Discovery and characterization of stable introns in yeast by
Jeffrey T. Morgan B.S., Biochemistry (2011)
University of Michigan
SUBMITTED TO THE DEPARTMENT OF BIOLOGY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF
DOCTOR OF PHILOSOPHY AT THE
MASSACHUSETTS INSTITUTE OF TECHNOLOGY SEPTEMBER 2018
c 2018 Massachusetts Institute of Technology All rights reserved
Signature redacted
Signature of Author: Jeffrey T. Morgan Department of Biology August 2, 2018Signature redacted
Certified by: David P. Bartel Professor of Biology Thesis SupervisorSignature redacted
Accepted by: Amy E. KeatingMASSACHUSETTS INSTITUTE Professor of Biology
OF TECHNOLOGY Co-Chair, Biology Graduate Committee
Discovery and characterization of stable introns in yeast by
Jeffrey T. Morgan
Submitted to the Department of Biology on August 2, 2018
In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Abstract
Spliceosomal introns are a defining feature of eukaryotes; they are present in all known
eukaryotic genomes, absent from all known non-eukaryotic genomes, and their accurate removal is essential for mRNA maturation. Although smaller ncRNAs can be processed from introns, the introns themselves are considered biologically inert byproducts of splicing; their collective fate post-splicing is to be de-branched and rapidly degraded.
This dissertation details the first described instance of a regulated fate and function for excised and de-branched introns in eukaryotes. We observed a set of introns in the budding yeast
Saccharomyces cerevisiae's transcriptome that, although rapidly degraded during log-phase
growth as expected, accumulate as linear RNAs under saturated-growth conditions and during inhibition of TORC 1, a key integrator of growth signaling. At least 34 introns-1 1% of the introns in S. cerevisiae-show this change in stability. We find no evidence that this stability can be attributed to intron retention in the mature transcript. Instead, introns that become stabilized remain associated with components of the spliceosome post-splicing, likely resulting in their protection from degradation. Compared to other yeast introns, these stable introns have no enriched sequence motifs but do share a short distance between their lariat branch point and the 3' splice site. Indeed, by manipulating this distance, we are able to show a causal relationship between branch-point position and stable-intron formation.
To test for cellular functions of stable introns, we created strains with precise intron deletions. We created 20 strains with combinations of up to five introns deleted, with the quintuple mutant eliminating >60% of the stable-intron molecules in the transcriptome. When these strains are challenged with the TORC I inhibitor rapamycin, their growth exceeds that of the parental strain, with a striking relationship (R2 = 0.9) between the fraction of SI molecules removed from the
transcriptome and the rate of growth under TORC 1 inhibition. Overexpression of native or engineered stable introns suppresses this aberrant rapamycin response. These results indicate that stable introns function within the TOR-mediated growth-signaling pathway of S. cerevisiae, and more broadly, excised introns can be stabilized and coopted to perform biological functions in eukaryotic cells.
Thesis Advisor: David P. Bartel Title: Professor
Acknowledgements
This work was possible because of the mentorship and trust of my advisor Dave Bartel. Dave has an endless ability to think critically and rigorously about the diverse projects in the lab. He taught me how to ask the right questions, how to conclusively answer them, when to focus on one experiment until you crack it, and when to seek outside input on a problem. He also tried to teach me a great deal about very specific aspects of grammar; I have assuredly erred in these aspects in the writing to follow. I was fortunate to land with great mentors since I started cold e-mailing Pls before my sophomore year of college: Steve Ragsdale and Li Yi at the University of Michigan, and Richard Leapman and Alioscka Sousa at the NIH. I did not fully appreciate them at the time, but I would not have ended up at MIT without their support and example.
I am grateful to many faculty members (and one fellow) of the MIT and Whitehead communities
for their insight over the years: Chris Burge, Gerry Fink, Dennis Kim, David Pincus, David Sabatini, and Phil Sharp. Gerry and Phil have been instrumental in the success of this project since its inception and have been constant sources of guidance during thesis committee meetings.
I additionally thank Gerry for his advice as I looked for postdocs, convincing me success lies
foremost in looking where others aren't already looking. I thank members of the Fink lab for input and discussion on my work during Fink group meetings, and a special additional thanks to David Pincus for a great deal of input and suggestions over the past few years.
I knew effectively nothing about what the Bartel lab studies when I joined. Because of the
creative and generous members of the lab, that quickly changed. I have overlapped with many amazing scientists in the lab: Weinberg, Igor, Vincent (during his brief return), Alex, Olivia, DK, Vikram, Sue-Jean, Katrin, Stephen, Junjie, Grace, Asia, Ben, Wenwen, Coffee, Jamie, Xuebing, Namita, Dan, Jarrett, Matt, Sean, Tim, Charlie, Danny, Kathy, Elena, Justin, Glenn, Thy. and
Emir. I am additionally grateful to Laura, the lab's administrative manager, for processing my
657 (and counting) orders, and for being an endless source of positivity in the lab. I must point to a few lab members in particular: Stephen for advice-both big- and small-picture; Alex for being an endless fount of timeworn impressions and timeless experimental designs; Olivia for guidance when I was starting out in the lab, and for the brief period when I was super into the Tour de France; Grace for always having the big soccer matches streaming on her computer for quick check-ins; Thy for many unsolicited pictures of her cat; and Sean for bringing his light-hearted presence, thoughtful opinions, and so much of the cafeteria's flatware to our bay.
I still believe a basic tenet of MIT Biology's recruitment pitch: it is special because of the focus
on one's cohort. Mine contained many amazing people. I especially want to thank Eric, Erik, Ian, Julie, Kevin, and Sahin for their friendship and support.
Finally, I thank my parents for supporting me, my education, and for pushing my siblings and I to follow our interests-even if those interests led us far from home. Many aren't lucky enough to do something they enjoy for a living, and I don't take that for granted. I thank my partner, Zo , for her support, putting up with my inability to accurately predict the time needed to finish up in lab, her love of Jeopardy! and national parks, making sure I eat decent food, and generally for making all aspects of my life much richer. Last but not least, I would like to thank our cat, Ollie, for being a good boy.
Table of Contents
A bstract... 3
A cknow ledgem nents ... 5
Ta ble of C ontents ... 7
C hapter 1. Introduction ... 9
Part 1. Pre-m R N A processing... 11
Intron recognition... 1 1 Chem istry of splicing ... 13
Figure 1. Tw o-step m echanism of pre-m RN A splicing... 14
The spliceosom e: a dynam ic ribonucleoprotein m achine... 15
Figure 2. Schematic view of the spliceosome cycle in S. cerevisiae... 18
Spliceosom e disassem bly and lariat-intron degradation... 19
Part 2. Introns qua introns... 21
Evolution of introns ... 21
Loss and gain of introns... 23
Figure 3. Intron density of eukaryotes ... 24
Function of introns in alternative splicing ... 25
Other functions of introns in m odern eukaryotes ... 26
N otable post-splicing fates of intact introns ... 28
Part 3. S. cerevisiae outside of log-phase grow th... 29
G row th phases... 30
Figure 4. G rowth phases of S. cerevisiae... 31
References... 34
Chapter 2. Excised linear introns regulate growth in yeast... 51
C hapter 3. Future D irections... 105
Chapter 1. Introduction
Jacques Monod's oft-cited axiom', "anything found to be true of E. coli must also be true of elephants," (Monod and Jacob, 1961) speaks to the long-discussed concept of biochemical unity of life on Earth: although organismal scale and complexity varies, the same elementary molecules and reactions underlie and unify all living things (Rubner, 1909; Kluyver and Donker, 1926; Friedmann, 2004). As science moves into the age of molecular biology, this unity could be
investigated in higher-order reactions. Seminal studies of the order and logic for transfer of genetic information from DNA to RNA to protein (Avery et al., 1944; Boivin and Vendrely,
1947; Watson and Crick, 1953; Mazia, 1956; Crick, 1958) found they operate, in a general sense, in bacteria as they do in eukaryotes. Importantly, analogous machinery (DNA-dependent RNA polymerase and messenger RNA-dependent ribosome) was identified as performing these operations in both bacteria and animals (Weiss and Gladstone, 1959; Hurwitz et al., 1960; Stevens, 1960; Brenner et al., 1961; Gros et al., 1961; Gierer, 1963; Warner et al., 1963; Wettstein et al., 1963). Although features of the 5' and 3' ends of eukaryotic messenger RNAs (mRNAs) indicated that there might be complexity of transcription and pre-mRNA processing not found in bacteria (Darnell et al., 1973; Brawerman, 1976; Shatkin, 1976), biochemical unity seemed as if it might apply to molecular biology: the details would differ, but the same elemental processes of gene expression would be shared by E. coli and elephants.
With this backdrop landed the discovery of mRNA splicing disrupted the notion of a unified molecular biology more than anything before it. First observed in adenovirus type 2 mRNAs produced late in the virus's infection cycle (Berget et al., 1977; Chow et al., 1977), this discovery revealed that the organization of genes in eukaryotes was profoundly different than that in bacteria. During the transfer of genetic information from DNA to mRNA, only select,
physically disconnected pieces ("exons") of a pre-mRNA are spliced together to form the mature mRNA while intervening pieces ("introns") are discarded. What was initially found for these viral mRNAs was shown to be characteristic of a diversity of eukaryotic organisms and mRNAs (Brack and Tonegawa, 1977; Breathnach et al., 1977; Jeffreys and Flavell, 1977; Gilmore-Hebert and Wall, 1978; Tilghman et al., 1978; Tonegawa et al., 1978; Weinstock et al., 1978).
Since the discovery of mRNA splicing 41 years ago, it has become clear that
spliceosomal introns (and the spliceosomal machinery to remove them) are a defining feature of eukaryotes: they are present in all known eukaryotic genomes and absent from all known non-eukaryotic genomes (Koonin, 2006; Irimia and Roy, 2014). Although a great deal has been
learned subsequently about the sequence determinants of splicing, the chemistry of splicing, the spliceosome, alternative splicing, and more, much is left to be discovered about this critical and ubiquitous step in eukaryotic gene expression. This dissertation describes the discovery,
characterization, and regulation of stable introns in the budding yeastS. cerevisiae. Although introns are generally viewed as inert and ephemeral by-products of splicing, the findings from this study add an unexpected dimension to possible fates and functions of spliceosomal introns within eukaryotic biology. Further, this dissertation emphasizes the benefit of considering
myriad environments relevant to the survival and evolution of a given species as opposed to focusing on only the most experimentally standardized and tractable conditions suitable for a laboratory. Our understanding of fundamental biological processes will remain incomplete if not considered in this light. This chapter introduces knowledge that contextualizes the research advances found in the rest of the dissertation. Therefore, the focus is on eukaryotic biology with a further emphasis on S. cerevisiae biology where appropriate2.
2 For instance, there is no discussion of the minor spliceosome, which is not extant in S. cerevisiae (Mewes et al.,
Part 1. Pre-mRNA processing
In eukaryotes, mRNA maturation requires three major steps. Upon the onset of RNA Polymerase 11 transcription, a 7-methylguanosine cap3 is added to the 5' end, introns (if present) are removed by splicing, and after transcription is complete, a string of adenosines are added to the 3' end (Proudfoot et al., 2002). Transcription termination and freeing of an mRNAs 3' end for polyadenylation involves sequence elements in the nascent RNA transcript recognized by trans-acting cleavage and polyadenylation factors. The stringency and specificity of this cleavage position varies greatly by organism and can be regulated to produce multiple mRNA isofonns through alternative polyadenylation (Proudfoot, 2011; Tian and Manley, 2013). These 5' and 3' modifications function to imbue stability and translational capacity to an mRNA during its life. Splicing, instead, is required to create an mRNA whose translation will generate the intended protein. Fidelity is absolutely vital; a skipped intron or inaccurate splice-site selection will result in aberrant protein products. This precision must be maintained despite the fact that intron lengths can vary by three orders of magnitude in a single organism (Garber et al., 1983; Hawkin,
1988; Michael and Manyuan, 1999). This section discusses in detail the recognition of an intron
within pre-mRNA, the chemistry of splicing, the machinery necessary to perform splicing, and the assembly and disassembly of this machinery throughout the splicing cycle.
Intron recognition
Only three sequences of a spliceosomal intron are universally required for its definition and recognition by the spliceosome: the 5' splice site (5'SS), the 3' splice site (3'SS), and the lariat branch point (BP) (Figure 1). Each of these three sequences has its own consensus motif
3 A noted exception: the nematode C. elegans, where most messages receive 2,2,7-trimethylguanosine caps through
trans-splicing of short splice-leader sequences (Blumenthal and Steward, 1997). Similar mechanisms are also present in less well-studied organisms (Hastings, 2005).
4 A noted exception: replication-dependent histones, which terminate in short stem-loops after endonucleolytic cleavage (Dominski and Marzluff, 2007).
generally shared between all spliceosomal introns, suggesting a single evolutionary origin
(discussed below). These sequences vary greatly in the strength of their conservation, being more strongly conserved in yeast and more weakly conserved in mammals. In yeast, the 5'SS hexamer is GUAYGU (Y being either pyrimidine ribonucleotide), the BP heptamer is UACUAAC
(branch point adenosine is underlined), and the 3'SS trimer is YAG (Langford and Gallwitz, 1983; Pikielny et al., 1983; Langford et al., 1984; Teem et al., 1984; Spingola et al., 1999; Davis et al., 2000). There are some additional features that aid in intron processing in yeast, such as a
pyrimidine-rich tract upstream of the 3'SS and an adequate distance between 5'SS and BP (Thompson-Juger and Domdey, 1987; Patterson and Guthrie, 1991). However, presence of the three motifs above is generally sufficient for splicing to occur.
In addition to consensus sequences being much more degenerate in mammals than yeast, a second fundamental difference in intron recognition between these eukaryotes is that
mammalian introns are often much longer than mammalian exons, whereas the inverse is true in yeast. Therefore, mammalian intron definition is in actuality often driven by the definition of
flanking exons (Robberson et al., 1990; Nakai and Sakamoto, 1994; Berget, 1995; Sterner et al., 1996). Myriad cis-regulatory elements are present in both exons and introns to promote
recruitment of spliceosome components to legitimate splice sites, which would be otherwise difficult to find using an intron-centric mechanism due to the degeneracy of splice-site sequences and widely varied intron lengths in mammals (Schaal and Maniatis, 1999; Fairbrother and
Chasin, 2000; Sun and Chasin, 2000; Fairbrother et al., 2002). The basic mechanism by which exon definition occurs is through initial recruitment of spliceosome components (U 1 snRNP
[small nuclear ribonucleo protein]) to the 5'SS of one intron, which in turn promotes recognition
5 Weak conservation could imply coupling between a given intron's motifs, i.e. intron-specific requirements for
particular 5'SS-BP-3'SS combinations. However, hybrid introns containing 5' and 3' splice junctions from separate genes can be processed as if endogenous introns (Chu and Sharp, 1981).
of the upstream intron's 3'SS by a distinct set of spliceosome components (U2 auxiliary factor [U2AF]) (Hoffman and Grabowski, 1992). In practice, this process is much more complex, being especially sensitive to intron and exon lengths, as well as which cis-regulatory elements are being actively utilized in a given cell at a given time at a given locus (Sterner et al., 1996;
Fox-Walsh et al., 2005). This regulation is central to alternative splicing, the prevalence and importance of which is discussed below.
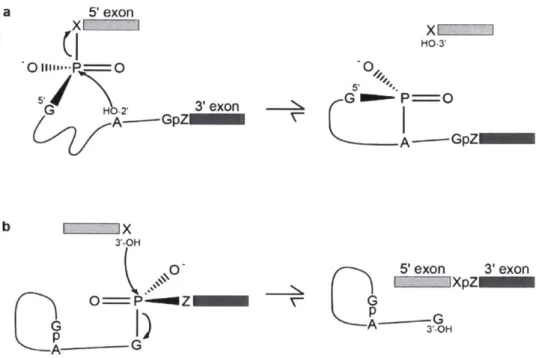
Chemistry of splicing
The fundamental sequence determinants of introns were by-and-large elucidated based on early in vivo experimentation. However, development of a splicing-competent in vitro system-as well system-as a system to produce sufficient quantities of desired, splicing-competent pre-mRNA substrates (Butler and Chamberlin, 1982; Kassavetis et al., 1982; Green et al., 1983; Melton et al., 1984)-was necessary for understanding how a splicing reaction proceeds from start to finish, and what steps in the reaction are blocked by particular mutations.
Splicing-competent extracts from HeLa cells (Hernandez and Keller, 1983; Hardy et al., 1984; Krainer et al., 1984) and yeast (Lin et al., 1985) did indeed enable rapid illumination of how a pre-mRNA is spliced. Splicing consists of two sequential transesterification reactions (Figure 1). In the first step, the 2'-OH of the branch point adenosine attacks the 5'SS, which ligates the 5'SS to the BP through a 2'-5' phosphodiester linkage6, and frees the 3'-OH on the 5'
exon (Figure 1 a). In the second step, the free 3'-OH on the 5' exon attacks the 3'SS, which ligates the two exons together, and releases the intron as a lariat with a free 3'-OH (Figure 1 b)
(Grabowski et al., 1984; Krainer et al., 1984; Padgett et al., 1984; Ruskin et al., 1984). The two
6 This peculiar branch structure (a nucleotide with both a 2'-5' and 3-5'
phosphodiester linkage) had been previously identified in bulk nuclear RNA (Wallace and Edmonds, 1983).
reactions are each SN2, and are not catalyzed as simply forward and reverse reactions in a single active site (Moore and Sharp, 1993). Intriguingly, both spliceosomal introns and group II self-splicing introns-a more ancient type of highly structured intron found in all three domains of life-were noted to proceed through the same intermediates (Sharp, 1985; Cech, 1986), and more recently have been shown to act through a common mechanism of RNA-mediated
positioning of divalent metals at catalytic sites (Sontheimer et al., 1997; Gordon et al., 2000; Fica et al., 2013). The potential of group I-like elements as the progenitors of spliceosomal introns is discussed below. a 5' exon HO-3' 54/
G n
G G 3'exonP=O A---GpZGpZ b X 3'-OH 0- 5'exon 3'exon XpZ pp AC GD )A-3OH
Figure 1. Two-step mechanism of pre-mRNA splicing.
a, First step of spliceosomal splicing, resulting in the 5'SS guanosine (G) ligated to the BP adenosine (A) through a 2'-5' phosphodiester linkage and a free 3'-OH on the 5' exon. b, Second step of spliceosomal splicing, resulting in the 5' exon ligated to the 3' exon and a lariat intron with a free 3'-OH. X and Z indicate the most 3' nucleotide of the 5' exon and the most 5' nucleotide of the 3' exon, respectively. (Modified from Moore et al., 1993).
The spliceosome: a dynamic ribonucleoprotein machine
Although the chemistry of splicing is relatively simple, the spliceosome is complex. It is a ribonucleoprotein complex made up of five small RNAs and approximately 100 proteins7. These components must assemble on each intron de novo and undergo a series of
ATP-dependent rearrangements to orient the requisite portions of intronic RNA such that the reactions described above can occur (Figure 2). During these rearrangements, individual proteins and multipartite snRNPs must dock and undock from the spliceosome in a specific order to drive the correct reaction to completion. Once complete, the spliceosome must release its two products-joined exons and lariat intron-and be disassembled so that its components may once again
assemble de novo on and catalyze removal of other introns.
Five snRNPs (U 1, U2, U4, U5 and U6 snRNPs), named after their respective small nuclear RNA (snRNA), are the major building blocks of the spliceosome8 (Brody and Abelson, 1985; Frendewey and Keller, 1985; Grabowski et al., 1985; Grabowski and Sharp. 1986). The discovery of snRNAs predates the discovery of splicing, although at the time these RNAs were only recognized for their distinguishing characteristics as abundant, stable, small, and nuclear
RNAs (Muramatsu and Busch, 1965; Hodnett and Busch, 1968; Weinberg and Penman, 1968; Weinberg and Penman, 1969; Ro-Choi and Busch, 1974; Zieve and Penman, 1976; Hellung-Larsen and Frederiksen, 1977). It was subsequently found that antibodies produced by
individuals with the autoimmune disease lupus react with snRNA-containing ribonucleoprotein complexes, allowing their immunoprecipitation and study (Mattioli and Reichlin, 1971;
Northway and Tan, 1972; Lerner and Steitz, 1979). These antibodies recognize similarly sized snRNPs in eukaryotes ranging from humans to fall armyworms (Lerner et al., 1980). These
7 The number is closer to 200 proteins in humans.
findings were extended to yeast by a concerted effort largely on the part of Christine Guthrie's lab because evolutionary distance precluded the use of human-derived antibodies to detect snRNPs, and minimal sequence conservation made 1:1 relationships with mammalian snRNA counterparts difficult to establish (Tollervey et al., 1983; Wise et al., 1983; Parker and Guthrie, 1985; Ares Jr, 1986; Kretzner et al., 1987; Parker et al., 1987; Patterson and Guthrie, 1987; Siliciano et al., 1987; Brow and Guthrie, 1988; Shuster and Guthrie, 1990)9.
The connection between snRNAs and splicing was initially proposed based on sequence complementarity between the very 5' end of U 1 and the 5'SS consensus sequence in mammalian introns (Lerner et al., 1980; Rogers and Wall, 1980)10. Experimental evidence followed showing immunodepletion of snRNPs, snRNAs, and RNase H-mediated removal of U I's 5' end are all sufficient to inhibit splicing (Padgett et al., 1983; Krimer et al., 1984; Rinke et al., 1984).
Similar depletion studies targeted at U2, U4, and U6 showed the essentiality of these components for splicing and spliceosome formation"1 (Kramer et al., 1984; Black et al., 1985; Chabot et al., 1985; Berget and Robberson, 1986; Black and Steitz, 1986).
Spliceosome function can be broken down into three major steps: recognition and assembly, catalysis, and release and disassembly'2. As discussed above, the U I snRNP first
recognizes the 5'SS (E Complex); this is mediated by direct RNA-RNA interactions between U I and the intron, as demonstrated genetically through compensatory base changes (Zhuang and Weiner, 1986; Seraphin et al., 1988; Siliciano and Guthrie, 1988). Similarly, the U2 snRNP is recruited via U2-mediated RNA-RNA recognition of the BP (Parker et al., 1987; Wu and
9 The interested reader should see (Guthrie, 2010) for a more complete story of this effort.
0 Initially, a "cross-over" model was proposed, where U I would pair with both the 5'SS
and 3'SS to align the two ends of an intron. However, while the 5'SS-complimentary sequence of UI is highly conserved, the 3'SS-complimentary sequence is not (Mount and Steitz, 1981).
" U5 is largely resistant to RNase H-mediated cleavage (Black and Pinto, 1989).
2 The disassembly step-as it most directly relates to intron stability and the fate of introns post-splicing-is
Manley, 1989; Zhuang and Weiner, 1989). U I recruitment is not dependent on ATP, while U2 recruitment is both dependent on ATP and the presence of U 1 (Bindereif and Green, 1987; Ruby and Abelson, 1988; Seraphin and Rosbash, 1989; O'Day et al., 1996)'1.4. This complex (A complex) is next joined by the pre-associated tri-snRNP of U4/U5/U6 (Bringmann et al., 1984; Hashimoto and Steitz, 1984; Bindereif and Green, 1987; Cheng and Abelson, 1987; Konarska and Sharp, 1987; Behrens and Lfihrmann, 1991; Stevens and Abelson, 1999). The ATP-dependent helicase activity of Prp28 disrupts U I-5'SS base pairing, resulting in U 1 snRNP eviction (B complex) (Staley and Guthrie, 1999). The final pre-catalytic step requires ATP-dependent helicase Brr2 to unwind U4-U6 pairing, which leads to dissociation of U4 and allows
U6 to make catalytically relevant interactions with U2 (Konarska and Sharp, 1987; Brow and
Guthrie, 1988; Lamond et al., 1988; Madhani and Guthrie, 1992; Sun and Manley, 1995;
Laggerbauer et al., 1998; Raghunathan and Guthrie, 1998). The catalytically active complex (B"' complex) is further stabilized by the NineTeen Complex (Ohi and Gould, 2002; Chan et al.,
2003; Chan and Cheng, 2005).
" In mammalian studies, U2 recruitment did not depend on an intact 5'SS (Ruskin and Green, 1985b). However, U I-then-U2 has been found to be the order of operations on intact introns (Bindereif and Green, 1987).
1 In most eukaryotes, U2 recruitment to the BP is preceded by U2AF association with the 3'SS and upstream pyrimidine-rich tract (Ruskin et al., 1988; Wu et al., 1999). However, this is not the case in S. cerevisiae. The
difference likely lies in the relative stringency of its BP sequence (UACUAAC in S. cerevisiae compared to YURAY [Y being a pyrimidine and R being a purine ribonucleotide] in humans) and inability for mammalian U2 snRNP to locate a degenerate BP motif without additional context clues.
'SS BP 3SS
EGUAUGU UACUAAC- YAG
F"
,-- ' \exonucleases Dbrl Prp43 +NTP -- I Prp ,- +ATP ,-' A A us 'us ~(L~j ~ pre-B Prp28 *ATP ligated exons Prp22 +ATP C* Pr Prp 6 +ATP Brr2 +ATP Bact /Prp2 +ATPFigure 2. Schematic view of the spliceosome cycle in S. cerevisiae.
Pre-mRNA (top) enters the splicing reaction, which produces ligated mRNA (left) and lariat intron (top left) as products. The snRNP particles (U 1, U2, U4, U5, U6) assemble on the pre-mRNA in an ordered manner. Solid arrows indicate the paths of the pre-mRNA and intron products. Dotted arrows indicate the paths of recycled snRNPs. Spliceosome assembly and fidelitous catalysis of the splicing cycle requires 5' splice-site, branch-point, and 3' splice-site sequences in the intron, as indicated on the pre-mRNA as 5'SS, BP, and 3'SS, respectively. The branch-point
adenosine is additionally indicated (red). For simplicity, the NineTeen Complex has been omitted. (Modified from Ruby and Abelson, 1991; Moore et al., 1993).
E
B
Catalysis occurs while retaining the remaining snRNAs (U2, U5, and U6) throughout the process. First, B is converted to the transient B* complex through juxtaposition of the 5'SS and the BP for branching, which then catalyzes step I of splicing (Warkocki et al., 2009). After step I
is complete (C complex), another ATP-dependent remodeling enables docking of the 3'SS into the active site (Schwer and Guthrie, 1992; James et al., 2002; Tseng et al., 2011). The 5' and 3' exons are aligned by the U5, and the resulting C* complex performs step II of splicing, joining the exons (Newman and Norman, 1992; Sontheimer and Steitz, 1993). At this stage, the P-complex spliceosome still retains its two products: joined exons and lariat intron. The mRNA is released through rearrangements driven by the Prp22 helicase (Arenas and Abelson, 1991; Schwer and Gross, 1998; Schwer, 2008). Release of the lariat intron is discussed below.
More than 30 years of genetic and biochemical study of spliceosome function led to the model described here. Recently, this model can be further refined in the light of high-resolution, stage-specific cryo-EM structures of both human (Agafonov et al., 2016; Bertram et al., 2017a; Bertram et al., 2017b; Zhang et al., 2017; Haselbach et al., 2018; Zhan et al., 2018) and yeast (Nguyen et al., 2015; Yan et al., 2015; Galej et al., 2016; Nguyen et al., 2016; Rauhut et al., 2016; Wan et al., 2016a; Wan et al., 2016b; Yan et al., 2016a, b; Bai et al., 2017; Fica et al., 2017; Li et al., 2017; Liu et al., 2017; Plaschka et al., 2017; Wan et al., 2017; Wilkinson et al., 2017) spliceosomes. These data will shape the future of research on this dynamic machine.
Spliceosome disassembly and lariat-intron degradation
Each newly assembled spliceosome is a single-turnover enzyme. Of course, a spliceosome that can only catalyze one splicing reaction is of little use for a cell. Just as the spliceosome undergoes dynamic changes during the splicing cycle, it must undergo a final set of
changes in order to dismantle its final form-the intron-lariat spliceosome (ILS)-into constituent parts. The DEAH-box NTPase Prp43 along with associated factors Ntrl and Ntr2 controls ILS disassembly (Figure 2) (Arenas and Abelson, 1997; Martin et al., 2002; Tsai et al., 2005; Boon et al., 2006; Pandit et al., 2006; Tsai et al., 2007; Fourmann et al., 2013). In vitro, incubation of purified ILS complexes with Prp43, Ntrl, Ntr2, and any NTP is sufficient to generate defined dissociation products: the intron-lariat, U6 snRNA, U2 snRNP containing SF3a/b, U5 snRNP, and the NTC (Fourmann et al., 2013). Some studies also implicate Brr2 and its associated GTPase Snul 14 in ILS disassembly (Small et al., 2006; Tsai et al., 2007; Hahn and Beggs, 2010), but their activities are not required for in vitro disassembly, as any NTP-not just ATP and/or GTP-supports disassembly (Fourmann et al., 2013). Abortive splicing events (such as spliceosomes stalled on suboptimal pre-mRNA substrates) are also rescued by this set of factors (Koodathingal et al., 2010; Mayas et al., 2010; Semlow and Staley, 2012).
Introns are generally degraded very rapidly after the completion of splicing (Ruskin and Green, 1985a; Arenas and Hurwitz, 1987; Sharp et al., 1987; Chapman and Boeke, 1991). In order for the intron lariat to be degraded, it must be debranched by the debranchase Dbrl
(Chapman and Boeke, 1991; Khalid et al., 2005). To be debranched an intron lariat must first be released from the ILS, indicating that ILS disassembly is required for intron degradation (Martin et al., 2002)". Without debranchase activity, intron lariats accumulate in S. cerevisiae, resulting
in a moderate reduction of growth rate. More severe phenotypes associated with loss of Dbrl-often embryonic lethality-are found in more intron-rich eukaryotes (Nam et al., 1997; Wang et al., 2004; Dickinson et al., 2016). So, in addition to recycling snRNPs, ILS disassembly is also vital for intron turnover, the loss of which is otherwise lethal.
15 This was rigorously shown for 1T's intron in log-phase yeast extract. Relevant for work presented below,
Part 2. Introns qua introns
Why are our genes in pieces? How and when did introns originate? What was their
selective advantage in our ancestors-or is that not the right question to be asking at all? These questions have driven a great deal of experimental, computational, and philosophical work since the discovery of splicing. Although these questions are impossible to answer definitively, this section first describes the current state of thought on the evolution of introns, their loss and gain over time, and their function in past and present eukaryotes. It concludes with a survey of the known diversity of intronic fates post-splicing, including examples of non-coding RNAs that are harbored within introns across eukaryota.
Evolution of introns
The debate over when introns arose in evolution (later termed "introns-early" vs. "introns-late" (Doolittle, 1987)) was contested for many years. Prominent early observers often favored an "introns-early" model wherein introns were relics of a primordial, pre-cellular gene structure. In this model, primordial exons were each independently functional; the function of interspersed introns was to allow exons to be easily joined and re-shuffled to produce new proteins without disrupting coding sequence (Gilbert, 1978, 1987). In turn, introns and the potential for "exon shuffling" were entirely lost in all modern bacterial lineages through extreme genome streamlining (Darnell, 1978; Doolittle, 1978). However, two major findings have largely pushed this model out of favor. First, the discovery of the archeal domain of life and
determination of the most parsimonious tree of life (an archeal origin of the last eukaryotic common ancestor [LECA]) requires of the "introns-early" model that all introns have been independently lost twice in all known lineages of bacteria and archea (Woese and Fox, 1977; Williams et al., 2013). Second, the "exon shuffling'" aspect of the "introns-early" model suggests
that introns would tend to fall between protein-domain boundaries, as it supposes pre-cellular exons would each encode a foldable, functional protein module. This is not convincingly borne out across the many sequenced eukaryotic genomes and transcriptomes (Doolittle, 2014).
What of the "introns-late" model, which suggests spliceosomal introns are not primordial but instead arose in eukaryotes alone (Cavalier-Smith, 1985, 1987)? This model took longer to fully mature, being especially supported by later findings of similarities between spliceosomal splicing and group II self-splicing (Sharp, 1985; Cech, 1986). Self-splicing introns serve as a much more concrete source for the origins of spliceosomal introns than a diverse collection of transposable elements-especially given the uniformity of splice-site sequences (Cavalier-Smith,
1978; Borst and Grivell, 1981; Cavalier-Smith, 1985). In this model, spliceosomal introns are the result of a massive invasion and proliferation of group Il-like introns introduced into proto-eukaryotes-potentially via the genome of cc-proteobacterium that became the mitochondria. How could this have happened? How would those invaded individuals pass on their genomes when one could imagine this irrevocably damaging their reproductive fitness? The spread on selfish, efficiently transposable genomic elements does not necessarily have to be beneficial or neutral to continue spreading in a population, and, as their frequency in the population increases, can become more and more detrimental to offspring viability 6 (Hickey, 1982). Therefore, this
scenario is compatible with the view that early introns lacked beneficial functions for eukaryotes, as one would expect given their evolutionary origin as selfish genomic elements. In all, the "introns-late" model as understood today proposes early eukaryotes did not acquire introns so that in a billion years we could have alternative splicing. Instead, they were likely forced upon
6 This is only true for sexually reproductive organisms, as selfish genomic elements only able to spread within a
clonal asexual lineage would certainly put that lineage at a unique disadvantage relative to the population (Cavalier-Smith, 1980). Intriguingly, this suggests such elements could have played a role in the evolution of sexual
the genome of the LECA and rapidly spread through the population. Some of the features that make eukaryotes what they are today (compact chromatin, a physical barrier between cytosol and genome, and RNAi to name a few) may have evolved in response to this invasion or ones like it (Madhani, 2013).
The evolution of the spliceosome likely resulted from the extreme pressures to maintain extensive sequence conservation of all of these newly acquired self-splicing introns. Unlike the minimal sequence requirements of spliceosomal introns, group I self-splicing introns have extensive RNA secondary structures that must be maintained for splicing to occur (Michel et al.,
1990). If too many mutations are introduced into a self-splicing intron intercalated between
exons of an essential gene, then the intron is retained in the mature mRNA, disrupts translation, and leads to an organism that is no longer viable. Expand this to the thousands of self-splicing introns littered throughout the LECA genome and it is easy to see where the evolutionary pressure came to develop trans-acting splicing machinery. An interesting possibility in the genesis of trans-acting machinery is that the five snRNAs present in modern eukaryotes arose from the fragmentation of self-splicing intron structures into "five easy pieces" (Sharp, 1991). These RNAs-along with the many proteins that became the spliceosome-could then act in trans to catalyze removal of all introns as long as enough sequence remained to define where introns' boundaries are. From there, each intron's sequence could rapidly diverge from the uniform precursor, allowing for the evolution of diverse intron functions we see today.
Loss and gain of introns
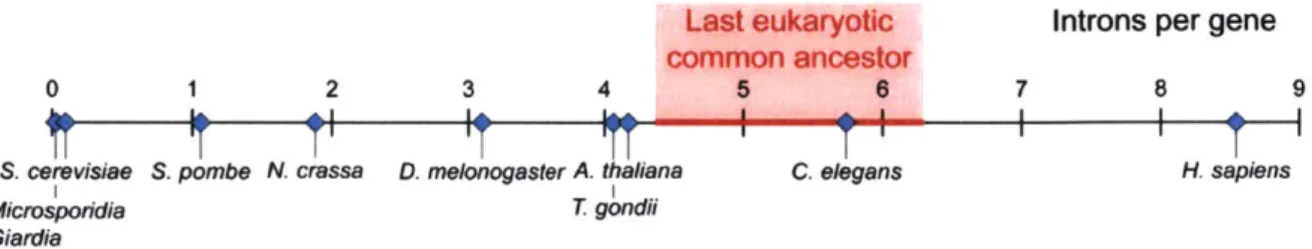
Introns have been lost and gained in various lineages since their initial proliferation. Although how these events occurred in the past cannot be started with certainty, hypotheses
about both events have been forwarded. Models based on modem eukaryotes estimate that the LECA contained between 4-6 introns per gene (Figure 3) (Koonin, 2006; Csuros et al., 2011). Therefore, while some eukaryotic lineages have doubled the number of introns per gene, intron
loss is the more dramatic and prominent event, with the S. cerevisiae lineage losing 99% of their primordial introns.
Last eukaryotic Introns per gene
common ancestor
0 1 2 3 4 5 6 7 8 9
S. cerevisiae S. pombe N. crassa D. melonogaster A. thaliana C. elegans H. sapiens
Micospoddla T gOndli
Giardia
Figure 3. Intron density of eukaryotes.
Shown are the introns per gene for example modem eukaryotes. These values range from -0.005 introns per gene (or 1 intron per 200 genes) in microsporidia and giardia to -8.5 introns per gene in humans. S. cerevisiae (left) has -0.05 introns per gene. The estimated intron density in the last eukaryotic common ancestor (middle, red) is 4.4-6.3 introns per gene (Modified from Csuros et al., 2011; additional data from Irimia and Roy, 2014).
A mechanism-driven hypothesis for intron loss in S. cerevisiae followed from the discovery that genetic Ty elements transpose through an RNA intermediate, necessitating an endogenous reverse transcriptase (Boeke et al., 1985; Garfinkel et al., 1985). If this activity exists in the cell, then intronless mature mRNA could be inadvertently reverse transcribed into cDNA; this cDNA could recombine into the genome, replacing the original, intron-containing genomic sequence (Fink, 1987). Because crossover events between genome and cDNA need to take place on either side of the intron to remove it, introns very close to the 5' end of the
transcript would be refractory to removal. Supporting this hypothesis, introns in S. cerevisiae and many other intron-poor eukaryotes are enriched in the very 5' end of transcripts (Fink, 1987;
Mourier and Jeffares, 2003)17. Although we cannot know how this processed happen throughout evolution, it has been shown that this mechanism is a viable pathway for intron loss in modern S.
cerevisiae (Derr et al., 1991; Derr, 1998).
Intron gain events are thought to be lineage-specific, dramatic, and rare (Fedorov et al.,
2003; Babenko et al., 2004; Coghlan and Wolfe, 2004). As with intron loss, mechanisms for
intron gain have been proposed (Yenerall and Zhou, 2012; Huff et al., 2016). A particularly intriguing finding related to intron gain is that both steps of spliceosomal splicing are reversible in vitro with appropriate salt and divalent cation conditions (Tseng and Cheng, 2008). In principle, this allows for the reverse splicing of intron lariats into new mRNA substrates. How reverse splicing would result in events of massive intron gain are unclear, and requires reverse transcription and recombination to enable genomic intron gain. That said, it has recently been shown experimentally that S. cerevisiae (Lee and Stevens, 2016) and single-celled algae
(Worden et al., 2009; Huff et al., 2016) can gain new introns, providing insight into these poorly understood phylogenetic events.
Function of introns in alternative splicing
S. cerevisiae contains only 300 spliceosomal introns-and only 14 multi-intronic
genes-across its 6,000 genes (Spingola et al., 1999; Davis et al., 2000; Juneau et al., 2007; Zhang et al.,
2007), making alternative splicing events few and far between (Juneau et al., 2009; Hossain et
al., 2011; Hossain et al., 2016). However, the function and utility of splicing in eukaryotes is often viewed through the lens of alternative splicing, warranting discussion of alternative splicing outside of yeast.
The first alternative splicing events were found relatively quickly after the discovery of splicing itself (Alt et al., 1980; Early et al., 1980). Today, we recognize that >95% of pre-mRNA
in humans have alternatively spliced isoforms (Pan et al., 2008; Wang et al., 2008). Alternative splicing serves as one of the major sources of transcriptome and proteome diversity in
multicellular eukaryotes. The utility of alternative splicing is particularly seen in developmental (Sanchez, 2004; Demir and Dickson, 2005) and differentiation-specific (Boutz et al., 2007; Makeyev et al., 2007) expression of specific isoforms.
Although there are instances when the molecular mechanism underlying the production of different isoforms have been elucidated (Siebel et al., 1992; Valca'rcel et al., 1993; Zuo and Maniatis, 1996; Sharma et al., 2008), in general, the totality of factors impinging on a given splicing event, in a given cell, with a given cellular history, in a given point in time, and in a given environment have made universal predictions of isoform usage challenging (Wang and
Burge, 2008). Nonetheless, it is difficult to overstate both the importance and complexity of alternative splicing in crafting the transcriptome of multicellular eukaryotes.
Other functions of introns in modern eukaryotes
Besides their role in alternative splicing, introns have other functions that manifest before, during, or after active splicing. In many multicellular eukaryotes, introns have been found to have prominent functions related to gene expression, translational yield of the mature mRNA, and ncRNA production. In many cases, the underlying mechanism is unclear, but
examples of these general functions are still illuminating for thinking about the roles introns play in modern eukaryotes.
There is a wealth of literature demonstrating coupling between transcription and introns (Moore and Proudfoot, 2009). In particular, 5' introns-those introns nearest the transcription start site-often contain regulatory elements that increase Pol 1l's initiation rate (Bornstein et al.,
1988; Vasil et al., 1989; Palmiter et al., 1991; Furger et al., 2002). In extreme cases, a 5' intron is required to produce detectable levels of a transcript at all (Buchman and Berg, 1988). Introns near the site of 3' processing may also modulate that process as well (Rigo and Martinson, 2008; Proudfoot, 2011). In metazoans, splicing results in the deposition of a complex at each exon-exon junction, which is appropriately named the exon-exon-junction complex (EJC) (Le Hir et al., 2000). The EJC remains on the mRNA until the pioneer round of translation in the cytoplasm, and is thought to serve as an indication that a given mRNA was processed correctly and should therefore be translated efficiently (Wiegand et al., 2003; Moore, 2005).
Introns often harbor smaller ncRNAs within their sequence, resulting in their incomplete degradation post-splicing as these processed remnants go on to outlast their ephemeral host
RNA18. The most prominent classes are small nucleolar RNAs (snoRNAs) and microRNAs (miRNAs). snoRNAs are required for ribosome biogenesis and direct two types of RNA base modifications, 2'-O-methylation and pseudouridylation, to 100-200 sites per ribosome as well as directing other aspects of rRNA trimming and maturation (Venema and Tollervey, 1999).
miRNAs are a class of small (-22 nt) RNAs that pair to partially complementary sequences within an mRNA and direct post-transcriptional repression of these target messages in diverse eukaryotic lineages (Bartel, 2018). miRNAs are processed from hairpin substrates, generally embedded within much longer primary Pol I transcripts (Lee et al., 2002; Lee et al., 2003; Cai et al., 2004; Lee et al., 2004). Approximately half of all human miRNAs are processed from introns through the canonical Drosha-mediated pathway (Baskerville and Bartel, 2005; Chiang et al.,
2010). In some cases, a full-length debranched intron will be both the correct length and have the correct, extensive base-pairing to resemble a Drosha-processed precursor miRNA. These
"mirtrons" are recognized by the downstream processing enzyme Dicer, bypassing Drosha (Ruby et al., 2007). Even in these cases, more than half of the excised intron will be catabolized during miRNA maturation. Other examples of functional ncRNAs processed from introns have been found in mammalian immunoglobin class-switch recombination (Zheng et al., 2015) and fly embryogenesis (Tay and Pek, 2017), with more likely awaiting discovery.
The function of individual introns has been examined thoroughly in S. cerevisiae, where because of the small number of introns and few alternative-splicing events the potential functions of introns outside of alternative splicing can be more readily recognized. Additionally, because 95% of S. cerevisiae genes do not contain an intron, it is unlikely for introns to have general functions related to mRNA quality control as they do in metazoans. For most introns, no growth phenotypes are detected when they are removed from the genome (Ng et al., 1985; Parenteau et al., 2008; Hooks et al., 2016). In a couple of cases, the presence of an intron in a given locus appears to have a function. In one case, the intron regulates expression of paralogous ribosomal-protein genes (Parenteau et al., 2011), and in another case the intron counteracts deleterious R-loop formation during transcription (Bonnet et al., 2017). As in other eukaryotes, some S.
cerevisiae introns are further processed into functional ncRNAs, such as snoRNAs, in which
case the flanking portions of the intron are rapidly catabolized (Qu et al., 1995; Petfalski et al., 1998).
Notable post-splicing fates of intact introns
There are very few known examples of full-length introns that persist post-splicing, and even fewer with known functions or regulation. Broadly, this category could include either
branched or linear introns. The most notable examples are the latency-associated transcripts (LATs): lariats produced during latent stages of herpes simplex virus I infections that may play a role in the maintenance of viral latency (Fraser et al., 1992; Wu et al., 1996; Cliffe et al., 2009). Lariats spliced from the T cell receptor-P pre-mRNA in mouse and human T cells have
developmentally regulated stability, although no function has been found (Qian et al., 1992). Trimmed circles (lariats with 3' tails removed) spliced from ANKRD52 may remain associated with their genomic locus and modulate transcription (Zhang et al., 2013). The sole example of a debranched, seemingly full-length intron is one produced by the Epstein-Barr virus in infected human B cells (Moss and Steitz, 2013). How this intron is protected from degradation is unknown.
Part 3. S. cerevisiae outside of log-phase growth
Cells-both within a multicellular organism and single-celled microorganisms-are not continually growing and dividing. In fact, most eukaryotic cells likely spend the majority of their lives in a resting, quiescent state exemplified by stem cells, neurons, eggs, and spores. (Werner-Washburne et al., 1996; Gray et al., 2004). This is especially true for organisms, such as S.
cerevisiae, that must respond to sub-optimal environmental changes without the benefit of locomotion. Waxing and waning of optimal and sub-optimal environments has likely been a consistent evolutionary pressure on S. cerevisiae's lineage. We see this reflected today in the species: yeast cells can remain viable in their quiescent state for months (equivalent to thousands of generation times for exponentially doubling cultures) (Lillie and Pringle, 1980; Granot and Snyder, 1993; Fuge et al., 1994), and likely for orders of magnitude longer under the right conditions (Prokesch, 1991).
Despite being among the most thoroughly studied eukaryotic organisms (Botstein and Fink, 2011), our knowledge of the molecular mechanisms of S. cerevisiae's environmental responses, state changes, and state maintenance is fragmented and incomplete. In turn, this lack of knowledge often leads to unfounded assumptions, based on the more experimentally tractable and standardized log-phase growth, which is presumed to hold for other growth conditions. Of course, yeasts in this growth phase are in their optimal environment, where the name of the game is presumably to produce as many offspring as possible. However, a culture of yeast cells is a dynamic entity with all growth phases able to provide relevant insights-especially in the case of phase-specific phenomenon-into the evolution and survival of the species. This section
provides an overview of how S. cerevisiae responds to changes in its environment. Focus is given to the growth phases transited by an otherwise unperturbed yeast culture, and what these growth phases can potentially teach us about S. cerevisiae's evolutionary interactions with its natural environments.
Growth phases
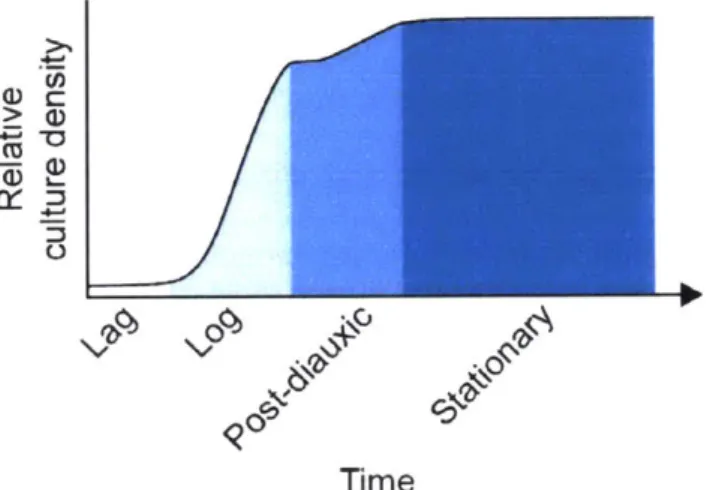
Yeast cultures are most often initiated by diluting from a confluent overnight culture into fresh media'9. First, the culture experiences a lag phase where yeast become increasingly
biochemically active, but do not yet divide (Figure 4) (Forsburg and Nurse, 1991). Next, they enter an extended state of exponential growth termed log phase. Log phase occurs while the yeast are sufficiently dilute such that their metabolism has little influence on overall nutrient
availability in the medium. In either of the two standard media for S. cerevisiae growth-rich, yeast extract-based media (YPD) or synthetic complete media (YSC)-the majority of cell
1) This style of culture is termed a "batch culture" due to all nutrients ever provided to the culture being provided in
one batch at the time of seeding. This is in contrast to less commonly used "continuous culture," which utilizes a chemostat to supplement media and maintain a constant growth rate.
division and increase in culture density occurs during the log phase when each doubling requires only 1.5-2 hours (under standard conditions). Since essential nutrients are finite in either media, the yeast will at some point sense some change in their environment and cease rapid divisions.
Surprisingly, this sensing can happen early in the rapid-doubling period. In particular, the ribosome-synthesis rate drops 50% early in log phase, even though multiple subsequent
doublings occur before the growth rate begins to decline (Ju and Warner, 1994; Warner, 1999). So, despite the apparent "full-throttle" nature of log phase, yeast in this phase are already beginning to estimate the future potential for growth in their environment.
Time
Figure 4. Growth phases of S. cerevisiae.
Shown are the growth phases that a newly seeded S. cerevisiae culture will transit if left unperturbed (x-axis labels) as well as the relative cell density in each phase (y-axis). The time axis is qualitative, as the length of each phase will depend on media used and initial cell density.
The type of media used to grow yeast will affect its growth rate as well as which essential nutrients are first to become limiting. In YPD media, the limiting nutrient is most often glucose (Lillie and Pringle, 1980). When glucose becomes exhausted, the culture undergoes a diauxic shift to begin utilizing other, non-fermentable carbon sources via respiration. This shift first results in a transient lag period with no growth while necessary enzymes are produced to utilize the new carbon source-not dissimilar to the lag phase discussed earlier (Monod, 1949). The
post-diauxic phase encompasses a slight increase in cell density until limiting nutrients are once again exhausted. It is worth noting that when grown in YSC, the limiting factor for growth is likely lipids and/or lipid precursors (Hanscho et al., 2012). Glucose, on the other hand, does not appear to be limiting; roughly half of the standard 20 g/L glucose is not catabolized by the time log phase ends (Ju and Warner, 1994; Hanscho et al., 2012). How much of a classical diauxic shift exists under these conditions is unclear.
Stationary phase is characterized by neither a marked increase nor decrease in culture density (Werner-Washburne et al., 1996). It is worth noting that stationary phase is distinctly a property of cultures, not individual cells. Stationary cultures are comprised of two primary populations: quiescent cells and non-quiescent cells (Allen et al., 2006; Aragon et al., 2008). Quiescent cells maintain viability, genome stability, ROS repression, and reproductive competency for much longer than non-quiescent cells. However, it is the non-quiescent population that continues to reproduce in the short term, and may be the source of new,
advantageous mutations in an altered environment (Longo et al., 1996; Allen et al., 2006). As the classification of distinct stationary-phase populations is relatively recent, there are still many unanswered questions about the interplay between quiescent and non-quiescent cells, and if there are quorum-like decisions that control the relative ratio of these populations during stationary-phase growth (Werner-Washburne et al., 2011).
Although some cells survive in stationary-phase cultures for extended periods of time (discussed above), the overall viability of the culture decreases over time. The study of chronological life span (CLS) and aging in yeast is often focused on this phase of growth. Of particular interest are genetic mutations that confer increased CLS. Genetic approaches have
DNA and damaged mitochondria, oxidative stress, cytosolic acidification, caloric restriction, and TOR signaling are among the most prominent (Fabrizio et al., 200 1; Powers et al., 2006;
Kennedy et al., 2007; Guarente, 2008; Seo et al., 2010; Hughes and Gottschling, 2012; Longo et al., 2012; Gottschling and Nystrom, 2017). In all, it is clear that diverse intrinsic and cell-extrinsic factors lead to the decline in a given cell's viability as well as the overall viability of a culture.
The question that initiated this dissertation was simply: Are there unknown, post-transcriptional gene regulatory regimes hidden outside of well-trodden environmental contexts? This dissertation describes one of many possible paths that led directly from that question: the discovery of regulated intron stability post-splicing and post-debranching in S. cerevisiae, and a function for these ncRNAs in TOR-mediated growth control. This regulated stability is not limited to one or two introns, but is characteristic of at least 34 introns, which is greater than
10% of all the spliceosomal introns in the species. Importantly, these introns like all other spliceosomal introns in S. cerevisiae are rapidly degraded in log-phase cultures, as expected based on many previous studies. Instead. stable introns are found in a variety of saturated-growth conditions, and specifically as a result of TOR inhibition. This work exemplifies a broader belief that focusing on a single environmental state can obfuscate fundamental aspects of biology, and serves as evidence that the post-splicing lives of introns may be much more complex than currently appreciated.
References
Agafonov, D.E., Kastner, B., Dybkov, 0., Hofele, R.V., Liu, W.-T., Urlaub, H., Lchrmann, R., and Stark, H. (2016). Molecular architecture of the human U4/U6. U5 tri-snRNP.
Science, aad2085.
Allen, C., Buttner, S., Aragon, A.D., Thomas, J.A., Meirelles, 0., Jaetao, J.E., Benn, D., Ruby, S.W., Veenhuis, M., Madeo, F., et al. (2006). Isolation of quiescent and nonquiescent cells from yeast stationary-phase cultures. The Journal of cell biology 174, 89-100. Alt, F.W., Bothwell, A.L., Knapp, M., Siden, E., Mather, E., Koshland, M., and Baltimore, D.
(1980). Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell 20, 293-301.
Aragon, A.D., Rodriguez, A.L., Meirelles, 0., Roy, S., Davidson, G.S., Tapia, P.H., Allen, C., Joe, R., Benn, D., and Werner-Washburne, M. (2008). Characterization of differentiated quiescent and nonquiescent cells in yeast stationary-phase cultures. Molecular biology of the cell 19, 1271-1280.
Arenas, J., and Abelson, J. (1991). Requirement of the RNA helicase-like protein PRP22 for release of messenger RNA from spliceosomes. Nature 349, 487.
Arenas, J., and Hurwitz, J. (1987). Purification of a RNA debranching activity from HeLa cells. Journal of Biological Chemistry 262, 4274-4279.
Arenas, J.E., and Abelson, J.N. (1997). Prp43: An RNA helicase-like factor involved in
spliceosome disassembly. Proceedings of the National Academy of Sciences 94, 11798-11802.
Ares Jr, M. (1986). U2 RNA from yeast is unexpectedly large and contains homology to vertebrate U4, U5, and U6 small nuclear RNAs. Cell 47, 49-59.
Avery, 0., Macleod, C., and McCarty, M. (1944). Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type Ill. The Journal of experimental medicine 79, 137-158.
Babenko, V.N., Rogozin, l.B., Mekhedov, S.L., and Koonin, E.V. (2004). Prevalence of intron gain over intron loss in the evolution of paralogous gene families. Nucleic acids research 32, 3724-3733.
Bai, R., Yan, C., Wan, R., Lei, J., and Shi, Y. (2017). Structure of the Post-catalytic Spliceosome from Saccharomyces cerevisiae. Cell 171, 1589-1598. e1588.
Bartel, D.P. (2018). Metazoan MicroRNAs. Cell 173, 20-51.
Baskerville, S., and Bartel, D.P. (2005). Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. Rna 11, 241-247.
Behrens, S.-E., and Ltihrmann, R. (1991). Immunoaffinity purification of a [U4/U6. U5] tri-snRNP from human cells. Genes & development 5, 1439-1452.
Berget, S.M. (1995). Exon recognition in vertebrate splicing. Journal of biological Chemistry
270, 2411-2414.
Berget, S.M., Moore, C., and Sharp, P.A. (1977). Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proceedings of the National Academy of Sciences 74, 3171-3175.
Berget, S.M., and Robberson, B.L. (1986). UJ, U2, and U4/U6 small nuclear ribonucleoproteins are required for in vitro splicing but not polyadenylation. Cell 46, 691-696.

![Figure 1. Some excised linear introns accumulate in yeast. a, Undetectable accumulation of the intron from the ACT] gene](https://thumb-eu.123doks.com/thumbv2/123doknet/14747884.578871/55.917.93.644.132.728/figure-excised-linear-introns-accumulate-undetectable-accumulation-intron.webp)