Publisher’s version / Version de l'éditeur:
American Ceramic Society Bulletin, 44, 2, pp. 151-155, 1965-06-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Hydration of gypsum plaster by the compact technique
Sereda, P. J.; Feldman, R. F.; Ramachandran, V. S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=65562e62-49a2-4a58-b994-7fb94195c3f0
https://publications-cnrc.canada.ca/fra/voir/objet/?id=65562e62-49a2-4a58-b994-7fb94195c3f0
Reprinted f r o m T h e A m e r i c ; ~ ~ ~ Cer;lmic Society Bulletin, Vol. -1-1, No. 2 . Felxuary 7 , 1065. Copyrigllt 1065 by T h e American Ceramic Socicty
A new method is described for following the hydration reaction of gypsum
plaster (hemihydrate). It involves the measurement of the length change of
a compact of unhydrated material when it is exposed to water in the liquid
or vapor phase. Advantages of this method over previous methods are
discussed and evidence presented to demonstrate them. By the compact
technique unhydrated particles can be brought very close together, resulting
in a very large decrease in the rate of hydration and an increase in the ap-
parent volume expansion accompanying the hydration. These effects can
be used to advantage in studying the mechanism of the reaction. This work
also shows the effect of temperature upon the expansion due to hydration.
Hvdration
of
Gvpsum Plaster
by
the Compact Technique
P. J. SEREDA, R. F. FELDMAN, and V. S. RAMACHANDRAN
National Research Council (Canada) Division o f Building Research, Ottawa
C,
XEAT
is evolved during the reaction of gypsurn plaster (hemihydrate) with water and there is a n absolute volume change-of the system as well as a variation of the apparent volume of the solid body. These effects have been observed and have been used to follow the rate of the reaction by many workers.Volume change has been used less extensively than the heat effect in studies of the hydration of gypsum plaster in spite of the fact that the shape of the rate curves of both eifects is very similar, as was shown by Chasseventl and An-
drews. "his is understandable, however, because the volume
change is much more difficult to observe than the tempera- ture rise, especially as the normal system is initially a plastic mass.
There are distinct advantages in following the reaction by means of volume changes, which reflect n o t only the chemical but also the physical changes and can give new information on the mechanism of the reaction. I n following the reaction by the heat eflect, the system must be allowed to increase in temperature, and this in turn may affect the reaction.
A variety of devices based on the dilatometer principle first conceived by Le Chatelier3 have been used t o study the absolute volume change resulting from the reaction of gypsum plaster with water. The increase in the apparent volume accompanying hydration is of great practical significance and was first ineasured by a n apparatus called an "extensometer"
developed by Gibson and J ~ h n s o n . ~ Cl~assevent made signif-
icant modifications and succeeded in obtaining results t h a t gave a comprehensive picture of this effect. He observed t h a t the contraction of the gypsuln plaster and water mix during setting could be eliminated by the addition of water t o the surface. He learned also t h a t the expansion due to hydration following setting was affected by the history during setting as well as by the ratio of plaster t o water and the type of plaster used. The effect of temperature upon expansion,
This paper is a contribution from the Division of Building Research of the National Research Council, Canada, and is published with the approval of the director of the Division.
VOL. 44, No. 2 (1965)
however, was not discovered because large samples were used in all this worlc and temperature control throughout the hy- drating mass was not achieved.
I t appeared t h a t new information about t h e process of hydration of gypsum plaster, especially from the first instant of wetting, could be obtained if the setting process was more defined and separated from the hydration process, and if the samples were small in a t least one dimension to allow control
of the telnperature throughout the mass. If a rigid porous
body is produced b y bringing unhydrated particles strongly into contact with each other, the process of setting can be ac- colnplished without water. The moment t h a t water is brought into contact with the surfaces of the particles the resulting physical and chemical effects are reflected iminedi- ately as an apparent volunle change. Such a system also permits the introduction of water in the vapor phase. Ap- parent volume change resulting from hydration with water in the vapor phase has not been possible by any of the methods used previously.
T h e experience of this Division in producing rigid porous systems by cornpaction of powdered materials was put t o use t o produce compacts of gypsuln plaster t h a t would meet the above recluirements for the study of the process of hydration. This paper describes the method for the measurement of the length changes of a rigid porous body (compact) of gypsum plaster formed by conlpaction of the powdered material, which is subsecluently exposed to water in the liquid or vapor phase. Preliminary results applying this method were pub- lished earlier in two notes dealing with gypsum plasterQnd
calcium oxide."esults are presented now to show the ad-
vantages of this method a n d t o indicate the new information t h a t can be obtained about the process of hydration.
Experimental
T h e procedure for the preparation of samples was the same as t h a t outlined in a previous publication? T h e modified
Tuckerman gage used as the optical exte~lson~eter for length
measurements has also been d e ~ c r i b e d . ~ , ~
Two types of gypsuln plaster (hemihydrate) were used in this study:
To Vacuum To S o u r c e o l Vialer
L
+
Fig. 1. Cell for exposing compacts to water in liquid or vapor phase for study of hydration.
1. Pottery plaster-produced by the "aridised" process
which' consists of heating gypsum with traces of cal- cium chloride,
2. "B-Base" Hydrocal-pure gypsum rock dehydrated in
the presence of steam.
*
When following hydration with water in the vapor phase the sample mounted in the extensometer was placed in a glass
cell equipped with an optical window (Fig. 1). The cell
was first evacuated and cooled to the desired temperature. T o achieve the desired relative humidity of approximately
100% ("saturated") or 95% the cell was filled with water or
sulfuric acid solution a t correct temperature to within a few millimeters of the sample and placed over a constant-tempera- ture plate controlled a t 3 j 0 , 50°, or 70°F as required. Ob- servations were made frequently at the start and continued as
*
Supplied by U. S. Gypsum Co., Chicago.I
I
P o l t e r y P l a s t e r - I\! -\
- -X"""
2:
\
*\. - 1 0 -\
-I
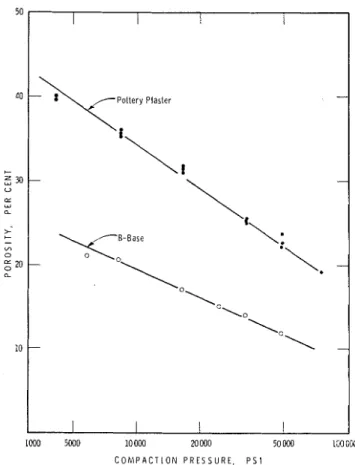
1000 5000 10000 M W O 50 000 lmC03 C O M P A C T I O N P R E S S U R E . P S IFig. 2. Effect of pressure on the porosity of compacts of two types of gypsum plaster.
often as required to give a rate curve until no further change was noted.
To follow hydration with water in the liquid phase the sample mounted on the extensometer was exposed to a vapor in the cell, as described for vapor hydration experiments, until expansion due to hydration began (usually 3 hours for unre- tarded plaster). More water at the correct temperature was then added to the cell until the sample was totally immersed. The quantity of water was limited to about 10 cm3 to avoid the dissolution of too much of the sample.
Results
The compaction properties of the two types of gypsum plaster were distinctly different. In Figure 2 the graph of the void fraction as related to the compacting pressure for pottery plaster is reproduced from a previous paper7 and compared with that for B-Base Hydrocal. These results show how samples having a two-fold variation in void fraction can be prepared from the one material and how the compaction characteristics can differ for two samples of the same chemical composition when they are prepared by different methods.
Figure 3 shows the calorimetric temperature rise curves with time that give the usual representation of the rate of reaction or reactivity curve as given by Ridge' and others. I t may be noted that there is a distinct difference in the reactivity of the two types of gypsum plaster.
When samples of pottery plaster are exposed to water vapor, a rapid expansion occurs which is completed in about 20 minutes followed by a dormant period. To explore the na- ture of the initial expansion of the compacts, cyclic experi- inents were conducted in which the samples were first exposed to saturated vapor for 3 hours, then placed over magnesium perchlorate for 21 hours. Results of these tests are given in
Fig. 4 and show that more than half of the initial expansion is
reversible. There is an irreversible portion of expansion added with each cycle, and every indication that this irre- versible portion would be much smaller if the time of ex- posure to vapor were shortened.
Figure 5 shows the expansion a t different temperatures
as related to the void fraction of the plaster compact before hydration. These results are for pottery plaster and B-Base
4.00 3.50 0 3.00 W V)
,
2.50 w ir 32
2.00 x w (L5
1.50 C I. 00 0.50 0.00 Fig. 3.I
I I I I I I II
0 10 20 3 40 50 60 70 80 TIME, MINUTESTemperature rise curve for two types of gypsum plaster when stirred with water in a calorimeter.
Dry rivet Dry Wet Dry Wet Dry Wet Dry
1 2 3 4
C Y C L E S
Fig. 4. Total expansion contraction during repeated cycling.
plaster hydrated in the licluid phase using both methods. Temperature as measured with a thermocouple placed inside the sample was controlled to within l/lO°C.
For the two types of plaster used the final expansion a t a given temperature was entirely dependent upon the void
fraction of the unhydrated compact. Figure G shows results
of expansion of samples of the two plasters compacted to the same void fraction. I t may be seen that although the reac- tivity of the two samples is very different, the total expansion was identical. Comparing the rates of hydration of the two samples of powdered gypsum plaster, when stirred with water as shown in Fig. 3, with the rates of expansion of compacts in
Figs. 6 and 7 , it is obvious that the time for hydration is
increased many tiines when the material is compacted. This is particularly true in the case of the B-Base plaster. The expansion due to hydration in the vapor phase seems to be a little greater than that for hydration in the licluid phase, although the rate is less (Fig. 8).
Figure 9 shows clearly the effect of void fraction (com-
pacting pressure) upon expansion. I t may be seen that the initial rates are similar and that only the final expansion is affected. To show that the expansion of the compacted
P O R O S I T Y , P E R CENT
Fig. 5. Expansion of pottery plaster as a function of porosity and tem- perature.
i
o ' l I ! ! i l i ; ~ l ~
0 1 ? 3 5 6 7 S Q l a 1 1 1 ?
i l : . l f . D A Y S
Fig. 6. Comparison of the expansion of compacts of pottery and "B- Base" plasters a t the same porosity when hydrated in liquid phase.
o f l l l l / ! ~ i i l !
0 1 2 3 ~ d 7 8 9 1 0 l i I ?
i l i . i i . D A Y S
Fig. 7. Comparison of expansion of compacts of pottery and "B-Base" plasters a t the same porosity when hydrated in vapor phase.
Fig. 8. Expansion of pottery plaster compacts a t different conditions of exposure to water.
Fig. 9. Expansion of "B-Base" plaster compacts made a t different pressures.
4 8 12 16 20 24 26
H Y D R A T E W A T E R , P E R C E N T
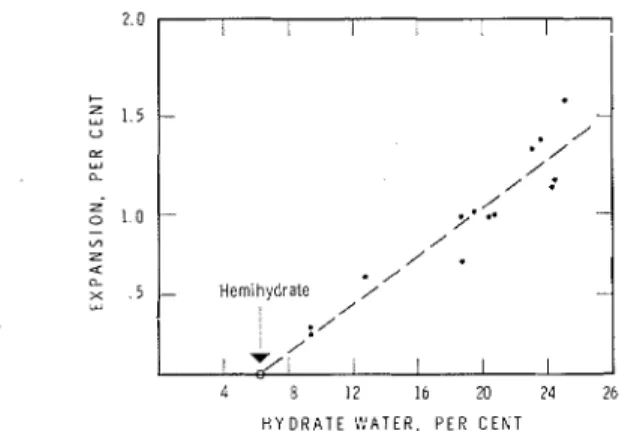
Fig. 10. Relation for expansion with extent o f hydration in liquid phase a t 70°F of pottery plaster compacts m a d e a t one pressure.
sample reflects the degree of hydration, the results of expan- sion of a number of compacts made a t one pressure and hy-
drated a t one temperature t o different times were ~llotted in
Fig. 10. Although a considerable scatter of results is evi- dent. a relationshin is indicated. T h e scatter of results is attributed to t h e Gocedure of stopping the reaction and de- termining the hydrate water.
Discussion
T h e results showing the different compaction values of t h e two types of plaster (Fig. 2) are difficult t o explain. Previous worklo has shown t h a t the two materials have dif- ferent physical characteristics such as surface area and ex- pansion due to adsorption, but i t is not evident how this can be used t o explain why the B-Base should compact more easily than pottery plaster. Optical examination a t 1000 magnification does not indicate a significant difference in particle size, although i t is possible that some agglon~erates
may not have been dispersed. T h e B-Base cryst. CL 1. 5 are re-
ported t o be ]nore ordered," but this does n o t suggest to the authors t h a t they should be easier to crush or more suhject to
*
Private comnluilication, U. S. G p p s u ~ n Co.The
P. J. Sereda obtained his Mas-
ters degree in chemical engineer- ing i n 1 9 4 4 from the University of Alberta. From 1 9 4 4 t o 1 9 4 8 he was employed by Atomic Energy of Canadd Ltd. at Chalk River, O n t . M r . Seredd then joined the Division o f Building Research o f the National Research Council o f Canada, w h e r e h e is hedd of the Inorganic Mdterials Section super- vising materials research.
R. F. Feldrnan received his B.Sc. i n pure science at the U n i - versity C o l l e g e of the West Indies,
P. J. Sereda London, d n d spent a further year w o r k i n g for his diplomd i n ceramic technology, w h i c h he received in 1 9 5 7 . H e then w e n t to the U n i - versity o f Toronto, receiving his M.A.Sc. i n chemical engineering i n 1 9 5 9 . M r . Feldman joined the Division o f Building Resedrch i n 1 9 5 9 ; he is n o w studying aspects pertdining to the chemical and physical properties of materials related t o building.
A t present V. S . Rarnachandran is a ~ o s t - d o c t o r d t e f e l l o w at the
rearrangement or plastic flow resulting i11 l ~ e t t e r packing as discussed by Cooper and Eaton.''
I t is an advantage, however, t h a t colnpacts with such a wide range of void fraction can be prepared, especially when this property seems to have such a great influence upon the expansion due to hydration.
Expansion began the inoinent t h a t water came into contact with the surface of the unhydrated particles, thus indicating physical changes. This was possible because the particles were in contact with each othcr. \\'hen gypsum plaster is inixcd with water into a paste a contraction occurs that 01,- scures the expansion produced lly wetting during the setting periocl. T h e initial rapid expansion was coinpletetl in allout 20 minutes and was followcd by an induction or dorlnant
period of several hours. There seems little d o ~ ~ l l t that this
expansion is related to the slight heat effcct observed in Fig. 3, and that new infornlation about the early stages of the process of hydration is revealed by this techniclue.
T h e most significant differencc between thc hydration of gypsum plaster in a mix with water (when stirred with excess
water or with water in the ratio :! : 1 plaster to water by ~veight)
and the hydration in the compact is the rate of reaction. This is undoubtedly related to the spacing of t h e particles. In the coinpact the particles are so close to each other t h a t the resulting fine pores impede the inovement of water a n d
ions a n d thus greatly reduce the rate of reaction. If the
fine pores are responsible for slowing the reaction I)y such a large factor, i t follows t h a t this fine pore systein can con- tribute to large ion concentration gradients, resulting iu varia-
tions in the degree of supersaturation. It must be the ion
concentration gradients and degree oi supersaturation re- quired in this system t h a t contribute chiefly to t h e slowing of the reaction.
T h e cyclic experiments involving exposure to "saturated" vapor followed by drying, demonstrated again t h a t a large portion of the initial expansion is due to adsorption on the large surface of the hemihydrate. The magnitude of this expansion c11ecl;s well with adsorptio~l expansion on t h e
same plaster rcported previo~~sly by Feldman and Sereda.lo
Only a portion of the expansion resulting from the exposure to "saturatecl" vapor for 3 hours is irreversible. This is a t -
trihuted t o soinc d i l ~ ~ d r a t e formation. Furthcr indirect evi-
dence t o support this proposition is provided 1 3 7 thc fact t h a t
Authors
resedrch pertdining t o clay mineralogy, lime dnd cement chemistry Dr. Rdmdchdndrdn holds B.Sc., M.Sc. dnd D.Phil. degrees from M y - sore, Bdndrds, dnd Calcutta Universities, respectively, i n India.
the conlpact will re~nain intact when inl~ncrsecl in water after preexposure to "saturated" vapor for 3 hours for the pottery
plaster sample and for about 6 hours for the B-Base sample.
Conlpacts of these samples will disintegrate whcn immersed in water \vithout prccxposure to vapor.
I n a previous papcr5 i t has Ixcn shown t h a t thc amount of expansion acconlpanying the setting of gypsum plastcr
varics greatly with tenlpcraturc Thcse rcsults \\,crc reportccl
for paste and for conlpacts ~ n a d c a t a prcssurc of about -I000
psi; it was sholvn t h a t this expansion was nlagnilicd in thc
conlpacts ovcr t h a t obscrved in thc paste, as would be ex-
pected from the diflercnce in the spacing ol' particlcs This
work has now bcen cstcndcd to includc compacts nlade in a
range of prcssurcs up to 75,000 psi
I t was first obscrved t h a t increasing t l ~ c load lor contpaction
increased the alnount ol' cspansion lor ally given tcmpcraturc.
This was most pronounced a t low ternpcratures llrhcn thc
results lor the two typcs ol' plastcr wcrc comparccl i t was realized t h a t thc expansions a t a given tcmpcraturc wcrc al- most idcntical if the two samples lvere colnpacted to thc sanle
void l'raction T o attain this thc pottery plaster was com-
pacted a t a load of 73,000 111 ancl 13-Basc a t 10,000 Ib. This
rcsult is clearly shown in Figs 6 and 7 , a ~ i d is in accord \\lit11
what would bc espectccl, the primary [actor determining cx- pansion cluc t o hydration should be tltc availahlc spacc hc-
t\\~ccn thc unhydrated particlcs
When comparing the vapor pliasc hydration \vivltli liquid phasc hydration a t roo111 te~nperaturc therc is a srnall in-
crease in thc expansion for the fornler (Figs ( i , 7 and 8 ) This
difference is s~wall when compared with thc cflect of change in
void fraction or change in tc~npcraturc
As previously mentioned, when plastcr is conlpactccl before
exposure to water a s vapor or lic~uicl for hydration, the cffect is
one of a very marked sloning of the rate ol' reaction This is
shown by co~nparison ol' the results of the timc for hydration
with those by 0'Kelly12 lor this pottery plaster, nlixcd a s
pastc, and also when stirred with water a s shotvn in Fig 3 What is, pcrhaps, even Inore significant is the observation that this cffect of slowing the rate of reaction is ~nagniliecl nlany
tinles when a less "rcacbvc" sample is used T h c T3-Base
san~ples are i~~clicatcd to be less reactive than pottery plastcr
by the calor~metric mcthod, a s uscd hy Ridge' and others, but
when the rates of hyclration reactions arc compared on the
basis of e ~ p a n s i o n of the compacts the difference in thc I eac-
tivity is magnilicd grcatly (Figs (i and 7 ) I t is suggested
t h a t such expansion curves ol' hydrating co~npacts night be
used nlore aclvantageously for clcfining reactivity of a sample
than the previous mcthods of heat cvolution T h c [act t h a t
the rate of expansion curvcs during carly hyclrat~on are si~nilar
lor compacts of cliflcrent void l'raction (Fig 9) lurther sup- ports the idea t h a t these might 1,e used to compare reactivity Perhaps the cffect of accclcrators and rctarclers can hc shown to bcttcr advantage than has 1)een achicvcd by o1)scrving the hcat cvolution. Rcsults of such a study will be presented in a separate papcr.
Conclusions
I t has bccn clcriionstratcd t h a t the cspansion ol' compacts
of gypsum plaster during hydration reflects the degree of hy- dration and provides a net\? method of following the reaction. This method cnal~lcs the observation of the physical and chemical cflects of wetting by water and hydration, a s re- flected by apparent volulne change, from thc momcnt t h a t water colnes in contact \vith thc surface of thc gypsum plaster. T h c mcthod is reasonably rcproclucible and allolvs for ac-
curate control of the te~nperaturc of hydration, which is shown
to be absolutcly essential because thc volume change is de-
pendent on temperature I t can be secn why some of the
previously recorded rcsults on volunle changc may not be ac-
curate; largc samples wcrc used, and cvcn if thc hyclration
had 1)ccn conductccl in a constant te~nperature bath there was
the possibility of large temperature gradients.
T h c hyclration of gypsum plaster in a compact procccds a t a
grcatly rcducecl rate This allolvs lor Inore precise observa-
tion of thc diflcrences of the rcactivity of thc samples, so t h a t rcsults of such work should be usel'ul for studying thc mecha- nism ol' hydration and should enable thc effects of accelerators or retarders to bc obscrvcd
Dcvelopecl originally for lollowing hydration of gypsum plaster, thc mcthod has nolv been applied with success to the study of the hydration of portland cclnent and calcium oxide
systems Results of this work arc in preparation for publica-
tion
*
Louis Chassevent, "\Tariatio~~ in the Volume of Plaster during
and after Setting," IZev. Ahterial~z- Constr2lctio17 Trav. Publ. ed.
C, No. 405, 188-94, S O . 406, 219-24, No. 407, 267-72, No.
408, 304-308 (1949).
H. Atidrews, "Thc Production, I'roperties a ~ l d Uses of Cal-
cium Sulphate Plasters," B I L . ; ~ ~ . Res. Congr., London, Div. 2 ,
D. 135 (1951).
I-I. 'Le ~ilatelier, "Revue dcs Materiaus cle Construction," KO. 227, (1927).
"
C. S. Gibson anclR.
W. Johnson, "Investigations of the Set- ting of Plaster of Paris," J. Soc. C ~ L ~ I I I . I n d . , 51 [ 4 ] 25-38 (1932); C e r a ~ ~ r . A hstr.. 11 161 344 (1932).P. J . ~ e r k d a , "Effect 'of Tkn~perature on the Expansion of
Setting of G y p s u ~ ~ ~ I'lastcr," iVfltlrre, 187 [4741] 929-30 (1960).
IT. S. R a ~ n a c l ~ a n c l r a ~ ~ , 1'. J. Sereda, and R. F. Feldman,
"Mecl1anis111 of I-Iyclratiot~ of Calcium Oxide," Submitted for publieation.
'
P. J. Sereda a11c1 I<. F. ITeIcIrnan, "Compacts of PowderedMaterials as Porous Bodies for Use in Sorption Studies," J.
n p p r . C I ~ ~ : I I I . , 13 [41 150-58 (1963).
fi I<. F. F e l t l l ~ ~ n ~ ~ , 1'. J. Sereda, ancl 17. S. K a ~ n a c h a n d r a ~ ~ , "A
StucIy of Length Changes of Compacts of Portland Cement During Their I-Iydration," Presented a t the Annual Meeting,
Highway Researcl~ Board, January 1964.
M. J. Ridge, "Aeceleration of the Set of Gypsum Plaster,"
Australian J. i l p p l . , Sci., 10 [ 2 ] 218-31 (1959); Ceraq~a. ..I hstr.,
1961, September, p. 204e.
lo R. I?. Feldnla.11 and P. J. Sereda, "The Use of Con~pacts to
Study the Sorption Characteristics of Powdered Plaster of
Paris," J. A p p l . Clte~iz. (London), 13 [ 4 ] 158-67 (1963).
l 1 A. R. Cooper, Jr., and L. E. Eaton, "Co~npaction Behavior
of Scveral Ccramic Powders," J. A m . Cerant. Soc., 45 [ 3 ] 97-101
11962).
l 2 B. M. O'KeIIy, "Pl~ysical Changes in Setting Gypsum Plas-
tcr," A S T f 1 I BILII. NO. 237, 55-62 (1959).