Cognitive resilience is mediated by the MEF2 network
in mice and humans
by
Scarlett J.V. Barker
B.S. Princeton University (2012)
Submitted to the Department of Brain and Cognitive Sciences in partial fulfillment of the requirements for the degree of
Doctor of Philosophy in Brain and Cognitive Sciences at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY February 2021
© Massachusetts Institute of Technology 2020. All rights reserved.
Author: . . . Department of Brain and Cognitive Sciences
November 30, 2020
Certified by: . . . Dr. Li-Huei Tsai Director of the Picower Institute for Learning and Memory Thesis Supervisor
Accepted by: . . . Dr. Rebecca Saxe Department Head of Graduate Education
Cognitive resilience is mediated by the MEF2 network in mice and humans
by
Scarlett J.V. Barker
Submitted to the Department of Brain and Cognitive Sciences on December 1, 2020, in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Brain and Cognitive Sciences
Abstract
Recent increases in human longevity have been accompanied by a rise in the incidence of dementia. While a large proportion of aged individuals display pathological hallmarks of neurodegenerative disease, a small number of these individuals are able to maintain healthy cognitive function even in the presence of extensive brain pathology. The molecular mechanisms that govern this neuro-protected state remain unknown, but individuals that exhibit cognitive resilience (CgR) represent a unique source of insight into potential therapies that could preserve brain function in the face of
neurodegenerative disease. Here, we employ a two-pronged approach to dissect the mechanism underlying CgR. First, using multiple integrated repositories of clinical and brain transcriptomic data we identified individuals who maintained normal cognition despite harboring a large burden of Alzheimer’s disease (AD) pathology. We observe significant up-regulation of MEF2 family members in these resilient patients when compared to patients whose cognition declined in the presence of pathology. Second, we utilize the only existing animal model of CgR – environmental enrichment – to investigate the molecular mechanisms involved in the induction of resilience. Accessibility of Mef2 binding sites, and expression of Mef2 targets are significantly increased upon enrichment. Additionally, knockdown of Mef2 family members just prior to the initiation of enrichment block its cognitive benefits, demonstrating the necessity of Mef2 activity for achieving the enhanced cognitive potential afforded by enrichment. Neurons lacking Mef2 are hyperexcitable, which is also one of the earliest pathological alterations observed in AD. These results suggest a potential mechanistic link between the Mef2 transcriptional network induced by enrichment and the prevention of disease-associated hyperexcitability. To determine the causal impact of Mef2 on cognition in the context of neurodegeneration, we use a viral approach to manipulate the PS19 mouse model of tauopathy. Remarkably, in the absence of enrichment, Mef2 overexpression alone is sufficient to improve cognition and reduce hyperexcitability in PS19 mice. Overall, our findings reveal a novel role for MEF2 transcription factors in promoting cognition throughout life, and maintaining cognitive resilience in the context of neurodegenerative disease.
Table of Contents
Acknowledgements………..7
List of Figures……….9
Chapter 1. Introduction………..….…..13
Chapter 2. Effects of an enriched environment on mouse behavior and the molecular properties of cortical neurons………...19
Chapter 3. Effects of Mef2a/c manipulation on mouse behavior and the molecular properties of cortical neurons………...53
Chapter 4. Cognitive resilience in humans is associated with a MEF2-transcriptional signature………..67
Chapter 5. Discussion………..93
Methods………103
Acknowledgements
I firmly believe that scientific advancement is not an individual endeavor, but one that relies on teams, mentorship, and the insight of past researchers. Therefore, this thesis represents the work of a team of people, the insight of an entire lab, and the refinement that comes from brainstorming with a community of scientists.
I am so grateful to my PhD advisor, Dr. Li-Huei Tsai, for allowing and encouraging me to join her lab. I learned more than I could’ve imagined, and was given the opportunity to pursue knowledge that might one day enhance the human experience. Li-Huei is the ultimate role model: 1) I have never seen her back down from a challenge, and no goal is out of reach for her or our lab. 2) Li-Huei is an extremely hard worker. She often comes in to lab on weekends — always with a box of tasty treats for everyone else — and prioritizes catching up with her students and post-docs from among her long list of responsibilities. I am amazed that she can head the Picower Institute, give lectures around the globe, manage a huge lab, and still always make it to lab meetings and happy hours on Fridays. I hope I can one day learn her time management skills. 3) Li-Huei is my inspiration to become a leader. She understands the importance of a
cohesive team, and she engenders so many qualities that young women can look up to. Li-Huei, I will always be grateful to you for changing my life for the better, especially during a difficult period.
I literally couldn’t have done this without my lab partner, Dr. Ravi Raju. We are such a good team! I loved all of our brainstorming sessions, debates, and latte conversations; I love that you are so “OCD” because I’m sure it enhanced the quality of the experiments we did together; I love that you see the value in life beyond science because it helped give me perspective. I know you’re going to be an amazing PI and, more importantly, live a happy life full of people who are better off because they know you. Thank you for everything.
One of my favorite parts of grad school was having the opportunity to mentor young scientists. I was so fortunate to work with one of the smartest, kindest, and most passionate undergraduates at MIT, Fatima. Shoutout to the rest of the EASE team, Noah, Karim, and Djuna, for making lab extra fun, and to all of my co-authors — I’m praying that our paper is published some day!
Thank you to my many scientific mentors: Dr. Marc Diamond, for helping me believe in my ability to pursue science; Dr. Manolis Kellis, for his never-ending enthusiasm for learning and discovery; Dr. Myriam Heiman, for pushing me to think about the details with her keen ability to identify the root of a question; Dr. Mriganka Sur, for his
invaluable and thought-provoking big-picture questions; and Dr. Elly Nedivi, for helping me become a better teacher and communicator from her example. I have had so many lab mentors throughout the years; I would like to name a few who went above and
Drs. Ram, Jay, and Chinna for your willingness to brainstorm with me, especially on late nights in lab. Thank you Dr. Hiruy Meharena and Dr. Asaf Marco for your scientific guidance and for being my lab family; I couldn’t have done it without you guys.
The most valuable parts of my life are the connections I make with other people, and I was so fortunate to have an amazing group of friends during my time at MIT. I can’t possibly name everyone who has shown me kindness on this journey. From the
philosophers I met early on, to my Cable neighbors (kfrey!) who became my Cambridge family and second home, to my lab friends who were the reason I started so many Saturday mornings (afternoons) sipping coffee in the break room and reminiscing about shenanigans, to my BCS friends who always said yes to an adventure (NP) or evening atrium chat when we should have been working, to my friends in different states who didn’t give up on me even when I was terrible at texting back — thank you all.
And finally, thank you to my family, both by genes and by circumstance. Natasha, Yael, Jonathan, Erick, Fatema — I hope you’re always in my life because you fill it with happiness. To my mom, dad, sister (Krizia), brothers (Coqui and Titi), niece (Camila), and nephew (Sebastian) — thank you for supporting and encouraging me from the start, for enriching my childhood, and for setting me on this path.
List of Figures
Figure 1. Modeling cognitive enrichment in mice
1a. Cognitive activity throughout life correlates with end-stage cognition 1b. Experimental design
1c. Housing conditions
Figure 2. Behavioral differences between mice housed in enriched vs. standard conditions
2a. Locomotion and anxiety measured via open field test
2b. Associative memory and cognitive flexibility measured via fear conditioning and extinction training
2c. Fear conditioning following no running wheel in the enrichment chamber 2d. Working memory measured via radial arm maze
Figure 3. Behavioral differences between mice housed in enriched, standard, and isolated conditions
3a. Anxiety measured via the open field test
3b. Associative memory measured via fear conditioning
3c. Behavior tests following environmental switch between enrichment and isolation
Figure 4. Differences in neuronal properties between mice housed in enriched vs. standard conditions
4a. Electrophysiological recordings from mice housed in enriched vs. standard conditions
4b. Immunohistochemistry of presynaptic signaling molecules, VGAT and VGLUT
Figure 5. Purifying neuronal nuclei for molecular analysis
5a. Flow cytometry assisted nuclear sorting 5b. Quantitative PCR of cell-type-specific loci
5c. Cell-type enrichment analysis of open regions mapped to nearest gene
Figure 6. ATAC-seq analysis in cortical neurons
6a. Principal component analysis of all ATAC-seq peaks
6b. Differentially accessible regions in cortical neurons from mice housed in enriched vs. control conditions
6c. Distribution of differentially accessible regions throughout the genome
Figure 7. Transcription factor motif analysis in differentially accessible regions (DARs)
7a. Overrepresented motifs between mice housed in enriched vs. standard conditions 7b. Mef2 motif abundance in DARs from enriched vs. standard-housed mice
Figure 8. RNA-seq analysis in cortical neurons
8a. RNA expression correlates with DNA accessibility and active histone marks 8b. Differentially expressed genes between mice housed in enriched vs. standard conditions
8c. Overlap between enrichment DEGs and transcription factor target genes 8d. Quantitative PCR validation in cortical tissue
8e. Immunohistochemistry of Mef2 nuclear protein levels 8f. Gene ontology enrichment analysis of cellular components
Figure 9. Olfactory receptor accessibility and expression in cortical neurons
9a. Sample ATAC seq traces from differentially accessible olfactory receptors between enriched and standard-housed mice
9b. In situ hybridization Olfr1396
9c. Quantitative PCR from bulk cortical tissue
9d. Differential expression of olfaction-related genes in cortical neurons between enriched and standard-housed mice
Figure 10. Knockdown of Mef2a/c in frontal cortex of wild type mice
10a. Mef2a/c knockdown via lentivirus
10b. Effect of Mef2a/c knockdown on body mass 10c. Effect of Mef2a/c knockdown on locomotion
10d. Effect of Mef2a/c knockdown on fear extinction training
Figure 11. Effect of Mef2a/c knockdown on cellular and molecular properties
11a. Electrophysiological recordings
11b. RNA in situ hybridization of Mef2 target genes
Figure 12. Effect of enrichment on neuronal properties of PS19 tauopathy mice
12a. Gene ontology enrichment among down regulated genes in neurons from PS19 mice
12b. Electrophysiological recordings from PS19 mice living in enriched or standard housing
12c. Immunohistochemistry of Mef2a nuclear protein levels
Figure 13. Overexpression of Mef2a/c in PS19 tauopathy mice
13a. In situ hybridization of GFP, Mef2c, and Mef2 targets after AAV injection 13b. Effect of overexpression on body mass
13c. Effect of overexpression on fear extinction training
13d. Effect of overexpression on electrophysiological properties
Figure 14. Analysis of RNA-sequencing from bulk cortical tissue
14a. Clinical and pathological distribution of patients in MSBB and ROSMAP cohorts 14b. Gene ontology enrichment for genes that correlate with end-stage cognition (Cog) and cognitive resilience (CgR)
Figure 15. Involvement of the MEF2 transcriptional network in human cognitive resilience
15a. Enrichment of transcription factor target genes in Cog and CgR gene lists 15b. MEF2C levels correlate with end-stage cognition
15c. Relative expression of Mef2a and Mef2c in mice and humans
15d. Coexpression correlation of specific transcription factors (Mef2a/c and immediate early genes ARC and FOS) with Cog and CgR genes
Figure 16. Direct comparison of resilient and non-resilient patients (MSBB)
16a. Patient information
16b. Transcription factors upregulated in resilient individuals
16c. Coexpression values between MEF2C and genes that are unregulated in resilient individuals
16d. Gene ontology enrichment analysis of differentially expressed genes between resilient and non-resilient individuals
Figure 17. Single-nucleus RNA-seq from resilient and non-resilient patients (ROSMAP)
17a. Patient information 17b. Cell type markers 17c. UMAP of all nuclei 17d. Cell type proportions
17e. Number of nuclei and DEGs identified for each cell type
17f. Gene ontology enrichment for resilience DEGs in each cell type
Figure 18. Subclustering major MEF2-expressing cell types
18a. MEF2 expression across cell types
18b. Subclustering of excitatory neurons, inhibitory neurons, and microglia 18c. Expression of MEF2A and MEF2C in resilient and non-resilient sub clusters 18d. Enrichment of MEF2 target genes in resilience DEGs across sub clusters
Figure 19. Excitatory neuron subcluster analysis
19a. Cortical layer enrichment among excitatory neuron subclusters 19b. Gene ontology enrichment of excitatory neuron subcluster markers
19c. Gene ontology enrichment of resilience DEGs in excitatory neuron subclusters
Figure 20. Mef2 in microglia
20a. Immunohistochemistry of Mef2a and Mef2c in Iba1-positivie cells in mouse cortex 20b. Effect of microglial activation on MEF2 expression in human induced-microglia
Chapter 1: Introduction
Cognitive resilience: Escaping the burden of aging and Alzheimer’s
Human lifespan has increased substantially – nearly a decade in the Unites States in the past fifty years – and great strides are being made to identify modifiable targets that might further enhance longevity (1). However, this extension of lifespan is often
associated with a decrease in quality of life (2), highlighting that measures to extend lifespan must also address how to maintain functional capacity as we age. One of the biggest obstacles to healthy aging is the significant burden of age-related
neurodegenerative diseases, which almost always result profound cognitive impairment and progressive decline. Here, we aim to identify molecular features that preserve cognitive function and could serve as therapeutic targets for neurodegenerative diseases and healthy aging.
Cognitively resilient individuals — those able to maintain healthy cognitive function despite harboring a large amount of brain pathology — represent a small subset of the population (3). This phenomenon was first described in the late 1980s, as physicians began to identify patients with no apparent behavioral signs of dementia who exhibited cellular and pathological alterations associated with Alzheimer’s disease (AD). AD is a neurodegenerative disorder characterized by the progressive accumulation of beta-amyloid plaques and neurofibrillary tau tangles, along with changes in cellular health
through mild cognitive impairment before exhibiting signs of severe dementia. In later stages of the disease, AD patients usually exhibit disruptive memory loss, personality changes, and difficulty completing tasks that require reasoning and decision making (5). Collectively, these cognitive and behavioral symptoms are profoundly life-altering, and can lead to a loss of independence for the affected individual. In addition to the obvious personal burden, AD has resulted in a substantial financial and psychological burden to caretakers and society in general. It is important to note, however, that the interpersonal and societal burden of this disease stems from its clinical manifestations. In other
words, the subset of individuals who accumulate neuropathology but are able to maintain cognitive function are spared from much of the suffering associated with AD.
Enriching lifestyle factors: preventative medicine for cognitive deterioration
The discrepancy between severity of insult and clinical outcome has been documented in a wide range of neurological conditions in addition to AD, including traumatic brain injury (6), Parkinson’s disease (7), multiple sclerosis (8), and stroke (9). The scope of this phenomenon suggests that the mechanisms enacted to confer cognitive protection against neural insult extend beyond AD-specific pathology and cellular changes. Early epidemiological observations identified a link between an individual’s risk of dementia and their level of education (10, 11). This association extends to various forms of
cognitive, social, and physical stimulation throughout life (12). As a whole, these healthy lifestyle factors, or forms of enrichment, are the most effective strategies that exist for enhancing cognitive function at baseline and enabling cognitive resilience (CgR) to multiple forms of brain pathology later in life. They represent the only known
preventative measure against aging- and disease-related cognitive decline. It is important to note that, for humans, “enrichment" is a broad term encompassing many stimulating activities. In addition to formal education, bilingualism (13), musical
attainment (14, 15), and frequency of cognitively engaging tasks such as problem solving, reading, and writing (11) have all been linked to cognitive protection in the context of aging or disease. Similarly, both social engagement (16) and physical activity (17) have been shown to promote cognitive health. Combinations of these variables may provide enhanced benefit (18), suggesting that distinct sources of enrichment potentially have additive or synergistic mechanisms of action. Even among twin pairs, greater midlife cognitive activity is associated with reduced risk of dementia (19), suggesting that potential confounding factors, such as genetic predisposition, are not solely responsible for the positive effect of enriching environmental factors in humans.
Rodent studies have further confirmed the causal role that an enriched lifestyle has in promoting CgR. In rodents, enrichment is classically modeled by providing multiple sources of stimulation from a variety of domains: littermates for social interaction, a running wheel for physical activity, a larger chamber containing multiple bedding materials for somatosensory and spatial exploration, and toys that are replaced regularly for novel investigation. Providing this type of environmental enrichment is sufficient to preserve cognition in the context of genetically-induced neurodegeneration (20, 21). As with humans, enrichment leads to beneficial cognitive outcomes for a variety of neurological conditions (22), including Alzheimer’s disease, Huntington’s
syndrome. Additionally, enrichment in rodents also reverses behavioral sensitization to cocaine (23), reduces symptoms of anxiety and depression after exposure to chronic stress (24), and can reverse adverse behavioral and physiological outcomes that arise from prenatal stress (25). The extensive positive effects of an enriched environment that manifest throughout life and in response to a variety of stressors underscore the need to understand the mechanisms through which this neuroprotection and resilience are afforded.
Neural correlates of cognitive health and decline
A limited number of studies have begun to identify targets and pathways that can be harnessed to confer CgR (26). Indeed, some of these biological processes, such as elevated expression of BDNF (27) or increased proliferation and survival of adult-born neurons in the hippocampus (28), are stimulated by environmental enrichment. One of the strongest neural correlates with cognitive decline is synaptic dysfunction (29), including synapse loss and deficits in synaptic plasticity. Neuronal hyperexcitability has also been shown to precede cognitive decline in a number of progressive neurological disorders. Clinical evidence suggests that AD patients often show signs of neural hyperexcitability prior to neurodegeneration (30). AD patients, both familial and sporadic, are at increased risk for developing seizures (31), and transgenic AD mice display epileptiform activity prior to the onset of neuron loss (32, 33). Hyperexcitability has been bidirectionally linked to both amyloid (34) and tau pathology (35, 36),
suggesting that a positive feedback cycle between neuronal excitability and protein pathology could contribute to early disease manifestations. Finally, reduced neural
excitation has been linked to enhanced longevity, further suggesting a detrimental role of hyperexcitability on neural health (37). Aberrant electrical activity could be caused by associated synaptic dysfunction, thereby linking the two mechanistically. However, the molecular drivers endogenously recruited to promote synaptic health in the context of disease remain largely unknown. Elucidation of these upstream mechanisms could lead to novel therapeutic targets for promoting cognitive health across the lifespan. In this thesis, I will describe our efforts to advance this goal.
Using a multidisciplinary approach, and based on a variety of experiments and
observations, we uncovered the Mef2 transcription factor family as a candidate mediator of CgR. Previous work on the Mef2 family (Mef2a-d) has examined their role in
neurodevelopment, implicating various members in maintaining appropriate neural transmission and regulating synaptic density (38, 39). In vivo studies have shown that knocking out Mef2 TFs in the mouse brain results in potentiated presynaptic release and synaptic transmission (39, 40), as well as increases in dendritic spine density (41). While Mef2 TFs are most highly expressed early in brain development, they continue to be expressed in the adult brain throughout life, in both excitatory and inhibitory neurons (42). Additionally, Mef2 transcriptional activity is induced by neuronal activity (43), highlighting the importance of this family in regulating molecular responses to neural stimulation. This functional property implicates the Mef2 family in synaptic plasticity, or the ability to dynamically strengthen or weaken specific synapses in response to external cues, such as those from a learning task. Interestingly, genetic variants within
implicating this family in the expression of higher order cognitive ability. These known features of Mef2 TFs make the family a particularly interesting target for linking a stimulating environment to cognitive benefit.
Thesis Aims
The work I present here is a compilation of three major lines of study: (i) Using a mouse model of environmental enrichment, we recapitulate the benefits of sustained cognitive stimulation in order to probe the molecular pathways associated with the induction of CgR, (ii) We interrogate the functional role of key molecular regulators using viral manipulation, and (iii) We analyze three cohorts of clinically-curated brain RNA-sequencing data to characterize the transcriptional underpinnings of CgR in human patients. We find that Mef2 transcription factors are induced upon environmental enrichment in mice, and associated with end-stage cognitive ability in humans. By altering Mef2 levels in mouse models of enrichment and neurodegeneration, we confirm their critical role as drivers of cognitive function and neuroprotection against AD-related phenotypes. Our findings extend the function of MEF2 beyond its well-studied roles in neurodevelopment and activity-dependent gene regulation by establishing it as a critical and specific mediator of cognitive health across the lifespan and in the context of
Chapter 2. Effects of an enriched environment on mouse
behavior and the molecular properties of cortical neurons
2.1 Modeling cognitive enrichment in mice
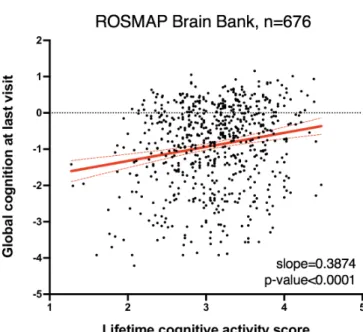
The strongest epidemiological predictors of CgR are mentally stimulating activities such as high educational attainment and cognitively demanding occupations (12). These forms of enrichment have been shown to afford neurological protection from the harmful pathology of neurodegenerative disease, delaying the onset of dementia by years. We examined clinical data from the Religious Orders Study (ROS) (45) and the Rush Memory and Aging Project (MAP) (46) cohort and found that among individuals spanning a range of dementia diagnoses and pathological burdens, there was a
significant positive correlation between frequency of cognitive activity (e.g. reading and writing) and end-stage cognition (Figure 1A).
Figure 1a. Lifetime cognitive activity score (frequency of participation in cognitively stimulating activities) plotted against global cognitive function at last visit (an average of 19 tests) for patients in the ROSMAP cohort (n = 676 individuals). Solid red line represents best-fit line; dashed red lines represent 95% confidence interval. Linear regression: Y=0.3874X – 2.1; p-value for slope<0.0001.
To study enriching lifestyle factors – including cognitive stimulation – in mice, researchers have employed models of environmental enrichment. As in humans, enrichment in mice has been shown to enhance cognition (47) and delay the onset of neurodegeneration in numerous disease models (20, 48). We hypothesized that the molecular effects of enrichment throughout life might underlie the neural processes that are induced and maintained in individuals who ultimately display CgR later in life.
We employed a paradigm in which wildtype Swiss Webster mice were weaned into environmental enrichment (EE) or standard housing (SH) cages and maintained in these conditions for one month prior to downstream molecular and behavioral analysis (Figure 1B). Additionally, we weaned a third group into isolation for a subset of
experiments in order to extend the spectrum of enrichment to a “deprived” condition, eliminating the component of social stimulation that is still present in standard group housing. While our main focus is on the comparison between standard housing and an enriched environment, the isolated mice provide insight into the compounded effects of various forms of enrichment, as they undergo greater deviation from environmental stimulation. EE chambers were large mouse cages containing two nesting/burrowing materials, a running wheel, and toys that were changed every 3-4 days for novelty and exploration (Figure 1C). By contrast, SH control mice were housed with an equal number of littermates (i.e. 3-4) in smaller, standard cages containing only one nesting material and no toys or running wheel. Isolation cages were identical to SH but housed mice individually. Mice were housed in these environmental conditions for one month before behavioral analysis or being euthanized for molecular experiments.
Figure 1b. Wildtype Swiss Webster mice were housed under standard conditions prior to weaning. At postnatal day 25 mice were weaned into either enriched environment (EE),
standard housing (SH), or isolated (SI) conditions. At P60, mice were subjected to molecular or behavioral analysis.
Figure 1c. Representative images of housing conditions.
1B. Experimental Design
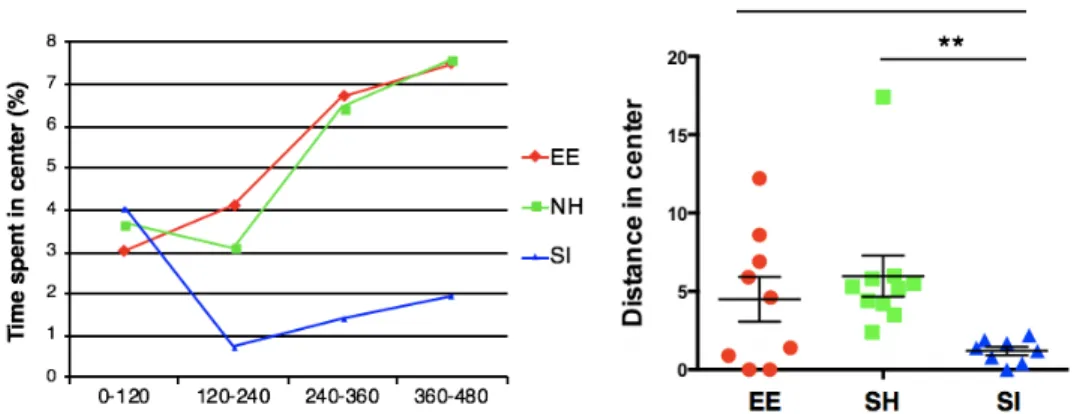
2.2 Environmental conditions have a profound effect on cognitive function To validate the cognitive impact of our enrichment model, we conducted a memory test based on contextual fear conditioning, a paradigm that involves foot-shocking each mouse in a chamber and probing the degree of freezing behavior the mouse displays in the same chamber the following day. Robustness of the fear memory is positively associated with the percentage of time the mouse spends freezing. As anticipated, EE mice spend more time freezing, thus displaying stronger associative memory compared to SH controls (Figure 2B, left). Importantly, EE mice display comparable levels of general locomotion and time spent in the center of an open chamber (Figure 2A), indicating that the observed difference in freezing cannot be attributed to a difference in baseline movement. To assess cognitive flexibility, we performed fear extinction training, in which each mouse is placed back into the original foot-shock chamber without
receiving any additional shocks for several days following initial fear conditioning. This task is highly dependent on the PFC and tests the animal’s ability to update their original fear association and re-learn that the chamber is no longer dangerous. As has been described previously (49), EE mice demonstrated significantly faster rates of fear extinction than SH animals (Figure 2B, right). We also performed a cued fear
conditioning test, which relies on neural circuits that are partially distinct from those involved in contextual fear conditioning. In this task, the mouse is foot-shocked in the presence of an auditory tone and the following day the same tone is presented without a foot shock. While the difference in time spent freezing was not statistically significant between the two groups, enriched mice trended toward higher levels of freezing (Figure
2B, center). Together, these results show that enrichment enhances the ability to both form new associations and update existing ones.
Figure 2a. EE and SH male mice show similar distances travelled (left) and time spent in the center of an open field (right) over a 10-minute period, demonstrating that they have no overt locomotor or anxiety deficits.
Figure 2b. Left: EE mice display enhanced associative memory in a contextual fear conditioning paradigm (n = 24 mice per group, unpaired t-test, p = 0.0012). Center: EE mice display a non-significant trend toward increased freezing in a cued fear conditioning paradigm (n = 24 mice per group, unpaired t-test, p = 0.075). Right: EE mice display enhanced rates of fear extinction (n = 10 SH mice vs. 9 EE mice, unpaired t-test comparing % freezing post 2-shocks and %
Previous research has elucidated the beneficial role of physical activity in supporting brain health and improving cognitive function (17). To determine if access to a running wheel is solely responsible for the observed improvement in associative memory, we reared mice in EE conditions identical to those described above but did not include a running wheel in the chamber. While we observed no difference in baseline locomotion between SH animals and EE animals housed without a running wheel (Figure 2C, left), the enriched group still showed greater freezing following contextual fear conditioning, indicating enhanced associative memory (Figure 2C, middle). These results underscore the potent ability of cognitive stimulation to improve memory performance, even in the absence of additional physical activity.
Figure 2c. When compared to SH mice, EE mice reared without a running wheel display no differences in locomotion (left), enhanced associative memory in a contextual fear conditioning paradigm (center; n = 8-13 mice per group, unpaired t-test, p < 0.001), and a non-significant trend toward increased freezing in a cued fear conditioning paradigm (right; n = 8-13 mice per group, unpaired t-test, p=0.107).
To determine the effect of enrichment on working memory, an executive function that is highly dependent on the PFC, we performed an 8-arm radial maze test. This maze is comprised of 8 identical arms arranged symmetrically around a center circle. Mice innately seek novelty and are thus incentivized to explore novel arms over arms they have previously entered. If an animal is unable to keep track of the arms it has entered, it might explore the same ones repeatedly by chance. While both groups ultimately explore the same number of arms (Figure 2D, left), EE mice outperform SH mice in this task by entering fewer repeat arms before exploring all novel arms (Figure 2D, right). Overall, these behavioral findings indicate that living in an enriched, stimulating environment has a beneficial effect on a variety of cognitive functions.
Figure 2d. In an 8-arm radial arm maze, EE mice enter the same number of arms in three minutes as SH mice, but make fewer repeat “errors”, i.e. enter more novel arms before repeat arms (n = 8-12 mice per group, unpaired t-test p < 0.05)
Social isolation is known to contribute to a wide range of negative health outcomes in social animals such as humans and rodents. To determine the cognitive effects of our
open chamber. Isolated mice spend significantly less time exploring the center of the open chamber (Figure 3A), which is indicative of anxiety-like behavior. Isolated mice also display deficits in associative memory compared to group-housed mice, but only when given two foot shocks rather than one (Figure 3B). In this fear conditioning paradigm, standard-housed mice display a stronger fear memory because the association is more aversive.
Figure 3a. Isolated mice explore the center of an open chamber much less than control mice (n = 8-10 per group; unpaired t-test p < 0.01).
significantly less than enriched mice (unpaired t-test p < 0.0001 for contextual, p < 0.001 for cued). Bottom: After two-shock contextual fear conditioning, isolated mice freeze significantly less than both standard-housed and enriched mice, suggesting impairments in associative memory only under specific conditions, when a highly aversive stimulus is used to increase freezing in control mice (n = 8-10 per group, unpaired t-test p < 0.05). After two-shock cued fear conditioning, however, isolated mice show comparable levels of freezing to both standard-housed and enriched mice, suggesting that some neural circuits that underlie memory formation are unimpaired after isolation.
We have demonstrated the ability to successfully model environmental conditions that produce distinct cognitive behaviors in mice. These results elucidate the power of the environment to generate neural changes that are substantial enough to yield behavioral differences in animals that are much more genetically similar than human populations. We next wondered whether a reversal of environmental conditions would be sufficient to alter behavioral differences. To test this, we compared enriched to isolated animals, as these environmental conditions are on opposite ends of the spectrum of cognitive stimulation. Half of the cohort was reared in either enrichment or isolation as previously described for 8 weeks. The other half were switched into the opposite environmental condition after four weeks. We performed this experiment in female mice because male mice are unable to be group-housed after a long period of isolation as they exhibit extreme aggression toward their conspecifics. The four groups displayed equivalent locomotion in an open field test (Figure 3C). While we did not observe significant differences in the latency to enter a lit chamber, mice reared in isolation for the entire experimental period, or those moved into isolation, show trends toward higher latencies, indicative of anxiety-like behavior. These trends are influenced by a small number of animals, suggesting that some animals may be exceptionally vulnerable to isolation. We replicated differences in freezing between enriched and isolated animals in both the
contextual and cued fear conditioning tests. In the contextual fear conditioning test the animals that were switched between conditions showed intermediate levels of freezing that were not significantly different from either of the two baseline groups (Figure 3C). Interestingly, in the cued fear conditioning test, isolated animals that were switched into enriched environments displayed significantly elevated levels of freezing, equivalent to those displayed by continuously enriched animals (Figure 3C). Enriched animals that were switched into isolation displayed significantly reduced levels of freezing,
comparable to those displayed by continuously isolated animals. Together, these results show that both enrichment and isolation are potent modulators of behavior, and suggest that the mechanisms that alter neural function in response to environmental stimulation must be actively maintained in the brain.
Figure 3c. Top: No significant differences in locomotion (left) or anxiety (right) between the four groups. Bottom: Enriched mice display greater freezing after one-shock contextual and cued fear conditioning, as anticipated (unpaired t-test p < 0.01). In the case of contextual fear
conditioning, mice that were switched from enriched to isolated conditions or vice versa display intermediate levels of freezing that are not significantly different from either the isolated or enriched group. In the case of cued fear conditioning, however, mice show a behavioral reversal — those switched from isolated to enriched conditions freeze significantly longer than isolated
mice (unpaired t-test p < 0.05), and those switched from enriched to isolated conditions show comparable freezing to isolated mice.
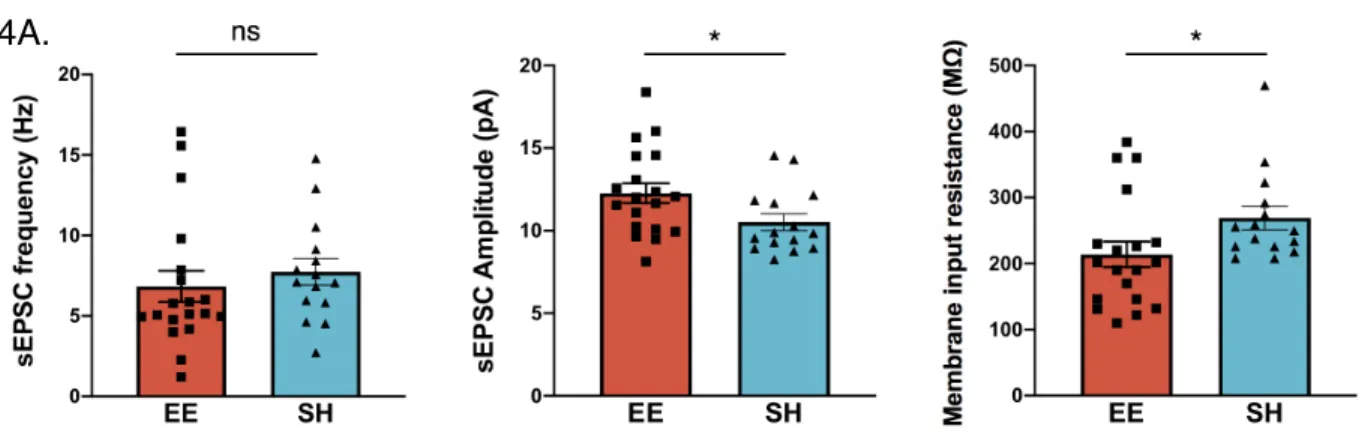
We next sought to determine the effect of enrichment on electrophysiological properties in the prefrontal cortex. Mice were sacrificed at 5 months of age, after several months of chronic environmental enrichment, and recordings were performed via patch clamping onto pyramidal neurons in middle-deep layers of prefrontal cortex. While we did not observe a difference in the frequency of spontaneous excitatory post synaptic currents, these currents had a greater amplitude in enriched mice (Figure 4A). One interpretation of this finding is that presynaptic excitatory output is unaltered, but postsynaptic
detection is heightened, possibly through the expression of more receptors. We also observed a decrease in membrane input resistance in enriched mice (Figure 4A, right), suggesting that they might have more open ion channels indicative of neural
transmission.
Figure 4a. Left: We did not detect a difference in the frequency of spontaneous excitatory post synaptic currents (sEPSC) of mPFC pyramidal neurons between wild-type enriched vs.
standard-housed male mice. Center: Enriched mice display greater sEPSC amplitude compared to standard-housed mice (n = 15-19 neurons from 3 mice per group, unpaired t-test p < 0.05). Right: Enriched mice display lower membrane input resistance compared to standard-housed mice (n = 15-19 neurons from 3 mice per group, unpaired t-test p < 0.05).
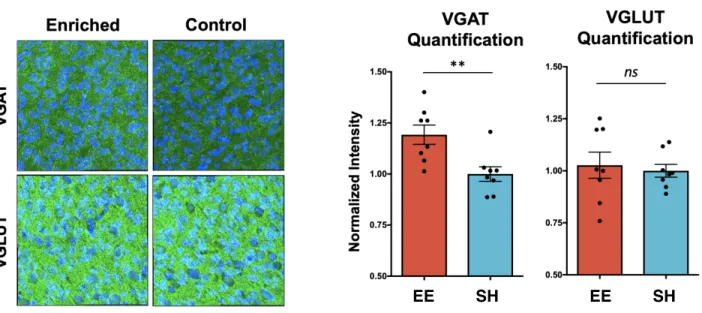
Figure 4b. Staining for VGAT, VGLUT (green) and DAPI (blue). Enriched mice show
comparable levels of VGLUT staining, but higher levels of VGAT staining in prefrontal cortex (n = 2 images from 4 mice per group, unpaired t-test p < 0.01).
We stained for the presynaptic synaptic signaling molecules VGAT and VGLUT. VGAT is a vesicular GABA transporter and VGLUT is a vesicular glutamate transporter, thus reflecting inhibitory and excitatory transmission, respectively. We did not observe a difference in VGLUT expression between enriched and standard-housed mice, which is consistent with our previous electrophysiological finding demonstrating comparable sEPSC frequency. However, we did observe significantly more VGAT in enriched mice (Figure 4B), which may suggest enhanced inhibitory transmission. We caution over-interpretation of this finding as these are individual signaling molecules in what is a complex web of synaptic machinery, but the disparity is intriguing and might suggest one mechanism by which enrichment is neuroprotective.
4B. Markers of synaptic signaling (immunohistochemistry)
2.3 Global changes in chromatin state in response to distinct environmental conditions
Previous work from our lab demonstrated that enrichment leads to chromatin remodeling and an increase in histone modifications associated with active
transcriptional states in neural tissue (50). However, the genomic loci and functional significance associated with this remodeling remain unknown. To unbiasedly assess the changes in global chromatin landscape that occur in the neurons of enriched mice, we performed ATAC-sequencing to identify regions of the genome that become more accessible after enrichment. Epigenomic accessibility reflects functional relevance, as these loci are more likely to be transcribed or operate as regulatory sites (51). We first used an antibody for Rbfox3 (NeuN) to sort neuronal nuclei from the dissociated frontal cortices of female mice reared in EE (n = 3 mice) or SH (n = 3 mice) conditions (Figure 5A). Microglia sorted on the basis of PU.1 positivity served as a negative control cell type. We validated proper isolation of neurons by 1) performing qPCR on putative open and closed regions (Neurod6 for Neurons, Aif1 for Microglia) before sequencing (Figure 5B), and 2) after sequencing, mapping all open regions to the nearest gene and
performing cell-type enrichment analysis using CTen microarray data (Figure 5C). As anticipated, CTen software revealed that our sorted Neurons most closely resembled the transcriptome of hippocampus and cerebral cortex tissue, while sorted Microglia most closely resembled the transcriptome of purified microglia and bone marrow macrophages.
Figure 5a. Representative image of a flow cytometry-assisted cell sorting (FACS) plot used for the selection of neurons and microglia based on relative levels of Neun and Iba1, respectively. Figure 5b. Quantitative PCR of promoter regions in a neuron-specific gene, Neurod6, and a microglia-specific gene, Aif1. Sorted neurons displayed greater amplification of the Neurod6 loci, whereas sorted microglia displayed greater amplification of the Aif1 loci.
Figure 5c. Unbiased enrichment analysis for all open regions mapped to the nearest gene using CTen software. Open regions in sorted neurons showed highly significant enrichment for neuronal-heavy tissues, including hippocampus and cerebral cortex. Open regions in sorted microglia showed significant enrichment for microglia and macrophages.
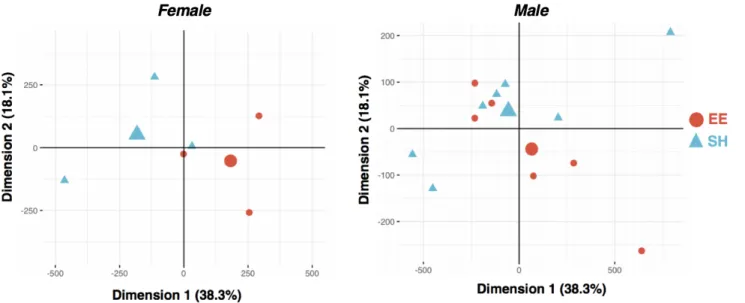
Principal component analysis performed on all ATAC-seq peaks showed modest separation of EE and SH samples (Figure 6A). We identified 675 differentially
accessible regions (DARs) between EE and SH female mice, 67% of which were more accessible in the enriched condition (Figure 6B, left). This is consistent with previous work from our lab showing an increase in active chromatin marks following enrichment (50) (Supplemental Tables 2-5). We repeated this experiment in male mice and similarly found more DARs in EE mice compared to their age-matched SH controls (Figure 6B, right).
Figure 6a. PCA of ATAC-seq peak counts in EE and SH females (left; n = 3 EE vs. 3 SH mice). PCA of ATAC-seq peak counts in EE and SH males (right; n = 7 EE vs. 6 SH mice).
Figure 6b. Left: Heatmap of significant (q-value ≦ 0.1) differentially accessible regions (DARs) as identified via ATAC-seq of neurons isolated from the frontal cortex of EE (red, 450 DARs) and SH (blue, 225 DARs) female mice (n = 3 mice per group). Right: Heatmap of significant (q-value ≦ 0.1) DARs as identified via ATACseq of neurons isolated from the frontal cortex of EE (red, 140 DARs) and SH (blue, 59 DARs) male mice (n = 6 EE vs. 7 SH).
To probe the functional significance of the genomic regions that became more
accessible after enrichment, we used multiple, complementary approaches to annotate all accessible regions and DARs identified across the genome. First, accessible regions in male and female neurons showed a characteristic distribution of openness in introns, intergenic regions, and promoters as is characteristic of published ATACseq datasets 6A.
(51). Interestingly in both males and females, regions that were more accessible in enriched neurons showed an over-representation of distal intergenic regions (Figure 6C, top), suggesting that distal enhancers and their associated binding proteins could be preferentially recruited in response to cognitive stimulation. Second, utilizing
ChromHMM, genome-wide chromatin states were inferred from existing ChIP-sequencing data of histone marks from adult mouse cortical neurons. This analysis revealed that DARs showed a significant enrichment for histone marks associated with enhancer sites (Figure 3C, bottom).
Figure 6c. Top: Pie chart distribution of genomic annotations for each set of ATACseq peaks in females (left) and males (right) for all peaks (top), gained EE peaks (middle) and gained SH peaks (bottom). Annotations are based on overlap with genomic reference annotations computed using the R package ChiPseeker and the UCSC reference of the mouse genome version mm10. DARs that are gained in EE are enriched for intergenic regions. Bottom: Fold Enrichment analysis of DARs within chromatin states inferred from existing Chip-seq data of adult mice cortical neurons (Halder et al. 2015) using ChromHMM.
Finally, DARs were assessed for statistical enrichment of transcription factor (TF) binding motifs using the software package Homer, which identified eleven motifs that were significantly overrepresented in the DARs of both enriched female and male mice (Figure 7A). This list could be broadly separated into the Mef2 family (Figure 7B) and members of the AP-1 complex, including JunB and Fosl2/Fra2. All Mef2 family members share the same binding motif, indicating potential overlap in function. Interestingly, only one TF from this list, MEF2C, has been causally implicated in both Alzheimer’s disease and general intelligence (as well as neurodevelopmental disorders) via genome-wide association studies (Figure 7A). Consistent with genomic annotation and chromHMM modelling that demonstrated differential openness of putative intergenic, enhancer regions in enriched neurons, Mef2 TFs have been shown to predominantly bind such regions (52).
Figure 7a. Left: Venn diagram of overlap between statistically overrepresented transcription factor (TF) binding motifs among DARs in male and female EE mice. Center: List of all
overlapping TFs along with their average rank (between male and female mice). Table indicating occurrence of TF in major neurocognitive-relevant GWAS studies. Right: Mef2 motifs are
enriched in the DARs that are gained in EE female and male mice.
Figure 7b. Left: Mef2a motif frequency (motif/bp/peak) plotted across the relative length of significant DARs gained in female and male EE (red) and SH mice (blue). Right: Mef2c motif frequency (motif/bp/peak) plotted across the relative length of significant DARs gained in female and male EE (red) and SH mice (blue).
7A.
Interrogation of chromatin state following EE led us to the conclusion that Mef2 transcription factors are critically involved in the neuronal response to enrichment. To determine if this effect is bidirectional, we performed ATAC-sequencing on cortical neurons purified from isolated mice and found that the Mef2 motif is enriched among regions that are more accessible in standard-housed mice compared to isolated mice (Figure 7C,D). This suggests a stepwise process in which regions containing Mef2 binding sites become increasingly more accessible in a manner that is dependent on the “dose” of cognitive stimulation. In other words, the transition from isolation to group housing induces opening of Mef2 binding sites, and the addition of cognitive stimulation in the case of enrichment induces further opening of these sites.
Mef2 transcription factors are highly expressed in both neurons and microglia. To determine if increased accessibility of the Mef2 binding motif occurs in a cell-type-specific manner, we again used Homer to identify motifs that were overrepresented in the open regions of EE microglia compared to SH microglia. Interestingly, there were negligible differences at the level of differential accessibility in microglia (data not shown), suggesting some neuronal specificity in terms of a sustained chromatin state response to enrichment. We speculate that microglia may show a more acute response to environmental stimulation that would be more accurately reflected in chromatin state immediately after the initial exposure.
Figure 7c. The Mef2 motif is among those significantly enriched in the DARs that are gained in SH mice relative to isolated (SI) mice.
Figure 7d. Left: Mef2a motif frequency (motif/bp/peak) plotted across the relative length of significant DARs gained in SH (blue) and SI mice (gray). Right: Mef2c motif frequency (motif/bp/ peak) plotted across the relative length of significant DARs gained in SH (blue) and SI mice (gray).
7C.
2.4 Environmental enrichment induces a transcriptional signature associated with Mef2 activity
In order to determine the transcriptional consequences of the altered chromatin
structure after enrichment, we performed RNA-sequencing from cortical neurons of both female and male mice and examined gene expression after one month of rearing in EE or SH conditions. Neurons were again isolated using an antibody for Rbfox3 (NeuN) to sort nuclei from dissociated frontal cortices. Nuclear RNA expression correlated highly with open regions from our ATAC-sequencing data (Figure 8A, left). Additionally, promoter regions of the most highly expressed genes were enriched for active histone marks from a dataset of cortical neurons (Figure 8A, right), indicating successful purification of neurons. We identified 679 and 657 differentially expressed genes (DEGs; FDR<0.1) in response to enrichment in female and male mice, respectively (Figure 8B).
8A.
Figure 8a. Left: Heatmap plot of the normalized ATACseq signal around promoters of 5% genes with highest (top) and lowest (bottom) expression levels. The top line plot shows the average ATACseq signal for the high (red) and low (blue) promoter groups. The blue heatmap on the right shows average expression values for the corresponding gene. Together, these plots show a high correlation between neuronal ATACseq and nuclear RNA signal. Right: Heatmap plots of normalized ATACseq and ChIP-seq signals around promoters of neuronal (top) and glial
(bottom) markers. ChIP-seq data of cortical neurons from adult mice and marker gene sets were obtained from Halder et al. 2015. These heatmaps show appropriate correlations between ATACseq signal (leftmost) and active marks (H3K4me3, H3K27ac) but not repressive marks (H3K27me3).
Figure 8b. Volcano plot of DEG analysis of nuclear RNA isolated from the frontal cortex of EE and SH mice. Left: female mice (n = 3 EE vs. 3 SH), showing 327 (red) and 348 (blue)
significantly up-regulated in EE (upEE) and SH (upSH) groups, respectively (significance cut-off q-value ≦ 0.1). Right: male mice (n = 6 EE vs. 6 SH), showing 280 (red) and 109 (blue)
significantly up-regulated in EE (upEE) and SH (upSH) groups, respectively (significance cut-off q-value ≦ 0.1).
Does differential accessibility of a TF binding motif after enrichment partially explain the set of DEGs between EE and SH mice? We compiled lists of target genes for the top TFs whose motifs were significantly enriched among regions that gained accessibility in EE mice: JunB (53), Fosl2/Fra2 (54), Mef2a (55, 56), and Mef2c (56, 57). We observed the greatest degree of overlap between enrichment DEGs and target genes for Mef2c in both sexes (Figure 8C). Additionally, enrichment DEGs in female mice showed a
significant degree of overlap with Mef2a targets. As there can be a discordance
between nuclear RNA expression and mature RNA transcript levels, increases in known Mef2-regulated genes (Zmat4, Astn2, Slc16a7, and Daam1) were also confirmed via qPCR of mature transcripts (Figure 8D) from an independent cohort of male mice (n=3 per group). Downstream targets of JunB or Fosl2 (Fra2), however, did not show a statistically meaningful overlap with enrichment DEGs in any sex (Figure 8C). Taken together, this data suggests that enhanced accessibility and transcriptional activity of the Mef2 family specifically – in particular Mef2c and Mef2a – are important features of
Mef2 family members exhibit activity-dependent regulation (38), and it could be
hypothesized that the benefits of enrichment are simply due to increased neural activity. To assess whether upregulation of the Mef2 network simply reflects a transcriptional signature associated with generic neuronal activity, or is a specific response to sustained enrichment, we compared enrichment DEGs to two published datasets of transcriptional output following neuronal activity. These datasets, termed scARG and ARG, were generated from single nucleus RNA-sequencing of dentate gyrus neurons after brief exposure to a novel environment (58), and bulk RNA-sequencing after stimulating neurons in vitro and in vivo with KCl and light stimulation (59), respectively. Interestingly, enrichment DEGs did not show significant overlap with generic neuronal activity DEGs, with the exception of the ARG list in female mice (Figure 8C). These results suggest that the type of neural activity arising from sustained enrichment leads to somewhat specific upregulation of the Mef2 transcriptional network, and cannot be explained by known transcriptional networks associated with generic neuronal activity alone.
Figure 8c. Overlap between EE DEGs (male mice=solid fill; female mice=checkered fill) and lists of TF target genes (Fosl2/Fra2, JunB, Mef2a, Mef2c) or activity-induced genes (ARG, scARG). Dashed line indicates statistical significance threshold via hypergeometric test.
Figure 8d. Quantitative PCR (qPCR) on RNA isolated from whole mPFC for a different cohort of animals to validate increases in known downstream targets of Mef2a (n = 4 male animals per group, 2-way ANOVA, p-value for group = 0.0002).
Nuclear localization of Mef2 TFs is important for the activation of their transcriptional network. We next assessed protein levels of Mef2a and Mef2c within the nuclei of prefrontal cortex neurons following enrichment and found that Mef2a was significantly increased in both male and female mice, and Mef2c was significantly increased in female mice (Figure 8E). This data is consistent with the notion that Mef2 activity is
are consistent with known functional roles of Mef2 TFs, we performed gene ontology analysis of enrichment DEGs. Genes that are altered in enriched mice are statistically enriched for cellular components localized to dendritic spines and synapses, including ion channels and receptor complexes (Figure 8F). This is consistent with previous work implicating various Mef2 family members in regulating synaptic density (38).
Widespread changes in the expression of synaptic and membrane proteins likely result in alterations at the level of neuronal transmission.
Figure 8e. Top left: Immunohistochemistry (IHC) from the frontal cortex of EE and SH female mice, staining for DAPI (blue), Rbfox3 (red), and Mef2a (green). Top right: Imaris quantification of IHC staining in top left with Mef2a/NeuN intensity plotted for individual nuclei across 3 EE and 3 SH mice. EE mice showed increased Mef2a intensity
(unpaired t-test, p-value < 0.0001) compared to SH mice. Bottom left: IHC from the medial prefrontal cortex of EE and SH male mice, staining for DAPI (blue), Rbfox3 (red), and Mef2c (green). Bottom right: Imaris quantification of IHC staining with Mef2c/NeuN intensity plotted for individual nuclei across 3 EE and 3 SH mice. EE mice showed increased Mef2c intensity (unpaired t-test, p-value < 0.0001) compared to SH mice.
Figure 8f. Gene set enrichment analysis (cellular component) for EE vs. SH DEGs in
female mice.
Unexpectedly, we found that genomic loci near olfactory receptor genes demonstrated increased accessibility in enriched mice (Figure 9A). Early research that identified olfactory receptors posited that these genes are not expressed in the brain (60);
however, recent studies have observed olfactory receptor expression in cases of neural injury, indicating that their expression may be induced in response to certain stimuli (61). To determine if increased accessibility was associated with increased expression we next performed RNA in situ hybridization in the frontal cortex using probes designed to detect Olfr1396. Enriched mice displayed significantly higher levels of Olfr1396 as measured by total signal intensity (Figure 9B). We also performed quantitative PCR on homogenized cortical tissue to measure relative levels of various olfactory receptor genes that showed increased accessibility via ATAC-sequencing: Olfr385, Olfr740, and Olfr1396. Increased accessibility around Olfr1385 was observed in an adjacent
intergenic region, while increased accessibility around Olfr740 and Olfr1396 were observed in genic regions (TSS and exon, respectively). Interestingly, normalized levels of Olfr740 and Olfr1396 were higher in enriched mice, but levels of Olfr1385 were comparable between the two groups (Figure 9C). A potential explanation for this
gene promoters versus intergenic regions. To acknowledge potential artifactual
explanations for the observed increase in Olfr expression, we compared threshold cycle values (Ct values) across three sample preparations: cortical RNA, cortical cDNA, and olfactory epithelium cDNA. Average Ct values for all olfactory receptors were higher in cDNA isolated from the olfactory epithelium, which served as a positive control,
compared to cDNA isolated from cortical tissue. Additionally, average Ct values for all olfactory receptors were higher in cortical cDNA compared to RNA from the same samples, confirming little genomic cDNA contamination in the extracted RNA. Finally, we performed RNA sequencing on mouse cortical neurons and compared levels of olfactory receptor expression between enriched mice and those housed in standard conditions. In the olfactory epithelium, each cell expresses only one of the >1000
olfactory receptors, which would yield low total expression on the population level of any given receptor. As anticipated, most olfactory receptors and olfaction-relevant genes (including vomeronasal receptors) showed minimal to no expression in all samples. We focused our analysis on olfactory genes that had a base mean greater than 10. From among these, six showed significantly greater expression in enriched mice while none were reduced in enriched mice. Together, these observations suggest that enrichment may result in the unique expression of a class of receptors not typically expressed in the brain. Intriguingly, mouse olfactory receptors have been shown to bind Mef2a at their promoters, and require this transcription factor for proper expression (62). While preliminary, these results are of great interest and should be investigated further, as they suggest a broadening of signal transduction in the brain following enrichment.
9A. Sample ATAC-seq traces from differentially accessible olfactory receptors
9B. In situ hybridization: Olfr1396
9C. qPCR from bulk cortical tissue
Figure 9a. ATAC-seq traces from two differentially accessible olfactory receptors, Olfr740 and Olfr1396.
Figure 9b. In situ hybridization of Olfr1396 in cortex shows much greater signal in enriched mice compared to isolated mice.
Figure 9c. Quantitative PCR of differentially accessible olfactory receptors and housekeeping genes from bulk cortical tissue of enriched and isolated mice. Enriched mice show trends toward increased amplification of Olfr740 and Olfr1396, two receptors that displayed increased accessibility in genic regions.
Figure 9d. RNA-seq from cortical neurons of enriched and standard-housed mice. Among all olfaction-related genes with a base mean greater than 10, six were significantly greater in enriched mice and the other 14 were comparable between the two groups.
Chapter 3. Effects of Mef2a/c manipulation on mouse
behavior and the molecular properties of cortical neurons
3.1 Loss of Mef2a/c in the frontal cortex impairs cognitive performance and leads to neuronal hyperexcitability in enriched wildtype mice
Epigenomic and transcriptomic profiling of enriched neurons suggests that Mef2 activity might play a role in mediating the phenotypic effects of EE. To causally link Mef2 and determine if increased Mef2 activity is required for the cognitive benefits of EE, we first used a lentiviral construct to generate short hairpin RNA (shRNA) that would reduce endogenous levels of Mef2a and Mef2c. Both family members were targeted because of our previous results showing an increase in nuclear Mef2a and Mef2c following
enrichment, along with previous work showing that they share a significant proportion of chromatin binding sites, indicative of redundancy in their targets and thus function (63). Control mice received a scrambled (Scrmb) shRNA construct. In vitro primary neuronal culture was used to demonstrate that the Scrmb virus did not affect levels of any Mef2 members, and that Mef2a and Mef2c shRNA constructs specifically knocked down only their targeted TF (Figure 10A, left). Bilateral lentiviral injections into the medial frontal cortices were performed on postnatal day 30 (P30), after which mice were reared in standard cages for two weeks to allow for viral shRNA expression. All mice were then moved to enrichment cages on P42 and remained in enriched environments for thirty days before undergoing fear extinction training and further experiments.
Figure 10a. Left: Quantitative PCR (qPCR) was used to determine efficiency and specificity of Mef2a-d knockdown in the presence of a Scrmb (red), Mef2a (solid green), or Mef2c (dotted green) shRNA cassette. Mef2a shRNA led to selective knockdown of Mef2a (unpaired t-test, value=0.0004). Mef2c shRNA led to robust knockdown of Mef2c (unpaired t-test,
p-value=0.0135) and a modest knockdown of Mef2a (unpaired t-test, p-value=0.0068) and Mef2d (unpaired t-test, p-value=0.0332). Right: Immunohistochemistry for DAPI (blue, top), GFP (green, top) and Mef2a (white, bottom), to show representative viral expression and Mef2 knockdown.
Injection of Mef2 viruses led to efficient knockdown (Figure 10A, right), and did not affect weight (Figure 10B) or locomotion (Figure 10C) of the animals. Both groups (Mef2a/c, Scmrb) displayed equivalent levels of initial memory acquisition, as there was no difference in the proportion of time spent freezing after receiving two foot shocks in the fear conditioning chamber (Figure 10D). However, Mef2a/c-knockdown in both males and females resulted in an impairment in memory extinction, as animals spent significantly more time freezing after multiple exposures to the chamber without foot shock (Figure 10D). These results suggest that the absence of Mef2a/c might impair the molecular processes required to effectively update previously formed associations. 10A.
Figure 10b. Mef2a/c and Scrmb shRNA-treated mice have similar body weights.
Figure 10c. Mef2a/c and Scrmb shRNA-treated mice show similar distance travelled in a
10-10B.
10C.
Figure 10d. Mef2a/c shRNA-treated mice show significantly impaired fear extinction. Left: male (n = 10 mice per group, unpaired t-test comparing % freezing post 2-shocks and % freezing on last day of extinction training, p=0.3047 for Mef2a/c KD and p=0.0101 for Scrmb); Right: female (n=12 Scrmb vs. 14 Mef2a/c KD; Scrmb t-test p-value = 0.0450 vs. Mef2a/c KD t-test p-value = 0.9635).
Previous studies have demonstrated that depletion of Mef2 family members has a significant impact on the electrophysiological properties of cortical excitatory neurons. Embryonic deletion of Mef2c in cortical excitatory neurons led to a significant decrease in cortical activity (57). In contrast, postnatal deletion of Mef2c led to increased
spontaneous firing of excitatory neurons in L2/L3 of the neocortex (64). To better understand the functional consequences of loss of cortical Mef2a/c postnatally in the context of an enriched enrichment, we performed electrophysiological recordings from pyramidal neurons harboring either Mef2a/c shRNA or Scrmb shRNA (identified by the presence of GFP) after mice were exposed to enrichment for thirty days. Mef2a/c-knockdown neurons exhibited greater membrane input resistance than control neurons (Figure 11A, top right). We also observed non-significant trends toward reduced action potential amplitude and reduced IPSC frequency (Figure 11A, bottom). The difference between a neuron’s action potential threshold and its baseline resting membrane potential was significantly reduced after Mef2a/c knockdown, indicating a lower threshold to fire (Figure 11A top right). These changes in membrane properties were accompanied by a considerable increase in hyperexcitability after Mef2a/c knockdown, evidenced by significantly more action potentials fired at a given current injection, compared to neurons containing the Scrmb virus (Figure 11A, top left). Together, these electrophysiological findings highlight Mef2’s critical role in tempering excitatory
modulated postnatally (64). They are also reminiscent of early electrophysiological changes that occur in numerous neurodegenerative disorders, including AD, in which neurons tend to show hyperexcitability in early pathological states, even before the onset of cognitive symptoms (65).
Several Mef2 targets are known to regulate the resting membrane potential of neurons (66), so we next examined how Mef2a/c knockdown in enriched animals affected downstream members of the Mef2 regulatory network to better understand the
mechanism by which hyperexcitability could be achieved. We focused on two of these genes, Scn1a and Gria4, which were also upregulated in cortical neurons of male, enriched mice. Scn1a encodes part of a sodium channel and is critically involved in maintaining appropriate excitability thresholds, as hundreds of mutations in this gene have been found to cause genetic epilepsy (67, 68). Similarly, Gria4 encodes part of a glutamate-gated ion channel, and loss-of-function mutations in this gene have also been associated with epileptic discharge (69). We used RNA in situ hybridization to measure transcript levels of these targets in GFP-positive neurons that received shRNA against Mef2a/c and confirmed that levels of Scn1a and Gria4 are decreased in neurons lacking Mef2a/c, suggesting that a Mef2-directed transcriptional program might contribute to the altered electrophysiological properties of these neurons (Figure 11B). Collectively, these results confirm that Mef2 activity is required during enrichment to enable cognitive benefits, and that blocking Mef2 activity conditionally leads to a hyperexcitability in cortical neurons.
Figure11a. Voltage-clamp recordings from pyramidal neurons (KD n = 11 cells from four animals; Ctrl n = 8 cells from 4 animals) in shRNA-expressing regions (identified by GFP fluorescence). Top left: Mef2a/c knockdown (green line) was associated with increased hyperexcitability, as measured by number of action potentials in 500 ms for a given injecting current (two-way ANOVA, p-value < 0.0001 for group and interaction). Top right: Neurons with decreased levels of Mef2a and Mef2c (green) showed increased membrane input resistance
(unpaired t-test, p-value=0.0010) and a reduced difference between baseline voltage and action potential threshold (unpaired t-test, p-value=0.0391).
Figure 11b. Left: RNA in situ hybridization for Scn1a (white) and Gria4 (red) in parallel with immunohistochemistry for GFP (green) in the frontal cortex of animals injected with Mef2a/c shRNA showed decreased levels of Scn1a and Gria4 transcripts in cells expressing GFP along with the shRNA construct (top, representative image). Right: Quantification of Scn1a (left; t-test p-value = 0.0168) and Gria4 (right; t-test p-value = 0.0015) intensity in either GFP positive (n=20 from four animals) of GFP negative (n=27 from 4 animals) cells.
3.2 Environmental enrichment rescues tauopathy-induced hyperexcitability, and is associated with increased levels of Mef2a
With human bulk and single-cell transcriptomic analysis pointing to a role for Mef2 transcriptional activity in excitatory neurons promoting cognitive resilience (CgR), we next asked if enrichment and Mef2 activity could actively rescue and promote resilience in the context of neurodegeneration. To do this we turned to the PS19 transgenic mouse model of tauopathy and neurodegeneration, which expresses the human tau protein harboring the P301S disease-causing mutation that leads to frontal temporal dementia (70). First, we examined RNA sequencing from neurons purified from 2-month-old PS19 mice and found that genes that are down-regulated in PS19 mice are enriched for components of the GABA receptor complex and inhibitory ligand-gated ion channels (Figure 12A, left). PS19 down-regulated genes are also significantly enriched for targets of the Mef2 family (Figure 12A, right). This data suggests that the transcriptional
network preserved in CgR might share salient features with the one that is suppressed in P301S-induced tauopathy.