Publisher’s version / Version de l'éditeur:
Materiaux et constructions. Materials and Structures, 19, 114, pp. 437-444, 1986
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Hydration kinetics and microstructural development in the 3
CaO.Al2-CaSO4.2H2O-CaCO3-H2O system
Ramachandran, V. S.; Zhang, C. M.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=8ee567f0-f52f-4a04-b2f1-11d8c38cd394 https://publications-cnrc.canada.ca/fra/voir/objet/?id=8ee567f0-f52f-4a04-b2f1-11d8c38cd394
Ser THl
N21d
no.
1438
National Research
Consell natlonal
c. 2
Council Canada
de recharches Canada
Institute for
lnstitut de
Research in
recherche en
Construction
construction
Hydration Kinetics and Microst~ctuml
Development
in the 3CaO.A I2O3-CaSO, .2H20-CaCO&O
System
by V.S. Ramachandran and Zhang Chun-Mei
Reprinted from
Materiaux et Constructions
Vol. 19, No. 114, 1986
p. 437
-
444
(IRC Paper No. 1438)
Price $2.00
NRCC 271 74
T h i s p a p e r i s b e i n g d i s t r i b u t e d i n r e p r i n t form by t h e I n s t i t u t e f o r R e s e a r c h i n C o n s t r u c t i o n . A l i s t of b u i l d i n g p r a c t i c e a n d r e s e a r c h p u b l i c a t i o n s a v a i l a b l e from t h e I n s t i t u t e may be o b t a i n e d by w r i t i n g t o t h e ~ u b l i c a t i b n s S e c t i o n , I n s t i t u t e f o r R e s e a r c h i n C o n s t r u c t i o n , N a t i o n a l R e s e a r c h C o u n c i l o f C a n a d a , O t t a w a , O n t a r i o , K l A OR6. Ce document e s t d i s t r i b u 6 s o u s forme d e t i r 6 - 3 - p a r t p a r 1 ' I n s t i t u t de r e c h e r c h e en c o n s t r u c t i o n . On p e u t o b t e n i r une l i s t e d e s p u b l i c a t F o n s d e 1 ' I n s t i t u t p o r t a n t s u r l e s t e c h n i q u e s ou les r e c h e r c h e s e n matiere d e b s t i m e n t e n G c r i v a n t B l a S e c t i o n d e s p u b l i c a t i o n s , Z n s t i t u t d e r e c h e r c h e en c o n s t r u c t i o n , G o n s e i l n a t i o n a l d e r e c h e r c h e s - - - K I A
0R6.
Hydration kinetics and microstructural development
in the
3
CaO
.
Al203-CaS04.2 HzO-CaC03-H20 system
V. S.
Ramachandran
Head, Building Materials Section, Division of Building Research, National Research Council of Canada, Ottawa.
Zhang Chun-Mei
Visiting Scientist, Nanjing Institute of Technology, Nanjing, The People's Republic of China.
Hydration and microstructural characteristics of mixtures containing C,A and CaS0,.2H20
(0, 12.5, 25%) with or without addition of CaCO, (0, 12.5, 25%) are followed by X-ray
dqfraction, dqferential scanning calorimetry, differential thermogravimetry, scanning electron microscopy and conduction calorimetry. Depending on the length of hydration, products formed at different periods from 5 min to 3 days consisted of hexagonal calcium aluminate hydrate, cubic calcium aluminate hydrate, calcium monocarboaluminate hydrate, ettringite, calcium monosulfoaluminate hydrate and possibly a solid solution of hexagonal calcium aluminate hydrate with monocarbo- and sulfo-aluminate hydrates.
Calcium carbonate retards or suppresses the formation of the cubic aluminate hydrate in the hydration of C,A. It accelerates formation of ettringite and its conversion to the monosulfoaluminate phase when added to the C,A
+
gypsum+
H 2 0 mixture.1. INTRODUCTION 2. EXPERIMENTAL PROCEDURE
Limestone dust produced in quarrying operations has been suggested for use as an additive in portland cement. The physical and mechanical properties of cement can be changed, however, by the addition of CaCO,, the extent depending on their composition.
The influence of CaCO, on early setting and harden- ing characteristics of cement depends on the amount of C,A and gypsum present. CaCO, forms complexes with C,A similar to those formed in C,A-gypsum mix- tures. In view of this, attempts have been made to substitute part of the gypsum by CaCO, [I, 21. The role of CaCO,, however, is incompletely understood. It was the objective of the present work to follow the hydration and changes in microstructural characteris- tics of individual cement components such as C,S, C,A and C,A
+
gypsum in the presence of CaCO, and relate the results to the behaviour of cement. Work-
on the C,S-CaC0,-H,O system has shown that C3S*. hydration is accelerated by CaCO,; microstructural changes are also affected [3]. This paper refers to the investigation of the C,A-gypsum-CaC0,-H20 system, with particular reference to determination of the various phases such as hexagonal aluminate hydrate, cubic aluminate hydrate, carboaluminate hydrate, and high and low sulfoaluminate hydrates formed at differ- ent periods of hydration.
2.1. Materials
Tricalcium aluminate was supplied by the Tetratech Co; gypsum and calcium carbonate (N, surface area 6.5 m2Ig) were supplied by Fisher Scientific Co. and Anachemia Chemical Co., respectively. Both were of reagent quality.
2.2. Sample preparation
In all, eight mixes were prepared: 1) C,A containing no gypsum, 2) C,A
+
12.5% gypsum, 3) C,A+
25% gypsum, 4) C3A+
12.5% CaCO,, 5) C,A+
25% CaCO,, 6) C,A+
12.5% gypsum+
12.5% CaCO,, 7) C,A+
25% gypsum+
12.5% CaCO, and 8) C,A+
25% gypsum+
25% CaCO,. The percentage of additives is based on the weight of C,A.The dry powders were transferred to capped vials, then glass beads were added and mixed for 3 min using a Spex mixer. Double-distilled water was added gradually and the mix was stirred at a water/C,A ratio of 4. At specified intervals, 5, 10, 20, 30 min, 1, 2, 4, 7 h, and 1, 2, 3 days, the samples were removed for examination; cold methanol was used to stop hydra- tion. The pastes were dried by two methods, i.e., at room temperature under vacuum, or at 50°C under vacuum.
Vol. 19 - No I 14 - Materiaux et Constructions
3. METHODS
3.1. Thermal analysis
DSC and TGA curves were obtained using the DuPont 1090 thermal analysis system. In each DSC experiment a 20-mg sample was heated from room temperature to 600°C at 20°C/min in a continuous flow of nitrogen gas. TGA was carried out by heating the samples to 1 OOO°C 131.
3.2. Conduction calorimetry
The calorimeter for measuring heat development was supplied by the Institute of Applied Physics, Delft, Holland [4]. Samples weighing 2 g were used for each experiment and water was added by syringe at W/C,A = 4.
3.3. Scanning electron microscope (SEM)
Microstructural examination was conducted on frac- tured pieces of specimen using a Cambridge Stereoscan Mark 2A. The specimens were given a conductive coating of carbon and gold.
3.4. X-ray diffraction (XRD)
X-ray photographs were obtained by a Norelco unit using a Debye-Scherrer camera. The relative intensities of the lines were obtained by Densitometer traces of the X-ray films.
4. RESULTS AND DISCUSSION
4.1. Hydration of 3 CaO
.
A1,0,Figure 1 shows DSC thermograms of C3A hydrated for periods varying from a few minutes to 2 days. Even at 5 min endothermal effects develop as a result of hydration products. The small peak below 100°C is caused by desorption of water; during the early periods peaks at about 145-150°C and 265-280°C represent the presence of a metastable hexagonal phase. As hydration progresses, the endothermal peak at about 150°C increases for up to 4 h; that at about 265-300°C conti- nues to increase for up to 2 days. The peak at about 300°C (4 h-2 d) signifies the presence of hexagonal and cubic phases (C,AH,). The exact time of conversion of hexagonal phase to cubic phase is not easy to determine because the two co-exist. At 2 days, however, there is strong indication that only the cubic phase is present.
XRD results indicate that C,A peaks decrease in intensity as hydration proceeds (Table I). The forma- tion of hexagonal phases at 10 min is confirmed. The cubic and hexagonal phases co-exist at 1 h, but at 2 days only the cubic phase could be detected.
Scanning electron micrographs of C,A hydrated for different periods are presented in figure 2. The unhy- drated C3A consists of irregular particles of various
sizes. Although the micrograph of the product at 10 min indicates the onset of hydration, it is only at 1 h that formation of hexagonal phases becomes evi- dent. At 4 h more hexagonal plates intermixed with the cubic hydrate may be seen, and at 2 days the sample contains only the cubic hydrate.
4.2. Hydration of 3 CaO
.
AI,O,-CaCO, mixture DSC curves of the products of hydration of C,A- CaCO, are different from those obtained by the hydra- tion of C,A. Two endothermal peaks at about 150- 160°C and 235245°C in the C,A-CaCO, mixture were also observed in the early periods of hydration of C3A without CaCO, (Fig. 1). These peaks represent calcium carboaluminate (or a solid solution ofC3A.Ca(OH)2.XH20-C3A-CaC03.XH20) 151. X-ray evidence reveals no hexagonal phase (C,AH, or C,AH,) in this system (Table I). The intensity of the
30 mln 4 h 2 d I t I l l 1 0 2 0 0 4 0 0 6 0 0 800 T E M P E R A T U R E . "C
Rg. 1. -DSC curves of C,A hydrated to different periods.
peaks increases with hydration, and there is indication at 2 h and beyond of a small endothermal effect at about 260-280°C. This may be attributed to the pre- sence of a small amount of the cubic phase. At 1-2 days it is intensified. At 2 days carboaluminate (solid solu- tion) and cubic phases co-exist. The addition of CaCO, to C3A seems to retard the formation of the cubic
phase [6]. The XRD results suggest that no cubic phase
.
is formed in the first hour; at 2 days, in the presence L of 12.5% CaCO,, the main phases are carboaluminateand cubic hydrates. Scanning electron micrographs I show that even at 10 min large plates form a network
(Fig. 3). These are attributed to carboaluminate or the solid solution. The microstructure of this product is different from that formed in C3A
+
0% CaCO, at 10 min (Fig. 2). At 7 h the plates increase in overall size, and at 2 days a mixture of plates and cubic hydrate is evident.V. S. Ramachandran - Zang Chun-Mei
TABLE I
X R D LINES OF THE PRODUCTS FORMED AT DIFFERENT TIMES IN THF C,A-CaC0,-CaS0,. 2 H,O-H,O SYSTEM
7
Components detected~ ~ ~ ~ ~ i ~ lHexagonal ~ Cubic Monosulfo- Carbo-
Period
C,A Gypsum CaCO, aluminate aluminate Ettringite aluminate aluminate
hydrate hydrate hydrate hydrate
10 min 1 *
-
- 3 --
-
l h 2 --
2 4 - - C3A . . . 7 h 2 - - 4 2-
A - 2 d 4-
- - 1 - --
C3A 10 min 1 - 2 - - --
2+
12.5%CaC03 . . . 2 d 4 - 5 - 2-
- --
I
1
C3A 10 min 2-
1 - ' 2+
l h 2 - 4 - - - 1 25% CaCO,.
. . .
3 d 5 --
- 4-
- 1 30 min 1 4 - --
4 - - I C3'4 l h 1 1 --
- --
3+
- - - 2 -I
7 h 3 4 4 - 12.5% gypsum...
5-
--
4 - 2-
30 min 1 - - - - 2 - - C3A l h 2-
--
2 --
+
7 h 4 - - --
- 2 - 25% gypsum . . ..
5 - - - --
1 - C3A 10 min 1 4 2 --
2 - 5+
20 min 1 - 2 - - - 4 5 12.5% gypsum l h 2 - 4-
- - 4 4+
7 h 2 - 5 - - - 4 2 12.5% CaCO, ..
.
3 d 5 - - - 3 2 C3A 30 min 1 - 3 - - 1 - -+
l h 3 - 4 - - 4 3 4 25% gypsum 7 h 3 - 5-
-
- 2 2+
3 d 5 - --
-
2 2 12.5% CaC03 .. .
* Numbers refer to relative intensities of peaks: 1, very strong; 2. strong; 3, medium strong; 4, weak; 5, very weak.
Differential thermogravimetric results are in agreement with those of DSC, XRD and SEM (Fig. 4). In mixtures containing 12.5% CaCO,, two peaks in addition to the two below 250°C at 10 min (caused by the carboaluminate) occur in the temperature ranges 700-750°C (due to decomposition of CaCO,) and 850- 900°C (due to decomposition of the carboaluminate) [7]. As the length of hydration increases, cubic alumi-
3 - ,
Fig. 3. -Micrographs of C,A
+
12.5% CaCO, hydrated for different klg. 2. -Micrographs of C,A hydrated for different periods. periods.Vol. 19 - No 1 14 - Materiaux et Constructions I I I I I I I I ' I l
-
-:E
0:
-
1 0 rnin-
- --
- - 0 1 0 0 2 0 0 3 0 0 4 0 0 5 0 0 6 0 0 7 0 0 PO0 9 0 0 1 0 0 0 1 1 0 0 T E M P E R A T U R E , "Gfig. 4. - Differential thermogravimetric CUNeS of C,A
+
12.5% CaCO, hydrated for different periods.nate seems to form at 7 h and 2 days, with a peak at about 300-315°C. The combined CaCO, in the car- boaluminate in the samples was indicated by the peak areas at 850-900°C; the amounts were 1.5, 6.0 and 8.2% at 10 min, 7 h, and 2 days, respectively, signifying a steady increase in the amount of carboaluminate as hydration progresses.
Larger amounts of carboaluminate are formed in the C,A-CaC0,-H,O system containing 25% CaCO, than in that containing 12.5% CaCO,, and there is no evi-
dence of cubic aluminate hydrate for up to 2 days.
XRD data confirm that only very small amounts of cubic aluminate hydrate exist at 3 days, the main phase being carboaluminate hydrate. Micrographs show clearly that hexagonal plates have formed even at 10 min, and that they increase as hydration progresses. Only very small amounts of cubic hydrate, if any, may exist at 3 days (Fig. 5).
The results for rate of heat development show that at
about 10 min peaks occur in C,A and C,A
+
CaCO,mixtures. Intensities are larger in samples containing
(4 (4
Fig. 5. -Micrographs of C,A
+
25% CaCO, hydrated for different periods.0 1 2 3
T I M E , h
fig. 6. -Conduction calorimetric curves of C,A hydrated with diffe- rent amounts of CaCO,.
CaCO, (Fig. 6) owing mainly to the formation of cal- cium carboaluminate (and/or solid solution). Larger heat evolution means that larger amounts are formed at higher CaCO, contents.
Comparison of the sequence of hydration products formed in the C,A-H20 and C,A-CaC0,-H20 sys- tems reveals that the formation of cubic aluminate hydrate is retarded by CaCO,. Under normal condi- tions of hydration the hexagonal aluminate hydrates are formed first and then converted to the cubic form. In the presence of CaCO,, calcium aluminate hydrate was not detected by DSC and X-ray techniques. The solid solution that forms (especially at higher CaCO, additions) may take longer to convert to the cubic form or may even be suppressed.
4.3. Hydration of 3Ca0.A120,-CaS0,.2H20 mixtures The sequence of hydration of C,A containing 12.5 or 25% gypsum was followed by DSC, scanning electron
micrographs (Fig. 7, 8), conduction calorimetry
(Fig. 9, lo), and XRD (Table I). The gypsum content decreases progressively and is almost consumed at 30 min, as is evident by the decreasing intensity of the endothermal peak at 130°C. The decrease in gypsum content should normally be reflected in the formation of ettringite. No clear peaks for ettringite are to be found, although the descending portion of the curve at 20 min and beyond is indicative of ettringite formation. Ettringite can easily lose water during the drying proce- dure, and this may explain the absence of a distinct peak in the series. Ettringite needles are evident, howe-
V. S. Rarnachandran - Zang Chun-Mei
Fig. 7. -Micrographs of C3A
+
12.5% gypsum hydrated for different periods.ver, in the micrographs at 30 min (Fig. 7). XRD results also indicate formation of ettringite at 30 min. After that the intensity of the endothermal peak at about 165-180°C increases substantially. This peak represents monosulfoaluminate hydrate. In other words, the ettringite appears to convert to monosulfoaluminate at 1 h. At this time a micrograph shows large plates of monosulfoaluminate. XRD data confirm the presence of monosulfoaluminate hydrate at 1 h and after. In addition, some hexagonal aluminate hydrate can be detected by XRD at 7 h, although its presence could not be confirmed by either DSC or SEM. From 7 h to 3 days monosulfoaluminate and some cubic phases are present (Table I).
In the presence of 25% gypsum DSC reveals the presence of ettringite even at 5 min, the amount increasing with time until at 1 to 2 h there is a sharp decrease. It is mostly consumed within 1 h. At 12.5% gypsum it is consumed within 30 min. XRD indicates the absence of gypsum, even at 30 min. Micrographs indicate the existence of ettringite needles up to 1 h
-
(Fig. 8).These results suggest that ettringite forms as early as 5 min for both gypsum contents, but at 25% gypsum it takes longer for complete consumption. The conversion of ettringite to monosulfate hydrates occurs more slowly in the presence of 25% gypsum. This is evident in the conduction calorimetric curves (Fig. 9, 10). Although at 12.5% gypsum some C,AH, is detec- ted, this phase is suppressed at 25% gypsum content.
Fig. 8. -Micrographs of C3A
+
25% gypsum hydrated for different periods.Only at 12.5% gypsum is there indication that the hexagonal aluminate hydrate has formed. Ettringite and monosulfate may co-exist, especially at early times. 4.4. Hydration of 3CaO.A1,O3-CaS0,.2HZO-CaC03
mixtures
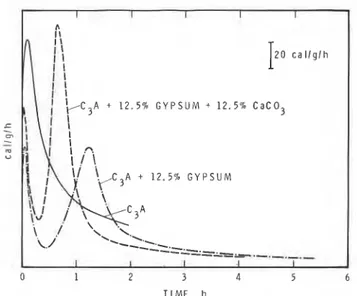
In the system containing 12.5% gypsum and 12.5% CaCO, DSC curves indicate that the rate at which gypsum disappears is accelerated by CaCO,. In the
I
2 0 c a l l g l h A + 1 2 . 5 % G Y P S U M t 12.5% C a C 0 3+ 1 2 . 5 % G Y P S U M
0 1 2 3 4 5 6
TIME, h
Fig. 9. - Conduction calorimetric curves of C,A
+
12.5% gypsum hydrated with and without CaCO,.V. S. Rarnachandran - Zang Chun-Mei
FFg. 12. - Micrographs of C3A
+
25% gypsum+
12.5% CaCO, hydrated for different periods.minate. There was some indication of carboaluminate in XRD for the mixture containing 12.5% gypsum
+
12.5% CaCO,, but this could not be confirmed in the 25% gypsum+
12.5% CaCO, mix- ture. Either the amount is too small or it is not well enough crystallized to permit detection by XRD. Micrographs of the C,A-gypsum-CaCO, system indi- cate that at 10 min plates and needles are formed (Fig. 12). Possibly the hexagonal plates represent cal- cium aluminate hydrate containing CaCO, incorpora- ted into it.4.5. Role of CaCO,
In the hydration of C,A at normal temperature a metastable, hexagonal aluminate hydrate forms initially and is subsequently converted to the cubic phase. Within a few minutes of hydration, C,A converts to the hexagonal phase and cubic hydrate is detected at 1 h or later. In the presence of CaCO,, carboaluminate hydrate or its solid solution with the hexagonal alumi- nate hydrate is initially formed. The cubic aluminate hydrate is retarded or even suppressed for up to several , days by CaCO,. It appears that the hexagonal alumi- nate hydrate containing carbonate is more stable than the normal calcium aluminate hydrate phase and, hence, does not easily convert to the cubic phase. This is analogous to the retarding effect of certain retarding admixtures that form complexes with the hexagonal aluminate and retard conversion to the cubic phases
[8]. The retarding effect of CaCO, on the cubic phase
does not necessarily mean that early hydration of C3A is itself retarded. In the early periods, hydration of C,A, or its consumption, is accelerated by CaCO, (Fig. 1, Table I).
Enhanced formation of ettringite in the presence of CaCO, can be explained as follows: In the C,A- gypsum-CaCO, mixture, CaCO, particles (6.5 m2/g) are much finer than either the C,A or gypsum (approxi- mately 0.5 m2/g). Thus, C,A is surrounded by fine particles of CaCO,. On contact with water, C,A reacts with CaCO, to form carboaluminate on the surface of C,A, producing a layer that may be less compact and more permeable to water than ettringite. It therefore interferes with the formation of a more rigid layer of ettringite. During hydration, continuous formation of carboaluminate hydrate with large hexagonal plates (not attached to the hydrating C,A surface) further promotes diffusion of H,O and through the C3A surface. It is known that conversion of ettringite to monosulfoaluminate hydrate takes place after the gypsum in the system has been exhausted. Calcium carboaluminate hydrate enhances the rate of consump- tion of gypsum and hence facilitates early conversion of ettringite to monosulfoaluminate.
5. CONCLUSION
In C,A-H20 mixtures conversion to C,AH, is either suppressed or retarded by the addition of CaCO,. This is attributed to the preferential formation of monocar- boaluminate hydrate.
The reaction between C,A and gypsum to form ettringite is accelerated by the addition of CaCO,. This is attributed to the reaction of CaCO, on the hydrating C,A surface, forming more permeable membranelhexa- gonal plates.
Conversion of ettringite to monosulfoaluminate hydrate is accelerated by CaCO,. This is related to the accelerated depletion of gypsum owing to the presence of CaCO,.
Under certain conditions, ettringite, monosulfoalu- minate, and carboaluminate may co-exist.
ACKNOWLEDGMENTS
The authors are grateful to J. Clark, G. M. Polomark, E. G. Quinn and P. J. Lefebvre for experimental assistance. This paper is a contribution from the Division of Building Research, National Research Council of Canada.
REFERENCES
[I] BENSTED J. - Some hydration investigations involving
portland cement-effect of calcium carbonate substitution of gypsum. World Cement technology, Vol. 11, No. 8, 1980,
Vol. 19 - No 1 14 - Matkriaux et Constructions
[2] BENSTED J. - Further hydration investigations involving
portland cement and the substitution of limestone for gypsum. World Cement Technology, Vo1. 14, No. 10,
1983, pp. 383-392.
[3] RAMACHANDRAN V. S., ZHANG C. M. - Influence of
CaCO, on the hydration and microstructural characteristics of tricalcium silicate. Submitted for publication.
[4] RAMACHANDRAN V. S. - Action of triethanolamine on the
hydration of tricalcium aluminate. Cement and Concrete Research, Vol. 3, No. 1, 1973, pp. 41-54.
[5] RAMACHANDRAN V. S. - Applications of differential
thermal analysis in cement chemistry. Chemical Publishing Co., Inc., New Y ork, 1969.
[6] JAMBOR J. - Influence of 3CaO.A1,O,.CaCO,.nH2O on
the structure of cement paste. 7th Int. Cong. Chem. Cem., Vol. IV, 1980, pp. 487-492.
[7] COLLEPARDI M., MONOSI S., MORICONI G., PAURI M. -
Influence of gluconate, lignosulfonate, and glucose admixtu- res on the hydration of tricalcium aluminoferrite in the presence of gypsum with or without calcium hydroxide.
Journal of the American Ceramic Society, Vol. 68, No. 5, 1985, PP. 126-128.
[8] RAMACHANDRAN V. S., FELDMAN R. F. - Effect of calcium
lignosulfonate on tricalcium aluminate and its hydration ,- products. Materials and Structures, Vol. 5, No. 26, 1972, pp. 67-76.
CinCtique de l'hydration et formation de la rnicro- structure dans le systcme 3 CaO
.
A1203-CaSO, .
2 H 2 0 - C a C 0 3 - H 2 0 . - O n ttu-die l'hydratation et les caracttristiques de la microstruc- ture de mtlanges renfermant du C,A et du CaS0,. 2 H 2 0 (0, 12,5 et 25% avec ou sans addition
de CaCO, (0, 12,5 et 25%) Li l'aide de la diffraction X, de la calorimttrie par balayage diffkrentiel, la thermogra- vimttrie difftrentielle, la microscopie klectronique d balayage et la calorimttrie par conduction. Selon la durte
de l'hydration, il se forme a difftrents intervalles allant de 5 minutes ci 3 jours de l'hydrate d'aluminate de calcium hexagonal, de l'hydrate d'aluminate de calcium cubique, de l'hydrate de monocarboaluminate de calcium, de l'ettringite, de l'hydrate de monosulfoaluminate de cal- cium et parfois une solution solide d'hydrate d'aluminate de calcium hexagonal avec hydrate de monocarbo et sulfoaluminate.
Le carbonate de calcium retarde ou empzche la forma- tion d'hydrate d'aluminate cubique duns l'hydratation de C3A. 11 acctltre la formation d'ettringite et sa conversion en phase monosulfoaluminate lorsqu'il s'ajoute au melange C 3 A