CELL-FREE FREEZE-DRIED SYNTHETIC BIOLOGY FOR WEARABLE
BIOTECHNOLOGY APPLICATIONS MASSACHUSETTSINSTITUTEOF TECHNOOGY by
Luis Ruben Soenksen Martinez
FEB 0 5 2020
LIBRARIES
B.S. Biomedical Engineering (2010)Instituto Tecnol6gico y de Estudios Superiores de Monterrey, Mexico City, Mexico
M.S.E. Bioengineering Innovation and Design (2012)
Johns Hopkins University, Baltimore, USA
Submitted to the Department of Mechanical Engineering in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Mechanical Engineering at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
,September2019 ei ) to 1
© 2019 Massachusetts Institute of Technology. All rights reserved.
Signature redacted
A u th o r...
Luis Ruben Soenksen Martinez Department of Mechanical Engineering Tuesday,,September 24, 2019
Signature redacted
Certifiedhvby .... ... ... .J... .etf
e . ... .... ... .. ..
Prof. mes J. Collins Termeer Professor of Medical Engiering & Science Massachusetts Institute of Technology; Broad Institute of IT and Harvard;
Wyss Institute for Biologically nsppi ering, Harvard University; Thesis advisor
Signature
redacted---A ccepted by ... .Accetedy...rof. Nicolas G. Hadjiconstantinoui~ ,i's.... . ....rof.N , " '" ~t i'n'*o u
Department of Mechanical Engineering, Graduate Officer MIT Co-Director, Computation for Design and Optimization Co-Director
a m m
Cell-free freeze-dried synthetic biology for wearable biotechnology applications by
Luis Ruben Soenksen Martinez
Submitted to the Department of Mechanical Engineering, on August 3 1st, 2019
in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Mechanical Engineering
ABSTRACT
Synthetic biology aims to develop modular genetic networks for computation, sensing, and control of biological systems, holding great promise for next-generation biosensing platforms. Similarly, advances in material sciences have allowed for the design of substrates and textiles engineered to exhibit novel mechanical, electrical, and optical properties for sensing and actuation. Wearable biosensors using synthetic biology principles and smart materials could expand on this potential, especially as solutions for continuous, fine-grained monitoring of physiological status, disease states, and pathogen/toxin exposure difficult to assess with other methods. Despite this, only few examples of synthetic biology sensors compatible with wearable use-cases have been described, all of which rely on the use of live engineered bacteria with sustainment limitations. Thus, we report on the development of novel shelf-stable, genetically-programmable, and highly sensitive wearable sensing platforms based on cell-free synthetic biology components freeze-dried into flexible substrates and textiles; as well as on a new class of smart programmable synthetic biology materials capable of reacting to environmental queues. These systems were designed to exhibit colorimetric, fluorescent, luminescence, electrical, or mechanical outputs that can be passively or actively interrogated within isolated modules or in larger-scale garments with wireless networking capabilities. We functionally validated such platforms using a variety of synthetic biology circuits for detecting several relevant environmental exposure targets such as metabolites, chemicals, and pathogen-associated nucleic acids. These findings suggest that cell-free synthetic biology tools have the potential to enable highly programmable wearable systems for rapid on-body detection or adaptation to external threats in first responders, warfighters or clinical personnel, as well as the assessment of athletic performance and monitoring to complex disease states
KEYWORDS:
Synthetic, biology, wearables, gene, network, sensing, monitoring, cell-free, freeze-dried, point-of-care, fabrics, textiles, sensing, electronics, internet-of-things, diagnostics.
Thesis supervisor: Prof. James J. Collins
Title: Termeer Professor of Medical Engineering & Science, Institute of Medical Engineering and Science, Massachusetts Institute of Technology.
U U m
Cell-free freeze-dried synthetic biology for wearable biotechnology applications
Luis Ruben Soenksen Martinez
Submitted to the Department of Mechanical Engineering, on August 3 1st, 2019
in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Mechanical Engineering
Domitilla Del Vecchio
Professor Mechanical Engineering, MIT Thesis committee chair
U m m
Cell-free freeze-dried synthetic biology for wearable biotechnology applications
Luis Ruben Soenksen Martinez
Submitted to the Department of Mechanical Engineering, on August 3 1st, 2019
in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Mechanical Engineering
Robert S. Langer
David H. Koch Institute Professor, MIT
Professor Mechanical, Chemical and Biological Engineering, MIT Harvard-MIT Division of Health Sciences and Technology, MIT
David H. Koch Institute for Integrative Cancer Research Thesis committee member
U m m
"I shall be telling this with a sigh Somewhere ages and ages hence:
Two roads diverged in a wood, and
I-I took the one less traveled by,
And that has made ALL the difference."
- Robert Frost, The Road Not Taken
ACKNOWLEDGMENTS
The content presented in this work would not have been possible without the help of many people and institutions. In particular, I recognize the support of my family, friends, faculty advisors, fellow students, administrative staff and the incredible community at MIT and Harvard during this journey. I want to thank my advisor, Prof. James J. Collins, for his support, guidance and keen insight while under his tutelage. I cannot underscore the importance that Prof. Collins has had in my research career, as well as the inspiration he has become personally and professionally. On the same lines, I would like to thank Prof. Domitilla Del Vecchio, Prof. Robert S. Langer, Prof. Linda G. Griffith, Prof. David L. Trumper and Prof. Edward Boyden for all their support during my PhD studies, and more broadly for being incredible role models in the pursuit of scientific knowledge and translatable inventions of the highest possible quality and ethical integrity. Moreover, I
thank the Departments of Mechanical Engineering (MechE) and Biological Engineering (BE) at MIT, as well as MIT'S Institute of Medical Engineering & Science (IMES) and the Wyss Institute at Harvard for providing me with an outstanding platform learn, ideate and execute this work as well as other projects.
I have been incredibly lucky on finding (or being found) by a myriad of good friends, labmates, collaborators, and administrative support over the years that have directly shaped; or indirectly affected, the ideas and direction of this work. I thank Peter Q. Nguyen, Max A. English, Nicolaas M. Angenent-Mari, Helena de Puig, Nina M. Donghia, Angelo S. Mao, Raphael V. Gayet, Ann Ran, Ally Huang, Thomas Ferrante, Carlos F Ng
and Tommaso Galbersanini for their direct input or collaboration in this work on synthetic biology. Furthermore, I thank Timothy Kassis, Pierre Sphabmixay, Brij M Bhushan, and Collin Edington for their support during my time developing organ-on-chip platforms at Trumper's and Griffith's Labs, as well as beyond. I also thank Prof. Martha L. Gray and the faculty at the Madrid M+Vision Fellowship within MIT's Research Lab of Electronics (RLE) for being the group who first saw in me the potential to succeed at MIT. Organizationally, I thank the broader MechE's faculty pool and especially academic administrator Leslie Regan for her moral and organizational support during my Ph.D. studies, as well as James Niemi for his kindness and encouragement to thrive within the Wyss Institute at Harvard University. I want to acknowledge the financial support from the
Mexican Council for Science and Technology (CONACYT), MIT, Harvard, DARPA, and Johnson & Johnson, who have made this work possible.
Finally, I would like; as well, to recognize my family and friends, who are my everything. Martha Tamez, Elizabeth Soenksen, Guillermo Villanueva, Laura Tamez, Ricardo Tamez, Martha Gonzalez, Carlos Castro-Gonzalez, Berta M. Fuster and every loved one that has walked with me and aid as I stumbled. To them, I thank for allowing my pursuits to always have a deeper meaning through you and your unconditional love.
0 N N
CONTENTS
Cell-free freeze-dried synthetic biology for wearable biotechnology applications.1
Abstract... 3
Keywords:... 3
Acknow ledgem ents ... 10
Contents...12
Thesis outline ... 15
CHAPTER 1 ... 16
Introduction ... 16
Background and Motivation ... 16
Solution strategies ... 18
State-of-the-art... 19
CHAPTER 2... 22
Objective ... 22
Project goals ... 22
Research Aims & Development plan ... . 23
CHA PTER 3 ... 29
Colorim etric W earable Synthetic Biology Platform ... 29
Motivation and General approach... . 29
Materials and m ethods... 31
Fabrication of colorimetric synthetic biology wearable device... 31
Validation of colorimetric wCF-FD-SB devices ... 33
CHAPTER 4... 48
Fluorescence / Luminescence Wearable Synthetic Biology Platform...48
Motivation and General approach... 48
Materials and methods... 49
Screening of textiles for freeze-dried cell-free synthetic biology ... 49
Single-thread testing for freeze-dried cell-free synthetic biology... 65
Fabrication of fluorescence/luminescence wearable textile for synthetic biology sensing d e m o n stra tio n s ... 6 6 Hardware / Software implementation of wearable POF spectrometer ... 70
assembly and operation of Textile-based wearable synthetic biology system ... 72
Validation of wCF-FD-SB devices in Fluorescence mode... 74
Validation of wCF-FD-SB device in Luminescence mode ... 80
CHAPTER ... 85
CRISPR-based Wearable Synthetic Biology Sensors ... 85
Motivation and General approach... 85
Validation of CRISPR-based wCF-FD-SB sensors ... 86
wCF-FD-SB CRISPR-based mecA sensor... 86
wCF-FD-SB CRISPR-based spa sensor ... 87
wCF-FD-SB CRISPR-based ermA sensor ... 87
Limit of detection and robustness of CRISPR-based mecA sensor ... 89
Orthogonality of CRISPR-based wearable sensors ... 91
CHAPTER 6...93
Program m able CRISPR-Responsive Smart Materials ... 93
Motivation and General approach... 93
Materials and methods... 95
Testing of catalytic properties of Casl2a ... 95
Selection of dna-Hydrogels for CRISPR-modulation demonstrations ... 98
Release of ssDNA-anchored molecular cargos from hydrogel matrices ... 98
Control of large-scale mechanical properties and release of viable cells from DNA-h y d ro g e ls ... 1 0 5 Synthesis of acrylamide-DNA gel precursors ... 109
Production of oligo-functionalized acrylamide polymers Ps-X and Ps-Y ... 110
Bulk degradation of polyacrylamide-DNA gels using FITC-dextran microparticles ... 110
Gold nanoparticle synthesis and PEG functionalization ... 111
Gold nanoparticle release from acrylamide gels ... 111
Preparation of polyacrylamide-DNA gels for bulk degradation using EvaGreen and cell re le a s e ... 1 1 2 Gelation of polyacrylamide (PA)-DNA with EvaGreen for bulk hydrogel degradation... 112
Primary cells for DNA-polyacrylamide gel release ... 113
Gelation of polyacrylamide (PA)-DNA with encapsulated cells ... 113
Cell release from DNA-polyacrylamide gel and viability analysis ... 113
Cell viability in Casl2a reaction conditions ... 114
Conductive DNA-based materials act as Casl2a-actuated electronic fuses ... 114
Synthesis of carbon black-DNA gels ... 117
Conductivity measurements of carbon black-DNA gels ... 119
In vitro reaction of Casl2a with carbon black-DNA gels ... 119
Casl2a-controlled hydrogel formation in a paper fluidic device enables diagnostic readouts . ... 1 2 1 Fabrication of CRISPR-gel pPad stop-flow system with electrical readout ... 129
Flow and conductivity measurements in pPad ... 130
RFID integration in CRISPR-mediated stop-flow pPad ... 131
Ebola diagnostic and EBOV RT-RPA CRISPR pPAD... 132
RFID readings of EBOV RT-RPA CRISPR pPAD ... 133
Utility of Programmable CRISPR-Responsive Smart Materials for wearable applications135 CHAPTER 7... ... ... 136
Garment-level integration of wearable synthetic biology sensors... 136
Motivation and General approach... 136
Materials and methods... 136
Design and validation of colorimetric wCF-FD-SB garment ... 136
Design and validation of fluorescence/luminescence wCF-FD-SB garment ... 137
CHAPTER 8 ... 145
Discussion ... 145
General achievements ... 145
Future environmental considerations ... 145
Future sam ple conditioning considerations... 146
Extension to wide-range detection of chemicals and toxins ... 147
Conclusions...147
Contributions...148
APPENDIX ... 149
Sensor and reporter sequences ... 149
Author biographies ... 153
List of figures ... 154
List of tables ... 162
BIBLIOGRAPHY ... 163
THESIS OUTLINE
This thesis consists of eight chapters. CHAPTER 1 (Introduction) gives the problem overview that this thesis is trying to resolve, as well as background information and the state-of-the-art regarding synthetic biology tools for wearable applications. CHAPTER 2 (Objective) defines the general solution approaches and aims selected towards the development of novel wearable sensing platforms based on cell-free synthetic biology reactions, which include systems freeze-dried into flexible substrates and textiles, as well as the generation of other associated smart materials. CHAPTER 3 presents the methods and validation results of a wearable colorimetric synthetic biology platform capable of reacting to specific metabolites, chemicals, and pathogen nucleic acid exposures. CHAPTER 4 presents the methods and validation results of a highly sensitive
textile-based wearable fluorescence/luminescence synthetic biology system containing inter-weaved polymeric optic fibers for detection of similar environmental molecular targets. CHAPTER 5 presents the methods and validation results of programmable CRISPR-based detection sensors freeze-dried into our textile-based fluorometric platform, enabling highly programmable nucleic acid detection limits that rival current laboratory-based methods such as qPCR. CHAPTER 6 expands on the use of CRISPR-based sensing systems to generate new classes of smart materials with mechanical and electrical modulation that allow for promising new uses in wearable applications. CHAPTER 7 then focuses on the integration of the developed wearable synthetic biology sensors within larger-scale garments with sensing electronics and wireless networking capabilities to provide a proof-of-concept for use in real-time dynamic monitoring of target exposure. Finally, CHAPTER 8, provides general conclusions on the results, as well as discussion on advantages, limitations and future direction of the developed technologies.
CHAPTER1
INTRODUCTION
BACKGROUND AND MOTIVATION
Synthetic biology is an expanding interdisciplinary field that employs engineering principles to create new molecular functions within bioinspired and natural living systems
[1]. It is also a field that has enabled unprecedented control over a variety of biological
parameters, which has led to transformational developments in biotechnology and medicine [2]. Among the many objectives of synthetic biology is the development of genetic regulatory networks [3], as well as modular elements for computation, interphase, and modification of biologically relevant models. These elements, originally inspired by electrical engineering, include oscillators, switches, logic gates, amplifiers, timers, counters, memories, sensors and even actuators [4][5][6]. A rich palette of modular biosensors, biologically embodied logic gates, and output effectors already populate the design toolkit for construction of custom biological circuits, all of which have enabled a new wave of fundamental discoveries in cellular biology [7]. Currently, synthetic biology is paving the way for new classes of inexpensive and rapidly deployable sensors, many of which are designed to enable screening, better diagnosis, and monitoring of disease states in a variety of real-world environments [1]. From the detection of pathological conditions to the exposure of chemical and biological threats, the number of sensing applications based on synthetic biology is steadily expanding thanks to the rapid incorporation of robust engineering tools within this field.
From a historical perspective, environmental and whole-cell biosensing is a centuries-old practice that can be traced back to the use of animal sentinels, such as caged canaries, which have been used for warning of toxic gases in coal mines [1]. Currently, hogs are still used in truffle searching, and trained dogs remain one of the most cost-effective options for the detection of illicit materials, explosives, and in some cases, even cancerous tumors [1][8]. During the last decade, the range and availability of such living sentinels have further expanded, including miniaturized systems made of native
cells and genetically modified bacteria capable of sensing a variety of molecular and environmental targets [9]. Such advancements in synthetic biology have validated the feasibility of interrogating previously inaccessible intra- and intercellular spaces to generate detectable (endogenous or engineered) downstream molecular activity. In fact, these new cellular sensors can now operate alone or even coupled to electronics to form exquisitely sensitive transducers useful in many valuable applications, from water quality assessment to toxin detection in war zones [8][10].
More recently, engineered bacteriophages and cell-free networks have taken these living sensing circuits out of the cell [11] and into human-made substrates such as paper
[12]. In particular, cell-free freeze-dried (CF-FD) synthetic biology networks appears to be
an attractive platform towards translational efforts for personal, clinical, and military use, mainly due to their environmental stability, safety profile, and promise of affordability. These advantages have driven the integration of CF-FD synthetic biology sensors into a
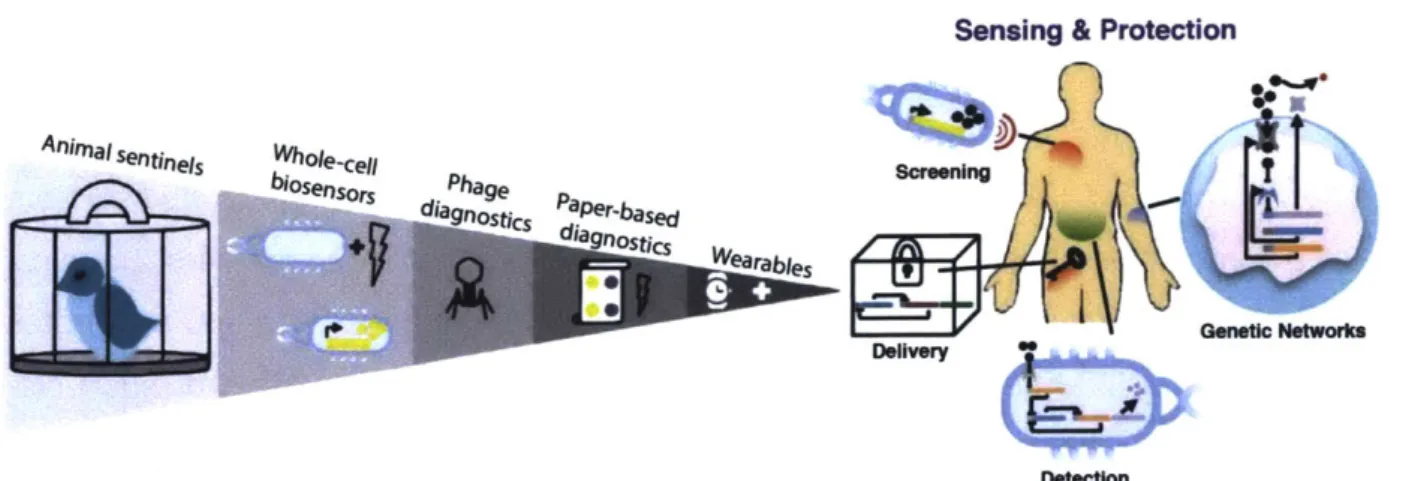
variety of formats, which lead to frontier of wearable applications in the near future (Figure 1) [1].
Sensing & Protection
Anhnasentines for solencee
ce from SScreening eors W oagtn Pape fa
digno iagn0 e
Deeffien
Figure 1: Historical progression and future directions of synthetic biology
networks for sensing and protection in human-centered devices. Adapted
from Slomovic, S., 2015. PNAS [1].
Similarly, fibers and textiles are among the oldest and most ubiquitous class of
materials in use today. From clothing to filtration, these types of substrates have adopted
a variety of forms and functions, many with great value to human society. Recent breakthroughs in fiber materials and manufacturing processes have allowed for the
design and production of fibers and fabrics with novel engineered mechanical, electrical, and optical properties marking the dawn of the wearable revolution and next-generation biosensing systems [13]. Wearable biosensors using synthetic biology principles and smart materials could greatly expand on this potential, especially as solutions for continuous, fine-grained monitoring of physiological status, disease states, and pathogen/toxin exposure difficult to assess with other methods. Despite this, only few examples of synthetic biology sensors compatible with wearable use-cases have been described, all of which rely on the use of live engineered bacteria for operation [14][15][16]. This available approach presents several limitations, particularly concerning the need for containing and sustaining potentially pathogenic or evolving organisms within these wearable devices for extended periods. Recognizing this gap, we propose to demonstrate the feasibility of incorporating cell-free freeze-dried synthetic biology sensors into selected materials, fibers, threads, weaves, and complex fabrics as a way expand the current synthetic biology toolkit into more convenient and safer parameter space for use in wearable devices.
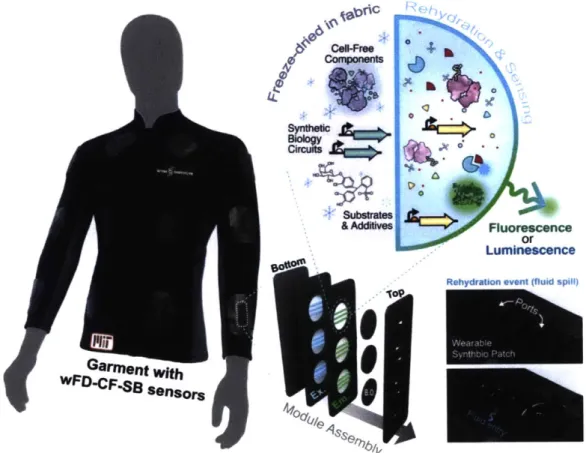
SOLUTION STRATEGIES
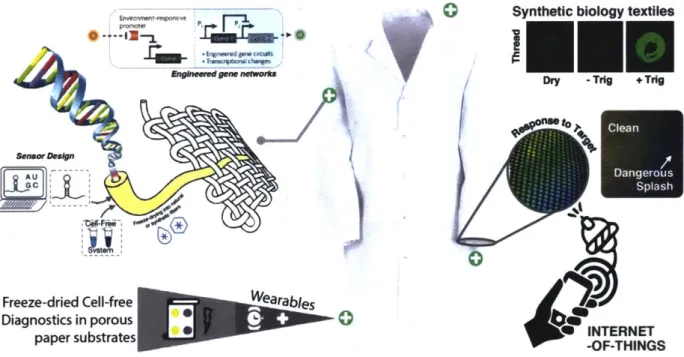
The goal of this work is to demonstrate a variety of wearable cell-free freeze-dried synthetic biology (wCF-FD-SB) platform technologies using genetically-encoded sensors that are compatible with the detection of bodily metabolites, pathogen markers, chemical toxins, and other environmental queues directly over synthetic and natural substrates such as polymer matrices and textiles (Figure 2). Moreover, the intent of such bio-reactive wearable materials and systems is to exhibit measurable optical, electrical, or mechanical outputs given the presence of the desired target, to be coupled to portable microelectronic interrogation systems for convenient data collection and wireless communication. Furthermore, we aim to provide new functionalities to these commonly use materials widening the range of valuable applications that these technologies can deliver. Indeed, if reactive to external stimuli, many of these novel wearable cell-free synthetic biology materials could provide a technological basis for real-time response to
biological and epidemiologic threats based on bio-sensor activation, as well as to drive decision making in disease and activity states.
E*-t-4i P En9MWed m bvmf -oi
0Syntl
Ii
fE
hetic biology textiles
I-Dry -Trig + Trig
to Freeze-dried Cell-free Diagnostics in porous paper substrates Wearables INTERNET -OF-THINGS
Figure 2: Base concept for wearable synthetic biology implementation. The schematic shows a model engineered genetic network with the optical output being embedded and
freeze-dried into textile substrates. This process leads to fabrics with shelf-stable
genetically encoded sensors that utilized purified transcription/translation cell-free
extracts and added substrates to achieve assessable outputs upon exposure to a target. Such is the case of the fluorescently-responsive threads (top-right) or fabrics(mid-right). These systems are also conceptualized to be compatible with
interrogation devices with wireless connectivity capabilities for integration into internet-of-things applications.
STATE-OF-THE-ART
To our knowledge, the area of wearable cell-free freeze-dried (wCF-FD) synthetic biology has not been previously demonstrated; however, two recent methods to integrate
bacterial biosensors and actuators into stretchable wearable substrates have been reported [14][15][16]. On the one hand, the work conducted by Wang et al. 2017 primarily
describes a wearable actuator based on the hygroscopic properties of an engineered
19
bacterial system deposited over an impermeable latex substrate [14]. On the other hand, Liu et al. 2018 describe the use of live whole-cell bacterial biosensors embedded into 3D-printable hydrogels that can be placed over the skin using a removable-tattoo film [15]. Even more recently, Moser et al. 2019, demonstrated the capacity to pattern engineered Escherichia coli (E. coli) into diverse materials including 3D printing polymers,
polystyrene, and cotton as a method to potentially impart self-healing and sensing functions into such substrates [17].
While seminal, such previous examples encounter several limitations and implementation barriers, particularly concerning the need for maintaining living organisms within these devices during extended periods. In practice, sustaining viability and function of living cells in wearable sensing systems would require nutrient delivery and waste extraction capacities, as well as temperature and gas regulation; all of which have not yet demonstrated and would involve significant engineering hurdles. Additionally, continually evolving cell populations would likely incur in mutational pressures during device use, which could result in loss of the genetic phenotype and sensing function [18]. Moreover, genetically engineered cells would present biocontainment and potential biohazard concerns, particularly if integrated into consumer-level garments. This situation could lead to stringent regulatory pathways in many critical applications, which is undesirable from a product development and commercial perspective. Thus, a new approach in synthetic biology is needed to resolve the mismatch between the practical requirements for wearable devices and available functional tools to construct engineered biological circuits for sensing and response of desired targets. Achieving this could open several new applications for synthetic biology, allowing operation in a wide range of wearable substrates, such as smart materials, functional fibers, and textiles; all of which could benefit from added capabilities for the detection of molecular targets challenging to assess through other technologies [1].
Recently, it has been demonstrated that cell-free synthetic biology reactions; which are self-contained abiotic chemical systems with all the biomolecular components required for efficient sensing, transcription, and translation, can be freeze-dried into a shelf-stable format using porous substrates [12]. Genetically engineered circuits encoded in deoxyribonucleic acids (DNA) or ribonucleic acids (RNA) can be added to cell-free
freeze-dried systems to be activated simply by rehydration in a variety of real-world situations. Furthermore, lyophilized cell-free synthetic biology reactions exhibit high stability during transportation and storage, which could allow for robust and portable use of such systems without specialized environmental control or biocontainment requirements.
CF-FD synthetic biology systems have already been used to develop inexpensive paper-based nucleic acid diagnostics [19], highly sensitive nucleic acid sensors [19][20], on-demand production of antimicrobials, antibodies, and enzymes [21]; and as low-cost educational kits for teaching synthetic biology in schools [22][23][24]. We consider the use of cell-free freeze-dried technology as a fundamentally advantageous feature for a new paradigm of wearable synthetic biology since it eliminates many of the potential safety concerns presented by other biosensing platforms using living cells. Specifically, cell-free synthetic biology removes most concerns involving pathogenicity and the capacity of undesired perpetuation of these circuits [25]. Furthermore, while all previous literature in whole-cell biosensors is relevant for our objective, none has demonstrated the integration of cell-free freeze-dried synthetic biology networks directly into smart materials, fibers or textiles commonly used in wearable applications, nor have demonstrated their integration into mobile electronic systems for monitoring.
Hence, we propose the use of cell-free freeze-dried genetic circuits in combination with flexible polymeric and textile substrates as the core concept for new classes of practical wearable synthetic biology sensors. We believe this path constitutes a valuable, exciting, and feasible pathway for the development of new synthetic biology applications and future augmentation of smart materials and wearable fabrics. We believe this development will have particular value in the creation of protective clothing for clinical and military use (e.g., lab coats, biohazard suits, gas masks), as well as for in-vivo and in-vitro molecular biosensing (e.g. diagnostics, organ-on-chips). We envision a future in which cell-free wearable synthetic biology clothing and systems act as biomimetic machines capable of sensing and reacting to us and our environment.
0 U M
CHAPTER2
OBJECTIVE
PROJECT GOALS
In this work, we selected three independent strategies to achieve the goal of developing wearable cell-free freeze-dried synthetic biology (wCF-FD-SB) systems for small molecule, nucleic acid, and toxin detection integrated into flexible substrates, textiles and other complex materials. These sensors and actuators were integrated using various relevant synthetic biology circuits, such as toehold switches, transcriptional factors, riboswitches, fluorescent aptamers, and Casl2a-based sensors to establish the modularity and potential of such a concept (Figure 3). Furthermore, we demonstrate that these wearable systems and materials are capable of generating measurable colorimetric, fluorescence, luminescence, mechanical, and electrical outputs upon exposure to specific targets of interest, many under real-world environmental conditions.
Wearable Synthetic Biology
connectivity Sensor(s) Textile
substrate (s) Transducers Electronics Interphases 1010 Batteryi Data Power processing 1 0 ---- ---- --- --- -
-iSynBio Circuit Use-Case Target POC
* RioswtchSmall Molecules
Riboswitch &Toxlns Theophylline
*
)~
Zika/Ebola IToehold Pathogens Z
(RNA/DNA)
Transcriptional Bh s OrganophosphateI/
I Circuits Metaboes DHB-1T / Lactate
I SHROKISSHERLOCK
Nucleic Acids MRSA
(CRISPR-Casl2a) (RNA/DNA)
Figure 3: Summary of an example implementation of wearable synthetic biology
presented in further chapters. A variety of modules, including riboswitches, toeholds, transcriptional circuits, and Casl2a-based sensors were included for use in small molecule, pathogen nucleic acid, and metabolite detection. SynBio= Synthetic Biology,
POC= Point-of-care, CRISPR = clustered regularly interspaced short palindromic repeat, Cas12a= CRISPR-associated 12a enzyme. DHBI-IT =
Hydroxybenzylidene)-1,2-dimethyl-IH-imidazol-5(4H)-one. MRSA = Methicillin-Resistant S. aureus.
RESEARCH AIMS & DEVELOPMENT PLAN
The present thesis proposes and investigates different approaches for realizing wearable synthetic biology devices, all of which are included as part of five primary research aims. These aims consider relevant criteria for design and development defined through analysis of the most promising use-cases for this type of technologies, as well as availability of materials, wearable substrates, and targets, detectable using synthetic biology principles (Figure 4).
Targets
Toxins
Design Criteria:
Pathogens 1. Readily activation (<2hrs after exposure)
2. Field stability (no incubation/refrigeration) 3. Sample capturing
teriads 4.Fluid confinement
.5. Low evaporation
6. Operation at body surface temperature (~30°C) E
,
t eri7. Compatible with sensors of varying complexity
8. Possble detection of:
.eagents- siaimolecules
rC- Complex analytes
- Pathogen or virus nucleic acid
- Multiplexed Targets
on
ionlo
Figure 4: Platforms, targets, and design criteria considered for conceptualization and
fabrication of synthetic biology devices. The intersection of targets and platforms define potential use-cases.
The five research aims of this work, as shown in our project development plan (Table 1) were as follows:
Aim 1. Demonstration of cell-free freeze-dried (CF-FD) synthetic biology circuit
activation in fiber-based substrates and textiles.
A standard CF-FD synthetic biology expression circuits using
p-galactosidase (LacZ) or green fluorescent protein (GFP) output should be tested in wearable paper formats and simple fiber materials. This first stage will focus on experimentation of simple expression assays using linear or circular plasmids containing the desired output gene preceded by a T7 promoter (a DNA
sequence-specific to the T7 polymerase that allows for controlled transcription of a desired 23
downstream gene). Commercially available solutions containing reconstituted E. coli protein synthesis extract with T7 polymerases are available based on the work published by Shimizu et al., 2001 [26]. We intend to use such commercial solutions for cell-free extracts in these new applications. Such cell-free extracts contain all the necessary components needed for in vitro transcription and translation in purified form [26]. Cell-free extracts with the designed expression circuits will be then deposited and freeze-dry to produce LacZ and GFP within the textile substrates (e.g., paper, mercerized cotton, or other thread-like substrates) to test compatibility of these model reactions with such substrates. The fabricated samples will be rehydrated for testing using a target plasmid to induce expression and assess efficiency of the reaction. The output of the system will be either a colorimetric change or a fluorescence signal depending on the selected plasmid. In the case of the colorimetric output, the expression of p-galactosidase catalyzes the hydrolysis of the galactoside analog chlorophenol red-P-D-galactopyranoside (CPRG), causing a change in color from yellow to purple, which can be interrogated visually. In the case of GFP expression, the fluorescent signal will be interrogated by exciting the sample with a 470nm illumination source, to them measure fluorescence around the 510nm emission peak of wild-type GFP. This aim is covered in CHAPTER 3 and CHAPTER 4.
Aim 2. Fabric surveying and optimization of substrate handling and preconditioning
for CF-FD synthetic biology reactions.
After demonstrating the feasibility of implementing genetic networks in a small set of porous and thread-like materials, we aim to test a sample genetic module within a variety of flexible fabric materials. This would demonstrate feasibility of integration for wearable cell-free freeze-dried synthetic biology (wCF-FD-SB) reactions into textiles. A variety of natural and synthetic fabrics will be identified for testing (e.g., silks, cotton, rayon, linen, hemp bamboo, wool, polyester, polyamide, nylon, and combination materials). These textiles will be then treated to eliminate impurities or potential non-specific binding sites. Bovine Serum Albumin (BSA) blocking will be explored to prevent detrimental substrate
interaction with the cell-free synthetic biology reagents included in the used canonical LacZ, or GFP synthetic biology reaction explored in Aim 1. Blocked and unblocked fabrics will be processed and tested in parallel using an optical plate reader to compare expression reaction kinetics. We propose to investigate approximately 100 different fabrics to evince feasibility in a wide range of textile substrates. The best performing fabrics according to the collected kinetic and endpoint data will be used for further tests. Other relevant properties of these fabrics will also be investigated, including fabric thickness, network density, and autofluorescence. This aim is covered in CHAPTER 4.
Aim 3. Development of example wearable CF-FD synthetic biology sensors for the
detection of bodily metabolites, pathogens, and chemical toxins.
• Bodily metabolite detection: As demonstration for monitoring bodily
metabolites, we propose to implement a cell-free freeze-dried synthetic biology aptamers for the detection of Difluoro-4-Hydroxybenzylidene)-1,2-dimethyl-1H-imidazol-5(4H)-one (DHBI-1T); an inducible transcriptional regulation system based on a tetracycline repressor (tetR), as well as modeling of lactate sensing circuits for embedding into selected textiles. This sub aim is covered in CHAPTER3andCHAPTER4.
• Pathogen product detection: We propose to implement a Zika virus sensor,
an Ebola virus sensor, Human Immunodeficiency Virus (HIV), Lyme disease and a Methicillin-resistant Staphylococcus aureus (MRSA) DNA sensor genetically encoded to operate within a subset of our materials and textiles to demonstrate pathogen detection systems for fluid splashes. In this investigation, MRSA is treated as a key target for detection, sue to being a leading cause of hospital-acquired infections and death. MRSA that has recently become a health priority for several agencies and ministries worldwide [27]. The mortality burden of MRSA infections in the USA currently exceeds those attributable to Alzheimer's disease, HIV/AIDS or even homicide [28]. The resistance to
p-lactam antibiotics, which defines most MRSA strains, can be detected by the presence of the mecA gene [29]. We propose to use this trait, in combination
with other common genes for resistance and virulence in S. aureus (e.g. ermA,
spa) as a desirable demonstration for genetically encoded MRSA sensors [30].
Sensing of such targets is designed specifically for highly sensitive programmable CRISPR-Cas2a-based sensors. This sub aim is covered in CHAPTER3,CHAPTER4,CHAPTER5and CHAPTER6.
•Chemical toxin detection: We also propose to demonstrate a genetic sensor encoded in fabrics capable of reacting to the presence of a toxin or harmful chemical in the environment with a fast reaction approach (e.g., harmful environmental molecules or nerve agents). To evince this application without the potential risk of testing such dangerous compounds, we propose to detect theophylline, a nontoxic molecule commonly found in tea and coffee. To provide a rapid alarm, the integration of riboswitches [31][32] into our sensing model is demonstration of this capacity, which for many targets are known to reach measurable outputs in as little as 5min. This sub aim is covered in CHAPTER 3
and CHAPTER 4.
Aim 4. Interrogation of wearable CF-FD with optical sensors and electronic devices
for mobile Interrogation synthetic biology network outputs
For this aim, we will demonstrate that wCF-FD-SB sensors can be integrated be interfaced with electronic systems (e.g., RFIDs & smartphones) through the use of electronic or optical interrogation. We also aim to demonstrate that these systems can be operated using mobile applications. To accomplish this, we intend to use fiber optic weaves to detect the fluorescence or luminescence signal from the activated synthetic biology sensors embedded into our selected fabrics, as well as direct interrogation of synthetic biology responsive materials. This aim is covered in CHAPTER 4, CHAPTER 5 and CHAPTER 6.
Aim 5. Garment-scale integration of wCF-FD for automatic in-vivo target detection
in textiles.
We will attempt to fabricate a variety of garments (e.g., armband, lab coat, sports shirt, long-sleeve, or mask) featuring the best performing wCF-FD-SB
sensors with optical or electrical interrogation. In these devices, we will attempt to confirm target specific outputs as well as automatically interrogate such sensors during simulated tests using an internet-of-things (loT) enabled
microcontroller unit.
Table 1: Gantt chart of project development for wearable cell-free freeze-dried synthetic
biology core technologies and demonstrations. The development timeline is divided into
6-month periods.
Aim 1. Demonstration of CF-FD synthetic biology functionalization of a natural fiber-based textile with a standard direct expression circuit using 0-galactosidase (LacZ) or Green Fluorescent Protein
(GFP)
Synthetic-biology fabric substrate survey X Testing of LacZ T7 and GFP fluorescent reaction on textile substrate X
as a standard model for synthetic- biology sensor.
Aim 2. Fabric surveying and optimization of substrate handling, storage, and preconditioning for CF-FD synthetic biology reactions.
Selection of best-performing fabrics for CF-FD synthetic biology X
according to kinetic runs.
Mechanical testing of best-performing fabrics X Optimization of washing procedure, BSA blocking, and pre- X
conditioning of selected fabrics
AIm 3. Development of example, wearable CF-FD synthetic biology
sensors for detection of bodily metabolites, pathogens, and chemical toxins.
Development of cell-free freeze-dried reactive sensor with colorimetric X
output for MRSA detection.
Development of cell-free freeze-dried reactive sensor with colorimetric X
output for lactate detection.
Development of cell-free freeze-dried reactive fabric using a X
theophylline synthetic riboswitch with a colorimetric output.
Aim 4. Interrogation of wearable CF-FD with optical sensors and electronic devices for mobile Interrogation synthetic biology
network outputs
Integration of optic fibers and optical sensors into CF-FD synthetic X
biology fabric to measure colorimetric output.
Integration of loT sensing and communication for wearable synthetic X
biology fabric using a portable microcontroller
Proof-of-concept of automatic sensing and notification push upon
target presence using appropriately programmed logic in the selected X
microcontroller platform.
AIm 5. Garment-scale Integration of wCF-FD-SBfor automatic in-vivo target detection
Fabrication of large-scale smart garment, shirt, or lab coat using best- X
performing wearable synthetic biology sensor and fabric investigated.
Validation of prototypes X
Compilation of results X
27
E
m U U
CHAPTER
3
COLORIMETRIC
WEARABLE SYNTHETIC BIOLOGY PLATFORM
MOTIVATION AND GENERAL APPROACH
For the first wearable cell-free freeze-dried synthetic biology (wCF-FD-SB) sensing platform demonstration, it was selected to embed colorimetric genetic circuits into a
cellulose substrate surrounded by an optimized fluid wicking and containment platform made of flexible elastomers (Figure 5).
1. Frem-Dried Synthetic DNA Reaction ON Susrae Contaffmaed spot 2. Rehydrated Reaction Translaon
-Coym
owretric upAWearable Synthetic Biology Yields Visual SignalPositive Detection
Figure 5: Schematic of the colorimetric platform for wCF-FD-SB sensors. Various
potential users are depicted (right). The basic principle of colorimetric wCF-FD-SB sensor operation, device, and method of activation is shown (right). Cell-free synthetic biology components, including genetic circuits, transcription/translation machinery, and
substrates are immobilized into a porous substrate (e.g. paper) and surrounded by flexible elastomer chambers to control rehydration conditions.
Using p-galactosidase as output for this colorimetric platform, a yellow to purple color change develops upon exposure to a fluid containing the target of interest. The design and fabrication of these wearable platforms followed from the inspection of
real-29 It.7,
world functional parameters. For instance, CF-FD components activated by exposure to an aqueous agent in the field will likely occur through a variable rehydration volume
(<100pQL) and under different environmental conditions, ranging from 20-40% relative
humidity (RH) and 20-37C. Therefore, we ought to design such wearable sensors considering methods to prevent genetic circuit inhibition due to underflow or excessive dilution of reaction components. Furthermore, we designed these devices to exhibit reduced evaporation rates after rehydration. To meet these requirements, we chose to create a three-layer flexible device with optimized reaction containment and fluid wicking properties (Figure 6). This device was iterated towards a design that directs fluid reactions to hydrophilic fiber networks (e.g., paper) enclosed within flexible impermeable chambers made of silicone elastomers. In this device model, genetically encoded circuits can be immobilized within the fibers of hydrophilic materials placed in internal reaction chambers. Then synthetic biology components can be made shelf-stable until rehydration
by fluid exposure through a freeze-drying process. The impermeable reaction chambers were designed to define a maximum rehydration volume (-50tL) and were tested to confirm reduced evaporation rates as compared to open reactions. The resulting devices were elastic and flexible (Figure 6c,d), permitting their potential use as wearable garments.
Figure 6: Assembly layout (a), as well as top view (b) of colorimetric wCF-FD-SB
device. Stretching (c) and twisting (d) of the device are shown to evince the elastic properties of the fabricated materials used. White bars indicate 15mm.
30
MATERIALS AND METHODS
FABRICATION OF COLORIMETRIC SYNTHETIC BIOLOGY WEARABLE DEVICE
In order to fabricate the colorimetric device shown in Figure 6a for testing with synthetic biology circuits, a series of casting and assembly steps were taken. First translucent (top) and opaque (middle and bottom) layers were made using Ecoflex@ silicone elastomer (Smooth-On, Inc, Macungie, PA), which was precast overnight at 650C
in flat plates with cardboard bottom coated with silicone release agents. Cured 2-3mm Ecoflex@ sheets were then laser-cut according to the layout shown in (Figure 7). Cut silicone layers were then released from cardboard backing and bonded together by depositing freshly-made liquid silicone elastomer at each layer interphase. Assembled layers with uncured elastomer were left overnight at 650C for assembled post-curing in a
well-ventilated oven. Clean WhatmanTM No. 4 0.5mm thickness filter-paper (GE Healthcare Lifesciences Inc., Chicago, IL) was cut to 10mm diameter disks (Figure 6a). This was done by using 10mm biopsy punches or laser-cut processes. Cut disks were then used to deposit the cell-free synthetic biology reactions containing the colorimetric reporting circuits. 5.19 mm 13 mm =I1.85mm 75mm >
0
0
4 90
30mmFigure 7: Assembly layers of the colorimetric wCF-FD-SB device. The layout of
elastomer layers in the colorimetric wCF-FD-SB device is shown.
Saturated reaction disks were then snap-frozen using liquid nitrogen and put in a
vacuum container to be dried for 4-8 hours using an SP Scientific Freezemobile yophilizer
(SP Industries, Inc., Warminster, PA). Freeze-dried reaction disks were then inserted
through the wicking ports of the elastomer chambers for final assembly before testing. The silicone elastomer chambers in the colorimetric device were made to have three 3x5mm curved wicking ports in each of the four wells. This design was confirmed to allow for entry of fluids such as dd-H20, while still delaying evaporation of cell-free reaction
after splash activation (Figure 8a) in at least 20% after 30 min. The bonded device chamber walls prevent flow or diffusion of the reaction after rehydration and appear to have minimum interaction with cell-free components. Fluid wicking at the entry ports after exposure with a contaminating spill is mediated by capillary action. This event then leads to rehydration of the reaction disk containing the chosen wCF-FD-SB system, which marks t=0 in further validation experiments(Figure 8a). A magnified view of an example activated Ebola virus DNA toehold wCF-FD-SB sensor within these chambers is shown in Figure 8b.
a
Dryb
Fluid Splashmin
60min
Figure 8: Sample activations of wFD-CF-SB colorimetric device for the detection of
Ebola virus RNA using toehold switches. (a) Port wicking into reaction disks using dd-H20 fluid splash. Rehydrated paper disks are visibly darker after fluid entry and wicking
into the substrate (t=Is after splash). (b) Activation of colorimetric Ebola virus DNA toehold switch wFD-CF-SB sensor using a 50pL splash of dd-H20 sample containing
300nM Ebola RNA trigger as compared to control (t=60min).
VALIDATION OF COLORIMETRIC wCF-FD-SB DEVICES
The versatility of the developed colorimetric cell-free freeze-dried synthetic biology sensor platform was further verified using four independent modules upstream to a lacZ
P-galactosidase
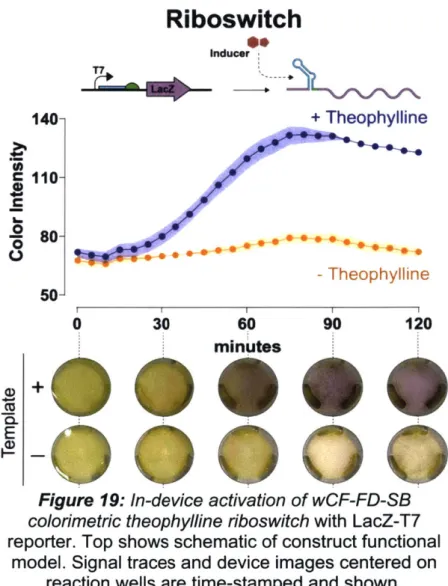
operon. These demonstrations include: (1) Sensing of a constitutive LacZ-T7 plasmid for expression (Figure 9 and Figure 10), (2) Detection of anhydrotetracycline (aTc) through a tetracycline repressor (TetR) transcriptional regulation circuit (Figure 11), (3) Sensing of Ebola virus RNA presence via a toehold switch (Figure 13); and (4) Identification of theophylline presence using a molecule specific riboswitch (Figure 19).CONSTITUTIVE LACZ EXPRESSION
Testing of constitutive expression using plasmids is a benchmark of basic operation for many cell-free synthetic biology systems. In the case of this demonstration, a series of experiments were conducted using fully assembling wCF-FD-SB devices with reconstituted cell-free protein synthesis extract from E. coli freeze-dried over the reaction disks. To test activation a fluid sample with constitutive LacZ-T7 plasmids at desired concentrations was prepared and splashed over the devices. Colorimetric change from yellow to magenta is indicative of transcription and translation activation within the system. The dynamics of such a reaction can be seen in Figure 9.
Constitutive LacZ-T7 expression reactions for wFD-CF-SB testing were prepared by combining 50pL of 1x NEB cell-free PURExpress@in vitro protein synthesis solution (New
England Biolabs, Inc., Ipswich, MA) with 0.5% RNAse Inhibitor and 5 ng/tL constitutive LacZ-T7 plasmid in positive (+) reactions or without for controls (-). Prepared reactions were quickly deposited in the pre-cut 10mm paper disks to be snap-frozen and lyophilized for 4-8hrs for later insertion into the device. Activation of these model circuit was achieved
by rehydration with a fluid splash of dd-H20 (50ptL). Dynamics of the reaction after
rehydration with splash were recorded to generate side-to-side traces and images depicting color changes within the reaction wells during the experiment.
Constitutive Expression
200~ + template C 150-10 0 -o - template 50 I I I I I 0 30 60 90 120 minutesFigure 9: In-device activation of wCF-FD-SB colorimetric constitutive
LacZ-T7 expression. Top shows schematic of construct functional model. Signal traces and device images centered on reaction wells are
time-stamped and shown.
All presented wFD-CF-SB colorimetric constitutive LacZ-T7 samples were done in triplicate and exhibited visible colorimetric changes within -60min upon exposure to sample containing such plasmid. Environmental conditions during activation were confirmed to be within 30-40% RH range at 300C. Further validation of this basic
expression system was done using additional replicates to verify the range of dynamic
behaviors presented by similar activation conditions, as seen in Figure 10.
In these tests, three positive replicates (5 ng/L of constitutive LacZ-T7 plasmid) and three negative replicates (0ng/pL of constitutive LacZ-T7 plasmid) were both
activated with fluid splashes at t=0. While signal for all of the positive replicates reached maximum signal by t=60min, some replicates exhibited visible color change within 20min,
suggesting that reaction optimization is achievable to reduce activation times of these
types of constructs. Subtle, but computer measurable color change in all replicates was observed at t=30min.
0
omin 15min 30min 45min 60min
Figure 10: Sample performance test ofcolorimetric wCF-FD-SB with constitutive
LacZ-T7 module. Activation of colorimetric prototype wells is shown using 5ng/pL constitutive
LacZ-T7 plasmid in a504Lrehydration splash as compared to rehydration with no
plasmid. Color change in one positive replicate was visible within 20min.
TRANSCRIPTIONAL REGULATION ATC SENSOR
Genetic circuits using transcriptional regulators are among some of the most common
types of synthetic biology switches. Here we report on the implementation ofan
anhydrotetracycline (aTc) sensor based on atetracycline repressor (TetR) circuit. The
TetR circuit isan interesting model to show the detection capabilities of our system since
TetR proteins play akey role in antibiotic resistance for several bacterial species [33].
Anhydrotetracycline constitutes aderivative of tetracycline that exhibits no antibiotic
activity, thus being acomplex, but controlled model molecule suitable for testing in our
cell-free platform. Thus, with this demonstration we aim to show that our colorimetric
wearable platform is suitable for other similar biomolecule detection applications.
Colorimetric change from yellow to magenta in Figure 11is indicative of aTc detection
within the system. The dynamics of such areaction can also be seen in Figure 11.
The TetR transcriptional regulation reactions used in Figure 11 were prepared in
50iL batches using 20pL of NEB cell-free PURExpress@ solA, 15ptLNEB solB, 2.0piLof
chlorophenol red-p-D-galactopyranoside (CPRG) at 30 pg/tL, 1.3tL murine RNAse inhibitorand 11.7pL of pJL1-tetO-LacZ sensor plasmid at 5 ng/pL. TetR was also obtained
by cell-free expression with subsequent purification and then integrated into the main
reaction. All reagents were resuspended in dd-H20up to final 50pL volumes. Prepared
sensor reactions (50pL per well) were then quickly deposited in individual 10mm paper reaction disks to be snap-frozen and then lyophilized for 4-8hrs. Immobilized freeze-dried reactions were then inserted within the device reaction well through the wicking ports. Sensor activations were achieved by rehydration with a fluid splash of dd-H20spiked with 25pg/mL anhydrotetracycline (aTc) trigger for the positive samples, while Opg/mL aTc trigger was used for controls. All wFD-CF-SB colorimetric transcriptional TetR-LacZ-T7 sensor tests were done in triplicate and exhibited visible colorimetric changes within -60min upon exposure to 25pg/mL aTc at 30-40% RH and 300C.
TranscriptionalRegulation
Inducer --- I TetR 170-+aTc * 140-C .2110- &-0 8 80- 50-0 30 60 90 120 minutes 0+ CL EFigure 11:
In-device activation of wCF-FD-SB colorimetric TetRtranscriptional regulation of LacZ-T7 expression aTc sensing. Top shows schematic of construct functional model. Signal traces and device images
centered on reaction wells are time-stamped and shown.
TRANSCRIPTIONAL REGULATION CIRCUIT FOR LACTATE SENSING
Apart from the previously described sensing targets, lactate constitutes an important biomolecule for sensing (e.g., body metabolite), which could become more readily monitored in the future using synthetic biology. Indeed, lactate is currently one of the most relevant biomarkers to assess oxidative stress in tissue and is a critical metabolite in monitoring physical performance [34]. Lactate is also a relevant biomarker to evaluate patient status in clinical settings [34]. During intense physical activity or stress, the usual aerobic metabolism in humans becomes incapable of satisfying the energy demand. This situation activates glycolysis, an anaerobic pathway that uses stored glycogen in muscle to produce energy. Lactate is generated as a sub-product of this reaction in a process known as lactate acidosis, which over time also increases the concentration of lactate in blood and sweat [35][36].
Given the importance of lactate monitoring in many clinical and sport-related applications, several commercial lactate sensors already exist, usually relying on finger-prick blood and electrochemistry equipment. While sufficiently sensitive, these devices are still too invasive and inconvenient for regular use, which limits their application in the detection of physiological stress inside and outside the clinic [34]. Here we report on a model lactate sensor with feasibility for wCF-FD synthetic biology platforms, which could be used through activation with human sweat. While not directly tested in our textile-based platforms, sensor development was conducted with encouraging results for future implementation as a full wCF-FD-SB sensor.
Our selected lactate biosensor builds upon those described in live E. Coli by Goers et al. 2017 [37]. In this model (Figure 12a), the absence of lactate causes dimers of the FadR-type regulator protein (LdR) to bind to the operator sites in the IldPRDp promoter of the sensor forming a tetramer that sequesters DNA. This prevents the transcription of the operon which reports on transcription events. When lactate becomes present in the network, it interacts with LIdR. Dissociating the LIdR-dimer bound to 02. This event then opens the LIdPRDp complex, which causes the dimer bound to 01, becoming a transcriptional activator that promotes transcription of the operon (Figure 12b).
a
dP pgTmSm~VA -MgjTWwt ~ "Ma a0 -LactatA asey A ~C. 7 7 c I -- 14.0 mM Lac 10 mMLac . 0.8 mM Lac 0.5 WMLac 0 2 mMLac 0 1 mMLac 0 05M Lac 0 mM Lac(Modfied from Goers et aL. 2017)
b
I
I
Unlocked
Locked
Unlocked
Lactate-(Added during cell-free protein synthesis)
Lactate sensor T7-IdPRD Promoter Testing at 300C
-6- pJL-T7-ldPRD::LacZ (20ng/uL)+ pJL1-fGFP (20nguL)
-- pJL1-T7dPRD::LacZ (20nguL)+ pJL1-UdR (20ng/uL)
.4.No Tmplate
4
4x
Repression
LidR must be added to CFPS
•Requires Ecoi RNAP.
Purify His-tagged LdR. • Pre-incubate stoichiometric
amounts of purified LIdR with the T71IdPRD Lactate
• Biosensor Template.
* LacZ output (convenient)
0:00 0:30 1:00 1:30 2:00
Tilms(hr)
Figure 12: (a) Model for synthetic biology Lactate sensor based on transcriptional
regulation, modified from Goers et al. 2017 [37]. (b) Mechanisms of sensor operation.
(c) Optimized cell-free lactate sensor behavior tested off-device.
38 m U S * urn is. zu
*
Lactate
3LIdR
C
5. 4- 3- 02-0X_
The transcription event accompanied by the presence of lactate can drive a colorimetric or fluorescence change depending on the reporting operon use. Furthermore, it was confirmed that the addition of Lactate dehydrogenase (LDH) to the reaction mix is capable of reducing the background though the base utilization of lactate in the sample. In the case of this model design, a genetically encoded sensor was experimentally achieved and was optimized to generate activity under physiologically relevant lactate concentrations (30mM), as seen in Figure 12c. However, this is only shown here as plate-reader data with dynamics of such sensor in absorbance (colorimetric) mode without direct implementation in our wearable platform. While the presence of lactate in combination with the T7-LdPRD appeared to generate signal as compared with no template controls in these tests, the experiments conducted in freeze-dried format did not achieve such a robust response. While unfortunate, the previous demonstration of TetR-based transcriptional regulation circuit suggests that upon further optimization the above-proposed lactate sensor model could be readily incorporated into our wearable platform.
TOEHOLD SWITCH EBOLA SENSOR
Toehold switches are another important class of highly programmable nucleic acid sensors that utilize hybridization to target RNAs to convert hairpin-locked transcripts into translationally-competent systems [4][38]. Figure 13 shows the use of an optimized Ebola virus RNA toehold sensor [12] within our platform, which suggests that other similar viral nucleic acid sensors with colorimetric output are achievable. Ebola RNA Toehold switch reactions for colorimetric wFD-CF-SB testing were prepared using 1x NEB cell-free PURExpress@ with 300nM Zaire Strain mRNA Ebola Virus Toehold sensor in dd-H20.
Prepared sensor reactions were quickly deposited in a 10mm paper disks to be snap-frozen and then lyophilized for 4-8hrs. Activation of sensors was achieved by rehydration with dd-H20spiked with 2pM of freshly made Ebola trigger RNA for the positive samples,
while OpM Ebola trigger RNA was used for controls. All reported wCF-FD-SB colorimetric toehold sensors for Ebola virus detection were done in triplicate and exhibited visible colorimetric changes within 60min of exposure to these relevant trigger conditions, at 30-40% RH and 30°C
Toehold Switch
Viral RNA e% 200- + Ebola RNA C SO C150 2100-a
-Ebola RNA50-0 30 60 90 120 minutes
Figure 13: In-device activation of wCF-FD-SB
colorimetric Ebola virus toehold switch with LacZ-T7
expression. Top shows schematic of construct functional model. Signal traces and device images centered on
reaction wells are time-stamped and shown.
TOEHOLD SWITCH MRSA SENSOR
Expanding on our results for the wearable Ebola Virus toehold switch sensor, it is
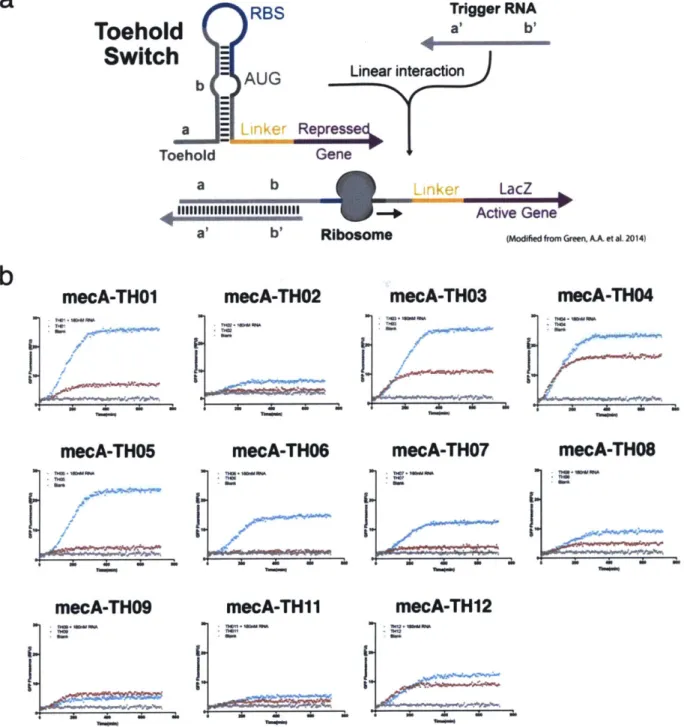
possible to design other similar circuits for the detection of free nucleic acids from pathogen presence in environmental samples [38]. In particular we propose the implementation of a freeze-dried toehold switch system capable of detecting the presence of Methicillin resistance genes in S. aureus, such as the mecA or ermA genes, or even the presence of S. aureus virulence through the spa gene. While not finally implemented as a sensor in our colorimetric wearable platform, we conducted design and validation tasks towards a functional toehold switch sensor for MRSA based on mecA trigger detection. We envision such toehold switches could be embedded and used in our
FD-SB colorimetric platform if needed after due optimization. A schematic of the proposed functionality of such toehold switches is presented in Figure 14a. Moreover, validation data acquired for eleven of such mecA-specific toehold switches in plate-reader conditions are shown in Figure 14b.
a
RBS Trigger RNAToehold
a' b'Switch
AUG Linear interaction
b a Linker Represse Toehold Gene a b a' b' Ri Linker LacZ b Active Gene'
(Modified fromGreen, A.et al. 2014)
bosome mecA-TH01 mecA-TH05 mecA-TH02 mecA-TH06 mecA-TH03 mecA-TH07 mecA-TH04 mecA-TH08
mecA-TH09 mecA-TH11 mecA-TH12
Figure 14: (a) General toehold switch model for the detection of nucleic acids via
colorimetric outputs, modified from Green, A.A. et al. 2014 [38]. (b) Experimental results of screening of colorimetric mecA-specific toehold switched for potential use in
wCF-FD-SB sensors. Sensor TH05 demonstrated the best performance from the tested set.
41