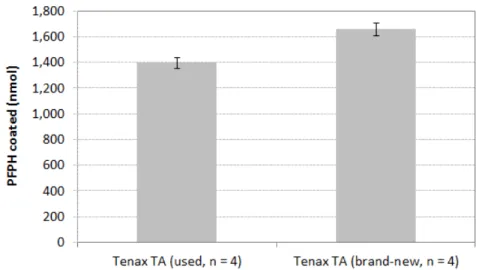
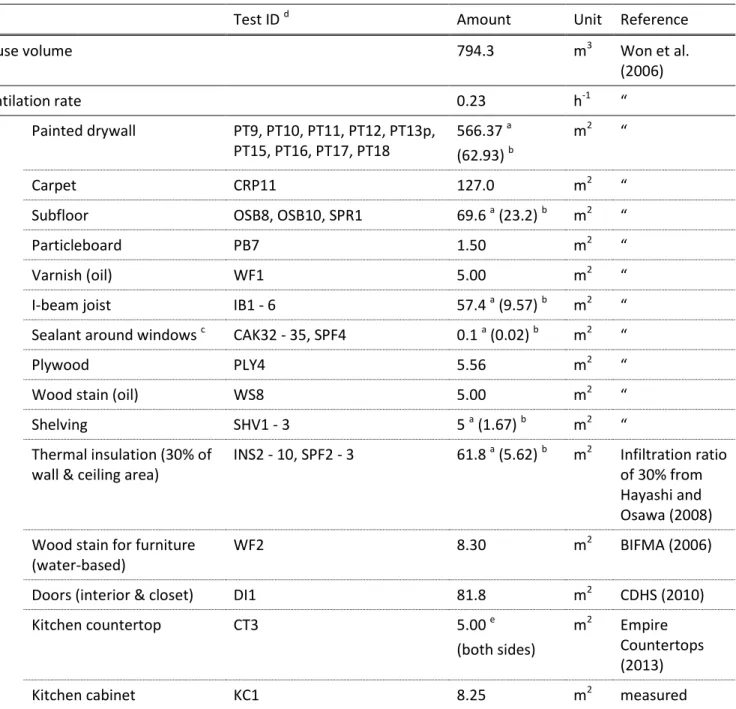
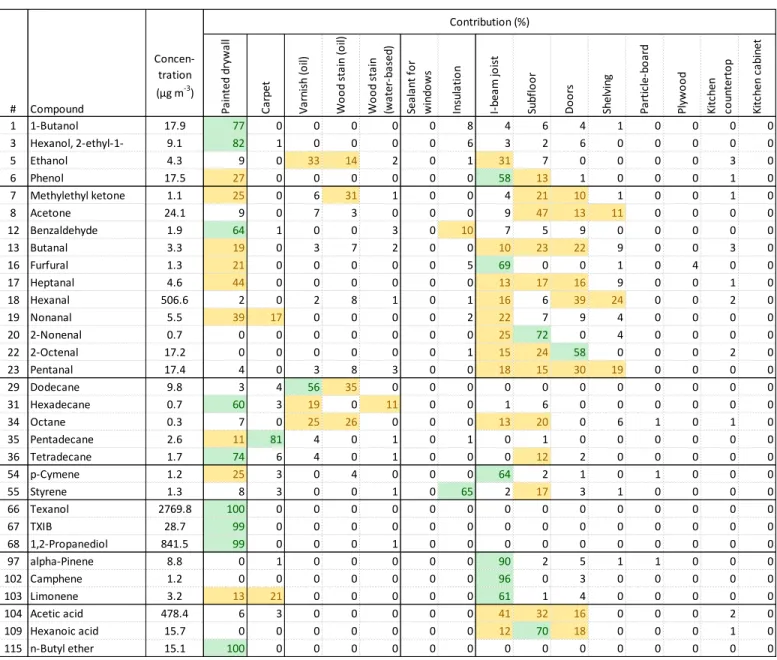
https://doi.org/10.4224/23002015
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez
pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the
first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien
DOI ci-dessous.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Material emissions testing: VOCs from wood, paint, and insulation
materials
Won, Doyun; Nong, Gang; Yang, Wenping; Collins, Peter
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=77464aff-3278-4693-9f2c-b87ba98a8f0e
https://publications-cnrc.canada.ca/fra/voir/objet/?id=77464aff-3278-4693-9f2c-b87ba98a8f0e
Material Emissions Testing:
VOCs from Wood, Paint, and Insulation Materials
Doyun Won, Gang Nong, Wenping Yang, and Pet er Collins
Prepared for: Healt h Canada
Report No.:
A1- 000342.2
Dat e I ssued: June 2014
This report may not be reproduced in whole or in part without the written consent of the
National Research Council Canada and the Client.
Material Emissions Testing:
VOCs from Wood, Paint, and Insulation Materials
Summary
A total of 30 building materials were tested for 121 volatile organic compounds (VOCs) in
small-scale (50 L) chambers in accordance with ASTM D5116-2010. The focus was on wood,
paint and insulation materials, which were identified as major sources of VOCs indoors or for
lack of information on VOC emissions in the previous study (Won et al., 2013). The emission
factors of 121 NRC target compounds are presented along with the empirical emission models
to describe the decay of emission factors. The data sets generated for these three groups of
materials in the previous study were also included in determining the mean emission factors for
each group of materials and the indoor air concentration based on a product usage scenario. In
total, the emissions data in this report represent 18 wood, 14 paint, and 17 insulation materials
tested between 2012/13 and 2013/14.
Terpenes and oxygenated compounds (e.g., alcohols, aldehydes, and acids) were detected
more frequently with high emission factors in wood products. Paint products also emitted
oxygenated compounds (1-butanol, acetone, formaldehyde, and acrolein) frequently.
However, the compounds with the highest emission factors and detection frequencies in paint
products were glycols and esters (e.g., Texanol and 1,2-propanediol), which are known to be
major ingredients of latex paints. While there were also various compounds detected in
insulation products (e.g., 1-butanol, 2-propanol, Texanol, acetaldehyde, and formaldehyde), the
emission factors of these compounds were relatively low.
Among
Health Ca ada’s priorit VOCs
1
, the following compounds were detected with a
detection frequency (DF) > 10%:
• O e al ohol: 2-propanol (DF above zero: 39%, DF above method detection limit: 33%)
• “i aro ati s: benzene (53%, 14%), xylenes (o-xylene: 45%, 6%, m&p-xylene: 71%, 14%),
ethylbenzene (71%, 12%), naphthalene (39%, 2%), toluene (76%, 24%)
• one chlorinated compound: chloroform (10%, 6%)
• Four aldehydes: acetaldehyde (88%, 86%), formaldehyde (96%, 94%), propanal (76%, 61%),
and acrolein (55%, 45%)
Two chlorinated compounds were detected in one or two materials included:
• T o hlori ated o pou ds: 1,2-dichloroethane (4%, 4%) and 1,4-dichlorobenzene (8%,
2%)
The compounds that were never detected or excluded were:
1