Publisher’s version / Version de l'éditeur:
Construction Technology Update, 2002-03-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Preventing concrete deterioration due to alkali-aggregate reaction
Grattan-Bellew, P. E.; Mitchell, L. D.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=2ce15a18-76b8-4bfa-b882-289afce402b8 https://publications-cnrc.canada.ca/fra/voir/objet/?id=2ce15a18-76b8-4bfa-b882-289afce402b8C o n s t r u c t i o n T e c h n o l o g y U p d a t e N o . 5 2
Definition of Alkali-Aggregate Reaction
Alkali-aggregate reaction is a chemical reaction between certain types of aggregates and hydroxyl ions (OH-) associated with alkalis in the cement. Usually, the alkalis come from the portland cement but they may also come from other ingredients in the concrete or from the environment. Under some conditions, the reaction may result in damaging expansion and cracking of the concrete. Concrete deterioration caused by alkali-aggregate reaction is generally slow, but progressive.
In Canada, crack-ing due to alkali-aggregate reaction generally becomes visible when con-crete is 5 to 10 years old. The cracks facilitate the entry of de-icing salt solu-tions that may cause corrosion of the reinforcing steel, thereby accelerating deterioration and weakening a struc-ture. Although there are no documented
cases of concrete structures in Canada failing due to alkali-aggregate reaction, alkali-aggregate reaction is a serious form of deterioration and measures need to be taken to minimize it.
Types of Reaction
There are two types of alkali-aggregate reaction:
Alkali-Silica Reaction
This is the most common form of alkali-aggregate reaction and results from the presence of certain siliceous aggregates in the concrete found in some granites, gneisses, volcanic rocks, greywackes, argillites, phyllites, hornfels, tuffs, and siliceous limestones. The product of the alkali-silica reaction is a gel that absorbs water and increases in volume. Pressure generated by the swelling gel ruptures the aggregate particles and causes cracks to extend into the surrounding concrete. Typically, alkali-silica reaction results in the formation of map-pattern cracking of the concrete (Figure 1).
Alkali-Carbonate Reaction
This is less common and cases in Canada to date are limited to parts of Eastern Ontario. With the alkali-carbonate reaction, certain dolomitic limestone aggregates react with the hydroxyl ions in the cement (or other
by P.E. Grattan-Bellew and Lyndon Mitchell
Alkali-aggregate reaction is a common cause of concrete cracking that results
in significant damage to concrete structures worldwide. This Update explains
the mechanisms of alkali-aggregate reaction, its effects on concrete and
methods for preventing deterioration from this cause in new construction.
Preventing Concrete
Deterioration Due to
Alkali-Aggregate Reaction
Figure 1. Typical map-pattern cracking due to alkali-silica reaction in a retaining wall in Ottawa, Ontario
2
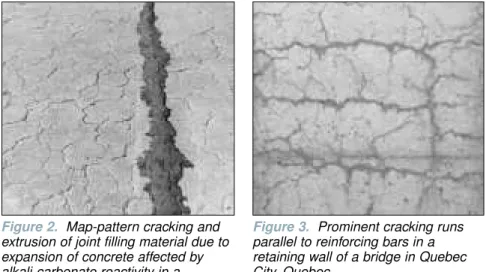
sources such as de-icing salts) and cause swelling. The swelling of the limestone particles causes the concrete to expand and crack. Despite 50 years of research, the mechanism of the reaction is still not well understood but it is known the alteration of dolomite to calcite is involved and clay minerals may also have a role in the reac-tion. The reaction results in cracks in the concrete (Figure 2) similar to those caused by alkali-silica reaction. It should be noted that limestone aggregates may be susceptible either to silica reaction, or alkali-carbonate reaction, or a combination of the two.
Typical Cracking Due to Alkali-Aggregate Reaction
Relatively equi-dimensional pattern cracking like that shown in Figures 1 and 2 is characteristic of non-reinforced or lightly reinforced concrete affected by alkali-aggre-gate reaction. But when there is a signifi-cant amount of reinforcing steel, cracking tends to be more prominent in the direction parallel to the reinforcement (Figure 3). However, map-cracking can also result from other causes such as drying shrinkage (sometimes referred to as plastic cracking), particularly in slabs on grade that were placed in hot windy weather without proper curing. This type of cracking appears within hours, or days after casting the concrete. In contrast, cracking due to alkali-aggregate reaction usually only becomes evident after 5 to 10 years.
Swelling of the Concrete Due to Alkali-Aggregate Reaction
Both alkali-silica and alkali-car-bonate reactions result in swelling of the concrete. The amount of swelling or expansion depends on the reactivity of the aggregates, the alkalinity of the cement solu-tion, and the ambient moisture conditions of the structure. Expansions of over 0.1% are not uncommon. An expansion of 0.1% would result in an increase in length of 1 cm for every 10 m length of an unreinforced structure. In many structures this amount of expansion may not cause problems, but in long sidewalks or median barriers, for example, the expansion may cause compression and heaving. Expansion in large structures like dams, powerhouses and locks can interfere with the operation of the flow gates or turbines.
Conditions that Cause Cracking and Expansion
Harmful alkali-aggregate reaction will occur when: A. the concrete aggregate is reactive and B. the alkali content of the concrete is high enough to sustain the reaction and C. enough moisture (greater than ~85% RH) is present to sustain the reaction. Usually, the alkali content must be less than 3 kg/m3to
prevent adverse reaction, but in mass struc-tures where the moisture content of the concrete remains high (dams, for example), harmful reactions have been recorded with alkali contents as low as 2 kg/m3. Most of
the alkalis originate from the portland cement. If a cubic metre of concrete contains 350 kilograms of cement with an alkali content of 0.9%, the alkali content will be 3.15 kg.
Locations of Potentially Reactive Aggregates in Canada
Potentially reactive rocks and documented cases of alkali-aggregate reaction in con-crete occur in virtually all parts of Canada. However, cases of alkali-aggregate reaction are less common in the Prairie Provinces and British Columbia because the alkali content of the cements produced there has
Figure 2. Map-pattern cracking and extrusion of joint filling material due to expansion of concrete affected by alkali-carbonate reactivity in a sidewalk in Kingston, Ontario
Figure 3. Prominent cracking runs parallel to reinforcing bars in a retaining wall of a bridge in Quebec City, Quebec.
3 traditionally been relatively low. The
dis-tribution of potentially reactive aggregate sources and documented cases of alkali-aggregate reactivity are shown in Figure 4. More information and up-to-date descrip-tions of alkali-aggregate reactivity in all parts of Canada can be found in references 1 and 2.
Test Methods for Determining Potential Reactivity of Aggregate
For new construction, an important means for establishing that an aggregate is non-reactive is to investigate its performance history in structures made with the same concrete mixtures and exposed to environ-ments similar to those of the proposed new construction. When this is not possible or not conclusive, laboratory test methods must be used. The Canadian Standards Association document CAN/CSA A23.1-00/A23.2-00 Concrete Materials and
Methods of Concrete Construction/Methods of Test for Concrete provides a number of
test methods for evaluating the potential reactivity of aggregates. Only the two considered to be the most effective are described here.
Accelerated Mortar Bar Test
The aggregate to be evaluated is crushed to sand size and mortar bars (25 x 25 x 285 mm) are made with it. After the mortar has set, the length of the bars is measured and the bars are stored, immersed in sodium hydroxide solution, at 80°C. The change in length of the bars is monitored periodically
for 14 days. Expansions greater than 0.15% at 14 days are considered to be indicative of potentially deleterious expansion of the aggregate in concrete having a high alkali content. This test is appropriate only for alkali-silica reactive aggregates. Although this mortar test does not exactly replicate how concrete will behave, the test is a good indicator of potential reactivity and has the great advantage of being quick. For details of the test procedure, consult CSA A23.2-25A.
Concrete Prism Test
The concrete prism test is applicable to both alkali-silica and alkali-carbonate reactive aggregates. The coarse aggregate is made into concrete prisms (75 x 75 x 275 mm). After de-molding, the length of the prisms is measured. They are then placed in sealed containers over water to maintain a humidity of over 95%. The containers are stored in an enclosure maintained at 38°C and the length of the prisms is monitored periodically for one year. Expansions greater that 0.04% at one year are consid-ered to be indicative of potentially reactive aggregates. For further details of the test procedure consult CSA A23.2-14A.
Preventive Measures when Potentially Reactive Aggregates Must Be Used
The best method for preventing premature deterioration of concrete due to alkali-aggregate reaction is to use alkali-aggregates that have a proven history of good performance in concrete, or have been shown by laboratory testing to be non-problematic. This is the only method that is applicable to alkali-carbonate reactive aggregates.
It may sometimes be possible to produce a non-damaging aggregate in a quarry containing reactive material by selective quarrying. However, good quality control coupled with periodic testing is essential to ensure that the aggregate continues to be non-reactive. When potentially alkali-silica reactive aggregates must be used, a number of methods have been developed to ensure the durability of the concrete. For general applications, use of a portland cement with an alkali content low enough to reduce the alkali content of the concrete below
Figure 4. Map of Canada showing locations of structures affected by alkali-aggregate reaction or sources of known reactive aggregate (adapted from Reference 2).
“Construction Technology Updates” is a series of technical articles containing practical information distilled from recent construction research.
For more information, contact Institute for Research in Construction, National Research Council of Canada, Ottawa K1A 0R6
Telephone: (613) 993-2607; Facsimile: (613) 952-7673; Internet: http://www.nrc.ca/irc © 2002
National Research Council of Canada March 2002
ISSN 1206-1220 3 kg/m3may prevent damaging
alkali-aggregate reaction in concrete. However, this measure alone will not always prevent adverse reactions because some aggregates may contribute alkalis to the concrete and over time increase the alkali concentration to a level that will cause alkali-silica reaction in the concrete.
The optimum method for minimizing the potential for expansion due to alkali-silica reaction in concrete is to replace a portion of the portland cement by a supplementary cementing material. Low-lime fly ash, ground granulated blast furnace slag, silica fume, meta kaolin and natural pozzolans used in the appropriate quantities have been found to be an effective antidote for alkali-silica reaction. Mixtures of two supplementary cementing materials with portland cement (so-called ternary mixtures) have also been found to be very effective in preventing deterioration due to alkali-silica reaction.3
In laboratory experiments, the addition of lithium salts to concrete prevents or minimizes expansion due to alkali-silica reaction. In the United States, at least one field test is underway on a section of highway that will help determine whether the benefits of the lithium are long-term, or whether the lithium leaches out.4 The
Canadian Standards do not yet endorse the addition of lithium so its use in Canada should be considered experimental for some time to come. A guide for the use of supplementary cementing materials to avoid deleterious expansion in concrete is provided in CSA A23.2-27A.
In the case of alkali-carbonate reactions, neither the use of low-alkali cement nor the use of a supplementary cementing material are effective in preventing deleterious expansion.
References
1. Canadian Journal of Civil Engineering, Vol. 27, No. 2, April 2000, Special Issue on Alkali-Aggregate Reactivity.
2. A23.1-00/A23.2-00, Concrete Materials and Methods of Concrete
Construction/Methods of Test for Concrete, September 2000, CSA International, 178 Rexdale Boulevard, Toronto, ON, M9W 1R3, 360 p. 3. Thomas, M.D.A., Shehata, M.H.,
Sashiprakash, S.G., Hopkins, D.S., Cail, K., 1999, “Use of Ternary Cementitious Systems Containing Silica Fume and Fly Ash in Concrete.” Cement & Concrete Research 29, pp. 1207-1214.
4. Johnston, D.P., Surdahl, R., Stokes, D.P., 2000, “A Case Study of a Lithium-Based Treatment of an ASR-Affected
Pavement.” Proceedings of 11th International Conference on Alkali-Aggregate Reaction, Quebec City, June 2000., Editors: M.A. Bérubé, B. Fournier, B. Durand, pp. 1149-1158.
Dr. Paddy Grattan-Bellew is a former research
officer in the Building Envelope Program of the National Research Council’s Institute for Research in Construction.
Dr. Lyndon Mitchell is a research officer in the