Publisher’s version / Version de l'éditeur:
ACI Materials Journal, 97, July/August 4, pp. 418-424, 2000-08-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Effect of polycarboxylate superplasticizer on contribution of interfacial
transition zone to electrical conductivity of Portland cement mortars
Xu, G.; Beaudoin, J. J.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=0d4751d6-995d-4833-8a02-3873baa88520 https://publications-cnrc.canada.ca/fra/voir/objet/?id=0d4751d6-995d-4833-8a02-3873baa88520
http://www.nrc-cnrc.gc.ca/irc
Effe c t of polyc a rbox yla t e supe rpla st ic ize r on c ont ribut ion of
int e rfa c ia l t ra nsit ion zone t o e le c t ric a l c onduc t ivit y of Port la nd
c e m e nt m ort a rs
N R C C - 4 3 1 2 4
X u , G . ; B e a u d o i n , J . J .
A u g u s t 2 0 0 0
A version of this document is published in / Une version de ce document se trouve dans:
ACI Materials Journal, 97, (4), July/August, pp. 418-424, August 01, 2000
The material in this document is covered by the provisions of the Copyright Act, by Canadian laws, policies, regulations and international agreements. Such provisions serve to identify the information source and, in specific instances, to prohibit reproduction of materials without written permission. For more information visit http://laws.justice.gc.ca/en/showtdm/cs/C-42
Les renseignements dans ce document sont protégés par la Loi sur le droit d'auteur, par les lois, les politiques et les règlements du Canada et des accords internationaux. Ces dispositions permettent d'identifier la source de l'information et, dans certains cas, d'interdire la copie de documents sans permission écrite. Pour obtenir de plus amples renseignements : http://lois.justice.gc.ca/fr/showtdm/cs/C-42
ACI MATERIALS JOURNAL
TECHNICAL PAPER
Title no. 97-M48
Effect of Polycarboxylate Superplasticizer on Contribution
of Interfacial Transition Zone to Electrical Conductivity of
Portland Cement Mortars
by
Guodong Xu and J, J. Beaudoin
The effect of a polycarboxylate high-range water-reducing admix· lure on the electrical conductivity and interfacial transition zone
(I1Z) characteristics of portland cement mortars was evaluated using AC impedance. spectroscopy, mercury intrusion porosimetry,
and two electrical conductance models: the inteifacial excess con-ductance and Bruggeman's asymmetrical effective medium
theo-ries. Mechanical tests were also performed. Mortar specimens used had sand-cement ratios (sIc) of 0.5, 1.0, and 1.5, aqueous
high-range water-reducing admixture dosages 0/0,0.3, and 0.75%
(by mass of cement) and a water-cement ratio (w/c) of0.35. The
results showed that the presence of the polycarboxylate high·range water·reducing admixture had positively modified the interfacial transition zone performance and the microstructural characteristics of the hardened mortars. It was found, however. that this high·range water-reducing admixture reduced the rate of hydration during the
first day,
Keywords: high-range water·reducing admixture; sand; water-cement ratio.
INTRODUCTION
High-range water-reducing admixtures, (HRWRAs) (also known as superplasticizers) are widely used in the concrete industry. They may be used either to increase the workability to produce a flowing or self-leveling fresh concrete for easy placement, or to reduce the mixing water or water-cement ra-tio(wlc) to achieve a densified paste structure and improved paste-to-aggregate interfacial bond. This increases the com-pressive strength of concrete and enhances the resistance to chemical attack.
The most commonly used HRWRAs currently are polynaphthalene sulfonate (PNS) and polymelamine sul-fonate (PMS) formaldehyde condensates. These two poly-mers have essentially the same dispersing mechanism. The main polymer chains adsorb to the surface of cement parti-cles; the cement particles become negatively charged by the negatively charged sulfonate groups of the side chains. Con-sequently, electrostatic repulsion occurs betweeu the ce-ment particles. This deflocculates the cece-ment particle agglomerates in a paste matrix by offsetting the interparticle attractive forces.
Polycarboxylate polymers are attracting increasing atten-tion because of their superior performance in dispersing ce-ment particles at smaller dosages and retaining concrete slumps without prolonging set times,I.2 compared to the PNS and PMS polymers. It is reported that electrostatic re-pulsion (zeta potential) appears to playa minor role in the dispersing mechanism associated with the polycarboxylate polymer. A significant steric hindrance effect, caused by the extension of their graft chains of polymers (for example, polyethylene oxide) away from the surface of cement parti-418
cles account for much of the dispersion capability,l.3-6 The dispersability increases as the polyethylene oxide graft side chains lengthen6 .7
Research on this type of HRWRA has mostly focused on dispersion mechanisms and hydration and setling effects.1-7
The objective of this study was to investigate the effect of this type of HRWRA on microstructural and interfacial transi-tion zone characteristics of portland cement mortars. Two ex-perimental techniques, the AC impedance spectroscopy and the mercury intrusion porosimetry, were employed in the study, Two electrical conductance models, the interfacial excess con-ductance modelS and Bruggeman's asymmetrical effective medium theory,9 were used to characterize the effect of the HRWRA on the electrical conductivity in the interfacial tran-sition zone of the mortars. Finally, compressive strength tests were performed to check if the electrical behavior and micro-structural characteristics influenced mechanical properties.
RESEARCH SIGNIFICANCE
Research on the use of polycarboxylate HRWRAs in ce-ment systems has focused primarily on the dispersion mech-anisms, and hydration and setting effects. Although there have been many investigations of the electrical properties of pastes and mortars, there is little published information re-garding the effect of HRWRA and especially polycarboxy-late HRWRA on the interfacial transition zone (ITZ) of mortars and concretes, This study addresses the effect of a polycarboxylate HRWRA on the interfacial zone electrical conductance and microstructural characteristics of portland cement mortars, by using AC impedance spectroscopy, mer-cury intrusion porosimetry, and two electrical conductance models: the interfacial excess conductance model and the Bruggeman's ideal conductivity model. Bruggeman's model has been used extensively in the area of applied physics. It is suggested that this model is relevant in assessing the effect of a HRWRA on the interfacial zone characteristics of a mor-tar system.
EXPERIMENTAL PROGRAM Materials and specimens
ASTM Type I portland cement was used. Its chemical composition is as follows: constitutes Si02 (20.11), Al20 3 (4_15), Fe203 (3.16), CaO (62.75), MgO (2.76), Na20
ACt Materials Journal,V. 97, No.4, July-August 2000.
MS No.99·083receivedj。ョオ。セ 3,1999,and revi,ewed under Institute publication policies. Copyright ©.2000,aュ・ョ」セョ sZPョセイ・エ・ iセウエャエオエ・N All rights reserved, includ-ing the makinclud-ing of copIes unlessセ・イュャウNウャoョ ISobtamed from the copyright proprietors. Pertinent discussionwillbepublished m the May·June 2001ACI Materials Journalif received by February 1, 2001.
ACI Materials Journal/July-August 2000
I
(I)
(2)
Electrode eHectCOl
(b)
Bulk paste effect
,, ,, ,, : R2 I
L
セ..._..
セ__ .J
(a) R,+R2 Real(ohms) Bulk paste effectR,
'Measuredbymercuryintmsionporosimetry. Proportion by mass
Portland Silica sand, Overall
Type of cement, grade Superplasticizer, porosity at mixtures ASTMType 1 20-30 in aqueous form Water 28 days, %
I 1 0.5 0 0.35 12.93 2 1 0.5 0.003 0.35 10.86 3 1 0.5 0.0075 0.35 11.55 4 I 1.0 0 0.35 12.87 5 [ 1.0 0.003 0.35 11.24 6 I 1.0 0.0075 0.35 10.53 7 1 1.5 0 0.35 11.79 8 I 1.5 0.003 0.35 10041 9 1 1.5 0.0075 0.35 9.24
Fig, l-(a) Schematic of AC impedance spectrum in real-imaginary complex plane for hardened cement paste; and (b) equivalent electrical circuit model.
Table 1-Mixture proportion of fresh mixtures and overall porosity of hardenedュッイエ。イウセ
419
where crmis the electrical conductivity of mortar; crpis the conductivity of cement paste; andVf,sandis the volume frac-tion of sand in the mortar.
Equation (2) is derived on the assumptions that the sand grains are spherical and nonconductive; the sand is ゥセエイッᆳ
duced into a continuous background of conductive cement where
0
is the thickness of transition zone; crt and crpare the electrical conductivity of transition zone and bulk paste, re-spectively; ris the average radius ofthe aggregate;k and b are constants determined by the relationship cr=kq>a+ b (where cr is the electrical conductivity of the mortar determined from the electrical resistance value at point R2on Fig. lea); and q>a the volume fraction of sand in the mortar specimen). Values ofcrt>crpore
> 0in Eq. (1) indicate a transition zone that is less dense than the bulk paste matrix; values of crt<crp ore
< 0indicate the opposite.Bruggeman's asymmetrical effective medium
theory-Bruggeman's effective media theorieslOhave been widely used in the area of applied physics for predicting the effec· tive conductivities of heterogeneous systems consisting of low conductive grains embedded in a high conductive ma-trix, or vice versa.14-16In the case of portland cement mor-tars, Bruggeman's asymmetrical equation, derived from an integrating procedure,17 can be expressed as
(0,22), K20 (0.87), and S03 (3.21). The sand was Ottawa Grade 20 to 30 silica sand (particle size, 600 to 850 11m in di-ameter) conforming to ASTM DesignationC 778-87. The HRWRA used was a polycarboxylate type with the appear-ance of an amber-colored liquid. The solid content of the ad-mixture, determined from the mass after drying the liquid admixture at105 C for 24 h, was approximately 31 %.
Mortar specimens with
sic
of 0.5, 1.0, and 1.5, and aque-ous HRWRA dosages of 0,0.3, and 0.75% (by mass of ce-ment) were produced. A wlc of 0.35 was used for all specimens that did not account for the small water portion from the HRWRA. Details of the mixtures are given in Table 1. The fresh mortar was cast in the perspex electrical conduc-tivity cells and compacted by mechanical vibration for 1 min. The cells had internal dimensions of77 x 77 x 50 mm with two opposite sides fitted with stainless steel electrodes. The specimens were cured in a 100% relative humidity room for 24 h and then immersed in saturated limewater for testing at selected periods of hydration.J.J.Beaudoin is a principal research officer at the Materials Laborarory ofthe
Insti-tute for Research in Construction, Narional Research Council, Canada. His research interests include the application of AC impedance spectroscopy In cement and con-crete structures.
Guodong Xuis a former research scielltisr at the Department oj Chemisty, Universitt! de Sherbrooke, Quebec, Canada. His research interests include the use ofchemical and mineral admixtures In cement and concrete, andfiber-reiliforced roofing materials.
ACI Materials Journal/July-August 2000
Methods
AC impedance spectroscopy-ACimpedance spectroscopy
is a well-known electrochemical technique of characterizing the electrical properties of cementitious materials.IO-l3A typ-ical impedance spectrum for a hydrating cement paste is plot· ted in the real-imaginary complex plane, shown in Fig. lea).It depicts a single arc in the high frequency range, with a small part of the second arc in a relatively low frequency region. It is suggested that the impedance behavior of solid-liquid in-terfaces in the cementitious system may be modeled as a col-lection of resistors and capacitors using an electrical equivalent circuit (Fig. l(b» whereR2,the high frequency arc (HFA) diameter represents the interfacial impedance be-havior, andR)
+
R2•the intercept of the HFA with the real axis, represents the overall bulk resistance of the speci-men.IO• 1The second arc is due to the cement-electrode in-terface capacitance effect. An impedance gain-phase analyzer was used for the impedance measurements at se-lected hydration periods of 5 h, 1, 4, 7, 14, 21, 28, and 56 days. Data were collected at frequencies ranging from 1 Hz to 15 MHz.Mercury intrusion porosimetry-Porosity and pore size
distribution of the specimens were determined using a mercu-ry intrusion porosimeter at pressures up to 380 MPa. Assum-ing a contact angle of130 degrees and a surface tension of 473 x 103
11m
2,the mercury can penetrate pores as small as 3.2 nm in diameter. Water-saturated samples approximately 2 mm thick were cut and immersed in excess isopropanol al-cohol for one week, followed by drying at60C for 24 h. Pre-treatment in isopropanol is thought to minimize the effect of drying on the pore structure. A typical sample weighing ap-proximately0.9 g was fractured into three or four pieces be-fore being placed in the penetrometer.Interfacial excess conductance, 8-This methodS has
been used to assess the relative difference in electrical con-ductance between the interfacial region and the bulk cement paste in a mortar system using the following equation ,
I
br 2000on
)f
sed on ets.I-7 ,'eet of transi-NO ex-nd the study. ,s eon-'ective of the ! tran-htests niero-les. mica! d20
3 'h20 -6The ft side ,lication iaclud-lrietors. 'lirnalif in ce- nech-there ies of In re- boxy-Z) of tof a ;trical 11!and . mer-;tance d the nodel ;, It is c:ffectmor--200 400 IJ. M·1 IJ. M·1 -150 [] M·2 -300 [] M·2
a
<> M-3a
0 M-3 ... Mp·1 [] Cl...
セ"
セ A Mp·1 セ -100 I!I MP-2 [] 0 t1l -200•
MP-2 c '0,.
MP-3 [] Cl 'OJ·
Mp·3 ell C1l .§.£
-50 -100 0 0 0 50 100 150 200 0 100 200 300 400 Real (Q) (b) 7 days Real (Q) (a) 1 day -500 ·600 ·400 IJ. M-l -500 IJ. M·l [] M·2 [] M-2a
" M-3a
-400"
M-3 ... -300 --セ 4 MP·1 セ..
MP-1 ell セ ·300•
MP-2 c II MP-2 'OJ '5 MP-3 Cll -200•
MP-:] ca•
EE
·200 -100 ·100ijNセ
セイャヲN
0 1J.1dIiIo. 0 0 100 200 300 400 500a
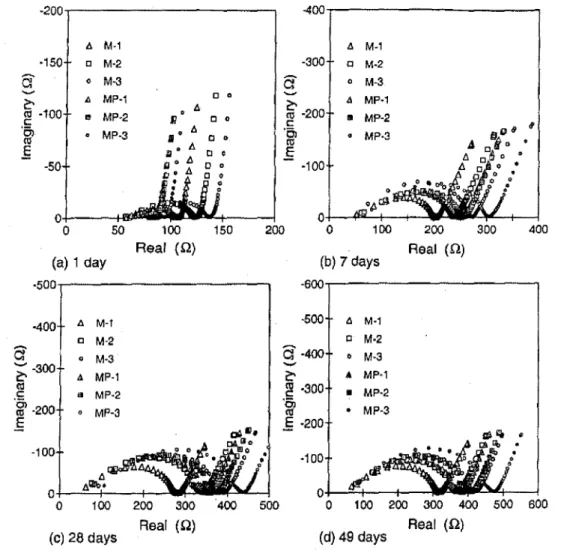
100 200 300 400 500 600 Real (0) (d) 49 days Real (0) (c) 28 daysFig. 2-AC impedance spectra of portland cement mortars in presence and absence of polycarboxylate superplasticizer with time of hydration: M-1 (sic ::: 0.5), M - 2 (sic
:::1.0),andM - 3(sic;;;;1.5) without superplasticizer; andMP-1(sic:::0.5), MP - 2(sic ;;;; 1.0), andMP - 3(sic=1.5)with0.75%superplasticizer;w/c= 0.35.
matrix; the conductivity of the cement matrix does not change with increasing volume fraction of sand; and there is no interfacial effect. These assumptions, however, are not completely true for practical mortars.
Nevertheless, it can be shown by plottingomlop(the rel-ative or normalized conductivity of the mortar am to the pasteセI againstVf,sandin Eq. (2) that the effective conduc-tivity for the mortar system decreases with increasing sand content. Noting that the theoretical curve represents the ideal-ized medium in which the ITZ effect on conductivity is zero, then the ITZ effect on electrical resisti vity of a practical mor-tar would be positive (that is, to reduce the conductivity of the paste-sand system) if the experimental data falls below the theoretical line (determined byEq.(2)). The opposite would be true if the experimental data lies above the theoretical curve.
The compressive strength test was carried out using a uni-versal testing machine.
RESULTS AND DISCUSSION Impedance behavior
It was observed during the preparation of the fresh mix-tures that the addition of the polycarboxylate HRWRA ap-peared to produce a more cohesive (and less prone to segregation) mortar mixture, compared to the PNS and PMS HRWRA adopted in an earlier research program.17-19 This
would indicate that the dispersion retention ability of the polymer is good.
Representative impedance spectra of the mortars with 0.75%
HRWRA are shown in Fig. 2. The effect ofthe HRWRA on the overall electrical resistivity of the mortars at hydration times of 5 h, 1, 7, ;md 49 days are given in Fig. 3. The overall electrical resistivity p was calculated from p
=
R • Ald. where A is the cross-sectional area of the specimen;d is the specimen thick-ness or the distance between the electrodes of the cell; andR is the bulk resistance(R=R t+Rz,
Fig. I(a)).The HFA or semicircle (Rt セ R
z,
Fig. lea)) did not appear at 5 h for all the mortars, and only part of the second real-imag-inary arc was observed. This was because the number of avail-able interfaces or internal surface area was too small to be detected at this stage of hydration, the pores between the solid particles were not yet filled by hydrates, and a rigid network structure was not yet fonned. The HFA was observed at hy-dration times of 24 h and beyond for all the specimens (Fig. 2). This was a result of microstructure and interface development relatedtothe cement hydration process.It is apparent on examination of Fig. 2(a), 3(a), and 3(b) that at 5 h and I day, almost all the HRWRA mortars exhibited a lower overall resistance compared to the corresponding con-trol specimens. This indicates that the addition of the polycar-boxylate HRWRA affectedthe hydration of cement and the overall hydration rate was reduced as a result. Such an effect at
600 4000
§
500 (a)5 hoursE
u (b) 1 day9-
g
3200.€'
400f
2400 .oe セ 300 '0)'"
2!.!:
1600 セ 200B
.;:: .t: セQPP U 800 2 ill ill 0 0 SlC=O.5 SlC=1.0 SlC=1.5 Type of mortars sヲcセoNU S1C=1.0 SfC..t.5 Type of mortarso
6000イイZZZ[[]ZZZ[MMMM[Zセ[[⦅ZZ[Z]⦅ᆳE
セ 5000 9-f4000 'l;; '0) 3000 l!!B
2000 't:j
1000 ill o 6000イ[Z[[ZZ[[[Z[Z[Z[Z[[ZZZZ[MMMエZセ[ZZZZMMML (c)7 days§
5000 9-セ 4000+
-セ Nセ 3 0 0 0 1 -S! セ 2000Y
1000 ill S1C..O.S S1C..1.0 S/C..1.5 SfC=O.S S1C..1.0 S!C=1.5Typeof mortars Type of mortars
Fig. 3-Elasticity resistivity values ofmortars with 0.3 and 0.75% polycarboxylate super-plasticizer (SP), in comparison to that without SP: wlc
=
0.35.50 -+-Cl%SP -o-Cl.3%SP --0-Cl.75%SP 0.00+---+--I--+--t--t--t---+--+----lf---;
o
0.16 - , - - - , 0.14 0.12"b
...
PNQセ
><. 0.08 セ:g
0.06 c:p 0.04 0.02 10 20 30 40Time of hydration (days)
Fig. 4-Effect of polycarboxylate superplasticizer on inter-facial excess conductance 0 of portland cement mortars with and without superplasticizer versus time of hydration: wlc
=
0.35; and sic=
0.5. 1.0, and 1.5.opposite. The
e
values versus time of hydration for mortars withsic
=0.5, 1.0, and 1.5 are plotted in Fig. 4, showing that thee
values for the HRWRA mortars are generally smaller than that for the plain mortar. although all the curves are above the horizontal axis (0>0). The smallere
values for the HR-WRA mortars, especially for the mortar with 0.3% HRHR-WRA, actually suggest a greater degree of improvement of the inter-facial zone relative to the corresponding bulk paste. This could be due to an improvement in the interfacial zone structure (po-rosity, pore size distribution, and disconnectivity) or a de-crease in the interfacial zone thickness. Thee
values for the mortars with 0.75% HRWRA were just marginally smaller than those without the additive (Fig. 4); the resistivity values were greater for the HRWRA mortars (Fig. 2). A plausible I day was not observed previously for mortars containingPNS or PMS HRWRAs with dosages up to 1.5% and the same Wlc.18,19 The overall electrical resistance of those mortars was generally greater than the corresponding control mortars without HRWRA. The electrical resistance values increased with increasing sand content because the sand particles are virtually nonconductive.
It can be seen from Fig. 2 and 3 that the overall electrical resistances measured at7 days and after for the HRWRA mor-tars were all greater than that for the control mormor-tars. The ad-dition ofHRWRA influenced both the cementfsand interfacial region and the bulk paste component in the mortar. The micro-structural changes of the interfacial zone itself are unable to be quantitatively measured using the impedance teclu)ique itself but can be inferred from the impedance data obtained. The greater bulk resistivity and HFA values for the HRWRA specimens are indicators of a denser interfacial transition zone and bulk matrix, finer pore distribution, decreased pore connectivity, and lower ionic concentration of the pore solu-tion, compared with the non-high-range water-reduced ad-mixture'specimens. The higher ionic concentration of the pore solution for the high-range water-reduced admixture system should have contributed to a reduction in the resistiv-ityand the HFA diameter. The greater resistance values ob-tained for the HRWRA specimens therefore must be explained by the improvement in the microstructure and po-rosity. This argument is supported by the results shown in the following sections.
Interfacial excess electrical conductance
The application of Eq.(1)in the present work established the relative difference in electrical conductance between the interfacial region and the bulk cement paste in the mortar system. As previously indicated, values of interfacial excess electrical conductance
e
>0 indicate an interfacial zone that is less dense than the bulk paste matrix;e
<0 indicates the ity of the lotappear eal-imag-rof avaiI-Jail to be Ithe solid Inetwork 'cd at hy-s(Fig. 2). elopment vithO.75% 'RAonthe mtimes of Ielectrical 'eAis the nen thick-ell; andR 13(b) that ;hibited a .ling con-, polycar-It and the Ieffect at0.5 (b) -O-O.OO%·14d _ _ O.30%·14d --O.75%-14d _ _ O.OO%-28d -.lr-O.30%·28d _ _ O.75%-28d 1 . 0 , . . - - - , 0.8 0.2 0.6 0.4 (a) Suparplasticizar dosage
/
-O-O.OO%·1d -'-O.30%.1d"\. 3'2 _ _ O.75%'1d """ (1-Vt•Slrd)-o-O.OO%-7d Hydration time ... O.30%-7d -.lr-O.75%-7d
セ
1.0 1:2" 0.8 セo E '0 0.6 セセ
0.4 'g8
セ 0.2 セゥセ
0.0+----.-"""T""-,....--r--,..---..,.-....--i 0.1 0.0KMMMイMセMMLイMMMイMMNNNMMMMイMNNLNMMMMャ 0.2 0.3 0.4 0.5 0.1 0.2 0.3 0.4. Volume fraction of sand Volume fraction of sand
Fig. 5-Comparison of relative electrical conductivities (mortar to paste) for mortars with and without polycarboxylate superplasticizer at various hydration times and volume frac-tions of sand: ASTM Type 1 portland cement; Ottawa Grade 20-30 silica sand; wlc = 0.35; and sIc
=
0.5, 1.0, and1.5by mass.explanation would be that the interfacial region and the bulk paste in the 0.75% HRWRA mortars were both proportional-ly improved.It should be noted that the
e
parameter represents relative values only; that is, the difference in electrical conduc-tivity between the interfacial region and the bulk cement paste in the mortar system. Equala
values for two different sys-tems do not necessarily mean equal resistivity. The interpre-tation of thee
values should, therefore, be made in combination with the impedance data or by other means.Bruggeman's asymmetrical equation
Figure 5 shows the relative or normalized conductivity of the experimental mortars,
crm!cr
p ,at typical hydration times of 1,7, 14, and 28 days, compared with Bruggeman's (l - Vf,sand)3f2theoretical curve(Eq. (2» for idealized mortar. At1day, the HRWRA mortars in general had greater values compared to the control mortars. The relative conductivity of the 0.75% dosage mortars was 1.27, 1.44, and 1.45 times greater than that of the corresponding control mortars(l day,Fig. 5(a»). This would suggest that the ITZ at 1 day had contributed negatively to the electrical resistance of theHRWRA mor-tars. At 7 days and beyond, all the data points for the mortars with the polycaboxylate HRWRA fall below the curves of the plain mortars, indicating that the HRWRA had positively modified the interfacial transition zone microstructure that, in
tum,contributed positively to (increased) the electrical resis-tanceof the system.
With increasing time of hydration, the data points of rela-tive conductivity were pushed toward the theoretical line. The values for HRWRA mortars with sand volume fraction of0.2(sic
=
0.5) fall below the theoretical curve, at 7 days or greater. This would imply that these mortars had either a denser ITZ or a more favorable ITZ microstructure, com-pared to their bulk matrix, or that the Bruggeman's equation (Eq. (2» is inaccurate for the mortar system. The majority of the experimental data falls above the theoretical curve. Thus, as a whole, the ITZ effect on electrical resistivity was negative. That is, the presence of the ITZ reduced the over-all resistivity of the mortars. The trend would appear to suggest that the greater the number of sand inclusions, the more pronounced the negative ITZ effect.PorosIty and pore size distribution
Figure 6 shows the curves for the cumulative pore size dis-tributions of the mortars at28 days. The largest porosity val-ue on a cumulative curve is an estimate of the total (apparent) porosity of the sample. The equivalent pore diameter corre-sponding to the initial point of the steepest slope on the cu-mulative curve is usually referred to as the threshold pore diameter at which large quantities of mercury start to pene-trate into the bulk of the specimen. The overall porosity data are listed in Table 1. The porosity data herein are presented on the basis of the total volume of the mortar.
The following information may be obtained from the pore size measurements shown in Fig.6 and Table 1:
1. All the HRWRA mortars had lower overall porosity (at 28 days), compared to the corresponding control mortars without the HRWRA. This would suggest that the HRWRA had positively modified the pore microstructure;
2. The overall porosity decreased with increasing sic. This is reasonable, as with the introduction of sand, the proportion of the paste in the sample decreases. The amount of pores should also decrease by virtue ofthe decreasing volume of paste. because the sand particles are relatively nonporous;
3. The pore volume of 100 to 1000 nm sizes increased with increasing sic;
4. For
sic
ratios of 1.0 and 1.5, mortars with 0.75% HR-WRA had the lowest porosity; and5.The threshold pore diameters were quite similar for all the mortars, that is, approximately 70 nm.
Compressive strength
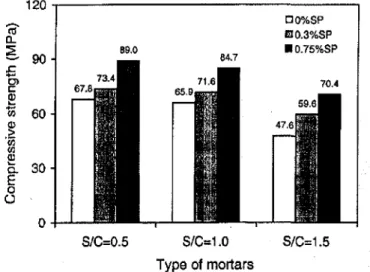
Compressive strengths of the mortars obtained at a hydra-tion time of 49 days are presented in Fig. 7, showing large strength gain for the mortars containing the polycarboxy-late HRWRA. The highest strength values were obtained for the mortars containing 0.75% HRWRA. The mechani-cal results are consistent with the conductivity and poro-simetry data previously shown.
CONCLUSIONS
The effect of a polycarboxylate HRWRA on the electrical re-sistivity and the interfacial transition zone characteristics of portland cement mortars with various sic ratios and HRWRA
15..,..---...,
S/C=1.5
S/C=O.5 S/C=1.0
Type of mortars
Fig. 7-Compressive strength of mortars at49days in pres-ence and abspres-ence
0/
polycarboxylate superplasticizer:wlc
=0.35; andsIc=
0.5, 1.0, and1.5by mass.o
Experimental values ofcrmlcrp'and the relative conductiv-ity (that is, the conductivconductiv-ity ratio of mortar and paste) of mortars are compared with the theoretical curve predicted by Bruggeman's asymmetrical effective medium theory. The majority of the experimental data falls above the theoretical line, indicating that as a whole, theITZeffect on electrical resistivity was negative (that is, to reduce resistivity). All the data points for the mortars with the polycaboxylateHRWRA fall bellow the curves of plain mortars at7 days and beyond, indicating that theHRWRAhad positively modified the in-terfacial transition zone microstructure that, in tum, contrib-uted positively to (reduced) the electrical conductance of the system. These are fully consistent with the impedance, poro-simetry,
a
parameter. and compressive strength results. It would appear that a plot of the relative conductivity values of the experimental mortars versus the volume fraction of ag-gregate (compared with Bruggeman's theoretical curve) is appropriate for assessing the effect ofHRWRAon the ITZ characteristics.A reduction in the hydration rate due to theHRWRA was observed for hydration times up toI day.
ACKNOWLEDGMENTS
This project received financial support from the NRC-NSERC-Industry program.
.REFERENCES
1. Shiba, D.; Kitagawa, K.;, Shimoda, M,; and Izumi, T., "Study on QUalities of High Flow Concrete Using New High-Range Water-Reducing Agent Polyether Type," 4th CANMET/AC//JC/ [nternational Conference
on Recent Advances in Concrete Technology-Supplementary Papers,
SP-179,V. M. Malhotra, ed., American Concrete Institute, Fannington Hills, Mich., 1998, pp. 71-85.
2. Jelnavorian, A. A.; Roberts, L.R.;Jardine, L.; Koyata, H.; and Dar-win, D.c.,"Condensed Polyacrylic Acid-Aminated Polyether Polymers as Superplasticizers fOF Concrete." Proceedings, 5th CANMET/ACI Interna-tional Conference, Superplasticizers and Other Chemical Admixtures in Concrete.V. M. Malhotra, ed., American Concrete Institute, Farmington Hills, Mich., 1997, pp. 54-81.
3. Collepardi, M.; Coppola, L.; Cerulli, T.; Pistolesi, C.; Zaffaroni. P.; Deroches. G.; and Drapeau, A., "Acrylic Based Superplasticizer," Fourth
CANMET/ACI International Conference on Superplaslicizers and Olher Materials, SP-148. V. M. Malhotra, ed.• American Concrete Institute.
Farmington Hills, Mich., 1994. pp. 1-18.
4. Uchikawa, H.; Hanehara, S.; and Sawaki, D.. "Effect of Electrostatic and Steric Repulsive Force of Organic Admixture on the Dispersion of Cement Particles in Fresh Cement Paste," Proceedings of the 10th
[merna-120 '@' ao%sp a. mO.3%SP :E 89.0 .D.75%SP
...
90 .c-
0 ) c: セ to:; 60 \l) > 'US <Il III 0. 30 E a 100 0 100 100 10 10 -6-0.00%SP -O-O.30%SP -'-O.75%SP (c)S/C=1.5 0.01 0.1 1 Pore diameter (J..tm) 0.Q1 0.1 1 Pore diameter (J..tm)ッlNNNNLNNNLNNLNNLNNNNNNNNNNNNLNLセAAAセセセセ
0.001o
lNNNLNNLNNLNLNLNMMNMNMNMセAAAセセセセ
0.001 15...- (alS/C",0.5 -II-O.OO%SP
::,!1 -a-O.30%SP 0 ... 12 --O.75%SP Nセ <Il
e
9 0 0.. CD .2: 6 «is
E 3 ::l 0°
0.001 0.01 0.1 1 10 Pore diameter (J..trn) 15 ;i (b)S/C=1.0 -A-O.OO%SP 0 --0:-0.30%6 P ... 12 --0.75%SP Nセ<Ile
9 a 0-CD > 6 セ "5 E 3 ::l Uセ
... 12 セ <Ile
98.'
g: 6 .'§ "5 E 3 ::l UFig. 6-Cumulative pore size distributions a/mortars in pres-ence and abspres-ence
0/
polycarboxylate superplasticizer at dos-ages 0/0,0.3, and 0.75%, at28days:w/c =0.35; andsic=
0.5, 1.0, and1.5 by mass.
dosages was evaluated using AC impedance spectroscopy, mercury intrusion porosimetry, and two electrical conduc-tance models: the interfacial excess conducconduc-tance model and Bruggeman's asymmetrical effective medium theory. Com-pressive strength tests were also performed..
Compared with the control mortars, the hardened HRWRA mortars (with dosages of 0.3 and 0.75%) had greater electrical resistivity, lower overall porosity, smaller interfacial excess conductance
a
values, and higher compressive strength. The smallere
values for the HRWRA mortars would suggest a greater degree of improvement of the interfacial zone rela-tive to the corresponding bulk paste. This could be due to an improvement in the interfacial zone structure (porosity, pore size distribution, and disconnectivity) or a decrease in the in-terfacial zone thickness.'5% HR-rosity (at mortars HRWRA sIc.This 'oportion of pores Jlume of IOroUS; lsed with Ithe pore esize dis-'osity val-apparent) rer corre-mthe cu-lOld pore tto pene-)sity data Jresented arforall a hydra-ng large 'arboxy-lbtained lechani-ld pora-trical re-[sties of lRWRA
tional Congress on Chemistry afCement,H. Justnes, ed., Amarkai AB and Congrex, Goteborg, 1997, pp. 3iiiOOL
5. Sakai, E., and Daimon, M., "Dispersion Mechanism of Alite Stabilized by Superplasticizers Containing Polyethylene Oxide Graft Chains,"
Proceed-ings, 5th CANMET/ACI International Conference, Superplasticizers and
Other Chemical Admixtures in Concrete, SP·173, V. M. Malhotra, ed., American Concrete Institute, Fannington Hills, Mich, 1997, pp. 187-201.
6. Yoshioka, K.; Sakai, E.; Daimon, M.; and Kitahara,A,"Role of Steric Hindrance in the Perfonnance of Superplasticizers for Concrete,"Journal of
the American Ceramics Society,Y.- 80, No. 10, 1997, pp. 2667-2671.
7. Ohta, A; Sugiyama, T.; and Tanaka, Y., "Fluidizing Mechanism and Application of Polycarboxlate-Based Superplasticizers,"Proceedings,5th
CANMET/ACI International Conference, Superplasticizers and Other
Chemical Admixtures in Concrete, SP-I73, V. M. Malhotra, ed., American Concrete Institute, Fannington Hills, Mich., 1997, pp. 359-378.
8. Xie P.; Beaudoin,J.J.; and Brousseau, R., "Flat Aggregate-Portland Cement Paste Interfaces-I: Electrical Conductivity Models,"Cement and
Concrete Research,V. 21,1991, pp. 515-522.
9. Bruggeman, D. A G., "Berechnung verschiedener physikalischer Konstanten von herogenen Substanzen: I. Dielektrizitatskonstanten und Leitfahigkeiten der Mischkorper aus isotropen Substanzen,"Annalen der
Physik" Y. 24, 1935,pp.636-664.
10. Gu,P.;Xie, P., Beaudoin, J. J.; and Brousseau, R., "A.C. Impedance Spectroscopy(I):A New Equivalent Circuit Model for Hydrated Portland Cement Paste," Cement and Concrete Research,V. 22, No.5, 1992, pp. 833-840.
11. McCarter, W. 1.; Gearing, S.; and Buzzed. N., "Impedance Measure-ments on Cement Paste,"Journal of the Materials Science Letters,V. 7, No. 10, 1988,pp.1056-1057.
12. Christensen, B. J.; Coverdale,R.T.; Olson, R. A.; Ford, S. J.; g。イセ
boczi, E.1.;Jennings, H. M.; and Mason, T.0.,"Impedance Spectroscopy of Hydratingc・ュ・ョエセb。ウ・、 Materials: Measurement, Interpretation, and
424
Application,"Joumal of the American Ceramics Society, V. 77, No. II, 1994,pp.2789-2804.
13. Xu, G.; Beaudoin. J. J.; Jolicoeur, C.; and Page, M., "Microstructural Investigation of Portland Cement Mortars Containing Varying Dosages of a Polynaphthalene Sulfonate Superplasticizer,"Proceedings of Second CAN-METIACI International Conference on High-Performance Concrete, and
Perfonnance and Quality of Concrete Structures,SP-186, V. M. Malhotra,
P. Helene,L. R.Prudencio, and D. C. C. Dal Molin, eds., American Con-crete Institute. Fannington Hills, Mich., 1999, pp,
253-274'-14. Meredith, R. E., and Tobias, C.
w.,
"Conduction in Heterogeneous Systems,"Advances in Electrochemistry and Electrochemictll Engineering,V. 2, Part II, C. W. Tobias, ed., Electrochemical Engineering, Interscience Publishers, 1962, pp. 15-47.
15. Landauer, R., "Electrical Conductivity in In homogeneous Media,"
Electrical Transport and Optical Properties of Inhomogeneous Media. 1. C.
Garland and D, B. Tanner, oos., American Institute of Physics. 1978, pp. 2-43. 16. McLachlan, D.S.;Blaszkiewicz. M.; and Newnham, R. E., "Electri-cal Resistivity of Composites,"Journal of the American Ceramics Society,
Y. 73, No.8, 1990,pp.2187-2203.
17. Xu. G.; Beaudoin, J. J.; Jolicoeur. C.; and Page, M., "The Effect ofa Polynaphthalene Sulfonate Superplasticizer on the Contribution of the Interfacial Transition Zone to the Electrical Resistivity of Mortars Contain-ing Silica and Limestone Fine Aggregate,"Cement and Concrete Research.
(accepted for publication)
18. Xu, G., "Effect of Varying Dosages of a Polymelamine Sulfonate Superplasticizer on the Properties of a Portland Cement Mortar,"Internal
ReportNo.2, 1998, 14 pp.
19. Xu, G.; Beaudoin, J. J.; Jolicoeur,
c.;
and Page, M., "Interfacial Transition Zone Characterization of Portland Cement Mortars Containing Relatively High Dosages of Polynaphthalene Sulfonate Superplasticizer,"Concrete Science and Engineering.(accepted for publication)