Analyzing the IT Architecture Landscape in the Biopharma Industries By
Sriram Balaji Bhashyam
B.E. ELECTRONICS & COMMUNICATION ENGINEERING
NATIONAL ENGINEERING COLLEGE, MS UNIVERSITY, 1996
SUBMITTED TO THE SYSTEM DESIGN AND MANAGEMENT PROGRAM IN PARTAL FULFILLMENT OF THE REQUIREMENT FOR THE DEGREE OF
MASTER OF SCIENCE IN ENGINEERING AND MANAGEMENT AT THE
MASSACHUSETTS INSTITUTE OF TECHNOLOGY JUNE 2019
2019 Sriram Balaji Bhashyam. All right reserved.
The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis in whole or in part in any medium now known
or hereafter created
Signature redacted
Signature of Author: --- -
--System Design & Management Program Mav 10. 2019
Signature redacted
Certified by:---Dr. Donna H Rhodes Principal Research Scientist Sociotechnical Systems Research Center
Signature redacted
Accepted by:---V
MASSACHUSETTS INSTITUTE Joan Rubin
OF TECHNOLOGY Executive Director, System Design & Management Program
JUN 2
7
2019
1
LIBRARIES
77 Massachusetts Avenue
Cambridge, MA 02139
MIT~ibarieshttp://Iibraries.mit.edu/ask
M
ITlibrarnes
DISCLAIMER NOTICE
Due to the condition of the original material, there are unavoidable flaws in this reproduction. We have made every effort possible to
provide you with the best copy available.
Thank you.
The images contained in this document are of the best quality available.
Analyzing the IT Architecture landscape in the Biopharma Industries
By
Sriram Balaji Bhashyam
Submitted to the Department of System Design and Management on May 10, 2019 in Partial Fulfillment of the
Requirements for the Degree of Master of Science in System Design and Management
ABSTRACT
As Charles Darwin explained his theory of evolution "It is not the strongest of the species that survive, nor the most intelligent but the one that is most responsive to change". This statement holds good for the role of IT in the Biopharma world. Today the role of IT is rapidly evolving, and the IT organization's role has shifted from being a technologist to the role of change agent. This is mainly driven by the technological advancements in finding new drugs at a faster pace. This is expected to undergo this transformation for a foreseeable future. The IT organization should ensure that it is led
by business priorities rather than delivering the business solutions and at a lower cost.
This can be done through the organizational design, process and governance. The IT strategy must function in lock steps with the business strategy. The biopharmaceutical industry is experiencing a strong growth over the past decade compared to the other industries. Almost all the pharmaceutical and biopharmaceutical companies are under tremendous pressure from the government agencies such as FDA and other financial regulations to ensure compliance as well as provide therapies to the patients at a reduced cost. With the new trends like predictive analytics, Artificial Intelligence,
Machine Learning, Digital medicine and Outcome based pricing models, it is very important for the IT function within the biopharma organizations to innovate itself
constantly. Maintaining the right balance between organizational performance and value delivery are the key drivers for architecting the IT enterprise.
The research explores different architectural approaches through various different lens from a business point of view, stakeholder point of view, regulatory point of view etc. to determine the optimal organizational structure for an enterprise IT group in a biopharma organization. The thesis also discusses the pros and cons of different types of IT
organizational models and provides a recommendation on the future IT enterprise.
Thesis Supervisor: Dr. Donna H. Rhodes
Acknowledgements
I would like to thank my Thesis Advisor Dr. Donna H. Rhodes, for providing me an
opportunity to work under her guidance. I am very grateful for her valuable insights and
feedback for my thesis.
I would like to thank System Design and Management department for guiding and
supporting throughout my graduate program at MIT
Finally, I would like to thank my family for supporting me through this educational
Sriram has over 23 years of extensive experience in consulting, Quality assurance, Program Management functions. He has worked with L&T, BaaN, GE and Infosys
earlier. He currently works as Associate Director at Biogen, where he is currently leads
the QA portfolio within the enterprise. Sriram earned is Engineering degree in 1996 at
National Engineering College in India. Sriram holds the Project Management
Professional (PMP) credentials from the Project Management Institute. Sriram enrolled
in the System Design and Management program at MIT in the fall of 2017.
Sriram currently lives in Westford, Massachusetts with his wife, Sumathi and daughter
Table of Contents
1. Introduction ... 10 1.1 Background ... 10 1.2 M otivation ... 13 1.3 Scope... 14 1.4 Research Questions ... 15 1.5 Research Approach ... 15 1.6 Thesis Overview ... 162. End-to-End View of the Business Landscape ... 18
3. Enterprise Landscape- Internal IT environm ent... 24
4. Biopharm a Enterprise IT Challenges ... 27
5. Force Field Analysis ... 33
5.1 Drivers for Change ... 34
5.2 Drivers against change:... 37
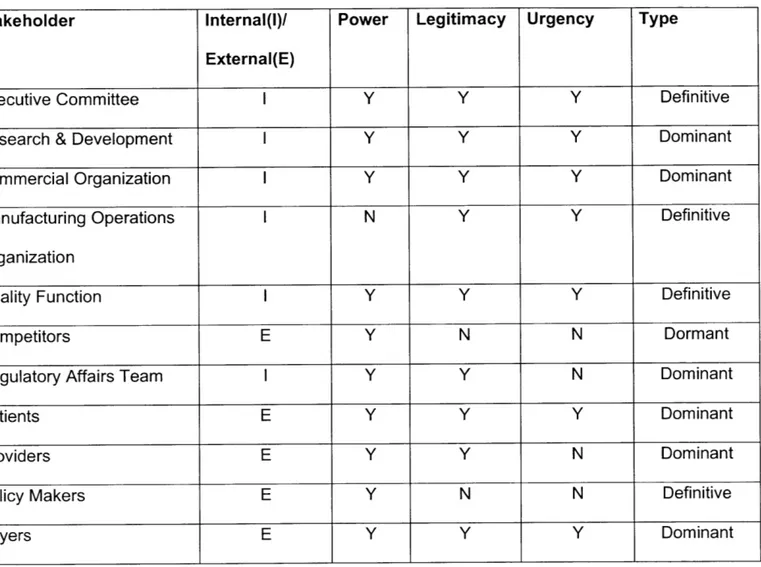
6. Perform ing Stakeholder Analysis... 41
6.1 Executive Committee ... 45
6.2 Research and Development Function... 45
6.3 Commercial Organization ... 46
6.4 M anufacturing Operations Organization... 47
6.6 Competitors ... 48
6.8 Patients ... 49
6.9 Providers ... 50
6.10 Policy M akers ... 51
6.11 Payers ... 51
7. Capturing the Current Architecture ... 52
7.1 Structure ... 52
7.3 Culture ... 60
7.4 M easures...61
8. Creating a Vision for IT organizations in Biopharma Industries... 66
8.1 COMMITMENT TO INNOVATION ... 67
8.2 PARTNERSHIP WITH THE BUSINESS TEAMS ... 67
8.3 ARTICULATING IT VALUE TO BUSINESS TEAMS... 70
8.4 IT LEADERSHIP TAKING CALCULATED RISKS... 71
9. Analyzing Alternate Architectures ... 73
9.1 CENTRALIZED IT ARCHITECTURE ... 74
9.2 DE-CENTRALIZED IT ARCHITECTURE ... 77
9.3 HYBRID IT ARCHITECTURE... 80
10. Architectural Options ... 83
11. Sum m ary and Conclusions ... 88
Table of Figures
Figure 1: Macro Trends in Pharmaceutical Innovation... 12
Figure 2: PharmaR&D expenditure in comparision with other industries ... 12
Figure 3-W orldwide Biopharma R&D expenditure... 18
Figure 4: External factors shaping Corporate Strategy... 20
Figure 5- Internal Value Creation ... 24
Figure 6: Interplay of Regulation in Drug Development... 35
Figure 7: External forces Influencing Corporate Strategy... 37
Figure 8: McKinsey Analysis - Cost Cutting with Limited Success... 40
Figure 9: Stakeholdermap ... 43
Figure 10: Organization Functions... 53
Figure 11: Optimizing Commercial Operations ... 56
Figure 12: Healthcare Decision Map ... 57
Fig u re 13 :X m atrix ... 6 2 Figure 14: Digital Health Startups... 69
Figure 15: Organizations Pathway ... 71
Figure 16:Technology Impact on Healthcare... 74
Figure 17:Organizational Priorities ... 76
Figure 18- Decentralization: Proposed Theoretical model... 78
Figure 19: Business Alignment Maturity ... 84
Figure 20: Information Technology Governance ... 86
Figure 21:Partnering for a successful Ecosystem ... 86
Figure 22: Theoretical framework for Hybrid Organization design ... 87
Figure 23: Challenges and Consequences of low R&D efficiency ... 89
List of Tables
Table 1: Analysis of External Factors ... 21
Table 2: Field Force Analysis... 33
Table 3 - Stakeholder Value... 41
1. Introduction
In role of IT function is changing with in the Biopharmaceutical industry. The IT function with in the biopharma industry are being transformed from being a technologist to a change agent. The biopharmaceutical industry in itself is undergoing major
transformation. The biopharma organizations are looking to accelerate the drug
discovery and increase the Research and Development (R&D) productivity. (Wise et al.,
2018b) discusses that the society is struggling to afford growing health care costs and
the ever-expanding pharmacopeia on offer from the biopharmaceutical industry. All the key stakeholders in the healthcare system (i.e. patients, prescribers, payers developers, medicines regulators, health technology assessment agencies and the
biopharmaceutical industry) need to recognize this. (Wise et al., 2018) points out to the fact that new drugs might need to be accompanied by new diagnostics. Almost all the biopharmaceutical organizations are under tremendous pressure from the government agencies such as FDA and other financial regulators to ensure compliance as well as provide cures for diseases at a reduced cost. The IT function has a key role to play in partnering with the business functions in helping them overcome the challenges. This IT function should ensure that it is led by the business priorities rather than delivering business solutions at a lower cost. The IT functions must innovate itself constantly by maintaining the right balance between organizational performance and value delivery. This can be accomplished through the right organizational design, governance
mechanisms and engagement framework.
1.1 Background
Biopharmaceuticals are one of the most advanced and sophisticated achievements in modern science. Biopharmaceutical drug product is manufactured, extracted from semi synthesized form of biological sources that are completely different from the typical
pharmaceutical development.(Sinskey, 2013)1, Professor of Biology at MIT calls the evolution of Biopharma industry as convergence of three disciplines, In his lecture on
How where and How are the drug derived, he explains the three disciplines .The three disciplines that converge are Organic synthesis for making compounds, Analytical
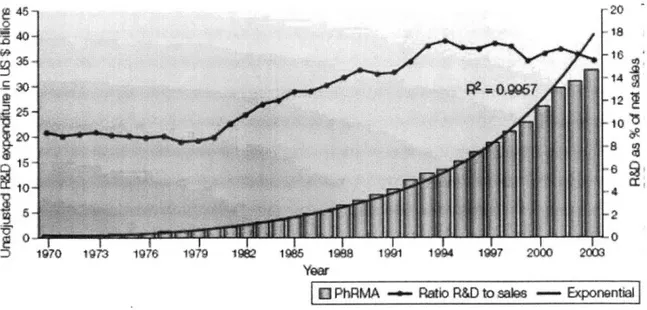
chemistry (for isolating and purifying compounds from Biological sources) and lastly pharmacology and physiology for establishing the models and testing the efficacy of new compounds. The biopharmaceutical industry has very rewarding benefits in terms of innovation and cure for some devastating diseases. In a paper by Fredric J. Cohen "Macro trends in pharmaceutical innovation2", Fredric J. Cohen explains the steady
increase of R&D investment by the pharmaceutical industry spending has increased in proportion to sales, As illustrated in the Figure 1. Figure 2 illustrates, the R&D
expenditure was compared with the other industries using National Science Foundation Survey 3 using Industrial Research and Development as the data source. Fedric J Cohen goes to state that the macro trends in pharmaceutical innovation points out to the fact that, Although the actual R&D spending and sales amount different between PhaRMA 4 and NSF surveys, patterns of R&D spending and sales over time are same.
1 httQs;//ocw. mitedL/courses/sloan-school-of-management/15-136i--principles-and-practice-of
drug-develooment-fall-2013/lecture-notes/MITI5 136JF13 Lec3 Hist pdf
2
https://www.researchgate.net/profile/Fred Cohen3/publication/45639378 Opinion Macro trends in pharmace utical innovation/links/5S8473d9O8aef58cO39b3cdf/Opinion-Macro-trends-in-pharmaceuticaI-innovation.pdf 3 National Science Foundation. Survey of Industrial Research and Development 1953-2003 [online], (2004)
Itps:/www. nsf. gov/statistics .
4 PhRMA represents the country's leading biopharmaceutical research companies and supports the search for new
46 20 1 740 li4
2-51s
~ 0 k YrCI PhWMA - Raoo R&D tosdlw - Epo tendal
Reprinted by permission from Nature Publishing Group: Nature Reviews Drug Discovery.
Source: Cohen F. 3. "Macro Trends in Pharmaceutical Innovation." Nature Reviews Drug Discovery (2005): 78-84.
Figure 1: Macro Trends in Pharmaceutical Innovation
14 4.5 12- -4.0 12 -3.5 0 -3.0 * 8 2.5 .2 6 -1-2.0 CT C 1.0 1968 1963 1968 1973 1978 1983 1988 1993 1998 Year
U All industries 1 Pharmaceutical industry - Ratio drug to all industries
Figure 2: PharmaR&D expenditure in comparision with other industries
According to an annual study of the members of PhRMA5, the biopharma companies report spending a record $71.4billion on research and development in 2017 and this number keeps growing every year. With this there tends to be a lot of M&A activities
s PhRMA represents the country's leading biopharmaceutical research companies and supports the search for new treatments and cures.
that keeps happening as organizations are trying to fill the gaps with their pipeline through asset acquisitions or through acquiring organizations.
With the background outlined in this section, IT function within the biopharma plays a very vital role in ensuring that it organizes itself and makes the right investments to support the business growth. The challenge for IT function is to bring in the process efficiencies and ensuring the right organizational structure and talent. The
biopharmaceutical business will continue to be fast paced with evolving technologies such as mobile, cloud, analytics, security etc. The ever-changing regulatory
environment will continue to be a challenge and hence the need for IT to be agile and nimble to those changes with the right organization structure, governance and process. This will ensure that the IT is providing the highest value at the pace required by the business functions and ultimately accelerating the drug development process.
1.2 Motivation
The design of the IT organizational model for biopharma organizations involves large and diverse teams working together to solve some of the challenges as highlighted in chapter 1. The biopharma business functions are becoming increasing diverse and
looking at digital technologies such as Artificial Intelligence (AI), Machine Learning to solve some of the complex problems in the drug research, manufacturing and
commercialization. (Birkinshaw, Visnjic, & Best, 2018) discusses the key strategic challenge the biopharma organizations have in responding to the emerging
technologies or business models that have the potential to make existing products and or ways of working obsolete. The IT functions in the biopharmaceutical industries
require sophisticated problem-solving skills and approaches to support the business needs. In many cases, the IT functions need to cope up with the new technologies and innovations in the healthcare technologies and challenges from new entrants to the incumbents. These factors present challenges for architecting the IT function effectively particularly as the organizations tend to grow at a much faster pace. The ability to
effectively address the opportunities posed to the IT function is also challenged by the traditional roles, organizational culture and the dynamics in the health care
stakeholders. Though the technologies are becoming available, the biopharma
organizations much change in order to harness the technology more successfully. This poses a very unique opportunity to be a differentiator in enabling the business teams to overcome challenges by leveraging the technology. As more biopharmaceutical
organizations are undoing digital transformation, this thesis focusses on IT architectures within the biopharmaceutical organizations. This author of this thesis analyzes different
architectural options and provides recommendation based on the current biopharmaceutical industry scenario.
1.3 Scope
In developing this thesis, the author focused in analyzing on the IT enterprise
architecture in the biopharmaceutical industry. This includes analyzing the biopharma organizations IT challenges. As pointed out by (Champagne, Hung, & Leclerc, 2015) from McKinsey, the biopharma organizations are running hard to keep pace with the latest changes brought about by the digital technology in comparison with other
industries such as Retail organizations, Banking , Communication sectors. However, at 20.4% the biopharmaceutical industry is one of the highest R&D spending as pointed out in the chapter 2. The scope of this thesis includes analyzing different IT
architectures from highly centralized enterprise architecture to de-centralized IT architecture as well as analyzing the hybrid IT architecture models. As part of this research, the author discussed the various advantages and disadvantages for each of the IT architectures in comparison with the emerging technologies in the biopharma organizations as well as the challenges faced by the biopharma industry from both external and internal factors.
1.4 Research Questions
Biopharma organizations corporate strategies are shaped by many external factors such as Economic factors, Global Presence, Competition, Markets, Quality &
Regulations and Emerging technologies. Each of the external factors has a direct or indirect impact on the IT functions within biopharma organizations. These factors pose opportunities for the IT function to be a change agent in this transformation. For
example (Reh, 2017), discusses the role of technology in accelerating the regulatory submissions for new drugs approvals by 12 weeks posing a Net present value (NPV) of $800mn USD. (Boni, 2018) discusses the emergence of digital medicine and its
transformations. In order for the IT functions in biopharma organizations to stay relevant, the author of this thesis analyzed the following areas
* How is the role of the IT function changing in order to meet the Business challenges?
" How are the IT functions engaging with the business teams in articulating the value to the business functions?
* What are the various considerations in choosing the IT Enterprise Architectures with in biopharma industries?
1.5 Research Approach
In developing this thesis, the author drew inspiration from two primary sources. First years of experience in IT consulting as well as lead roles in the different industries such as Retail, Consumer Packaged Goods (CPG) and Biopharma organization led the author to identify the Information technology (IT), domain knowledge and need for this thesis. Second the course work within the MIT System Design and Management
program such as "Systems Architecting applied to Enterprises" providing frame work for ecosystem analysis, stakeholder analysis, enterprise architecture and design. The approach for this thesis is as follows
* Define a clear need for the IT functions in biopharma organizations to look at business challenges and IT challenges that needs a change in the existing organizational architecture and processes to stay relevant to the business needs.
" Research the various enterprise architectures through knowledge gathering with biopharma industry leaders
" Analyzed various scientific papers and articles from various global research and advisory organizations that provides insight and tools for leaders in IT function.
" Apply enterprise architecting frame works such as Force Field Analysis,
Stakeholder Analysis, capture strong and weak alignments through X matrix and analyzing alternate architectures.
1.6 Thesis Overview
This document has been organized to present the thesis to the reader in a structured approach. First, Chapters 2 through 4 will introduce the reader to the end to end view of the biopharma business landscape followed by the internal IT landscape and the
biopharma enterprise IT challenges. Chapter 5 through 9 will present the central thesis. Finally, Chapters 10 and 11 will present the readers with architecture options and conclusions.
Brief overview of the thesis has been highlighted below:
" Chapter 2, "End to End View of the Business Landscape" introduces the business landscape of the Biopharma Industry and some of the recent
advancements as well as technology impacts. This chapter provides some of the trends that are shaping the Biopharma industry.
" Chapter 3, "Landscape- Internal IT environment" provides a view of the IT functions within the biopharma organizations. This chapter also introduces the readers with the opportunities for the IT function with in the biopharma
organizations.
" Chapter 4, "Biopharma Enterprise IT Challenges "discusses the challenges for the IT function within the biopharma organizations
* Chapter 5, "Force Field Analysis" provides the readers with some of the key drivers for change as well as drivers that are working against the change to overcome some key challenges for the biopharma organization as well as role of IT function in enabling the changes.
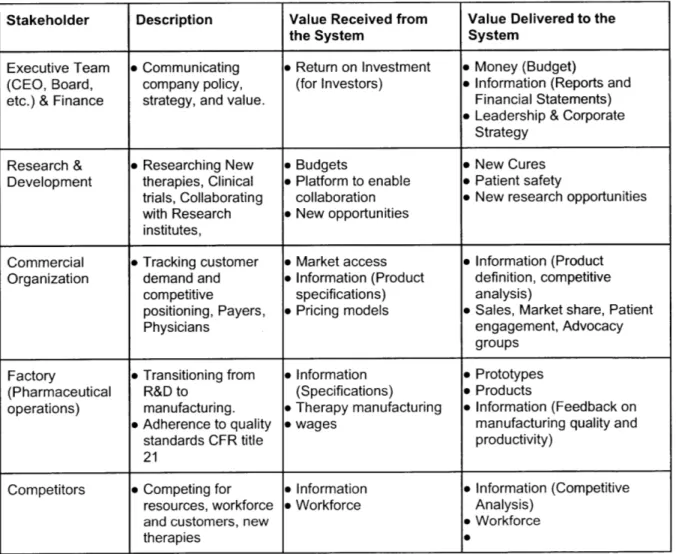
* Chapter 6," Performing Stakeholder Analysis" looks at key stakeholders for Biopharma organizations as well as does a deep dive in-terms of value received as well as value delivered to the system.
* Chapter 7," Capturing the Current Architecture", discusses the current state architecture within the biopharma organizations. This chapter analyzes various dimensions such as structure, behavior, culture, measures,
" Chapter 8," Creating Vision for IT organizations in Biopharma Industries"
discusses the key vision elements that are key for the biopharma organizations in midst of the emerging technologies and digital transformations.
* Chapter 9," Analyzing Alternate Architectures" discusses the alternate architectures for the architecting future enterprise.
" Chapter 10," Architecture Options" discusses the future state architecture for the biopharma organizations
* Chapter 11," Summary and Conclusions" provides a complete research summary of the thesis as well as insights to future research topics.
2. End-to-End View of the Business Landscape
The Biopharma Industry as a whole is undergoing many transformations, as explained in the paper (Birkinshaw, Visnjic, & Best, 2018)6 discusses two dramatic developments in the last 50 years -the molecular and cellular biology revolution and the genomics revolution is paving way for the convergence revolution that is taking place. In another study by The Association of the British Pharmaceutical Industry7, The worldwide Biopharmaceutical companies see their R&D spends steadily increasing. The study points out that Global Pharmaceutical Industry invested over $1.36 trillion USD in R&D spend. At an 20.4%, The pharmaceutical industry is one of the highest R&D measures of any sector globally.
Worldwide BioPharma Companies R&D expenditure
180 25% 160 156.7 24% a 140 136.3 135.5 137.8 . Y 23% 128.3 126.5 128.4 1201198 22% 100 U 20% 80 19% 60 18% ~ 40 17% 20 16% 0 ... 15% 2006 2008 2010 2012 2014 2016
0 Pharma R&D Spend -0- R&D as a share of worldwide sales
SOuRC: Evat atePharma. WORLD PREVIEW 2017: OUTLOOK TO 2022, p. 19 Figure 3-Worldwide Biopharma R&D expenditure
The Biopharma organizations are constantly under pressure to find new therapies and innovative cures in a faster pace due to competition. There is also an increasing
6 http://www.aplu.org/projects-and-initiatives/research-science-and-technology/hibar/resou rces/MITwhitepaper.pdf
7
complexity in clinical trials and lower productivity due to the nature of research. The biopharma organizations are partnering with the research institutions and Universities to accelerate the drug discovery process. Few examples include, GSK- Harvard,
AstraZeneca- Columbia, Pfizer- University of California, Monsanto- University of Washington and Hoechst- Massachusetts General Hospital'. In addition to the
academia collaborations biopharma organizations are looking to collaborate with their peers to find cure for some devastating diseases. For example, Pfizer9 is partnering with Kineta, Biogen etc. to collaborate and bring new therapies to the patients. The large biopharma's are looking for partnerships and there is lot of consolidations happening in this area. In 2018, Takeda's takeover of Shire pharmaceuticals, is one such example, There are many of these mergers and acquisitions happening at a very rapid pace. "(Richman, Mitchell, Vidal, & Schulman, 2017)10 discusses the Pharmaceutical M&A, deal values reached almost $400 billion in 2015, for deals with reported value. The Biopharma organizations are trying to consolidate the research portfolios as well as narrow the research areas. These kinds of specialization help the Biopharma
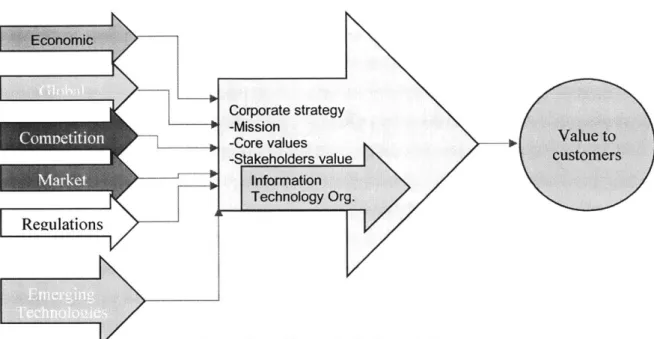
Organizations in expanding the research pipeline to a specific disease area such as Neurology, Oncology etc. Focused research areas help the organizations acquire assets in different stages of research phases as Phase I, Phase 11, Phase Ill studies etc. The research strategies and the production methods are technically very diverse, all these adds up to the costs due to the complexity. The biopharma organizations are trying to expand globally, and competition is increasing every day with new entrants and increasing competition. To better understand the Biopharma organizations corporate strategy and shaping IT function's strategy, the external factors play a very important role. External factor influencing the corporate strategy is portrayed in the Figure 4. The IT function should play a critical role
in enabling the business functions to accomplish the strategy and most importantly should have an organizational model that adapts to the changing needs.
8 https://doi.org/10.1080/17460441.2017.1318124
https://www.pfizer.com/partners
Econmic
Corporate strategy
-Mission Vlet
i -Core values Vaiueto
-Stakeholders value customers
WL k Information Technology Org.
Regyulations
Figure 4: Externalfactors shaping Corporate Strategy
The major takeaways are
- There are many external factors that influence the biopharma industries, but the
information technology function plays a very vital role in responding to those external factors
- The IT function formulates its own internal strategy to support the overall
corporate strategy.
Table 1 shows the detail analysis of the external factors and the associated impact. The IT function has a role in each of those external factors as described below. It should be noted that these are some of the factors and there could be mucb more in ever
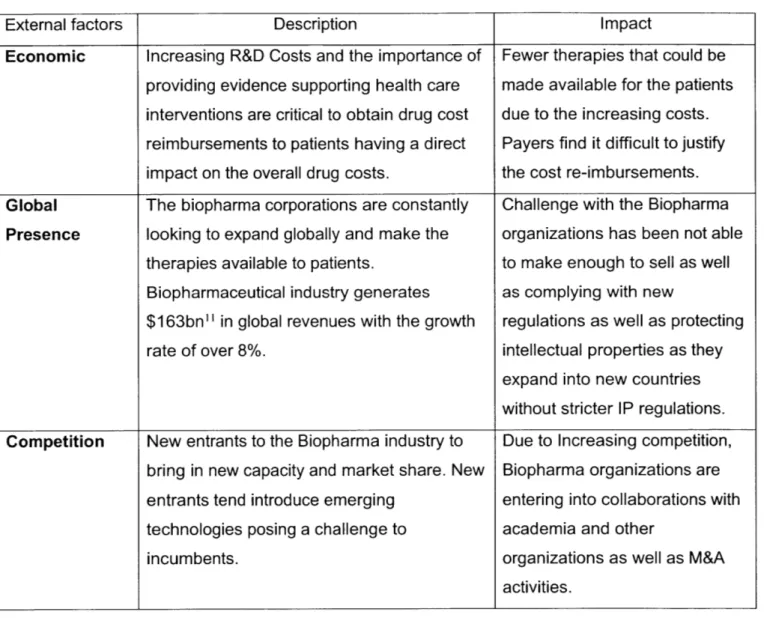
Table 1: Analysis of External Factors
External factors Description Impact
Economic Increasing R&D Costs and the importance of Fewer therapies that could be providing evidence supporting health care made available for the patients interventions are critical to obtain drug cost due to the increasing costs. reimbursements to patients having a direct Payers find it difficult to justify impact on the overall drug costs. the cost re-imbursements.
Global The biopharma corporations are constantly Challenge with the Biopharma
Presence looking to expand globally and make the organizations has been not able therapies available to patients. to make enough to sell as well Biopharmaceutical industry generates as complying with new
$163bn" in global revenues with the growth regulations as well as protecting
rate of over 8%. intellectual properties as they
expand into new countries without stricter IP regulations. Competition New entrants to the Biopharma industry to Due to Increasing competition,
bring in new capacity and market share. New Biopharma organizations are entrants tend introduce emerging entering into collaborations with technologies posing a challenge to academia and other
incumbents. organizations as well as M&A
activities.
11
Effective launch of biopharma has never been so challenging. There are many hurdles to market access. With the increasing costs, The Biopharma organizations should look at
new markets through partnerships for increasing market share and penetration.
Quality function within the Biopharma
organizations are finding it difficult to keep up with the rising demand of the regulators (US Federal Drug Administration or its equivalent in other countries). This complexity is
increased with the Biopharma organizations having global presence.
I I
As new technologies are emerging, The Biopharma organizations are looking to leverage Artificial Intelligence (AI), Machine Learning (ML), Big data, Analytics in the product development lifecycle. For example: Role of Artificial Intelligence, Machine learning in R&D, manufacturing operations and Patient engagement.
New pricing models such as performance-based contracts, outcome-based pricing etc. to gain entry into new market areas. Impact of Cost effectiveness and clinical
effectiveness are on the raise to
gain market access.12
The Biopharma organizations could have an adverse impact in case of non- compliance to the regulations. Examples:
GlaxoSmithKline fined $3bn in fine, Pfizer fined $2.3bn
etc.("Top 10 Biggest Biopharma Marketing Fines," 2014).
Biopharma organizations need to adopt and embrace new technologies to stay relevant. There is a threat from new entrants to the incumbents.
The takeaway from this analysis is that the IT function should remain as a strategic partner for the business functions. All the external factors highlighted in the Table 1, poses a unique opportunity for the IT function. Some of the opportunities include
12 https://www2.deloitte.com/insights/us/en/industry/ife-sciences/big-pharmas-market-access-mission.html Market Quality and Regulations Emerging Technologies I I
- Leveraging technology in reducing the costs. (Reh, 2017) points out that the
regulatory submissions could be accelerated up to 12 weeks posing an Net Present value (NPV) of $800mn to pharmaceutical organization
- (Harrington, Phillips, & Srai, 2017) discusses Recent advances in novel
technologies ('Continuous' processing, printing, sensing and diagnostics) provide global pharmaceutical value chain network through targeted technology
interventions
- (Boni, 2018) discusses the emergence of digital medicine to transform and
disrupt the healthcare over several decades. This includes the collaboration of various players such as patients, payers, physicians, providers and partners. Technology will play a key role in solving some of the challenges as well as convergence of technology industry in bringing new perspectives to the
healthcare industry.
The IT function need to pay close attention to the constant changes that can potentially have an impact the business. It is very critical that the IT enterprise develops the right vision, strategy, partnerships and organization model to help support the business overcome the external challenges highlighted in Table 1. (Woerner & Weill, 2019)13 discusses the IT function that are digitally savvy can provide leadership to the entire enterprise. This leadership in the IT function involves reinforcing actions
* CIO's work with the Company executives on the role of digital to Business * Improving external Customer engagement
" Relentlessly delivering Operational efficiencies.
The IT function has a significant role to play in addressing some of the external challenges through close partnerships with the business functions.
3. Enterprise Landscape- Internal IT environment
---
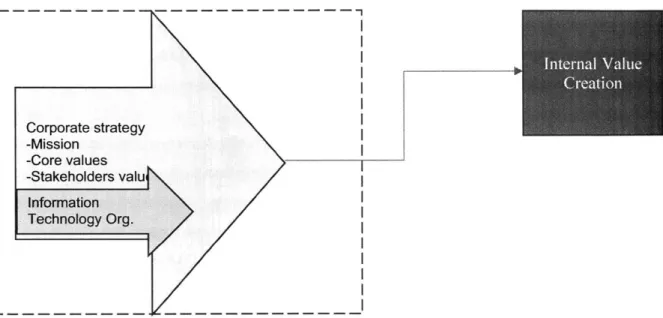
1 I Corporate strategy I-Mission -Core values I-Stakeholders valu I Information ITechnology Org.I L------Figure 5- Internal Value Creation
The IT function within biopharma plays a very important role in enabling the business functions from Drug development, manufacturing, commercilization. More Biopharma organizations rely greatly on the Enterprise IT organization to bring in innovations and latest technologies to reduce the R&D cycle time and increase patient safety. In an article in Nature'4, an estimated USD 2.6bn is the price tag for developing an treatment,
A lot of that money is spent on clinical trials, regulatory approvals etc. This includes the
cost of the nine out of ten drug research failing to meet the end point or the research goals. (Schuhmacher, Gassmann, & Hinder, 2016) discusses the challenge related to
high R&D spend is mainly attributed to low success rates, cost of the failed drug . The R&D efficencies of major research based pharmacutical companies range from USD
3.2-32.3 billion from 2006 tO 2014, (Schuhmacher et al., 2016) discusses the
organizations focussing on activites to increase the innovation potential by looking to do things differently, in a faster and cheaper way. Technology is being looked at as an
enabler to address the challenges. (Fleming, 2018b), looks at some leading biopharma corporations focussing on emerging technologies as part of drug development. For
example Pfizer is partnering with IBM Watson, to use machine learning to search for immuno-oncology drugs. Sanofi has signed a deal with Al start up to hunt for an metabolic disease therapies, Roche is leveraging Al for finding cures. These are some of the examples of where technology playing a vital role.
The role of IT functions are changing rapidly, Gone are those days where Enterprise IT fucntion is viewed as an cost center and are typically aligned with the business
functions . The IT function was widely perceived as a support function to the business .
The IT function is often compared with the procurement and finance teams. The
positioning of the enterprise IT structure is of the paramount importance to the CIO's of the biopharma organizations.
" Understanding and reacting to the network effects of the internal and external factors are very critical for the enterprise IT.
" Innovation is very critical to the enterprise IT organizations, a lack of innovation can be very crippling, therefore it is very important for these organizations to look at the right strategies , balanced staffing (pyramid structure), right set of tools and processes as well as aligning with the business priorities. The enterprise IT organizations should foster a climate for development and exchange of new ideas and to build the network to commuincate, spread and internaize the new ideas through the entire organization
* Very often Enterprise IT lacks the business knowledge and acumen. Having the right talent pool, capabilities and potential are very critical to the enterprise IT organization within biopharma organizations. This organization should embrace the right vision, strategic objectives that aligns with the business priorities and innovation that can be leveraged by the business to gain the competitive edge.
Creating the right organization structure in place is very critical for the enterprise IT organizations to support the business. This can become a big cost center and
most importantly articulating the strategy. As pointed out in the book by (Nightingale & Rhodes, 2015), it is no surprise that the enterprise that fail to keep up with the changing world will sooner or later be doomed to failure. This statement is very relevant in case of IT functions within biopharma organizations. IT functions must react to the changes very quickly by adopting the organizational structures in lieu of the changes. (Steinberg, Horwitz, & Zohar, 2015) discusses the evolution of tech sector has upended the old, familiar business models opening up new avenues for innovation. The new approaches include gathering health and disease data quickly at high volume and in real time. These innovations challenge the biopharma organizations that are slow to change since the biopharma industry is heavily regulated and the organizations do not want to take risks and are slow to adopt. In order for the IT functions to be successful in supporting the corporate strategy and aligning with business goals, (Weill & Woerner, 2018) point to some key questions such as Creating an new standard of customer experience ,
Driving Digital Strategy, Collaborate with business to develop new business models by harnessing technology, Optimize the capital allocation and obtain an unrivaled
efficiency. These questions are key to the IT functions that are taking up the planned organizational transformational initiatives to stay relevant. While the IT function continue to be ready for the future transformation initiatives, the stakeholders and the ecosystem play a very important role in shaping the future enterprise.
4. Biopharma Enterprise IT Challenges
"The Beginning is the most important part of the work"- Plato
The process of enterprise transformation is no simple feat. It starts with identifying all the challenges that the organization or a group with in an enterprise face. This is very true with the enterprise IT within the biopharma industries.
In a McKinsey 11 study, (Champagne et al., 2015) describes how the pharmaceutical companies are running hard to keep pace with the latest changes brought about by the digital technology. Compared to the other verticals like Retail, Banking, communication sectors, the enterprise IT function within Biopharma /pharma industries tend to be less mature / rather take a very conservative approach in bringing in new technologies primarily due to the regulations by the FDA and other international regulatory bodies. Typically, these regulations in the biopharma industries are driven by CFR part 11. Title 21 CFR Part 11 16 is part of the Title 21 of the code of Federal Regulations that
establishes the United States Food and Drug Administration (FDA) on the electronic records and electronic signatures, audit trails, data storage, data archiving and other regulatory requirements that the biopharma organizations need to comply. The FDA audits are often very stringent since these are directly associated with the patient safety. The IT function continues to battle with the regulatory requirements in ensuring the IT platforms within the landscape stay compliant and does not disrupt the business. They often tend to take a very conservative approach in introducing new processes or technologies unlike other verticals like Retail, Banking etc.
On the contrary the Biopharma and Pharma executives are often times challenged with disruptive technologies and the benefits that these technologies offer in addressing some of the problem areas such as R&D, Supply chain, Patient engagement,
"s
https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/how-pharma-can-win-in-a-digital-world
Commercialization etc. While these technologies drive the most value in the business functions and often times the IT side of the house to has much more challenges in enabling these technologies due to the regulations, Infrastructure and Architecture.
The R&D costs in the pharma industries are very expensive, the pharma organizations are often times looking to reduce the cycle time from research to development to launch and IT organizations within the pharma industries are under constant pressure to bring in the right arsenal to enable this. For example, the digital discovery and the testing of the molecules with the advanced modeling and simulation techniques will be common place. Another relevant example could be physiological simulation to accelerate the product development and 3-D modelling using computer simulation for potential toxicity etc.
Enabling the Marketing and field force team is another big challenge for the IT
organizations, there is a constant competition from the new entrants in the biopharma space and the business teams are looking at IT to enable the field force to adapt right strategies from therapy positioning, market positioning, new market access. The global markets functions are looking to have and omni channel conversations with physicians and patients. The IT function is often times challenged in enabling technology to
achieve the business goals. Enabling the field force teams engage with medical science liaisons, providers, payers and patient advocacy groups who can influence the
recommendation of a particular therapy or a drug by using technology. The field force team also leverages of social media, patient portals, apps to fill scripts, to engage patients, payers, advocacy groups.
Data is the next big thing in the Biopharma space, one of the publications from Boston Consulting group17, the cost of genome sequencing has dropped from $1Omn USD 15
years back to $1000 today. The genomic data is available in the biopharma R&D has increased exponentially. At the same time, the volume of patient, disease, and
17 htvso!/wwwcga ena
treatment -related information has expanded dramatically. The IT function need to enable platforms to harmonize the data, create the right infrastructure for the scientist to run experiments. The IT organizations needs to effectively organize itself and create the right infrastructure to accomplish this goal. (Tao et al., 2019) discusses risk of data breach, compromised data collection. 73% of the organizations in Pharmaceutical and health care industry are set to begin or increase the investment in big data. Estimates suggest that investments in pharmaceutical industry was accounted for nearly $4bn
USD. Led by the opportunities for health care providers, payers and government
agencies these investments set to grow by 15% over next three years. Cyber-attacks are big concern in the pharmaceutical industry. (Tao et al., 2019) points out that cyber security specialists express cyber criminals are progressively perusing over $3 trillion
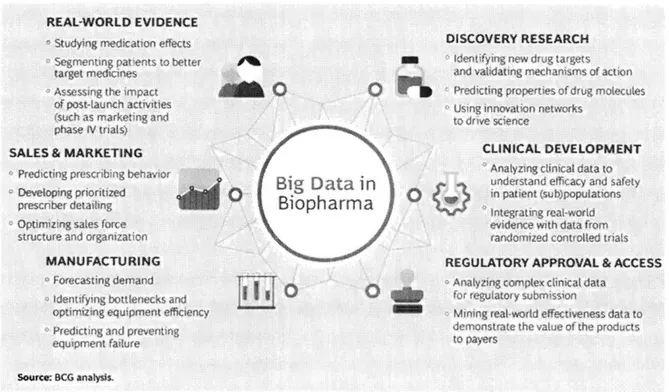
US health care industry. Figure 6 demonstrates the impact of data on every aspect of
the Biopharma industry. With the new regulations such as GDPR, Data privacy etc. the expectation from IT to play a key role supporting the business as an enabler.
REAL-WORLD EVIDENCE
e rtdvg med'n nefcs
aget edciLs
0f post- taujnC acv1 e
ph"ase N' t aa1 s) SALES & MARKETING
e~ Deeing pio itiz
Q
prescnedtatimgMANUFACTURING
jForeas in de
ed id fing ot. enecksad o tiirng euipmen eUciency
Predic ingadrenng
equi rment adue
Bg
DataJ
Biopharm
DISCOVERY RESEARCH
and andtmgmechnum 1 oract ion
ae ctng Wpmso rog olue
fo drv science CLINICAL DEVELOPMENT n t J su ppl atns "'andw e o ve!ati a 2 I
REGULATORY APPROVAL & ACCESS
nng "reat world eff -ctvenes data ro
nra the va ue o)f the Ucts
to Payers
Soumr BCG anays
SOURCE: BCG point of view on "Making Big Data Work: Biopharma" Figure 6- Making Big Data Work: Biopharma
As called out in the paper by BCG, the potential of data is real and the organizations that reap the benefits needs to have clear goals, right strategy and execution. The IT organizations that are deemed successful does the following
- Focus on delivering Value- The IT organizations needs to focus on delivering the right value
- Strategic thinking about capabilities- The Biopharma IT CIO's needs to have the strategic thinking, focus on right capabilities and right organization structure.
- Develop Operating models that work- The paper points out that the successful organizations need to think outside the box and traditional organizational models
are unlikely to work.
The IT functions are often encountered with two groups challenges with one part of the organization wants to run at much faster pace, while the other part of the organization managing the legacy state for cost and reliability and architecture runs at a much slower
pace due to the platform level constraints
(van Nooten et al., 2012) discusses the importance of evidence generation to
commercialize a drug in the market and it discusses various challenges during the drug development process such as Market access, role of government agencies, internal
challenges and external challenges. While discussing the internal challenges like the siloed organizational structure within the biopharma organizations resulting in lack of communication, collaboration, resource challenges, interactions with research
organizations, with clinical trials to commercial group. The IT organization plays right in the middle of these verticals and often times challenged due to the siloed behavior. Another important challenge faced by the IT function is the cyber security, In June 2017,
Merck was one of the more than dozens of business attached by massive ransom ware that ended up effective organizations all over the world. In 2017, one study revealed that more than 54%18 of the organizations experienced one or more attack that compromised the data. In the Biopharma organizations hackers are looking to take
more sensitive and valuable data. Regardless of the size of the organization IT
functions should plan for the unknows. (Souza, 2018) points to the fact that the Merck's breach could have arguably attributed to the cultural flaw and their siloed IT overlooked it.
(Souza, 2018) discusses the biopharma organizations often times fail to believe that IT function and planning for growth area should be immediate focus. Not only the IT function can empower the growth of the organization if architected correctly but built for
agility and long-term organizational goals.
(Boni, 2018), discusses some of the principles that are critical for the IT function in order to overcome the challenged discussed in this chapter. Operate lean and agile
development processes, Scaling both the technology and business teams adaptively as the markets are developing and lastly Create and Grow innovation teams.
(Wang, 2017) compares Biopharma organizations with the Tech Industry to bring the much-needed efficacy, efficiency in the Biopharma organizations to overcome the slow pace of biopharma organizations. The paper points out the challenges faced by the Pharma organizations that are typically organized through vertical integration. IT functions tend to align with the business functions vertically causing silos within the IT
organization ending up with redundant technology and platforms that tend to become difficult to maintain. (Wang, 2017) points to the fact that Technology industry is moving more towards horizontal integration enabling the collaboration with the internal and external stakeholders. Horizontal Integration brings in more efficiencies in terms of minimal infrastructure costs, enabling faster decision making and reducing the time to market.
Lastly, another very important challenge for the IT functions are Cyber security and data protection. (Souza, 2018) discusses the importance of cyber security in the
Pharmaceutical organizations in particular due to few key reasons, In an 2017, study revealed about 54% of the organizations experienced one or more successful attacks that compromised the data or the IT infrastructure.(Souza, 2018) points to another study
by Deloitte , the pharmaceutical industry is the number one target for cyber-attack due
to the Intellectual property. The challenge for the IT organization is to ensure that organization is protected from cyber-attacks.
To summarize, this chapter discussed some of the key challenges of the IT function in the Biopharma organizations, It is important to layout the challenges to architect an
5. Force Field Analysis
(Lapalme et al., 2016) discusses that in the foreseeable future, organizations will face the ever-changing technologies, increasing level of complexity and uncertainty. While many believe that Enterprise Architecture will be a solution to the problems helping organizations navigating the difficult terrain by guiding the design of adaptive and resilient enterprises. Enterprise Architecture can play a key role in helping to design the enterprises of the future. (Boni, 2018) discusses the Digital transformation in the
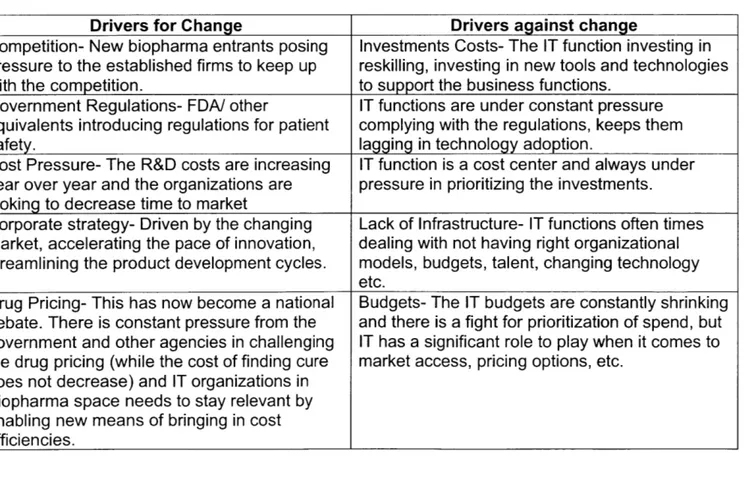
Biopharma space at an accelerated pace, leading to the Convergence: where health care, traditional biopharma and technology (the tech industry) collaborate as the industry moves towards the user centric- business model. In the Table 2, force field analysis helps to understand the drivers for the change as well as drivers against the change. While the drivers for the change provide significant opportunities for the IT function, On the contrary plenty of threats include privacy, data security, technology adoption and regulatory requirements for the IT function to be successful.
Table 2: Field Force Analysis
Drivers for Change Drivers against change
Competition- New biopharma entrants posing Investments Costs- The IT function investing in pressure to the established firms to keep up reskilling, investing in new tools and technologies
with the competition. to support the business functions.
Government Regulations- FDA/ other IT functions are under constant pressure equivalents introducing regulations for patient complying with the regulations, keeps them
safety. lagging in technology adoption.
Cost Pressure- The R&D costs are increasing IT function is a cost center and always under year over year and the organizations are pressure in prioritizing the investments.
looking to decrease time to market
Corporate strategy- Driven by the changing Lack of Infrastructure- IT functions often times market, accelerating the pace of innovation, dealing with not having right organizational streamlining the product development cycles. models, budgets, talent, changing technology
etc.
Drug Pricing- This has now become a national Budgets- The IT budgets are constantly shrinking debate. There is constant pressure from the and there is a fight for prioritization of spend, but government and other agencies in challenging IT has a significant role to play when it comes to the drug pricing (while the cost of finding cure market access, pricing options, etc.
does not decrease) and IT organizations in Biopharma space needs to stay relevant by enabling new means of bringing in cost
5.1 Drivers for Change
Competition- As previously discussed, there are new players every year, there are many alternate ways of finding cure for disease areas, any new therapies for an organization is constantly challenged by an alternate way of finding cure for the same disease. (Schuhmacher et al., 2016) points to the fact that, The Center for Medicine Research International (CMR) reported in its pharmaceutical R&D factbook 2014 an average success rate of 4.9% from first toxicity dose to market approval with the phase success rates of 66, 44, 26, 72 and 91 % from first toxicity dose to first human dose. (Birkinshaw et al., 2018) discusses the key strategic challenges for established firms in responding to the emerging technologies while the new entrants are able to embrace and adopt the new technology posing challenges to the incumbent firms. (Boller, 2017), discusses the regulatory system in the U.S is designed in principle, to enable vigorous and effective competition that will have impact on the margins on the large players. Competition from new entrants is seen as one of the main drivers for the incumbent biopharma organizations to re-look at their operating models. This drives the M&A activities as highlighted by (Barak Richman et al., 2017).
Government regulations- Quality functions within the Biopharma organizations are finding it difficult to keep up with the pact of regulations. As more disruptive technologies are introduced in the drug discovery and manufacturing process the
FDA / other equivalent regulatory bodies in other countries are trying to regulate
the industry with newer regulations. The government regulations apply across various phases from drug discovery through the commercialization. (Attridge,
2008) discusses the areas of government regulations and incentives which effect
each of them. These are science and medicines, public policy and funding, patent laws and exclusivity regulations The paper points to the fact that
FDA-EMEA (European Medicine Agency) review process is currently underway and
aimed to streamline the drug development requirements. (Rose, 2014) points that despite the fact that there was no explicit change in the regulations , the cost
of new drugs have continued to outpace the Consumer Price Index (CPI), However undocumented change in the regulatory requirements have occurred. (Boller, 2017) discusses the regulatory barriers to competition. The FDA controls the entry of both innovator and follow on pharmaceutical treatments to ensure that they are safe and effective. The paper points to the fact that U. S Regulatory framework governing biologics have experienced numerous delays .The IT function need to work closely with the quality organizations in ensuring the regulatory standards are followed all through the life cycle. The Figure 6 shows the interplay of regulation during the drug development process
Activity
Academic, Lob. preclinincal
cluster development, Clinical 1, 11, 111
SMEs, Manufacture
multinationals
EU, national, public and industry Research funding of bioscience and medicine
European patent low,
S supplementary protection
Product
Patents Prdc GLP GCP, GMP animial regulation, development
0 E prgesa and licensing
develpent
GLP - good laboratory practice; GCP - good clinical practice; GMP - good manufacturing practice
Figure 6: Interplay of Regulation in Drug Development
Cost Pressure- This is driven by the R&D costs and drug development costs and the life cycle of drug discovery. (Wise et al., 2018a) discusses the prices of biopharmaceutical products are under scrutiny especially by the payers and the Health Technology Assessment agencies. Real World data (RWD) are being used to understand the disease. Real World Evidence (RWE) that underpins the
economic case for innovative medicines. (Wise et al., 2018a) calls out that total prescription drug spending has increased by 9% to USD $324 billion, By 2020 the health care costs are projected to reach 19.8% of GDP. The biopharma organizations are looking at technology as an medium to reduce the costs. (Boni,
2018) with digital medicine and digital health, there is an evidence of
convergence of technology and medicine. As an example Artificial Intelligence, Machine Learning, Digital imaging, digital health (consumer-facing wearables) ,
Open Innovation Partnerships such as Google/ Alphabet Nerily in partnership with J&J, GSK and Sanofi. IBM Watson partnering surrounding AI/ML, Watson
for Oncology, drug delivery patches, Brain computer Interface to connect the visually challenged are some of the example of where technology is being looked at to reduce the cost.
Corporate strategy- Reacting to market reality, need to be faster, nimble, most
efficient and more innovative. (Osterwalder, Pigneur, & Hoboken, 2010) discusses the need for continuous scanning is more important because the growing complexity of the economic landscape. The book points to the fact that it will be helpful for the organizations to conceive the external environment as sort of the design space, by which adopting the business models taking into account the design drivers such as customer needs, new technologies etc. Figure 7 shows the various forces that influence the corporate strategy. In order for the IT function to align with the corporate strategy, it need to have a very close
alignment with the business functions. A good understanding of the business models will help the IT function consider the kind of organization structure and the investments.(Osterwalder et al., 2010) points out that the pharma industry will undergo an substantial transformation and come up with the disruptive business models and these will be through technology led transformations.
REGULATORY TRENDS TECHNOLOGY TRENDS S.
SUPPLIERS AND OTHERV
CHAIN ACTORS STAKEHOLDERS * COMPETITORS (INCUMBENTS) NEW ENTRANTS 4 (INSURGENTS) SUBSTITUTE PRODUCTS AND SERVICES
SOCIETAL AND CULTURAL TRENDS
10 0 SOCIOECONOMIC TRENDS tALUE K MARKET ISSUES SWITCHING COSTS 4
GLOBAL MARKET CONDITIONS CAPITAL AARKETS
k, 0
CO
ECONOMIC INFRASTRUCTURE MMODITIES AND OTHER RESOURCES
Source: Business Model Generation: A Handbook for Visionaries, Game Changers, and Challengers Figure 7: Externalforces Influencing Corporate Strategy
5.2 Drivers against change:
(Rose, 2014) discusses the pharmaceutical industry being characterized as unusually high costs and the R&D cost of bringing a new compound to market was estimated to be at $1.3bn in 2005. To some extend these high costs are attributed to pre-clinical testing, human clinical trials, high failure rates and the opportunity costs tied up during the eight to twelve years of drug development.
However, the regulations require extensive prelaunch clinical trial data on safety and efficacy. In United states, the statutory regulation of pharmaceutical through
FDA is in addition to and uncoordinated with the increasing level of indirect
regulation through tort liability, market access regulations etc. One of the key drivers against this change is the IT budgets, (Bishop & Jones, 2018) from
MARKET SEGMENTS
NEEDS AND DEMANDS
Gartner discusses the impact to IT function due to the increased R&D costs, The article points to the fact that in spite of the emerging technologies IT budgets will prioritize project and product delivery based on the extent of medical and /or administrative cost savings. Innovation and modernization initiatives that
leverage intelligence to automate decision making will supplant the budget traditionally allocated to incremental refactoring of the costly manual process.
Regulations play a very important role in the Biopharma organizations. IT Functions are often governed by CFR Part 11 regulations by FDA19. These regulations have impact on multiple areas within IT function. FDA regulations cover different areas with the IT such as Controls for Closed systems, Controls for Open systems, Regulatory requirements, Electronic signature components and controls and controls for audit trials in the IT applications. (Hodgson, Taylor,
& Taylor, 2017) from Deloitte discusses the challenges that Biopharma
organizations face as the compliance needs have grown organically from a plethora of disconnected rules and regulations having an impact on the industry. These rules are addressed in silos of individual operational or business functions and as a result discrete compliance agenda. When IT functions start to engage with the platforms that are deemed as GxP 20 type of applications, then IT
program teams tend to take much longer time in completing these programs and often times run for a longer duration and end up cost overrun as well as
increasing the time to market. (Paul, 2008) discusses the pharmaceutical
industry as highly regulated and Regulators and best-practice framework require a lot of high-quality standards of records for which the real-world pharmaceutical
industry has some challenges. Research and Literature in this field is not yet advanced as one could think may be due to lack of international communication across the industry.
(Hodgson, Payne, Maini, & Chan, 2017) from Deloitte, discusses the recent and ongoing European regulatory changes having impact on the Biopharma
19 https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=11
20 GxP is an general abbreviation for "Good Practice" quality guidelines. The x stands for various fields such as c for
organization, which in turn has a direct or indirect impact on the IT functions. Identification of Medicinal Products (IDMP) is being developed and implemented
by the International Organization of Standardization (ISO), The clinical trials
regulations mandate the biopharma organizations to deliver, update and maintain a number of IT platforms and systems to improve how the clinical trials are applied for assessed and monitored. The European union regulatory landscape is evolving quickly, It is important for the organizations to monitor and comply with the new and changing requirements. From the IT budgets stand point IT organizations are posed with the challenge to prioritize the next generation initiatives (like Al/ ML/ Big data etc.) to the regulatory environments and often times end up deferring some of the next generation initiatives causing them to lag when compared to other industry verticals like Retail, Banking, Energy sectors (Gyurjyan, Parsons, & Thaker, 2011) discusses the change in the organizational health from a sample of pharma organizations suffering from a lack of external focus and overemphasis on near-term delivery. This is causing to be one of the drivers against the change. (Gyurjyan et al., 2011) discusses that pharma organizations announcing the cost reduction measures are largely driven by the
paten cliff and IT functions typically happen to be a biggest victim in the process. Figure 8 shows that, Over the past 20 years R&D expenses have grown 40 to 60 percent faster than revenues however organizations have limited success with cost cutting measures. As the Biopharma organizations tend to prioritize the budgets, the IT function is often constrained with the budgets and often end up with supporting the current business processes instead of taking on new transformation initiatives.
Revenues Earings before interest and tax Depreciation and amortization % of revenues 103 Research and development * SG&A
Cost of goods sold
-$1 ,027b CAGR, 6.4 6.2 40 20 0 -199 91 92 93 94 95 96 97 98 99 2000 01 02 03 04 05 06 07 08 09 10 2011 Figure 8: McKinsey Analysis - Cost Cutting with Limited Success
McKinsey study (Gyurjyan et al., 2011) discusses that apart from budgetary pressures, bioph'arma organizations are adjusting to complex stakeholder environment, greater difficulties gaining market access and stringent regulatory
requirements . This setup makes it very challenging for the IT functions to create the right infrastructure that can be leveraged by the business teams.
To summarize, while some of the drivers against the change that was discussed in this chapter are critical, it is important for the IT leadership to find new ways to complete their planned transformations, architect the enterprise, reinvigorate the organization and enable the business. The IT functions should remain resilient and adaptable and able to think the way of engagement with various
stakeholders.