,
Murray,
A. W.& Kirschner, M. W.(1991)
Nature(London)
349,132-138.27. St.John, T.,
Gallatin,
W.M.,Siegelman,
M.,Smith,
H.T.,Fried,
V.A. &Weissman, I.L.(1986)
Science231,
845-850. 28. Rennie,M.J.(1985)
Br.Med. Bull. 41,257-264. 29. Rennie,M.J. &Harrison,R.(1984)
Lanceti,323-325. 30. Mitch, W.E., Medina, R., Grieber, S.,May,
R.C.,England,
B.K., Price,S.R.,
Bailey,
J. L. &Goldberg,
A. L.(1994)
J.Clin. Invest.93,
2127-2133.31. Ilian,M. A.&
Forsberg,
N. E.(1992)
Biochem.J.287,
163-171. 32. Medina, R.,Wing,
S.S.&Goldberg,
A. L.(1995)Biochem.
J.307,
631-637.
33. Llovera, M., Garcia-Martinez, C.,
Agell,
N., Marzabal, M.,Lopez-Soriano,
F. J. &Argiles,
J. M.(1994)
FEBS Lett. 338,311-318.
34.
Wing,
S.S., Haas,A. L.&Goldberg,
A. L.(1995)
Biochem.J.307,639-645.
35.
Mykles,
D.L. &Haire,M. F.(1991)Arch.
Biochem.Biophys.
288,543-551.
36.
Taylor,
R.G.,Tassy,
C., Briand, M., Robert, N., Briand, Y. &Ouali,A.
(1995)
Mol. Biol.Rep.
21,71-73.37. Rock,K.L.,Gramm,C., Rothstein, L.,
Clark,
K.,Stein,
R.,Dick, L.,Hwang,
D.&Goldberg,
A.L.(1994)
Cell78, 761-771. 38. Gibson, J. N.A.,Halliday,
D., Morrison,W.L., Stoward, P.J.,Hornsby,
G.A., Watt, P.W., Murdoch, G. &Rennie,
M. J.(1987)
Clin. Sci.72,
503-509.39. Taillandier, D., Aurousseau, E.,
Meynial-Denis,
D.,Bechet, D., Ferrara, M.,Cottin, P.,Ducastaing,
A.,Bigard,
X.,Guezennec,C.Y.,Schmid,H.-P. &Attaix,D.
(1996)
Biochem.J., inpress.
40. Price, S.R.,England,
B.K.,Bailey,
J.L., VanVreede,
K. &Mitch,W.E.
(1994)
Am.J.Physiol.
267,C955-C960.41. Dardevet, D., Sornet, C.,Taillandier, D.,
Savary,
I.,Attaix, D., Grizard,J.(1995)
J. Clin.Invest.96,2113-2119.42. Flores,E.A.,
Bistrian,
B.R.,Pomposelli,
J.J.,Dinarello,C.A., Blackburn, G.L. & Istfan, N. W.(1989)
J. Clin. Invest. 83,1614-1622.
43.
Tsujinaka,
T.,Ebisui, C.,Fujita,
J.,Kishibuchi,
M.,Morimoto, T.,Ogawa,
A.,Katsume, A.,Ohsugi,
Y.,Kominami,E.&Monden,M.
(1995)
Biochem.Biophys.
Res. Commun. 207,168-174. 44. Ebisui,C.,Tsujinaka,
T.,Morimoto, T., Kan, K.,Iijima,
S., Yano,M.,Kominami, E., Tanaka,K.&Monden,M.
(1995)
Clin.Sci.89,431-439.