Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Analytical Chemistry, 2019-08-29
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC : https://nrc-publications.canada.ca/eng/view/object/?id=eaa20df3-9323-4f48-92e2-47d6d1795f5d https://publications-cnrc.canada.ca/fra/voir/objet/?id=eaa20df3-9323-4f48-92e2-47d6d1795f5d
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1021/acs.analchem.9b02615
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Selective gas chromatography mass spectrometry method for
ultratrace detection of selenocyanate
Selective Gas Chromatography Mass Spectrometry Method for
Ultratrace Detection of Selenocyanate
Enea Pagliano,
*
Kelly L. LeBlanc, and Zoltán Mester
National Research Council Canada, 1200 Montreal Road, Ottawa, Ontario K1A 0R6, Canada
*
S Supporting InformationABSTRACT: The recent interest in the determination of selenocyanate (SeCN−) in wastewater systems has spurred the development of
analytical methods for its determination at the ultratrace level. Since most of the current procedures require complex and costly instrumental configurations, we have developed a simple and rapid gas chromatog-raphy tandem mass spectrometry (GC/MS/MS) method able to detect SeCN−in water samples with a LOD of 0.1 ng/g Se. A 1 mL volume of
aqueous sample was buffered with sodium bicarbonate and treated with triethyloxonium tetrafluoroborate for conversion of the analyte into volatile EtSeCN. The derivatization yield was higher than 90%, and it
could tolerate concentrations of chloride or sulfate up to 2%. The EtSeCN was extracted in chloroform and could be detected in electron ionization and also in negative chemical ionization mode with a further gain in signal-to-noise ratio by a factor of 2. The method was applied for the analysis of natural waters with quantitation of SeCN− in the low ng/g region. The Se13C15N−
internal standard could be used for isotope dilution. Quantitative spike recoveries of 1 ng/g Se were obtained from seawater and river water, and 1 ng/g Se could be quantified within a standard uncertainty of 15%.
A
lthough selenium (Se) is an essential nutrient for human health, it is typically considered a toxin in freshwater aquatic environments where, when present at concentrations slightly above background levels, it can have devastating effects due to its teratogenicity to predatory fish and waterfowl.1 As we continue to improve our understanding of the bio-geochemical cycling of Se, allowable limits laid out by various regulatory bodies have become increasingly strict.2The United States Environmental Protection Agency (USEPA), for example, has updated their water quality criterion for Se to include (fish) tissue-based limits and overall lower allowable aquatic Se concentrations.3As the toxicological effects of Se are dependent on its chemical form, speciation analysis is relevant for understanding the health of an aquatic system and to plan and implement remediation strategies. Selenocyanate (SeCN−) is one of the
Se compounds emerging as an analyte of interest in wastewater analysis. SeCN− is formed through the combination of
elemental Se0and free cyanide (CN−) and has been observed
in various types of waters in concentrations that span a broad range. Trace levels of SeCN−(0.2 ppb or 1% of total dissolved
Se) have been found in a Se-contaminated eutrophic river system,4 and this species accounts for up to 10% of the Se in
some flue gas desulfurization waters5and is the major form of Se in wastewaters from certain gold mining operations.6
To comply with environmental regulations, industries must treat Se-containing wastewater before it can be released back into an aquatic system. The efficiency of a removal procedure is largely species dependent. For example, traditional precipitation methods for Se removal are not effective for SeCN−.7,8 On the other hand, methods specific for direct
conversion of SeCN−to Se0are not applicable for Se(IV) and
Se(VI).9 Furthermore, novel biological treatment systems for Se seem likely to be inefficient for SeCN−removal from waters,
on the basis of current knowledge.10−12
In the context of different requirements for wastewater treatment, there is a need for simple analytical methods capable of measuring SeCN− at low concentrations and with
high degrees of accuracy. Classical methods used for SeCN−
quantitation are not without shortcomings. For example, selective sequential hydride generation (with atomic absorb-ance or fluorescence detection) involves the use of operation-ally defined fractions, Se(IV), Se(VI), and reduced/organic Se,13−15the last of which includes SeCN−as well as a variety of
other species.16Additionally, the presence of certain transition metals (as would occur in many industrial effluents) has been shown to cause interferences in hydride generation through their action as catalyst in the decomposition of the analyte hydrides.17 Therefore, separation methods have been
devel-oped involving anion exchange chromatography (AEC). SeCN− is retained strongly on AEC columns, meaning that
careful column and eluent selection is vital to ensure optimal chromatographic peak shape. Conductivity detection is commonly employed with AEC but is fairly insensitive for SeCN− determination.18 Inductively coupled plasma mass
spectrometry (ICPMS) is a much more sensitive detection system and can be coupled to AEC either directly19 or via a
Received: June 7, 2019
Accepted: August 29, 2019
Published: August 29, 2019
pubs.acs.org/ac Cite This:Anal. Chem. XXXX, XXX, XXX−XXX
Published XXXX by the American Chemical
Society A DOI:10.1021/acs.analchem.9b02615
hydride generation system using online pre-reduction.6,16 However, detection of Se by ICPMS is penalized by polyatomic interference and requires the use of collision cell technology to reach the environmental concentrations. This type of specialized instrumentation can be prohibitively expensive for applications such as the monitoring of SeCN−
in industrial wastewaters.
In this study, we developed a rapid and simple method for the quantitation of SeCN− in water by gas chromatography
mass spectrometry (GC/MS). The novel approach is based on a simple aqueous derivatization of SeCN− into EtSeCN by
triethyloxonium tetrafluoroborate,20,21which can be quantified by GC/MS at the sub part-per-billion level.
■
EXPERIMENTAL SECTIONReagents and Materials. Potassium selenocyanate (KSeCN, 99%) and isotopically enriched potassium seleno-cyanate (97% KSe13C15N with 99%13C and 98%15N), sodium
bicarbonate (NaHCO3, 99.5%), anydrous sodium sulfate
(Na2SO4, 99%), chloroform (CHCl3, GC grade), hexane
(C6H14, HPLC grade), acetonitrile (MeCN, HPLC grade), and
triethyloxonium tetrafluoroborate (Et3OBF4, 97%) were
purchased from Sigma-Aldrich. The purity of KSeCN and KSe13C15N was verified by ICPMS against the NIST 3149
primary standard. As reported in Section S2, the purity for KSeCN and KSe13C15N was 77.9% and 78.3%, respectively.
Ultrapure water was generated in-house with a Thermo Scientific GenPure UV xCAD plus system (18.2 MΩ cm at 25 °C). A solution of Et3OBF4 in MeCN was prepared by
dissolving 5 g of reagent in 5 mL of MeCN precooled at −20 °C. This solution was stable for more than 1 month when kept at −20 °C.
Tap water was obtained locally in Ottawa; river water was sampled from the Rideau River on April 13, 2019, and coastal seawater was sampled from the Atlantic Ocean (Halifax, February 23, 2017). These matrices were filtered at 0.45 μm and spiked with SeCN− (1 ng/g Se) before analysis. Other
water samples were prepared in-house following the cultivation of Chlorella vulgaris in a growing medium containing 10−25 μg/L Se(VI) as described inSection S4. The Chlorella vulgaris could convert in situ some Se(VI) into SeCN−, which could be
detected by gas chromatography tandem mass spectrometry (GC/MS/MS).
Sample Preparation.A single-step aqueous derivatization was employed to convert SeCN− into volatile EtSeCN. A 60
mg aliquot of NaHCO3 was transferred in a 4 mL glass vial
with 1 mL of aqueous sample (or standard) at 4 °C and 50 μL of an Et3OBF4/MeCN solution. The reaction was completed
within 1 h at room temperature. Into the same vial, 0.5 mL of CHCl3was added, and the mixture was manually shaken for a
few seconds. This simple liquid/liquid extraction allowed the migration of EtSeCN into CHCl3. The organic layer was
transferred with a glass pipet into a 4 mL vial containing 250− 300 mg of anhydrous Na2SO4and analyzed by GC/MS.
GC/MS Instruments. The EtSeCN derivative could be measured by GC/MS in both electron ionization (EI) and negative chemical ionization (NCI) mode. The EI experiments were performed using an Agilent 7000 triple quadrupole GC/ MS in single ion monitoring (SIM, m/z 134.96 and 136.96) and in multiple reaction monitoring (MRM, m/z transitions 134.96 to 106.93 and 136.96 to 108.93) modes. NCI detection was obtained on a Hewlett-Packard 5973 single quadrupole GC/MS in SIM mode (m/z 105.9 and 107.9). The separation was carried out on a DB-5.625 column (30 m length, 0.250 mm, 0.25 μm) using standard instrumental settings as reported inSection S1.
Safety Considerations. Triethyloxonium tetrafluorobo-rate is a strong ethylating reagent, which was handled in a fume hood wearing nitrile gloves.21 Leftovers of the Et3OBF4/
MeCN solution were hydrolyzed before disposal.
■
RESULTS AND DISCUSSIONDerivatization and Extraction. The reactivity between Et3OBF4and SeCN−was studied in aqueous medium at room
temperature. As reported for other inorganic anions,20,21 this chemistry yields the analyte ethyl-derivative (EtSeCN), which is a molecule suitable for gas chromatographic analysis. It was observed that EtSeCN is not sufficiently volatile for static headspace sampling and required liquid−liquid extraction into an organic solvent. Derivatization conditions and extraction were optimized.
The hydrolysis of Et3OBF4 releases H+(aq), turning the
sample pH acidic. In these conditions, SeCN−is not stable and
degrades to Se0.4,20For this reason, the samples were treated
with NaHCO3 before derivatization. The analytical response
was monitored as a function of NaHCO3. When NaHCO3was
omitted, a 25% decrease in the signal was observed along with an RSD of 36% on the peak area of three replicates (Figure 1). When 1 mL of standard was treated with 20 to 100 mg of NaHCO3 and 100 μL of Et3OBF4/MeCN, the signal was
stable with an RSD below 5%.
The response was further optimized by varying the amount of Et3OBF4/MeCN solution used for derivatization. As
reported inFigure 1, no sharp variations in the signal intensity were observed when 1 mL of standard with 60 mg of NaHCO3
was treated with 20 to 120 μL of Et3OBF4/MeCN and no Figure 1.Derivatization of 1 mL of SeCN−standard 50 ng/g Se: variations of the analytical signal. Left: effect of NaHCO
3 with 100 μL of
Et3OBF4/MeCN solution (180 min reaction time). Center: effect of Et3OBF4/MeCN solution with 60 mg of NaHCO3(180 min reaction time).
Right: Effect of the derivatization time with 60 mg of NaHCO3and 50 μL of Et3OBF4/MeCN.
Analytical Chemistry Technical Note
DOI:10.1021/acs.analchem.9b02615 Anal. Chem. XXXX, XXX, XXX−XXX
signal was recorded if the reagent was omitted. A 50 μL volume of Et3OBF4/MeCN was adequate to ensure a large
excess of reagent for complete derivatization.
The effect of the derivatization time on the signal response was also investigated.Figure 1shows that, from 30 to 180 min, there were no significant variations in the signal intensity. This optimization suggests that 1 mL of sample should be treated with 60 mg of NaHCO3and 50 μL of Et3OBF4/MeCN and
left to stand for 1 h at room temperature.
After derivatization, the EtSeCN was extracted in 0.5 mL of organic solvent. Although the volume of extracting solvent could be further reduced, a 0.5 mL volume ensured simple liquid transfers by common glass pipettes. Initially, the extraction was performed in hexane; then, it was noticed that chloroform allowed for a five times better recovery (Figure S4).
The method efficiency was also evaluated. The derivatization procedure was applied to a 1 mL volume of SeCN−standard
(750 ng/g Se). After chloroform extraction of the EtSeCN derivative, the aqueous phase was diluted and analyzed for residual Se by ICPMS as reported in Section S2. Less than 6.5% of the total initial Se was found in the aqueous phase,
demonstrating that the overall efficiency of the derivatization/ extraction procedure is higher than 90%.
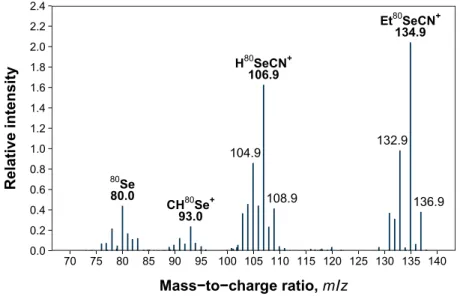
GC/MS Detection. The EtSeCN was suitable for GC/MS analysis. The single quadrupole EI mass spectrum of this molecule shows similarities with the EtSCN spectrum22 (Figure 2). The molecular ion at m/z 134.9 is the most intense signal (Et80SeCN+) followed by its fragment at m/z
106.9 (H80SeCN+). As expected, the triple quadrupole product
ion mass spectrum from m/z 134.9 is dominated by m/z 106.9 (Figure S2). For GC/MS/MS acquisitions, the optimal collision energy for this transition was 5 eV. The EtSeCN could also be detected in single quadruple NCI mode. In the EtSeCN NCI mass spectrum, the only detected ion cluster was the one related to the SeCN−ion (Figure S3).
In all acquisition modes, SeCN−could be detected below 1
ng/g Se. A 0.3 ng/g Se standard generated a chromatographic peak with a signal-to-noise ratio (S/N) of 13.5 (EI MRM, m/z transition 134.9 to 106.9, Figure 3a). 0.3 ng/g Se was established as the conservative LOQ and corresponded to a LOD of 0.1 ng/g Se. The loss in sensitivity observed in single quadrupole EI SIM mode (m/z 134.9) was minimal: 0.3 ng/g Se was detected with a S/N of 6.5 (Figure 3b). The NCI SIM
Figure 2.EI GC/MS mass spectrum of the EtSeCN derivative generated by an aqueous reaction between Et3OBF4and SeCN−. EI GC/MS/MS
and NCI GC/MS spectra are reported inFigures S2 and S3.
Figure 3.Determination of SeCN−at the detection limit. 0.3 ng/g Se was detected by (a) EI GC/MS/MS on m/z transition from 134.96 to 106.93
(S/N = 13.5), (b) EI GC/MS on m/z 134.96 (S/N = 6.5), (c) NCI GC/MS on m/z 105.9 (S/N = 23.8), and (d) IC ICP-DRC-MS on m/z 77.9 (S/N = 23).
DOI:10.1021/acs.analchem.9b02615 Anal. Chem. XXXX, XXX, XXX−XXX
detection was more sensitive than EI MRM, and for the standard at 0.3 ng/g Se, a S/N of 23.8 was observed (Figure 3c). Most notably, the disposition of the EtSeCN derivative to generate negative ions may open the avenue for electron capture detection (ECD) for inorganic and organic selenocya-nates, the latter of which are becoming increasingly popular in research into chemopreventive agents.23,24
The performance of the GC/MS methods was compared to an ion-chromatography procedure with dynamic reaction cell inductively coupled plasma mass spectrometry (IC ICP-DRC-MS, described inSection S3). 0.3 ng/g Se could be detected by IC ICP-DRC-MS with a S/N of 23 (Figure 3d), in line with GC/MS results. However, the IC ICP-DRC-MS method required a large-volume injection (500 μL) and used H2in the
dynamic reaction cell in order to quench polyatomic interference. With a more modern ICPMS/MS instrument, like the Agilent 8800, Se detection in H2mode on the Se+ >
Se+transition only produced a gain in sensitivity by a factor of
2.5 with respect to the ICP-DRC-MS. The novel GC/MS method could reach a similar performance using a more economical and simpler instrumental platform. Furthermore, the absolute detection for the GC/MS method was more than 2 orders of magnitude better than the IC ICP-DRC-MS (1 μL injected in the GC/MS vs 500 μL injected in the IC ICP-DRC-MS).
The GC/MS method was proven to be linear from 0.3 to 100 ng/g Se (Figure S6), and the repeatability injection RSD for a standard at 50 ng/g Se was 3% for n = 5 (Figure S5). Finally, the derivative eluted within only 5.3 min, and there was no deterioration or bleed of the analytical column after 500 injections.
Application to Natural Waters.The method was applied for the quantitation of SeCN−in tap water, river water, and
seawater. The blank matrices were filtered and spiked with 1 ng/g Se before analysis. The samples were then analyzed by GC/MS/MS using both external calibration without internal standards and isotope dilution (ID) quantitation.25The same
samples were also analyzed by the IC ICP-DRC-MS control method, and all results are presented in Table 1. No SeCN−
was detected in tap water, and the IC ICP-DRC-MS experiments (Figure S9) demonstrated that the interaction of SeCN−with residual oxidants present in tap water resulted in a
complete conversion of the analyte into Se(IV). Such an effect was not observed in the river water and seawater where
quantitative recoveries of SeCN− were obtained by ID GC/
MS/MS. Quantitation of 1 ng/g Se was obtained within a standard uncertainty of 15%. The isotope dilution quantitation was compared to an external calibration without internal standard. In the case of seawater, no differences between the two calibration strategies were observed. This result demon-strated that a high-saline matrix did not influence the method performance. On the other hand, for the river water sample, the results by external calibration GC/MS/MS were under-estimated by 30% with respect to the gravimetric data. This matrix effect was compensated when quantitation was performed by ID GC/MS/MS using Se13C15N− as internal
standard.
Finally, the method was employed to detect the conversion of Se(VI) into SeCN− in the growing media of Chlorella
vulgaris algae. Starting from 10 to 25 ng/g Se(VI), the algae promoted formation of SeCN− in the amount of 0.35−0.50
ng/g (Table 1). This value is consistent with the concentration range reported in other studies using similar conditions.4
Wastewater and Matrix Interference. The determina-tion of SeCN− is mostly performed for quality control of
wastewater generated by gold mining, petroleum, and coal industries. Such effluents are notoriously rich in sulfate whose concentration can exceed 2%.26Methods based on ICPMS are penalized by sulfate interference, and ion chromatography does not efficiently separate a large excess of SO42− from SeCN−
causing shifts in the retention times. The effect of sulfate on the GC/MS/MS method was studied. The derivatization of 6 ng/g Se was carried out in a solution with Na2SO4. The sulfate
did not interfere significantly with the yield of derivatization/ extraction (Figure S7), and no perturbation on the GC/MS/ MS chromatogram was observed. With 2% sulfate, the matrix suppression was only 5%. This result further confirms that the GC/MS/MS method can efficiently handle samples with a high salt content. A wastewater sample from a gold mine facility was also analyzed. While no incurred SeCN−was found,
we obtained a quantitative spike recovery without observing suppression from the wastewater matrix. Although the Et3OBF4 can ethylate other anions/nucleophiles typically
present in water samples, no reduction of derivatization yield was observed in seawater and wastewater media where a large excess of alkylable substances is present. Such an observation confirms that ethylation of SeCN− is kinetically favored and
tolerates conditions that may be detrimental to other methods Table 1. Quantitation of SeCN−in Natural Waters by GC/MS/MS (External Calibration and Isotope Dilution) and by IC
ICP-DRC-MSa
matrix SeCN added GC/MS/MS ID GC/MS/MS IC ICP-DRC-MS tap water 1.229 ± 0.001 <LOD <LOD <LOD tap water 1.229 ± 0.001 <LOD <LOD <LOD tap water 1.229 ± 0.001 <LOD <LOD <LOD river water 1.188 ± 0.001 0.81 ± 0.14 1.49 ± 0.31 1.04 ± 0.04 river water 1.188 ± 0.001 0.78 ± 0.13 1.44 ± 0.27 1.04 ± 0.04 river water 1.188 ± 0.001 0.78 ± 0.14 1.45 ± 0.21 1.08 ± 0.04 seawater 1.239 ± 0.001 1.33 ± 0.17 1.55 ± 0.18 1.21 ± 0.05 seawater 1.239 ± 0.001 1.48 ± 0.15 1.28 ± 0.18 1.24 ± 0.05 seawater 1.239 ± 0.001 1.35 ± 0.16 1.30 ± 0.19 1.27 ± 0.05 algae water n/a 0.38 ± 0.15 n/a n/a algae water n/a 0.36 ± 0.15 n/a n/a algae water n/a 0.49 ± 0.15 n/a n/a algae water n/a 0.49 ± 0.15 n/a n/a
aResults are reported as ng/g Se, and the standard uncertainty was evaluated by error propagation.
Analytical Chemistry Technical Note
DOI:10.1021/acs.analchem.9b02615 Anal. Chem. XXXX, XXX, XXX−XXX
like the ones base on liquid chromatography and hydride generation.
■
CONCLUSIONA selective method for the determination of selenocyanate in natural waters was developed and validated. Simple aqueous chemistry allowed for quantitative conversion of SeCN−into
volatile EtSeCN, which could be detected by GC/MS with a LOD of 0.1 ng/g Se starting from 1 mL of aqueous sample. The novel procedure makes use of a standard instrumental setup available in most laboratories and allows for high sample throughput. These methodological qualities make our method a valid alternative for SeCN− quantitation with respect to
current procedures, which are mostly based on costly instrumental hybrids. The method is minimally affected by high-saline matrices and could be used in conjunction with isotope dilution for robust quantitation and for metrological applications. This study was meant to demonstrate the proof-of-concept of this novel analytical approach within the simplest sample preparation design. In this vein, the application of standard preconcentration and microextraction techniques, with higher sample volumes, will further improve an already competitive detection power and may avoid the use of organic solvent for extraction of the EtSeCN derivative.
■
ASSOCIATED CONTENT*
S Supporting InformationThe Supporting Information is available free of charge on the
ACS Publications website at DOI: 10.1021/acs.anal-chem.9b02615.
Method optimization and experimental details (PDF)
■
AUTHOR INFORMATION Corresponding Author *E-mail:enea.pagliano@nrc-cnrc.gc.ca. ORCID Enea Pagliano: 0000-0001-9755-8737 Kelly L. LeBlanc: 0000-0001-6544-5850 Zoltán Mester: 0000-0002-2377-2615 NotesThe authors declare no competing financial interest.
■
ACKNOWLEDGMENTSMitchell Bordash and Paramee Kumkrong are greatly acknowl-edged for contributions in sample preparation and for the donation of wastewater samples.
■
REFERENCES(1) Janz, D. M.; DeForest, D. K.; Brooks, M. L.; Chapman, P. M.; Gilron, G.; Hoff, D.; Hopkins, W. A.; McIntyre, D. O.; Mebane, C. A.; Palace, V. P.; Skorupa, J. P.; Wayland, M. Ecological Assessment of Selenium in the Aquatic Environment; CRC Press, Taylor & Francis Group, 2010; Chapter 6, pp 141−231.
(2) Kumkrong, P.; LeBlanc, K. L.; Mercier, P. H. J.; Mester, Z. Sci. Total Environ. 2018, 640−641, 1611−1634.
(3) U.S. EPA Aquatic Life Ambient Water Quality Criterion for Selenium − Freshwater; EPA 822-R-16-006; Office of Water; Office of Science and Technology; United States Environmental Protection Agency: Washington, D.C., 2016.
(4) LeBlanc, K. L.; Smith, M. S.; Wallschläger, D. Environ. Sci. Technol. 2012, 46, 5867−5875.
(5) Petrov, P. K.; Charters, J. W.; Wallschläger, D. Environ. Sci. Technol. 2012, 46, 1716−1723.
(6) Wallschläger, D.; Bloom, N. S. J. Anal. At. Spectrom. 2001, 16, 1322−1328.
(7) Balistrieri, L. S.; Chao, T. Geochim. Cosmochim. Acta 1990, 54, 739−751.
(8) Meng, X.; Bang, S.; Korfiatis, G. P. Water Res. 2002, 36, 3867− 3873.
(9) Vohra, M. S. Fresenius Environ. Bull. 2015, 24, 1108−1118. (10) Ye, Z. H.; Lin, Z.-Q.; Whiting, S. N.; de Souza, M. P.; Terry, N. Chemosphere 2003, 52, 1571−1579.
(11) Simmons, D. B. D.; Wallschläger, D. Environ. Sci. Technol. 2011, 45, 2165−2171.
(12) Tan, L. C.; Nancharaiah, Y. V.; van Hullebusch, E. D.; Lens, P. N. L. Biotechnol. Adv. 2016, 34, 886−907.
(13) LeBlanc, K. L.; Kumkrong, P.; Mercier, P. H. J.; Mester, Z. Sci. Total Environ. 2018, 640−641, 1635−1651.
(14) Chen, Y.-W.; Zhou, M.-D.; Tong, J.; Belzile, N. Anal. Chim. Acta 2005, 545, 142−148.
(15) Cutter, G. A. Science 1982, 217, 829−831.
(16) Wallschläger, D.; London, J. J. Anal. At. Spectrom. 2004, 19, 1119−1127.
(17) Dedina, J.; Tsalev, D. L. Hydride Generation Atomic Absorption Spectrometry; John Wiley & Sons: New York, 1995.
(18) Xu, S.; Zheng, M.; Zhang, X.; Zhang, J.; Lee, Y.-I. Microchem. J. 2012, 101, 70−74.
(19) Wallschläger, D.; Roehl, R. J. Anal. At. Spectrom. 2001, 16, 922−925.
(20) D’Ulivo, A.; Pagliano, E.; Onor, M.; Pitzalis, E.; Zamboni, R. Anal. Chem. 2009, 81, 6399−6406.
(21) Pagliano, E.; Campanella, B.; D’Ulivo, A.; Mester, Z. Anal. Chim. Acta 2018, 1025, 12−40.
(22) Ammazzini, S.; Onor, M.; Pagliano, E.; Mester, Z.; Campanella, B.; Pitzalis, E.; Bramanti, E.; D’Ulivo, A. J. Chromatogr. A 2015, 1400, 124−130.
(23) Ghose, A.; Fleming, J.; El-Bayoumy, K.; Harrison, P. R. Cancer Res. 2001, 61, 7479−7487.
(24) Sharma, A. K.; Sharma, A.; Desai, D.; Madhunapantula, S. V.; Huh, S. J.; Robertson, G. P.; Amin, S. J. Med. Chem. 2008, 51, 7820− 7826.
(25) Pagliano, E.; Mester, Z.; Meija, J. Anal. Bioanal. Chem. 2013, 405, 2879−2887.
(26) Gitari, W. M.; Petrik, L. F.; Key, D. L.; Okujeni, C. Int. J. Environ. Sci. Technol. 2010, 7, 519−534.
DOI:10.1021/acs.analchem.9b02615 Anal. Chem. XXXX, XXX, XXX−XXX