READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Pore structure, permeability and diffusivity as related to durability =
Influence de la structure des pores, de la perméabilité et de la
diffusivité sur la durabilité
Feldman, R. F.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=a3a34b01-c3a9-4841-8001-810f6abf8d2d
https://publications-cnrc.canada.ca/fra/voir/objet/?id=a3a34b01-c3a9-4841-8001-810f6abf8d2d
Ser
N2ld
INatlonal Research
Consell national
no.
1508
1+1
Councll Canada
de recherches Canada
c. 2
BLDG
Institute for
lnstitut de
Research in
-
Construction
construction
recherche en
Pore Structure, Permeability
and
Diffusivity as Related to Durability
by R.F. Feldman
Reprinted from
8th International Congress
on the Chemistry of Cement
Rio de Janeiro, Brazil, September 22
-
27, 1986
p. 1-21
(IRC Paper No. 1508)
Price $3.00
NRCC 28687
Ce
document
h t edes
m m s
de pumdabilitt
aux
liqaides,
A
Seau
et
3
l'oxygtne
tffecmtes sur Ic
cimcnt
PortIand pur
et
nu
mQange
de cimenr
Portland
hydratd.
La
@paration des tchantilIons de
mimeque
le mode ophtoirc de l'e@rience
sontvitaux.
L'auteu
discute
des diverses
dthodes u d i s hpour
di5tnmincrla m m
desp o ~ s
ainsi
qut des
travaux.portant
surla
rchtion pcm6abilit6-saucm des
ports.Les
d t h d e s de
mesure
de
diffusion t~
la
fois des ions
et
de
l'oxygtne y
sont @sen&s
etassocih
&
la
protccnon de racier
d'armam
par la matrice de Mton. Les mkthodes
de
conae-diffusion
utilisant
Ic ranplacement de l'eau par divers fluides y sont compan5es. La
pertinence
de
plusieurs techniques concernant I'information diffuste
sur la
rksistance du
Mton
diverse$
formes d'attaque
y est
mise
en
question.
R.F. Feldman, Division of Building Research, National Uesearch Council Canada
POHB STHUCTURE, PERMEABILITY AND UIFeusIvIn AS RELATE? TO DURABILITY
INFLUENCE I)Y. LA STRUCTUIE DES PORES, DE
LA
P E ~ A B I L I T E ET DE LA DIFPUSIVIT~ SURLA
DURABILIT~SUUMARY: This paper discusses permeability measurements m d e by fluids, water and oxygen, on both normal and blended hydrated portland cements. Sample preparation and experimental procedure are vital considerations. Pore structure determinations by several proceduree are discussed together with work relating it to permeability.
Methods of diffusion meanurenents of both ions and oxygen are presented and related to the protection of reinforcing steel by the concrete oatrix. Counter-diffusion mzthods of water replacement by different fluids are compared. The relevance of several techniquee to giving informtion with regard to resistance of concrete to various forms of attack is debated.
R.F. Feldman, Division of Building Besearch, National Remearch Council Canada
INTRODUCTION
Host physical and engineering properties of hardened cement pastes and concrete6 depend on their microstructure. Relationships between coapresaive strength and porosity have been researched greatly and have been adequately reviewed (1). However, several studies on durability have indicated that perwability is probably the most important factor in relation to durability (2-9).
The objective of durability studies is to predict performance in various environments. Some of these environments cause chemical changes in concrete which depend on the permeation of the reacting species (10). The permeation may be in the liquid state. e.g., water containing various ions, or in the gaseous state, such as oxygen or carbon dioxide (11.12). In many instances the transport mechanism may involve diffusion, such as in the case of the penetration of chloride or sulfate ions (7.13.14). but the diffusivity of a body may be related to similar pore structure parameters, as is permeability (11).
Accurate measurement of permeability is important for characterizing materials in nuclear waste isolation and other applications (15-19), and this has led to a renewed effort in understanding permeahillty-pore atructrtre relationships (20-25). Recently, there has been increasing concern over the factors that control the durability of exposed, reinforced concrete structurea (6.8.26-33). Whereas it is recognized that good quality concrete provides adequate protection under normal
circumstances, it is also clear that in certain structures, such as bridges and parking garages, and some in contact with sea water, where chloride ion6 are present in sufficient quantity, new approaches are needed (34-44).
Specifications for concrete have often been based on its 28day strengths, but it is now apparent that quantitative information is required on the effect of a range of parameters such as the waterfcement ratio, the binder content, curing conditions, effect of cement additives and
replacements on the durability and thus permeability of concrete (44-45).
H e w studies have revealed that durability of cement and concrete can be improved by the
incorporation of materials such as pulverized fuel ash (fly-ash), condensed silica fume, rice husk ash, and other pozzolanic materials (34-44). Although it is known that these materials react with calcium hydroxide produced by portland cement hydration, little in known about the changes in structure brought about by this interaction. Similar results with regard to the permeability of mixtures of portland cement and ground granulated blaet furnace slag have been observed (36.46). Some workers have correlated these properties vith the Ca(OH)2CSH interface (37.47) and others vith the apparent increased volume of small pores (36).
Despite several investigations of the effects of supplementary cewnting materials on perwability and the relation of theme to pore mtructure, the factors controlling petaability are mtill not clearly understood (24.36.44). Ihim is partly due
to the methods of obtaining pore-size distribution data. Several investigations have shown that the poreaize distribution curves obtained from mercury intrusion porosimetry (the main mettlod for obtaining poresize distribution data) are subject to details of the specimen preparation prior to the
experiments (48-50).
In recent years, investigators have used different methods of drying specimens before making pore structure measurements (51-55). These nethods include the use of solvents, such as methanol, which has recently been shown to react with CS(OH)~ and possibly CSH (56-58). and isopropanol (50). which appears to show more promise since it is relatively non-reactive. Some of these methods have been compared with direct oven drying, and differences in porosity have been used to develop explanations for permeabilities of both blended and plain
pastes (25,49,53).
Permeability and diffusivity related to durability of mortars and concretes involve more parameters than those involving paste, such as the nature of the aggregate paste bonds and interfaces. Greater difficulties than with pastes may also exist vith cracking on drying, due to the restraint of the aggregate.
The objective of this review is to identify the basic factors relating pore structure to
permeability (and diffusivity) and how they affect durability.
EQUATIONS USED TO CALCULATE PERMEABII.ITY Permeability to Water
The permeability coefficient is most commonly calculated through a modified D'Arcy equation:
vhe re
Q
-
flow through the specimen (m3/s)K
-
permeability of medium (mfs)A
-
cross-sectional area through which flow is occurring (a2)dh
-
hydraulic gradient across the specimen and is dxequal to PIP@
L
-
thickness of the specimen, P is the applied pressure and p is the density.Permeability to Oxygen
Contrary to the case of water, oxygen is compressible and the pressure gradient through the concrete or cement paste pores will be considerable; the .ass flow rate of gas, howver, m a t be the.same through the concrete specimen length when a steady flov ha# k e n achieved.
During experillental determination, gas flow rate, It (cd/s) is measured at an absolute pressure of P bar, with an input presmure of P bar. Thus, in t d DIArcy equation, the flov rate £8 taken at the average pressure (P2
+
P1)/2, while the preseure gradient acroms the mpmcimen of thicknessL
im Pi-
P1. k a u d n g the vimcosity of oxygen to beR.F. Feldman, Division of Building Research. National Research Council Canada
2 . 0 2 x Ns/m2 at 20°C. the permeability roefftclent in rnlcalated as:
4.04.R*P1.1.* 10-16
K m2/m
A * ( P ~
-
p2 ) ( 2 )2 1
Diffusion of Water Vapour
vhere
Df
-
diffurivlty in free Itquidc
-
v0111me tof yore f rartlon T-
turtttaity6
-
constrictivlty, vhich depends both on the porous medium and the diffusion epeciee mien saturated nl~rclmenn of paate or concreteare dried under controlled conditions, the aimple meant~rement of weight loss with time can ive an
S
estimate of the diEfuaion constant "D" (m /a), am shown Ln the following equation for
emall-tomoderate times:
where Ut 1s the wight loae at time, t(s), and W, ir the ultimate wight loss. L(n) is half the
thicknesn of the specimen.
Diffusion of Oxygen Through Saturated Speciuns A slmple diffueion equation based on Pick's law ia usually employed in calculating DN, the average effective dlffuatvity (02/e).
where
QA
-
molecular flov of oxygen (ml/a)L
-
thickness of the rpeci.cn (a)S
-
crosaaectional area (m2)R
-
gar conetant (nJ atn/K -1)-
8.206 x T-
absolute temperatureCI and C2 are the mole fractions of oxygen in the gaaeous phases in contact with opposing facer of the specimen.
Diffuaion of Ionr Through Saturated Cement Pamtea If a specimen of cement paate of thickners "1' and area "A" separatea two compartments where in one an ionic species ham concentration C and in the other a concentration C
,
where C,>h
C,
C, im effectively conetaat ani C2 increases ltnearly, than a quari-oteady state eximta and the activity of chloride ion does not change at a11 pointa in the speciman.vhere
V
-
-
volum of c o q a r t m n t 2time taken for ataady-.tat@ conditions to occur
~ ( c m ~ / # )
-
effective diffumirity of the ion. Thia diffwirity la almo called the intriauic diffumivity (Di) and experiuntally it is w l a r to w r k in t e r m of the average flux per unit area of the medium rather than the liquid. I b w n r ,c6/r2 iu known as the diffusibility of the medium. Mffurion Coefficient of Chloride Ions from Non-Steady State Values
The diffueion coefficients may be calculated for chloride ions by measuring the quantity of chloride penetrated at varioue diatancee within the specimen:
~~.(cm~/a)
-
dif fusion coefficientx
-
distance (cm) from the boundary t-
time (a)dx/dc ie calculated from the slope of the c loride concentration vr. distance plot and
xdc im ca1cuLated from the area under the curve.
SAMPLE PREPARATION FOR MEASUREMENT OF PBRnEMILlTY Water Permeability
b n y reeearcherr have comented upon the length of time taken for steadyatate flow conditione to be eotabliahed when water at high prereure ia forced through the pores of concrete or pastes
(25,35,45,48,49). In fact, in sole instances a reverse flov has k e n observed.
)(any explanations have been offered:
a) continued hydration during the tert; b) calcium hydroxide transport and crystallization in larger porea, causing blocking; c) formation of calcite in porer; d) changing composition of pore solution; e) lack of initial saturation of the concrete; f) effect of vatec prersure on etability of pore structures. b y et a1. questioned whether total mteady rtate vould, in fact, be attained, and defined ateady atate ar the change of petraability coefficient in onm day, being lera than
0.1
*
10-l2 m/m (68). Mort vorkerr have eqhasicrd the need to vacuum maturate rpeciun. before the teat; D.y at a1. (48) reported cerultr in uhich vacuum maturation procedurem were performed over a period of 20 day. without an appreciable change in weight, but the permability test remulted in anincreaae in maqle night of 25 to SOX-of the might of water thich flowed through the coacrete. It is apparent that satimfactory remulta cannot be
obtained on maatorated specimeno, and to maturate m p e c i m r by vacuum im difficult.
C a n r u t be taken to avoid crackm in the concrete due to drying or to have ruptunm occurring at the -1 (the side of the mpecimn uham it la held i n tbm appmratw). t a k a also d l 1 give totally arroluoum ralwm.
R.F. Feldman. Division of Building Research, National Reeearch c o u n c i l Canada
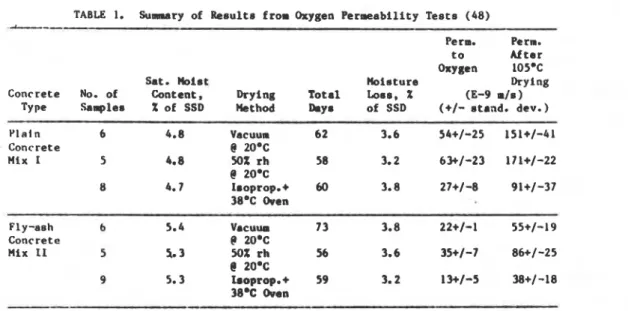
TABLE 1. Suarary of R e s u l t s from Oxygen Permeability T e s t s (48)
- - - - - -
Perm. Perm.
t o A f t e r
Oxygen 105OC
Sat. Moist Moisture h.ying
Concrete No.of Content, Drying T o t a l L o s s , % (E-9 m/s)
Type S a l p l e r % of SSD Method Days of SSD
(+I-
stand. dev.)P l a i n 6 4.8 Vacuum 62 3.6 54+/-25 151+/-41 Concrete @ 20°C Mix I 5 4.8 50% r h 58 3.2 63+/-23 171+/-22
e
20°C 8 4.7 Lroprop.+ 60 3.8 27+/-8 91+/-37 38.C Oven Fly-ash 6 5.4 Vacuum 7 3 3.8 22+/-1 55+/-19 Concrete Q 20°C n i xr r
s
5.3 50% r h M 3.6 35+/-7 86+/-25e
2omc 9 5.3 180ptop.+ 59 3.2 I%/-5 38+/-18 38% Oven Oxygen PermeabilityOxygen permeability w a s u r e m e n t r a r e quick s i n c e r e l i a b l e a t e a d y - s t a t e v a l u e r a r e a t t a i n e d r a p i d l y , and a p p l i e d p r e s s u r e r can be low (11). Hovever, i n o r d e r t o llrake a meaeurenant, t h e specimen m a t he d r i e d , and changes i n t h e pore s t r u c t u r e v i t h r e s p e c t t o s a t u r a t e d c o n d i t i o n s w i l l probably r e s u l t . The r e e u l t r of oxygen p e r m e a b i l i t y experiments performed by Day e t a1. (48) on c o n c r e t e a r e presented i n Table 1. Three procedure8 of d r y i n g were used: ( i ) vacuum d r y i n g a t 20°C; ( i i ) drying a t 53% RH a t 20°C i n a gloved box; ( i i i ) isopropanol s o l v e n t replacement followed by drying i n an oven a t 38OC.
After permeability neasurementr were msde, a 1 1 specimens were oven d r i e d a t 105.C and p a r e e a b i l i t y t o oxygen was again measured. The r e s u l t s i n d i c a t e t h a t t h e drying aethod had a l a r g e i n f l u e n c e , w i t h t h e isopropanol replace.ent y i e l d i n g t h e lowest p e r m e a b i l i t i e a . Oven d r y i n g i n every c a r e increamed t h e ~ > c . r w a h i l l t leu r o n s l d e r a b l y but i t i r p o r r i b l e
t l l n l pnt t ul LIIIH m y be due t o d c r o c r a c k i n g of t h e concrete. The o t h e r d i f f e r e n c e r a r e thought t o be due t o pore s t r u c t u r e c o l l a p s e and c o a r r a n i n g of t h e pores l e a d i n g t o h i g h e r p e n a b i l i t i e r . It ha8 k e n suggested by Day e t a l . (48) and Narrh (25) t h a t t h e isopropanol d r y i n g causes t h e l e a r t d i r r u p t i o n t o t h e pore s t r u c t u r e . Work by P a r r o t , u r i n g methanol and o t h e r organic r o l v a n t r , l e a d r t o t h e m a r conclueion (51).
PORE STRUCTURE DETERHIUTIONS
Permeability h a s been rhovn t o be dapendent n o t s o much upon p o r o a i t y , an arm n o r mechanical p r o p e r t i e s , but on p o r e - o i r e d i r t r i b u t i o n r (20.21)- Three methods a r e m i n l y urad f o r r u u r i n g p o r e l i z e d i s t r i b u t i o n : (A) r r c u r y i n t r u r i o n p o r o s i m t r y ; ( 0 ) g a r a d r o r p t i o n d e r o r p t i o n , u r ~ l l y performed with n i t r o g e n o r butane; (C) a l e c t r o n microecopy and l u g e a l u l y a i r ; thore i r f o u r t h method,
(D)
Yaod'r metal i n t r u r i o a , u h i c hi r a i d l a r
t o A.
A
-
I n o r d e r t o measure t h e p o r e s i z e d i s t r i b u t i o n of a body, t h e rample m a t be d r i e d , andd c r o r t r u c t u r a l p r o p e r t i e s of cement p a r t e a r e h i g h l y dependent upon t h e d e t a i l s of drying. Narrh et a1. (50) have meamured p o r e a i z e
d i r t t i b u t i o n s f o r propan-2-ol-dried specimens and f o r i d e n t i c a l rpecimens d r i e d d i r e c t l y i n t h e oven a t 105'C. The r e r u l t s f o r o r d i n a r y p o r t l a n d cement p a s t a and a f l y a r h bland a r e p r e r e n t a d i n Fig.. 1 and 2, r e r p e c t i v e l y , each f o r t h e two d r y i n g p r o c d u r e s . The t h i c k n e r r of t h e specimens ured i n t h e r e d r y i n g procedure# war n o t reported.
For t h e o r d i n a r y c e r n t , a f t e r two months h y d r a t i o n , t h e r e i s a reduced r l o p e f o r o v e n d r i e d specimens below 80 A pore r a d i u s , but o v e r a l l t h e d i s t r i b u t i o n curves a r e n o t l a r g e l y d i f f e r e n t . However, f o r t h e o n e d r y specimen (Fig. 1) and t h e f l y - a s h blendr (Fig. 2) t h e r e a r e l a r g e d i f f e r e n c e r , a s t h e r e a r e a l s o f o r t h e t h r e r h o l d d i a m e t e r r ,
Rt,
i n Fig. 2. Pore v o l u m r a t p o r e r a d i i e q u a l t o and g r e a t e r than 350 A and two w n t h r h y d r a t i o n a r e not t o o d i f f e r e n t f o r t h e o r d i n a r y cement, but
d i f f e r e n c e r a r e g r a a t e r f o r t h e blended paste; t h i r r u g g e r t r t h a t d r y i v d g h t a f f e c t t h e p e r m e a b i l i t y of blended p a r t a r but not t h a t of o r d i n a r y pastern.
Feld-n (37.M.47) r a r u r e d pore-size
d i r t r i b u t i o n r of o r d i n a r y p o r t l a n d cement, f l y a s h , .lag and s i l i c a fume blendr. Drying i n t h e s e c a r e r war done by e v a c u a t i n g t h i n r p a c i a n r ( 1 u t h i c k ) f o r 15-24 hours b e f o r e h e a t i n g t o 105.C. R e r u l t r are rhoun i n Fig.. 3 and 4. I n t h e c a r e of o r d i n a r y p o r t l a n d cement, i n t r u r i o n c u r v e r a r e of d a c r e a r i n g a l o p e (concave
t o
the pore-oire a x i r ) , while f o r t h e blend8 of r l a g and fly-arh t h e curver k c o m convex t o t h e p o r e - r i t e a x i r ( i n c r e u i n g r l o p e ) between 28 day8 and t h r w m n t h r hydration. I n t h e care of rilica f u n , t h a blendr become concave b t w o e n reven and f o u r t e e n day. h y d r a t i o a (Pig. b ) , depending on t h a c o n c a n t r a t i o n of r i l i c a fume. 'Ihe f l y - m r h .ampler of Narrh and OIy, d r i e d by r o l v o n t r e p l a c e m n t , a l r o r h w ' t h i r f e a t u r e (50). 'Ihirremlt
har b a n r e l a t a d t o t h e C.(OH) content ofthe
body (37) (Fig.k).
Ih. r l o p e08
t h ad i a t r i b u t i o a
curve at
u x i u m i n t r u d e d preOBUte war p l o t t e d a g a i n s t h y d r a t i o n t i r i n Fig. Sb. IheRDINARY PORTLAND CEMENT PASTE
wrc
n
47 350cCUE
-
OVEN-DRIED
80
i
PORE RADIUS,
fi
F I G U R E
1
T V P l CAL MERCURY P O R O S I M E T R Y RESULTS FOR OVEN-DR IED
A N D SOLVENT-REPLACED O R D I N A R Y PORTLAND CEMENT PASTE
*
50 d3
4 0
2
03 0
SOLVENT-REPUED
o MRESHOlORADIUS
!i
5
2 0
-
WL
5
10
35
00
l o 1
1 02
PORE RADIUS,
1
F I G U R E 2
T Y P I C A L M E R C U R Y P O R O S I M E T R Y RESULTS FOR O V E N - D R I E D
A N D SOLVENT-REPLACED BLENDED CEMENT P A S T E
EQUIVALENT PORE DIAMETER, microns 50
20
2 0.2 0.020.002
40
r) 20 3 MONTHS 0 6 MONTHS 10 0 1 ,112 YEARS 0 50 Z 040
-
vl a E*
30
z
-
14 D A Y S*
20
6 28 D A Y Sz
3 MONTHS3
0>
'O 0 5040
v 8 D A Y S 8 14 D A Y S a 28 D A Y S 20 3 MONTHS 0 6 MONTHS 10 0 I 10 100laa
lo om
lnr
t
w
ABSOLUTE PRESSURE, p s i F I G U R E3
CHANGE I N P O R E - S I Z E D l S T R l B U T l O N OF'CEMENT PASTE AND CEMENT BLENDS W I T H H Y D R A T I O N T I M E A t 21°C
R.F. Feldman, Division of Building l s e a r c h , N a t i o m l l s e a r c h Council Ceqada 4 2 l c l .;P b. SILICA fWlE. Wllc + 111
.
0.45 36 30 24 10 12 6 0lo5
lo4
10 lo0PORE DIAAlETER. nm
F I G U R E 4
CHANGE I N P O R E - S I Z E D I S T R I B U T I O N FOR CEMENT P A S T E wlrn AND W l T n o u r S I L I C A FUME
Ca(OH) content is a l s o p l o t t e d agairrst hydration
tia (pig. 5.). It m y be obaerved t h a t where t h e
Ca(OHIZ content is low o r decreasing, t h e s l o p e is high o r increasing. This is f o r the c a s e r of the blended cements.
The e f f e c t of t h e m r c u r y i n t r u s i o n process on t h e m i c r o s t r u c t u r e h a s been i n v e s t i g a t e d (60). Specimens t h a t have been i n t r u d e d with mercury umre
reintruded. This war performed by r e w v i n g t h e r r a u r y by extended d i s t i l l a t i o n . Ih. repeated i n t r u s i o n shoved t h a t , i n
most
sup108 of p l a i n p o r t l a n d cement p a s t e , t h e pore-ire d i s t r i b u t i o n 'did not change u c h but f o r a11 the blends n j o r d i f f e r e n c e s occurred. The r e s u l t shown is Pig. 6 is l change i n c h a r a c t e r of t h e curve from convex t oconcave t o t h e pore d i a r t e r axes, and a n i n t r u e i o n
t h r e r h o l d a t .a m c h higher pore diameter. Thie was explained (60) on t h e b a s i s t h a t t h e hydrated blended c e u n t pore s t r u c t u r e was ~ d up of e r e l a t i v e l y l a r g e , but discontinuous, t h i n r a l l e d pores. During r r r c u r y i n t r u s i o n . t h e s e poree were d i s r u p t e d a t high p r e s s u r e and intruded. Repeat i n t r u s i o n would r e s u l t i n t h e s e pores being f i l l e d a t u c h lower pressures. The h a r s h e r technique of d i r e c t oven d r y i n g might r e s u l t , i n some i n s t a n c e s , i n t h e same damage t h a t occurred during mercury i n t r u s i o n . Thie was r h o m t o be t h e c a s e by Harsh and b y (49); t h e y compared helium p o r o s i t i e s aeasured f o r o r d i n a r y o r blended p o r t l a n d cement p a r t e s d r i e d by d i r e c t oven h e a t i n g and by
prop~n-2-01 t r e a t l e n t . R e s u l t s i n Pig. 7 show t h a t h i g h e r p o r o s i t i e e a r e obtained f o r t h e blends by t h e d i r e c t o v e n d r y i n g technique.
The presence of Ca(OH)2 i n hydrated o r d i n a r y p o r t l a n d cement p a s t e s has been used by Feldman (37) t o e x p l a i n t h e r e l a t i v e c o n t i n u i t y of t h i n pore s t r u c t u r e ae c o q a r e d t o t h a t of blends. The wark of llrrmh a t a l . (50) h a s rhown g r e a t e r s i m i l a r i t y i n their p o r e - a i z e d i s t r i b u t i o n (Figs. 1 and 2) when s o l v e n t o r d i r e c t oven dried.
Roy and Parker (36) prepared cements, Type I1
and 60:40 blend of s l a g with t h e cement, f o r mercury i n t r u a i o n . They d r i e d them by t h r e e d i f f e r e n t methods: f r e e z e d r i e d , vacuum d r i e d a t 80°C f o r 2 hour., and d r i e d with acetone followed by room t e a p e r a t u r e vacuum drying. Samples from t h e t h r e e methods were r e p o r t e d t o show l i t t l e d i f f e r e n c e i n pore s t r u c t u r e s . These a u t h o r s a l s o found t h a t t h e c r i t i c a l radium ( r a d i u s a t maximum dV/dP) of a cement p a s t e may correspond with t h e m i c r o s t r u c t u r e of the m a t e r i a l . In t h e p a s t e s made with a w/c of 0.5 and 0.6, they found t h a t t h e c r i t i c a l r a d i u s may be resolved by microscope. In well-developed g e l s . t h e c r i t i c a l diameter was about t h e d i s t a n c e between t h e f i b e r s observed i n SEII micrographs. Slag-cement p a s t e s were concluded t o have a f i n e r s t r u c t u r e than p o r t l a n d cement p a s t e s from t h e o b s e r v a t i o n of a s m a l l e r c r i t i c a l pore radius.
Nidgley and I l l e t o n (53) d r i e d p a s t e s f o r mercury i n t r u s i o n measurements i n vacuum a t 105OC and f o r e l e c t r o n microecopy by s o l v e n t replacement with e t h e r . Comparisons made of pore r a d i i by t h e s e techniques found s u r p r i a i n g equivalence of pores above 100 A r a d i u s , s u g g e s t i n g t h a t d i f f e r e n t d r y i r y techniques may mainly a f f e c t pores l e r s t h a n 100 A
radius.
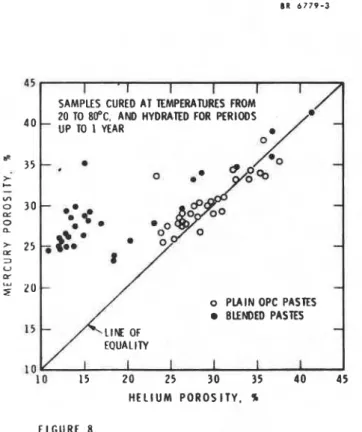
T o t a l p o r o s i t i e s measured by mercury i n t r u s i o n a r e a180 i n d i c a t i v e of t h e m i c r o s t r u c t u r e of hydrated p a r t e s . Am ddicussed above, t h e f i n e pore s t r u c t u r e is s e n s i t i v e t o drying techniques and t h e p r e s s u r e used i n t h e mercury porosimtters. Several workers (47.49.61) have found t h a t t h e p o r o s i t i e s of p l a i n p a r t e s measured by mercury porosimetry and by helium p y c n o n t r y g i v e s i m i l a r valoes. h r s h and b y (49) have found t h a t t h i s is t r u e f o r d i r e c t l y o v e n d r i e d specimens and f o r those which were rolvent-replacmd (propan-2-01) and t h e n oven d r i e d (Pig. 8). However, f o r u t u r e o v e n d r i e d f l y u s h p a s t e s , t h e helium p o r o s i t y is an much ae 102 by volume lers than mercury p o r o s i t y (47.49.61) and f o r s o l v e n t 7 e p l a c e d r p e c i r n s t h i s d i f f e r e n c e is A 8
u c h a s 20% less (48). Other r e s u l t s by l l r r s h 8nd
R.F. Qeldwn. Division of Building Barearch, National Rarearch Council Canada
1 1 1 1 I I 1- 1 1
C t M k N l PAS1$$ W / S 4 U.4) O IA
-
CEMLNI M)rl6 I@ - W l l H 35". EDMONTON FLY ASH IC -WITH 35% BOUNDARY DAM FLY ASH. I D
-
WITH 3%"' OTTAWA GROVND SAND-
f -, D IC -a 1,-
0 IE I TIME. days F I G U R E 5CHANCE I N ( a l Ca10H12 CONTENT AND ( b l SLOPE AT M A X I M U M MERCURY I N T R U S I O N PRESSURE FOR V A R I O U S PORTLAND CEMENT BLENDS
r p e c i a e n r , e r p e c i a l l y f o r blended c e m n t r , a r e lnuch higher than helium p o r o r i t i e r f o r propan-2-01- replaced s p e c i m n r (Fig. 7).
Mnreh end b y (49) conridered t h e m a n u r e of t o t a l p r o r i t y of propmn-Z-ol-tmplaud spa elm^& am r good i n d i c a t o r of r c e a r a i b l e porwity and thum r e l a t e d t o perllaabkliry (25). A p l o t of t h i r p o r o s i t y us. t h e p e m b i l i t y of w a t m r a a t u r a t r d pants s p . c i m n a i a pmmantmd
in
Pig. 9. Dcopite tlw v i d e r c a t t e r . t h e r e apparrm t o b. a c o r r e l a t i o n . B-
Nitrogen a d r o r p t i o n ha. k e n ured u c h i n the p a s t but r y r t e u t i c v a r i a t i o n i n d r y i n g p r o c e d u p r and comparison with a molecule i n v o l v i n g p a r e l y physical i n t e r a c t l o n e have not been previouely done. Lawrence (62,63) ha8 concluded t h a t n i t r o g e n and butane i n t e r a c t i o n s a r e p h y r i c a l i n nature. I n a d d i t i o n , he concluded t h a t hardened p a r t e r , when r a p i d l y d r i e d , c o n t a i n r l i t - 8 h a p . d p o r e r w i t h width8 chat vary i n mire from d c r o t o macro. Averago pore r l i t v i d t h r l i e h a t m e n 2 and 4 M, while t h eh y d r a t i o n product8 approximate t o p l a t y aggregation8 w i t h o v e r a l l average thickneesee between 30 and
1000 nm. Slowly d r i e d p a r t e r have m c h reduced r u r f a c e a r e a r and wider, . o r e rymmetrical porer. Pore e n a l y r i r of t h e m r o p o r a r u r f a c e f o r r a p i d l y d r i e d partem ha8 yimldpd r u r f a c e @ t e a 8 w l l b . 1 ~ BET v a l u e r , i n d i c a t i n g t h a t a c o n r i d e r a b l e f r a c t i o n of t h e o u r f a c e a r e a war contained i n d c r o p o r e r . Evidence a l r o e x i r t r of restricted admorption a t r e l a t i v e p r e r r u r o r above 0.6, i n d i c a t i n g 8 preponderance of s l i t - s h a p e d pore.. b t h . o o l d r y i n g appear8 t o r h i f t the p o r e ~ i z e d i r t r i b u t i o n of r p e c i w c u t o muller r a d i i am c o p a r m d w i t h W r y i n g . Pbre r e r e a r c h
ir
w e d e d on t h e r e l a t i o n e h i p botvaan the change i n t h e micro porer w i t h a g i v e n d r y i n g techniqcu t o t h e change i n t h e u c r o porer.mince
t h e l a t t e r p o r r rare . o r e r e l e v a n t t o
p r o p . r t i e 8 dependent on p e t w a b i l i t y of t h o u t e r i a l .R.P. Peldoan, Mvision of Building Research, National Research Council Canada 0
1st
I N T R U S I O N.
C
l 2nd I N T R U S I O Nd
D E R I V A T I V E E Q U I V A L E N T P O R E D I A M E T E R . D. nm F I G U R E 6 H g I N T R U S I O N A N D D E R I V A T I V E FOR F L Y - A S H A N D C E M E N T B L E N D H Y D R A T E D F O R 2 Y E A R S A T 3 5 O CC
-
A method for large pore analysis has bewdeveloped by Jennings, Parrott and cororkers (54). Samples are dried by solvent replacement and vacuum treatment at room temperature. These samples are then impregnated under vacuum with a low-viscosity epoxy resin; after the resin hardened, the iqregnated ealples were ground flat and polished. They were then etched with 50% hydrochloric acid
+
50% water to yield a resin replica of the iqregnated pores. The polished surface of the r&in replica was viewed with a scanning electron microscope and f ields of aboutlod
m2 werephotographed. These twodimensional representstions of the pore structure were analyzed, ueiag an image analyzer.
Ihc
areas analyzed vere *elected to krepresentative of a larger field of viev. Sow
oubjective decisions were requirrd in deciding where s pore-solid interface should be drawn, but
differences ktveen individuals vere not great. Poraeity maps w r e constructed and some results are illustrated in Table 2. lhe open porosity
deterdaed from microscopy v u estimated by ;xcluding isolated and singlesntrance pores that
wre thought not to be able to cuatribute to flow through the channels io the t w d i a n S i 0 ~ 1 luge. Zhis is extrarly speculative since in three d i m ~ i o l u the situation m y be entirely different.
The impregnated porosity from the microscopy measurement is shown to be similar to large pores .50 no diameter obtained from volumetric
wasurements (difference between total porosity determined by water or methanol and the volume of pores penetrated by butane at a relative pressure of 0.95). However, it appears as if the estimate of the small pores by butane determined on dried samples would be considerably leas than those occupied by water or merhanol from the wet state, since they both occupy interlayer positions. Thus, this mthod of determining pores >SO nm diameter should lead to a coneiderable overestimate. The technique of helium pycnometry would be a better method to determine the volume of larger, continuous pores.
Despite the fact that some of the assumptions in this technique may be difficult to confirm, it does seem to show that the decreaee of larger, continuous pores with degree of hydration is luch -re abrupt than merely the decreaee of large pores
(Table 2).
D
-
Wood '6petal intrusion. Recently, severalworkers have claimed that cement paste has
SAMPLES CURED AT IEMPERARIRES FROM I 1 I I
V)
20 TO 8O"C. AND HYDRATED FOR PERIODS o PUlN OPC PASTES
40
-
UP TO I YEAR a. 00 V)s
. s , , O 0z
3 5 --
P:-
1E . 10-12; 0 O 0 05
10-13-
0 0 > 0 -4-
.
m-
d
1614 5-
nz l 0 . W-
"
lo:";
o..:0 PLAIN OPC PASTES
.
l CURING AT TEMPERATURES8 BLENDED PASTES
lo-1b r
• • FROM 20 TO 80eC PERMEABILITY-
AN0 POROSITY MEASUREMENT• AT AGES UP TO 1 YEAR
1
I I I II
110 15 20 25 30 35 40 45
10 I 5 20 25 30 35 40 45 HELIUM POROSITY. ISOPROPANOL PREPARED. %
HELIUM POROSITY, ISOPROPANOL PREPARED, %
FIGURE 9
FIGURE 7 PLOT OF HELIUM POROSITY v r PERMEABILITY OF
WATER-SATURATED PASTES COMPARISON OF MEASURED HELIUM POROSITIES
FOR DIFFERENT SPECIMEN DRYING METHODS O R 6 7 7 9 - 5
8R 6 7 7 9 - 3
TABLE 2. R e s u l t s of M f f u s i v i t y and Porosity P r o p e r t i e s f o r A l i t e Hydrated st Various Times (54)
--A_--
-Hydration time (days) 1 3 10 28
Degree of hydration 0.22 0.30 0.50 0.83
M f f u s i o n time, e t i (min) 30 34 74 142 SAMPLES CURED AT TEMPERATURES FROM
Miscroscopy r e s u l t s Impregnated p o r o s i t y 0.50 0.42 0.26 0.25 Open p o r o s i t y 0.44 0.38 0.15 0.12 Open pores ( X ) 8 8 9 0 5 8 48 > Volumetric e s t i m a t e s C
-
T o t a l p o r o s i t y 0.61 0.58 0.55 0.49 v,-
Porosity >50 nm wide 0.51 0.44 0.34 0.27-
s p h e r i c a l pores which a c t a s c r i t i c a l f l a w s (64.65). 3u These pores a r e s t a t e d t o be g r e a t e r then 15
urn
andn-
-
a s l a r g e a s LOO0urn.
In a d d i t i o n , i t han been emphasized t h a t t h e s e pores cannot be observed by o PLAIN OPC PASTES mercury i n t r u s i o n becauee e n t r a n c e i n t o t h e s e pores 8 BLENDED PASTES may be l i m i t e d by t h e f a c t t h a t t h e i r e n t r a n c e s a r e-
s m a l l e r than can be e n t e r e d by mercury a t t h e maximum pressure. Wood's metal is a four-conponeat f u s i b l e a l l o y which melts a t 70°C, t h e composition being bismuth 50%, cadmium lo%, t i n 13.3%. and l e a d10 20 25 30 35 40 45 26.7%. It has a c o n t a c t a n g l e of 102O with cement
HELIUM POROSITY, % paste. Rehman (66) w e d dood's metal a e a
poroeimetric f l u i d and a f t e r impregnating a t p r e s a u r e s of 0.92 and 40 MPa (400 and 10.6 nm pore
FIGURE 8 d i a m t e r s , r e s p e c t i v e l y ) and allowed any i n t r u d e d
COMPARISON OF MERCURY AND HELIUM POROSITIES metal t o f r e e z e w i t h i n pores. Rehman (66) examined
FOR SPECIMENS PREPARED BY THE SOLVENT- f r a c t u r e d s u r f a c e s and found t h a t t h e r e was very
REPLACEMENT METHOD l i t t l e i n t r u s i o n of Wood's metal a t a p r e s s u r e of
0.92 W a , even though pores of about 100 um diameter BR 6 7 7 9 - 4 e x i s t e d . At t h e h i g h e r p r e s s u r e , some of t h e s e
R.F. Q e l d ~ n , D i v i s i o n of B u i l d i n g Rsaearch, Nation01 R e a m r c h C o u n c i l ~ d a
Kahnen (66) c o n c l u d e s t h a t these amcropores c o n t r i b u t e s i g n i f i c a n t l y t o t h e t o t a l p o r o s i t y and
nf f e c t p u r e - s i z e d i s t r t b t ~ t i o n . Other work
(47.5Y.60). r n p e r t n l l y on blended cementa. i n d i c a t e d t h a t Home of t h e s e poren do e x l s t and, i n f a c t , d e n s i t y measurements by helium pycnometry c o n f i r m t h l s b u t , on t h e o t h e r hand, on p l a i n p a s t e s w i t h W / C of 0.4 o r h i g h e r , helium and mercury i n t r u s i o n p o r o s i t i e s a r e s i m i l a r and d e n s i t y v a l u e s a r e c o n s i s t e n t . N e v e r t h e l e s s , t h e s e p o r e s , i f p r e s e n t I n s i g n i f i c a n t p r o p o r t i o n , c o u l d e v e n t u a l l y a f f e c t t h e ~ ) e r m e a h i l i t y o f a body i f c o n t i n u i t y between t h e s e and o t h e r l a r g e p o r e s o c c u r r e d due t o c r a c k s formed by d r y i n g o r o t h e r h a r s h t r e a t m e n t . Water a s F l u i d i n P l a i n P a s t e s The d e s i g n o f e q u i p r e n t t o w a e u r e t h e p e r m e a b i l i t y of p a s t e o r c o n c r e t e t o l i q u i d water h a s a long h i s t o r y (16.35). It i s clear t h a t t h e c r i t i c a l element was t h e p r e v e n t i o n of l e a k a g e around t h e specimen. Many workers (18,35,48) cast a n epoxy r l n g around t h e specimen w i t h t h e s e a l .dependent upon t h e epoxy-concrete bond. Other
workers have used a c o n i c a l specimeh w i t h a s i l i c o n e rubber seal (25). b r a r e c e l t l y , h w r e n c e , I b y and c o r o r k e r s have used a s y s t e m whereby a t r a n s v e r s e p r e s s u r e , g r e a t e r t h a n t h a t of t h e p e r m e a t i n g f l u i d , c o u l d be a p p l i e d t o a s e a l i n g nembrane (45,48). With such a syutem, H i l l s found a s t r o n g dependence of p e r m e a b i l i t y on c o n f l n t n g p r e s s u r e on c o n c r e t e (16). T h i s e f f e c t nay be a t t r i b u t e d t o o p e n i n g and c l o s i n g of c r a c k s i n t h e specimen, and i l l u s t r a t e d t h e d i f f i c u l t i e s i n v o l v e d w i t h t h i s measurement. Harsh (25) measured w a t e r p e r m e a b i l i t y of o r d i n a r y p o r t l a n d c e r r n t p a s t e p r e p a r e d a t w/c of 0.67 and c u r e d at 20, 35. 50 and 65.C. Ihe 2 8 d a y and h o n t h specimens c u r e d a t 20% y i e l d e d v a l u e r of a p p r o x i m a t e l y 1.5 x 1 0 - l ~ m / s , whereas t h e 35, 5 0
and 6S°C p a s t e s had v a l u e s of b e t w e n 4 and
9 x 10-12 RIB. Hooton (35). u s i n g a
s u l f a t e - r e s i s t i n g cement mixed a t w/c of 0.36, found d e c r e a s e s from 340 x 10-Id t o 1 x 10'~' m/s d u r i n g h y d r a t i o n from 7 t o 180 days. Coto and Roy (23) compared p a s t e s c u r e d f o r 28 d a y s a t d i f f e r e n t w/c's end t e m p e r a t u r e s . At 27.C and w/c of 0.35 t h e p e r m e a b i l i t y was 0.6 x 10-l6 m / s , w h i l e a t 60°C, a t t h e same w/c, t h e p e r m e a b i l i t y was I x 10'12 m/s. Nyatne and I l l s t o n (21.22) and k h t a and I k n w h a n (20) a l s o meaeured p e r m e a b i l i t i e s of o r d i n a r y p o r t l a n d c e a e n t a t s e v e r a l wfc and c u r i n g t i n e ; v a l u e s o b t a i n e d by Nyame and I l l s t o n (21.22) v a r i e d from 5 x l o 4 m / s a t 2 d a y s h y d r a t i o n and w/c of 1.0 t o 5 x 10-15 m/a a t 10 months h y d r a t i o n and w/c of 0.23 (Pig. 10). Ihese a u t h o r s found a r e l a t i v e l y s m a l l but s i g n i f i c a n t d e c r e a s e i n p e r m e a b i l i t y w i t h i n c r e a s i n g p r e s s u r e a p p l i e d d u r i n g t h e experiment and a t t r i b u t e d t h i s t o some c l o g g i n g of p o r e s by t h e i n c r e s s e of p r e s s u r e i n a d d i t i o n t o c o n t i n u e d h y d r a t i o n . Water as F l u i d i n Blended P a s t e a
Marah (25) and c o r o r k e r a (49) meaaured p e r m e a b i l i t i e s of p a s t e s where 30% of cement w u r e p l a c e d w i t h f l y - a a h . S i g n i f i c a n t d e c r e a a a a
i n
p e r m e a b i l i t y were observed. Hornvor, a t a
" 1 2 3 7 14 28 10 20 d a y s months T I M E OF H Y D R A T I O N F I G U R E 10 EFFECTS OF H Y D R A T I O N O N P E R M E A B l L t T Y OF H A R D E N E D C E M E N T P A S T E S OF D I F F E R E N T W I C R A T I O S t e m p e r a t u r e of 20°C, d e c r e a s e s i n p e r m e a b i l i t y r e l a t i v e t o o r d i n a r y cement were n o t obaerved u n t i l a f t e r 28 daya h y d r a t i o n ; p e r n c a b i l i t i e a f a l l from 7 x 10-l2 m/a a t 28 d a y r t o 6 x 10'15 m / s a f t e r 3 months h y d r a t i o n . S i m i l a r r e d u e t i o n e i n
p e r m e a b i l i t y were obaerved a t 3s0C between 7 and 28
day6 h y d r a t i o n , v h i l e a t 50 and 65OC t h e ' b l e n d e d p a s t e s a c h i e v e d low p e r m e a b i l i t y by 7 daye. Hooton (35) o b s e r v e d d e c r e a s e d p e r m e a b i l i t y w i t h a i l i c a fume a d d i t i o n and bnmohan and M h t a ( 2 ) obaerved similar t r e n d s w i t h a d d i t i o n s s u c h as rice husk a s h , f l y - a s h and b l a a t f u r n a c e s l a g . Roy and P a r k e r (36) a l a o found p e r r r a b i l i t l e a of slag-cement b l e n d p a a t e a t o be c o n s i d e r a b l y l o v e r t h a n o r d i n a r y p o r t l a n d remnt p a a t e a . S . q l e s were nude a t w/c'a of 0.30, 0.40, 0.50 and 0 . 6 0 and were c u r e d a t 27, 45, 60 and 90% f o r p e r i o d s from 14 t o 28 dayr. P e r m e a b i l i t i e s i n c r e a s e d w i t h c u r i n g t e m p e r a t u r e and
w/c
r a t i o .h t m e a b i l i t i e a of blended cement c o n c r e t e s wre w a a u r e d tj h w r e n c e (44). He found t h a t mature
c o n e r e t e a c o n t a i n i n g 35% r e p l a c e m e n t of cement by fly-ah were two t o f i v e tiwa lees permeable t h a n o r d i n a r y p o r t l a n d cewnt o r a l a g % e m n t c o n c r e t e when co.parad a t t h e a a m 2 8 d a y cube strength. n i l l a (16) a l s o o b o e r w d t h a t p e r m e a b i l i t y of
concrete
w.8 a l g n i f i c a n t l y d e c r e a s e d by p a r t i a l a u b r t i t u t i a n of p o r t l a n d cement w i t h f l y - u h .12
R.F. Feldman, Division of Building Reeearch, National Rerearch Council Canada
Oxygen a s Fluid 10-l4
I
1
1
Car flows u c h more r e a d i l y through c o n c r e t e rnnples than doe. water, and a o prerrurem a r e u c h l o v e r and tlme nrpded t o a t t a i n r t e a d y r t a t e is u c h lenn. However, t o mearure p e r m a b i l i t i e a . t h o specimen m a t be d r i e d . Reeultr i n Table 1 (48) nhow t h a t f o r a c o n c r e t e of v / c of 0.47. t h e r t h o d r
of drying a r e very i a p o r t a n t . ' h e e e have been lo-*
-
discussed i n s e c t i o n 3b. Crube and Laurence (67)reported i n i t i a l r e e u l t r of p e r m e a b i l i t y r a r u r e m n t N
f r o n a c o o p e r a t i v e program undertaken by raven E
l a b o r a t o r i e s (8). Oxyeen p e r r a b i l i t i e r l a y i n t h e
<
range 0.001 t o 30 x 1 0 - l ~ m2. S p e c i w n r w r e d r i e d c-
between 50 and 651 RH. Conridering mixer c o n t a l n i n g a ld16
-.
/'
-
J
300 kg ce*cnt/m3. i t vae found t h a t i n c r e a e i n g c u r i n g time from one t o t h r e e day. reduced
permeability by a n average of f i v e time, while a
z
=
Wf u r t h e r i n c r e a s e i n c u r i n g t i r t o 28 days r e e u l t e d o
i n a f u r t h e r d e c r e a s e i n p e r m e a b i l i t y by n i n e t i m e .
.%..
Change i n w/c r a t i o , on t h e o t h e r hand. hae a l e r e e rthough s t i l l important e f f e c t ; reducing v / c r a t i o by
-
0.05Other reduced works obtained p e r m e a b i l i t y r i d l a r 1.6 r e s u l t o timer on (68). average. h v r e n c e
-/Ia=
( 4 4 ) extended h i s study t o blended cementa and found
t h a t c o n c r e t e epeci.ene t h a t had 352 r e p l a c e u n t of 0 1 D A Y cement v i t h fly-ash and t h a t v e r e d r i e d t o 55% IUI 28 D A Y S
had p e r m e a b i l i t i e s about f i v e t i m e l e a n than
ordinary cement o r elag. S i m i l a r r e r u l t r had
I
1
previouely been reported f o r undriod r p e c i v n r u r i n g 10'"
water r r f l u i d (2,251. lo4
16'
10-I lodDIFFUSIVITY, n2
White e t a l . (19) and M c h n i e l (4) mearured
permeability of mortars uaing n i t r o g e n gae. F I G U R E 11
Specimens v e r e p r e - d r i e d t o conetant w i g h t a t R E L A T I O N BETWEEN O X Y G E N D I F F U S I V I T Y A N D 110.C. Uaing t h e Klinkenberg c o r r e c t i o n , they found P E R M E A B I L I T Y
t h e t values obtained by v o t e r and t h o r e by n i t r o g e n
were comparsble. &a b 7 7 v - 7
A major problem of c o n c r e t e d u r a b i l i t y is t h e c o r r o s i o n of reinforcement s t e e l . The r t e e l i n normally i n a p r e a i v e s t a t e , but t h i n may change am a r e s u l t of t h e d i f f u s i o n of carbon d i o x i d e o r c h l o r i d e ions. Rates of wbeequent rteel c o r r o r i o n may depend on t h e r a t e of d i f f u r i o n of oxygen t o t h e s t e e l , e s well am d i f f u e i o n of v a r i o u r ionr.
Knowledge of t h e parameters c o n t r o l l i n g diffumion r a t e s a r e thue very important.
Oxygen Dif f ueion
R e l a t i v e l y f e v s t u d i e s of g a r d i f f u r i o n i n c o n c r e t e have been c a r r i e d o u t . Some e a r l i e r , comprehensive work wen c a r r i e d o u t by Zagar (69) and Darr and Ludwig (70). and o t h e r 6 have provided I ~ l t c r e ~ t i n g d e t n (71). Darr e t e l . (70) c a l c u l a t e d a n 01-dinary ef f a c t i v e d i f f u r i o n c o n e t a n t , while I*lvrc~nrr ( I I ) cal c t ~ l a t e d a n average d i f f u s i o n c o n s t a n t . which LH an approximation of t h e value given by Darr and Ludwig (70). Crube and Lavrence
( 7 2 ) extended previous work by u s i n g a wider range of mixre and d i f f e r e n t c u r i n g c o n d i t i o n s (20 houre nr 28 days) followed by d r y i n g t o 55% RH. They observed t h a t once a c e r t a i n degree of d r y i n g had been achieved. t h e d i f f u r i v i t y of oxygen r o u i n e d reasonably u n a f f e c t e d by further drying. ' h u t t i h u shown that d i f f u e i v i t y i e very e o n r i t i v e t o d r y i n g humidity (12). In h i e vork, Larrence a h w e d t h a t oxygen d i f f u e i v i t y and p e r m a b i l i t y are r e l a t e d
l i n e a r l y (Fig. 11) (11). I& f e l t t h a t t h t ~ r e l a t i o n e h i p m y be u r e f u l i n developing a r i m p l e r technique f o r t e e t i n g d u r a b i l i t y . He a180 rhovcd t h a t t h e d i f f u e i o n r a t e of COP i n f u l l y carbonated c o n c r e t e can be emtimated from t h e d i f f u a i o n r a t e of oxygsft.
C a l c u l a t i o n r by Lawrence (11) u s i n g e x p r e a r i o n r d e r i v e d by Crank (73) f o r d i f f u r i o n p l u s r e a c t i o n have h e n given (10). A l i n e a r i n c r e a r e of carbonation depth8 with r q u a r e r o o t of tin i r
p r e d i c t e d and obrerved.
As d i r c u r r e d above, t h e r e l a t i v e humidity o r t h e degree of f i l l i n g of t h e pore r t r u c t u r e w i t h i n t h e c o n c r e t e v i t h aqueoue r o l u t i o n w i l l g r e a t l y a f f e c t d i f f u e i o n proceree6 and t h i s e t a t e i r one t h a t o f t e n e x i r t s . Da Wind (74) hae euggerted t h a t f o r a given c o n c r e t e , t h e r a t i o of oxygen
d i f f u e i v i t y i n t h e d r i e d e t a t e t o t h a t i n t h e s a t u r a t e d e t a t e should approximate t o t h o r a t i o of t h e bulk d i f f u e i v i t i e e of oxygen i n a i r and l i q u i d water and vould be about lo6. This c o n t e n t i o n i n v o l v e r many a a e u q t i o n r . Previour measurenentr (11.12) of d i f f u e i v i t y on c o n c r e t e d r i e d t o W 6 0 X RH v e r e about
lo-@
m 2 / r , t h u r oxygen d i f f u s i v i t i e e through s a t u r a t e d concrete. rhould be of t h e o r d e r of 10'14 m2/r. F u r t h e r work by Lavrence (75) on a v a r i e t y of e a t u r a t e d c o n c r e t e r obtained v a l u e r t h e m a j o r i t y of v h i c h f e l l b a t w e n 1 and 8 x 10-l3$18.
Theoe a r e conoiderably a u l l e r than t h o s e r e p o r t e d by ' h m t t i (12) and r u g g e r t t h a t c o n c r e t e o f f e r s a a r c h m r e r e r i r t a n t barrier t o t h e p u r a g e of OXygen
H.F. veldman, Division of Building Basearch, Nmtional P.rerrch Council Canada
than does liquid water. This is contrary t o the concluatons of s o w other workers (70).
haut~mtng Lawrence's measuremnta a r e accurate
( I S ) , ;I e t n t e of a c t i v e corrosion is possible f o r
rc-lnt~brctng s t e e l i n good q u a l i t y , maturated 1:0)11crete since too l i t t l e oxygen w i l l penetrate t o form n p n ~ n l v a t t n g layer on anodic areas. However, n low rrlte of corrosion due t o the low r a t e of oxygel~ reduction a t the cathode a l r o r e s u l t r , but the overnll e f f e c t i r beneficial.
Ionic Diffusion
'Ihe f a c t o r s c o n t r o l l i n g corrosion of
retnforcing s t e e l i n concrete have been investigated
hy sev.?ral workers recently (6-8.12.2633). Hoarson e t a l . (8) have concluded t h a t the i p o r t a n t factor. ;)re: (1) the CL- and OH' content of the pore s n l r ~ t i o n ; ( i i ) t h e porosity and pore-sire d t s t r l h ~ t i o n of the matrix material; ( i i i ) the r e e l a t i v i t y of the matrix. The corrosion r o t a , i n addition t o the above factor., i r controlled by t h e a v n t l n b l l i t y of oxygen.
Work by Page e t a1. (19,27,28) and by Treadaway c t , , I . (29). using paster with micro-oilicr
teplncement. measured corrosion behaviour of embedded s t e e l . Corrosion p o t e n t i a l and l i n e a r polnrization measurements were made; pore rolutiona were analyzed. This work showed t h a t l e w l r of cnrronion protection cannot be accurately predicted ~ o l e l y on the basis of data r e l a t e d t o the pore u o l u t i o ~ ~ cnmposition. auch a s the r a t i o r of f r e e chloride t o hydroxyl ion concentrationo. This ir
ntgnificant because a number of i n v e s t i g a t i o n r
( 1 2 . 7 6 ) i n simulated concrete pore r o l u t i o n r cnntainlng diaaolved oxygen and varioue l e v e l r of chloride and hydroxyl ions has suggertrd t h a t a c r i t i c a l r a t i o f o r CL'/OP of 0.6 e x i r t r . Knowledge of the r e l a t i v e d i f f u r i v i t i e r of chloride and o t h e r
lons i n the various matrices is a l r o required. Hnnswon e t a1. (8) have ohom t h a t the W. quantity of CL' added t o ordinary portland cement w r t a r ha. d i f f e r e n t e f f e c t s when added a8 CaCL
,
NmCL, o r KC*.The corrorion r a t e is strongly dapenient on t h e cation, CaCL having a ouch l o r e d e l e t e r i o u r . e f f e c t than e i t h e r
k
CL or KCL. There vorkerr ( 8 ) obmervedH coarrrning of the pore r t r u c t u r e i n tha 100 t o
LOO0 nm range by w r c u r y p o r o r i m t r y m d ruggerted t h a t the increased s e v e r i t y of W L 2 addition
i r
due t o a combination of t h e pore a t r u c t u r e change and decreaee i n the pH, but i r not r e l a t e d t o the concentration of CL' remaining i n t h e pore rolution. Preace (33) showed t h a t a cemnt-mlag mortar witha
s i g n i f i c a n t l y f i n e r pore r t r u c t u r e than n o r u l cemnt mortar had an e l e c t r i c a l r e r i r t i v i t y t e n times higher and chloride d i f f u s i v i t y ten times lower than the n o r u l c e w n t wrtrr; i n addition. the i n i t i a t i o n of chloride-induced corrooion and t h e subeaquent r a t e r of corrorion -re both lawor i r l
theslag ~ r t a r .
There have been only a fow r t u d i e r of the d i f f u r i o n of ionr through water-maturated cement reported i n t h e l i t e r a t u r e . Koado e t a1. -7) measured e f f e c t i v e d i f f u r i o n c o a f f i c i e n t r by placing s o l u t i o n s a t u r a t e d with C.(OH)2 on'one r i d e of
r
epeciwn i n a d i f f u a i o n c e l l , a d a chloride solution r a t u r a t e d with C.(OHI2 on the other. Coefficientr ware determined f o r ion. vhrn UCL, NaCL. KC;, b C L 2 and U&L2 ware rued. Mxed
rolutione were a l s o used. F i f t y t o one hundred hours were required before a steady-state condition was attained. ' h e diffueion of C T ion combined with divalent c a t i o n war g r e a t e r than t h a t combined with mnovalent cation8 and i t decreased i n the order of DCL' (HgCL )
>
DCL- (CaCL2)>
DCL' (LiCL) 3DCL' (KCL) > DCL' ( L C L ) . These values varied from 18.3 t o 6.25 x 10-l2 m 2 / r , while values f o r No+, I(+
and ~ i + were 1.70. 2.95 and 1.70 x 10-l2 m 2 / r ,
rerpectively. Kondo e t a l . (77) pointed out t h a t t h e w l c n t paste behaved M an electro-positive, red-permeable membrane. The f a c t t h a t CaCL2 and &CL2 r e a c t physically and chemically with the cement paste probably has some influence on t h e high c o e f f i c i e n t values f o r CL- when these s a l t s a r e ured. Urhiyama e t 01. (78) investigated the d i f f u r i o n r a t e s of SO; and found t h a t CL- d i f f u s e s 10 t o 100 timer f a s t e r than t h a t of SO; but t h e value of the l a t t e r WM dependent on the pH of the
r o l u t i o n and appeared t o be affected by e t t r i n g i t e formation. Coto a t a l . (79) measured the e f f e c t i v e d i f f u r i o n c o e f f i c i e n t from t h e penetration depth of CL- and obtained a value of 3.4 x 1 0 - l ~ m2/r. It war ruggerted t h a t c o r r e c t i o n s should be prde f o r t h e chloride reaction with aluminate phases and a value of 230 r 10-l2 m 2 / r war given. These vorkerr r l r o pointed out t h a t the diffueion of C&' ion war f a r t e r with Mg* than i n NO+. Midgley and I l l o t o n (80) a l r o measured the chloride d i f f u s i o n
c o e f f i c i e n t r by meaouring chloride concentrations i n rpecimene t h a t were roaked i n NaCL s o l u t i o n f o r s i x monthr o r one year. Beeultr were obtained f o r w/c r a t i o r of 0.23. 0.47 and 0.71. Values a f t e r one year were 0.56. 0.85 and 2.17 x 10-l2 m 2 / s , rerpectively. Coto and Roy (80) used a diffueion c e l l t o measure t h e d i f f u r i o n c o e f f i c i e n t of both Na+ and CL' or a function of temperature and w/c r a t i o . Daionixed water was i n the o t h e r s i d e of t h e c e l l . They found no pronounced e f f e c t s of w/c r a t i o o r curing c o n d i t i o m , and they found t h a t t h e CL- had a higher d i f f u s i o n coef f i c i e n t than Na+, about 6.9 x 10-l2 m 2 / s and 1.5 x 10-l2 m2/a, rerpectively, a t a w/c r a t i o of 0.4 f o r a Type I cement a t 27.C.
Ilowver, a c t i v a t i o n energies f o r the d i f f u s i o n of N.+ and C4' were 20 and 12 kcal -1-1, rerpectively. Bnkker (46) a l a o manured c o e f f i c i e n t s of d i f f u s i o n of N.+ and K+ ione. These were e a s u r e d f o r a w r t a r of w/c r a t i o of 0.5 and sand/cem~nt ra t i o of 2.0, uring both ordinary portland and b l r r t furnacr r l a g c a w n t . Valuer a f t e r 14 dry8 f o r t h e ordinary portland c e m n t a r e r i d l a r t o those obtained by Goto and Roy (81); valuer f o r portland and b l a s t furnace m r t a r s f o r
N.+
ion8 a f t e r 14 days hydrationwere
2.38 and 0.10 x 10'12 m2/r, rerpectively. Page e t a l . (7,13,82,83) mearured e f f e c t i v e d i f f u r i v i t i e r of CL' uring IU NaCL i n s a t u r a t e d C.(0H)2 and r a t u r a t e d Ca(Otl)2 i n t h e o t h e rc o q a r t m n t . lhay mearured values a s a function of t m e r a t u r e and v / c r a t i o . An Arrhenius p l o t f o r ordinary portland c e r n t of d i f f e r e n t w/c r a t i o s i r
r h o m i n Fig. 12 (82). Rorultr of Collepardi (84) a r e r l r o plotted. Activation m e r g i e r a t w/c of 0.4 a r e LO k c a l / w l e i n a s i m i l a r range an Coto and
Roy (81). Vrluer of e f f e c t i v e d i f f u r i v i t i e s of CL' i n o t h r r c a m t paster w r e r l r o meaaurad by Page e t 01. (83) and a r e ohown i n Tmble 3. There authorr (57,13,82,83) w a r u r e d porn d i r t r i b u t i o ~ by orrcury i n t m r i o n and ware not able t o oboerve r i g n i f i c a n t d i f f e n n c a r i n t h e m i c r o r t ~ c t u r e ; p o r o r i t i a r w r e , i n f a c t , l a r g e r f o r the fly-arh parte. J h a v e r , mince recant d a t a (60) have r h o m t h a t porQ of